Abstract
An intraluminal thrombus (ILT) forms in the majority of abdominal aortic aneurysms (AAAs). While the ILT has traditionally been perceived as a byproduct of aneurysmal disease, the mechanical environment within the ILT may contribute to the degeneration of the aortic wall by affecting biological events of cells embedded within the ILT. In this study, the drained secant modulus (E5 ∼ modulus at 5% strain) of ILT specimens (luminal, medial, and abluminal) procured from elective open repair was measured and compared using unconfined compression. Five groups of fibrin-based thrombus mimics were also synthesized by mixing various combinations of fibrinogen, thrombin, and calcium. Drained secant moduli were compared to determine the effect of the components' concentrations on mimic stiffness. The stiffness of the mimics was also compared to the native ILT. Preliminary data on the water content of the ILT layers and mimics was measured. It was found that the abluminal layer (E5 = 19.3 kPa) is stiffer than the medial (2.49 kPa) and luminal (1.54 kPa) layers, both of which are statistically similar. E5 of the mimics (0.63, 0.22, 0.23, 0.87, and 2.54 kPa) is dependent on the concentration of all three components: E5 decreases with a decrease in fibrinogen (60 to 20 and 20 to 15 mg/ml) and a decrease in thrombin (3 to 0.3 units/ml), and E5 increases with a decrease in calcium (0.1 to 0.01 M). E5 from two of the mimics were not statistically different than the medial and luminal layers of ILT. A thrombus mimic with similar biochemical components, structure, and mechanical properties as native ILT would provide an appropriate test medium for AAA mechanobiology studies.
Keywords: aneurysm, intraluminal thrombus, AAA, compressive modulus, fibrinogen
Introduction
Abdominal aortic aneurysms (AAAs) affect a significant portion of the population in developed countries, and the incidence is believed to be increasing as life expectancy increases. AAAs are present in 4% to 8% of men over 60 and 1% to 3% of women over 60 [1]. The long time course of the disease results in the formation of an intraluminal thrombus (ILT) in most AAAs [2]. Some investigators have discussed the mechanical role of the ILT, with this tissue primarily acting as a mechanical shield, thus decreasing the stresses acting on the AAA wall [3, 4].
The ILT has traditionally been perceived as a byproduct of aneurysmal disease. However, some investigators have hypothesized that the ILT may play a more active role in the pathophysiology of AAA development [2]. The mechanical environment within the ILT and the AAA wall (e.g., matrix strain) changes as the aortic wall dilates. We hypothesize that this mechanical environment may affect the biological activity of cells embedded within the ILT and therefore contribute to the degeneration of the aortic wall. The mechanical properties of the ILT have been investigated by a number of investigators. Many of these experimental investigations have been based on the tensile behavior of the ILT [5-7]. These tests have primarily been on the luminal and medial layers of the ILT, as gripping the abluminal layer is often times impossible. The hydraulic permeability of the ILT was investigated by Adolph et al. [8], however no distinction was made between how these values vary through the thickness of the thrombus. To our knowledge, little information exists in the literature regarding the compressive mechanical behavior of the ILT, especially for the abluminal layer. Such information may be important not only in identifying how stress distributions are distributed within the ILT and AAA wall, but also may be helpful in future studies on ILT mechanobiology.
Development of a thrombus mimic with similar components, structure, and mechanical properties as native ILT would facilitate a more controlled environment for AAA mechanobiology studies. Such a mimic would be ideal for in vitro testing as the number of native ILT specimens available for testing will likely continue to decrease with the increasing popularity of endovascular repair. Fibrin-based constructs present an attractive option for the mimic because they can be constructed from blood proteins and components present in the clotting cascade, and thus have a similar chemical structure as native ILT. The mechanical properties of these fibrin-based constructs (e.g., elastic modulus, permeability, and strength) can also be manipulated by varying the concentrations of fibrinogen [9-12], thrombin [12], factor XIII [9, 13], fibronectin [14, 15], platelets [11], and calcium [9, 11-13, 16, 17].
The purpose of this study was threefold: first, to measure the drained secant modulus at 5% strain (E5) of the different layers of ILT taken from open repair; second, to measure how the concentrations of fibrinogen, thrombin, and calcium affect E5 in fibrin based mimics; and third, to determine the apparent differences in properties between the developed mimic and native ILT. In addition, preliminary information on the water content and microstructure of the ILT and mimics was assessed. Such information will serve as a baseline dataset for future studies investigating the pressure distribution within the ILT and how the ILT may play a role in aneurysmal mechano-pathophysiology.
Methods
ILT Specimens
ILT specimens were procured from the elective open AAA repairs of six patients at the University Medical Center, Tucson, AZ in accordance with the Human Subjects Protection Program at the University of Arizona. General information about the patients is shown in Table 1. Specimens were placed in an isotonic phosphate buffered solution (PBS) immediately after extraction from the aorta and stored at 4°C until testing. All tests were performed within 48 hours of the surgery. Gentle physical manipulation was used to separate the ILT specimens into three layers based on relative position and color: abluminal, medial, and luminal (Figure 1).
Table 1.
Information about AAA patients from which ILT specimens were obtained. *Smoker: Yes indicates patient previously or currently smokes. **Comorbidities: coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), hypercholesterol (HC), hyperlipidemia (HL), and hypertension (HT).
| Patient | Age | Gender | AAA Diameter (cm) | *Smoker | **Comorbidities |
|---|---|---|---|---|---|
| 1 | 76 | M | 6 | Yes | HC, HT |
| 2 | 72 | M | > 7 | Yes | CAD, HT |
| 3 | 70 | F | 4.9 | Yes | COPD, HL |
| 4 | 72 | M | 6 | Yes | HT |
| 5 | 60 | M | 5.3 | Yes | CAD, HT |
| 6 | 85 | M | 5.7 | Unknown |
Figure 1.
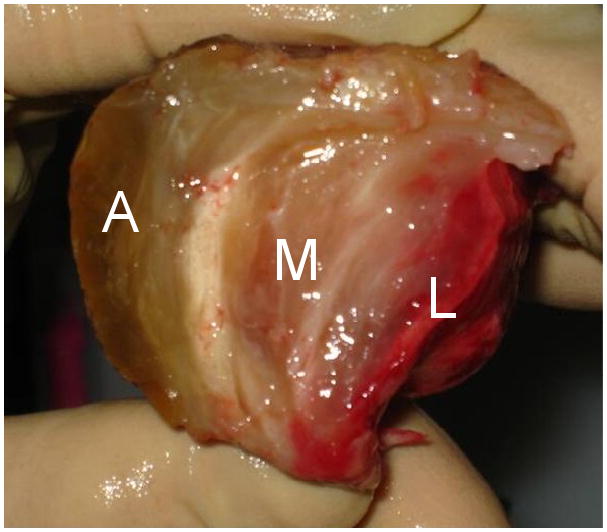
ILT specimen with abluminal (A), medial (M), and luminal (L) layers.
Thrombus Mimics
Bovine fibrinogen (Sigma, F8630) was dissolved in Hank's Balanced Salt Solution (HBSS, Mediatech, 21-020-CV) to concentrations of 40 and 80 mg/ml, bovine thrombin (Sigma, T6200) was dissolved in water to a concentration of 100 units/ml, and calcium chloride was dissolved in HBSS to yield a calcium rich HBSS solution (1 M calcium). Five groups of mimics were constructed by mixing the solutions, additional HBSS, and water with various ratios in well plates to yield the final concentrations listed in Table 2. Mixed solutions were set in an incubator (37°C, 10% CO2) overnight to gelate. The concentrations in M1 were chosen as a baseline, and in groups M2, M3, and M4, the concentration of one component (fibrinogen, thrombin, or calcium) was varied from the baseline to determine the effect of that component's concentration on E5. In M5, the concentrations of thrombin and calcium that yielded the stiffest mimics were used and the fibrinogen concentration was increased to 60 mg/ml in attempt to create an even stiffer mimic.
Table 2.
Final component concentrations of thrombus mimics with number of samples tested (n).
| Group | Fibrinogen (mg/ml) | Thrombin (NIH units/ml) | Calcium (M) |
|---|---|---|---|
| M1 (n=9) | 20 | 3.0 | 0.10 |
| M2 (n=5) | 15 | 3.0 | 0.10 |
| M3 (n=5) | 20 | 0.3 | 0.10 |
| M4 (n=8) | 20 | 3.0 | 0.01 |
| M5 (n=5) | 60 | 3.0 | 0.01 |
Drained Secant Modulus
A circular biopunch (8 mm diameter) was used to cut cylindrically shaped samples from each layer of the ILT and the mimic well plates. The thickness of each sample was measured by taking the average of five measurements using a caliper. There were 14, 15, and 18 ILT samples isolated from the abluminal, medial and luminal layers, respectively (thickness = 3.0±0.8, 4.5±0.9, and 4.3±1.2 mm, respectively) and 32 mimic samples (thickness = 3.9±0.6 mm). Mimics were set in a bath of PBS for at least one hour to allow fluid saturation.
Unconfined-compression stress-relaxation tests were performed on the samples in a bath of PBS at 37°C using a dynamic mechanical analyzer (Perkin Elmer, Pyris Diamond DMA) which had a spatial resolution of 1 μm and a load resolution of 0.5 μN (a schematic of unconfined-compression testing is shown in Figure 2). Stress relaxation tests were performed by applying and holding a 5% compressive strain for 20 minutes while measuring the corresponding decrease in compressive load over time. Strains of 10% and 15% were also subsequently applied for 20 minutes each. Since the samples did not equilibrate after 20 minutes, the equilibrium load was determined by fitting the data to a 3-term sum of exponentials equation:
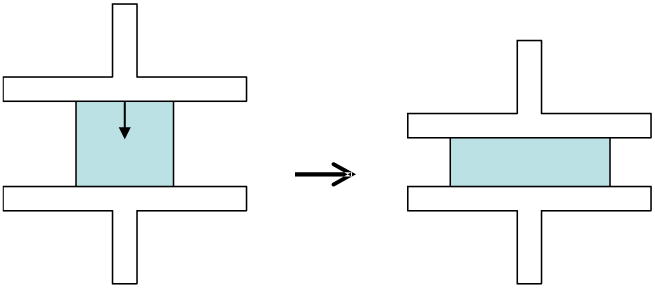
Figure 2.
Schematic of an unconfined-compression test. The undeformed sample is on the left and the deformed sample is on the right. Step compressive strains of 5, 10, and 15% were applied to each sample, and the time dependent load relaxation was recorded.
| (1) |
where L is instantaneous load, t is time, A is equilibrium load, and Bi and Ci are constants. A, Bi, and Ci were fit to the data using Marquardt-Levenberg nonlinear regression technique within the commercially available software package SigmaStat. Drained equilibrium stress (T∞) was calculated as the equilibrium load divided by the initial cross sectional area. E5 was calculated as T∞ at a 5% compressive strain divided by -0.05.
Water Content and SEM
As a step towards our long term goal, preliminary water content data and SEM images were recorded for the ILT and mimics. The initial water content of all layers of the ILT from one patient and from each of the mimics was measured. The volume of samples saturated with PBS was obtained by measuring the volume of fluid they displaced in a graduated cylinder. The PBS-saturated samples were then weighed, set in a biosafety cabinet to dry, and weighed 16 hours later. Initial water volume was calculated as the change in mass multiplied by the density of water. The reported value of water content is given as the water volume divided by the PBS-saturated sample volume. Water content of M5 was not measured.
M1 was prepared for SEM imaging as follows. The sample was fixed in gluteraldehyde, formaldehyde, and 0.015% ruthenium red. It was then fixed in osmium tetraoxide. The sample was dehydrated in ethanol and quenched in liquid nitrogen, which broke the sample into pieces to allow us to image an inside area instead of the surface. It was dried in CO2 to its critical point, gold coated, and then SEM imaged (Hitachi S-3400N).
Statistical Analysis
Pair-wise comparisons of the drained secant moduli of the ILT layers and the mimics were performed with a Mann-Whitney rank sum test using the software program SigmaStat. A non-parametric test was used due to non-normal distributions of the sample groups. Significant difference was determined by a p-value < 0.05.
Results
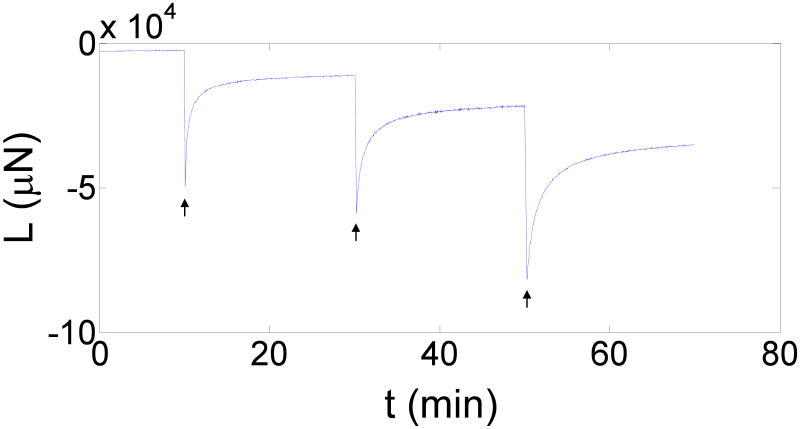
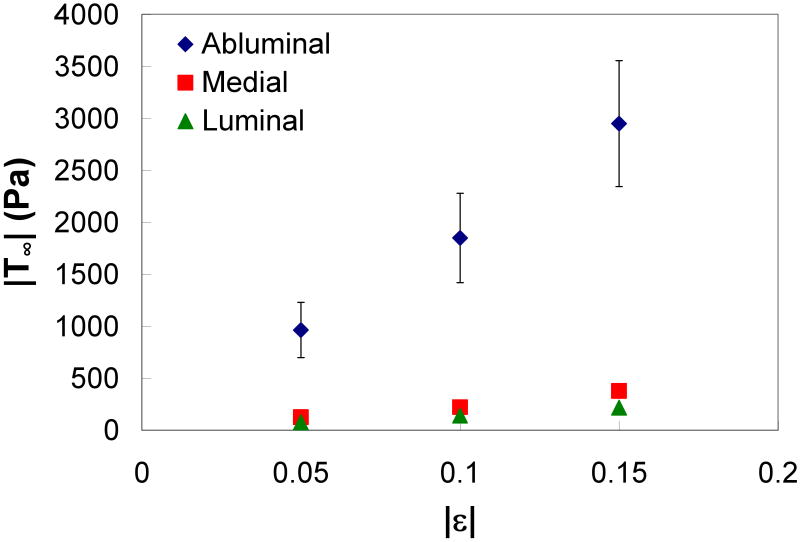
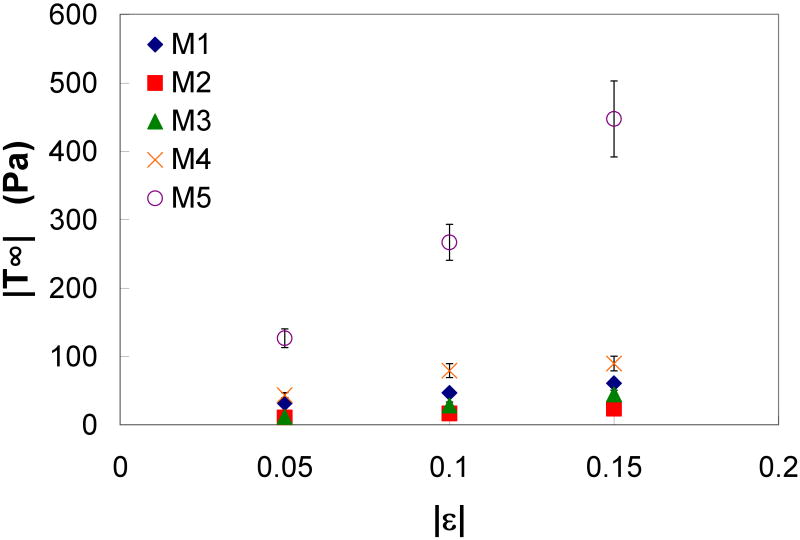
A representative ILT sample is shown in Figure 1. Figure 3 shows the load response over time for a typical unconfined-compression stress-relaxation test. T∞ at each strain of the three ILT layers is shown in Figure 4. The luminal and medial layers appear to have a similar stiffness, while the abluminal layer had a much stiffer response. Figure 5 shows the T∞ at each strain of the mimics. M5, which has the highest fibrinogen concentration and the lowest calcium concentration, appears to be the stiffest, while M2, which has the lowest fibrinogen concentration, was the most compliant.
Figure 3.
Load (L) output over time (t) for an unconfined-compression stress-relaxation test. Arrows indicate where strains of -0.05, -0.10, and -0.15 were applied.
Figure 4.
Equilibrium stress (T∞) versus strain (ε) of the ILT layers (with standard error bars).
Figure 5.
Equilibrium stress (T∞) versus strain (ε) of the thrombus mimics (with standard error bars).
Mean and standard error values of E5 for the ILT layers and mimics are reported in Table 3 along with statistical comparisons between each mimic and ILT group. The abluminal layer was found to have a significantly greater E5 than all other samples. There was no significant difference between the E5 for the medial and luminal layers. The E5 of M4 was not significantly different than the luminal layer, and the E5 of M5 was not significantly different than the medial layer. All other mimics were significantly less stiff than the ILT layers. The differences between the baseline mimic (M1) and the other mimic groups (M2, M3, M4, and M5) indicate that the stiffness of the mimic can be manipulated by adjusting the concentration of fibrinogen, thrombin, or calcium. For example, a decrease in fibrinogen (20 to 15 mg/ml) or thrombin (3.0 to 0.3 units/ml) decreases E5, while a decrease in calcium (0.10 to 0.01 M) increases E5.
Table 3.
Compressive drained secant moduli (E5) with standard error (SE) and statistical difference of E5 between abluminal (Abl), medial (Med), and luminal (Lum) layers and mimic groups (M1, M2, M3, M4, M5). Yes signifies a statistical difference between the two samples (p < 0.05).
| E5 (±SE) | Abl | Med | Lum | M1 | M2 | M3 | M4 | M5 | |
|---|---|---|---|---|---|---|---|---|---|
| Pa | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 | p<0.05 | |
| Abl | 19300 (5300) | -- | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Med | 2490 (540) | -- | No | Yes | Yes | Yes | Yes | No | |
| Lum | 1540 (220) | -- | Yes | Yes | Yes | No | Yes | ||
| M1 | 635 (81) | -- | Yes | Yes | Yes | Yes | |||
| M2 | 216 (50) | -- | No | Yes | Yes | ||||
| M3 | 232 (47) | -- | Yes | Yes | |||||
| M4 | 868 (74) | -- | Yes | ||||||
| M5 | 2540 (270) | -- |
Water content for the ILT layers and mimics is reported in Table 4. Water content of the ILT ranged from 0.77 to 0.83 and that of the mimics ranged from 0.93 to 0.96. Figure 6 is an SEM image taken of M1. While there appears to be significant degree of nonhomogeneity in mimic structure, the fibrinous network of the clot is qualitatively similar to results reported by Wang et al. for native ILT [5].
Table 4.
Water content of ILT layers and mimics.
| Sample | Water Content |
|---|---|
| Abluminal | 0.83 |
| Medial | 0.77 |
| Luminal | 0.78 |
| M1 | 0.93 |
| M2 | 0.95 |
| M3 | 0.94 |
| M4 | 0.96 |
Figure 6.
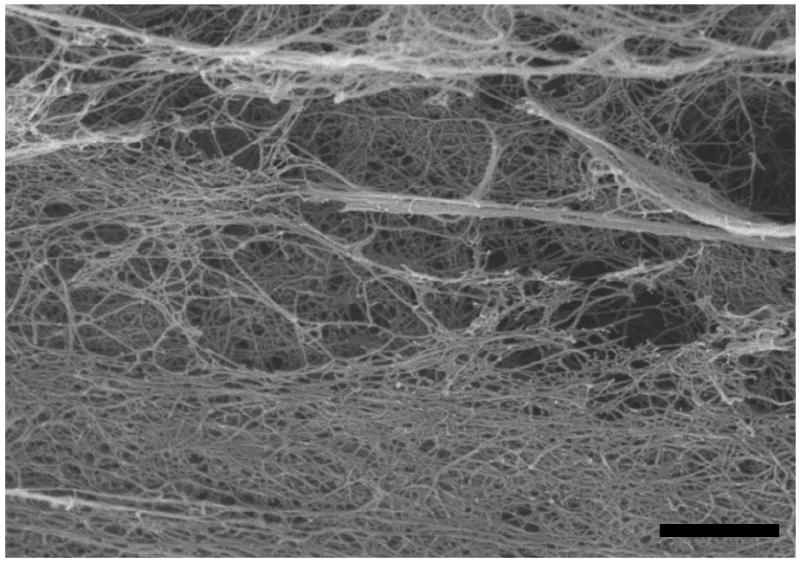
SEM image of M1. The scale bar is 20 microns.
Discussion
In this study, the compressive behavior of the luminal, medial and abluminal layers of the ILT and five thrombus mimics were investigated using an unconfined-compression setup. We report a higher compressive stiffness for the abluminal layer compared to the medial and luminal layers, suggesting a nonhomogenous mechanical behavior of the ILT in compression. Our results also indicate that a mimic can be constructed whose mechanical properties can be modified controllably using variations in calcium, fibrinogen, and thrombin concentrations. In particular, we present here mimics which behave mechanically similar to the luminal and medial layers of native ILT.
Several other groups have investigated the mechanical properties of the ILT. Boschetti et al. reported the compressive elastic modulus of the ILT luminal, medial, and abluminal layers at 45% compressive strain to be approximately 11, 20, and 22 kPa, respectively [18], which is higher than what we report (1.5, 2.5, and 19 kPa) at a 5% compressive strain. This increase is likely due to the difference in applied compressive strains. However, in both cases the data show a trend of increase in modulus from the luminal to the abluminal layers. Hinnen et al. reported an ILT elastic modulus of 35 kPa based on a cyclic shear strain on the order of 0.01. However, in this study the ILT was not divided into layers [19]. This is higher than what we report, which is likely due to the difference in methods—Hinnen et al. measured the shear modulus and converted it to elastic modulus using the Poisson's ratio. They also report the development of a mimic with a similar elastic modulus as the ILT, but no mention is made of addressing the biological composition and structure of the ILT. Similarities in these properties will also likely be important in developing an in vitro test setup for future mechanobiological studies.
Our results demonstrate that the E5 of the mimics can be adjusted by changing the concentration of fibrinogen, thrombin, or calcium. Carr and Carr showed that the elastic modulus of a thrombus increased with an increase in fibrinogen (1 to 4 mg/ml) and decreased with an increase in calcium (5 to 20 mM) [11], which is consistent with our results. Ryan et al. modeled fibrin-based constructs as a viscoelastic material and found similar relationships between storage modulus and the concentrations of fibrinogen and calcium [12]. However, they also found that the storage modulus decreases with an increase in thrombin concentration (0.1 to 5 units/ml), which is opposite to the relationship we found. This difference may be due to their difference in methodology—they used cyclic shear tests while we used stress-relaxation compression tests.
In addition, Ryan et al. examined the effects of fibrinogen, thrombin, and calcium concentrations on the structure of the fiber network in fibrin-based constructs by analyzing computerized three-dimensional models based on SEM images from the constructs. They found that increasing fibrinogen, increasing thrombin, and decreasing calcium decreases fiber length and diameter and increases fiber density and branch points in the fibrin network. They concluded that maximal stiffness is established in fibrin networks that consist of a balance between large fibers and high branching. These results suggest that there is an optimal concentration of fibrinogen, thrombin, and calcium for making a mimic of maximum stiffness. A fibrinogen or thrombin concentration that is too high or a calcium concentration that is too low may decrease the stiffness of the resulting mimic because, although the branching may be great, fiber size would be too small.
Two of our mimics (M4 and M5) were not statistically different than the ILT luminal and medial layers, respectively. The long-term goal of our group is to develop a mimic which mechanically, structurally, and biochemically behaves similar to the ILT. Our results suggest that our current mimics may be adequate for mimicking the luminal and medial layer compliance, while the abluminal layer will require further modification. Increasing the E5 of the ‘abluminal’ mimic may be accomplished by further adjusting the concentrations of components already stated or through the addition of additional components. For example, the stiffness of thrombus has also been shown to increase with the presence of factor XIII [13] and fibronectin [14]. Furthermore, platelets may be added to the mimic in future studies, as they are a major component of thrombus and have also been shown to increase stiffness of fibrin-based constructs [11]. Addition of such biologically active components may also provide a mechanism by which the aging of the ILT can be modeled.
While not the focus of the current work, determination of the hydraulic permeability of the ILT and our mimic is ongoing within our laboratory. Specifically of interest will be how these values may change as a function of location in the ILT as well as progression of the disease (thick vs. thin ILT). This information can also be used in future studies to model the convective transport in this tissue, which will be essential in assessing the effectiveness of drug delivery in. Development of a pharmacological treatment has been suggested as an alternative treatment of AAA which have been detected but not progressed to a stage where invasive procedure (i.e. open surgery or endovascular repair) is warranted [20]. In other words, when an AAA is detected, treatment to inhibit (or slow) the progression of AAA can be employed without delay, instead of employing a period of watchful monitoring. Doxycycline, an MMP inhibitor, is one such drug currently under investigation [21, 22].
One of the limitations of this study is the number of ILT samples tested. For example, the reported water content data only had one sample for each group. Current research in our laboratory is aimed at further identifying differences in water content and structure for the ILT layers and our mimics. For example, while we did find a qualitative similarity in our SEM mimic structure compared to ILT structure reported elsewhere, it is not immediately clear whether the non-uniformity in structure shown in Figure 6 is real, or simply an artifact of the SEM specimen preparation methods. Another possible limitation of our study was the length of time in which samples were stored prior to testing; specifically as such storage may result in degradation and changes in mechanical behavior. However, our data suggest that there was no significant difference in E5 for samples tested within 24 hours of the AAA repair versus 24 to 48 hours after the repair. It is also important to note that the E5 reported here is based on compression at 5% strain, whereas it may not be accurate at larger strains. In fact, our results suggest that the drained properties above 5% may be mildly nonlinear (Figure 4). Other researchers have similarly reported increasing stiffness at higher strains of thrombus and fibrin-based constructs [23-25].
As previously stated, our goal is to develop a mimic which has similar properties to the ILT, and the immediate future goal of our lab is to measure and compare permeability between the groups by analyzing the time dependent change in load of our unconfined-compression tests. Finite element analysis can also then be used to model the ILT layers and mimics as poroelastic materials. Further investigations will also include computationally modeling the fluid movement through the ILT during the heart cycle and investigating the potential of different drug delivery strategies. Additionally, cells typically found in the ILT (e.g. macrophages and neutrophils) can be embedded into the mimics to study how the ILT may be involved in the mechanobiology and progression of AAA.
Acknowledgments
The authors would like to thank Dr.'s Joseph Mills, Son Duong, John Hughes, and Kay Goshima of the Vascular Surgery Department for their help in procuring ILT samples. Funding for this work was provided by the NSF (CAREER 0644570 – JPVG) and the NIH Biomedical Cardiovascular Training Grant (HL007955 – BME GIDP).
Footnotes
Conflict of Interest Statement: The authors of this paper have no conflict of interest that would inappropriately influence this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Omran M, Verma S, Lindsay TF, Weisel RD, Sternbach Y. Clinical decision making for endovascular repair of abdominal aortic aneurysm. Circulation. 2004;110(23):e517–23. doi: 10.1161/01.CIR.0000148961.44397.C7. [DOI] [PubMed] [Google Scholar]
- 2.Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci. 2006;1085:133–8. doi: 10.1196/annals.1383.044. [DOI] [PubMed] [Google Scholar]
- 3.Wang DH, Makaroun MS, Webster MW, Vorp DA. Effect of intraluminal thrombus on wall stress in patient-specific models of abdominal aortic aneurysm. J Vasc Surg. 2002;36(3):598–604. doi: 10.1067/mva.2002.126087. [DOI] [PubMed] [Google Scholar]
- 4.Hinnen JW, Koning OH, Visser MJ, Van Bockel HJ. Effect of intraluminal thrombus on pressure transmission in the abdominal aortic aneurysm. J Vasc Surg. 2005;42(6):1176–82. doi: 10.1016/j.jvs.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Wang DH, Makaroun M, Webster MW, Vorp DA. Mechanical properties and microstructure of intraluminal thrombus from abdominal aortic aneurysm. J Biomech Eng. 2001;123(6):536–9. doi: 10.1115/1.1411971. [DOI] [PubMed] [Google Scholar]
- 6.Di Martino E, Mantero S, Inzoli F, Melissano G, Astore D, Chiesa R, Fumero R. Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: experimental characterisation and structural static computational analysis. European Journal of Vascular & Endovascular Surgery. 1998;15(4):290–9. doi: 10.1016/s1078-5884(98)80031-2. [DOI] [PubMed] [Google Scholar]
- 7.Vande Geest JP, Sacks MS, Vorp DA. A planar biaxial constitutive relation for the luminal layer of intra-luminal thrombus in abdominal aortic aneurysms. J Biomech. 2006;39(13):2347–54. doi: 10.1016/j.jbiomech.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25(5):916–26. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- 9.Alston SM, Solen KA, Broderick AH, Sukavaneshvar S, Mohammad SF. New method to prepare autologous fibrin glue on demand. Transl Res. 2007;149(4):187–95. doi: 10.1016/j.trsl.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Blomback B, Okada M. Fibrin gel structure and clotting time. Thromb Res. 1982;25(1-2):51–70. doi: 10.1016/0049-3848(82)90214-6. [DOI] [PubMed] [Google Scholar]
- 11.Carr ME, Jr, Carr SL. Fibrin structure and concentration alter clot elastic modulus but do not alter platelet mediated force development. Blood Coagul Fibrinolysis. 1995;6(1):79–86. doi: 10.1097/00001721-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77(5):2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladner JA, Nossal R. Effects of crosslinking on the rigidity and proteolytic susceptibility of human fibrin clots. Thromb Res. 1983;30(3):273–88. doi: 10.1016/0049-3848(83)90081-6. [DOI] [PubMed] [Google Scholar]
- 14.Kamykowski GW, Mosher DF, Lorand L, Ferry JD. Modification of shear modulus and creep compliance of fibrin clots by fibronectin. Biophys Chem. 1981;13(1):25–8. doi: 10.1016/0301-4622(81)80021-x. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, Blomback B, Chang MD, Horowitz B. Fibronectin and fibrin gel structure. J Biol Chem. 1985;260(3):1811–20. [PubMed] [Google Scholar]
- 16.Okada M, Blomback B. Calcium and fibrin gel structure. Thromb Res. 1983;29(3):269–80. doi: 10.1016/0049-3848(83)90039-7. [DOI] [PubMed] [Google Scholar]
- 17.Shen LL, Hermans J, McDonagh J, McDonagh RP, Carr M. Effects of calcium ion and covalent crosslinking on formation and elasticity of fibrin cells. Thromb Res. 1975;6(3):255–65. doi: 10.1016/0049-3848(75)90073-0. [DOI] [PubMed] [Google Scholar]
- 18.Inzoli F, Boschetti F, Zappa M, Longo T, Fumero R. Biomechanical factors in abdominal aortic aneurysm rupture. Eur J Vasc Surg. 1993;7:667–74. doi: 10.1016/s0950-821x(05)80714-5. [DOI] [PubMed] [Google Scholar]
- 19.Hinnen JW, Rixen DJ, Koning OH, van Bockel JH, Hamming JF. Development of fibrinous thrombus analogue for in-vitro abdominal aortic aneurysm studies. J Biomech. 2007;40(2):289–95. doi: 10.1016/j.jbiomech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Rentschler M, Baxter BT. Pharmacological approaches to prevent abdominal aortic aneurysm enlargement and rupture. Ann N Y Acad Sci. 2006;1085:39–46. doi: 10.1196/annals.1383.003. [DOI] [PubMed] [Google Scholar]
- 21.Petrinec D, Liao S, Holmes DR, Reilly JM, Parks WC, Thompson RW. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: preservation of aortic elastin associated with suppressed production of 92 kD gelatinase. J Vasc Surg. 1996;23(2):336–46. doi: 10.1016/s0741-5214(96)70279-3. [DOI] [PubMed] [Google Scholar]
- 22.Bartoli MA, Parodi FE, Chu J, Pagano MB, Mao D, Baxter BT, Buckley C, Ennis TL, Thompson RW. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Ann Vasc Surg. 2006;20(2):228–36. doi: 10.1007/s10016-006-9017-z. [DOI] [PubMed] [Google Scholar]
- 23.Roberts WW, Lorand L, Mockros LF. Viscoelastic properties of fibrin clots. Biorheology. 1973;10(1):29–42. doi: 10.3233/bir-1973-10105. [DOI] [PubMed] [Google Scholar]
- 24.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–4. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 25.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5 1:116–24. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]