Abstract
Severe visual field constriction (tunnel vision) impairs the ability to navigate and walk safely. We evaluated Trifield glasses as a mobility rehabilitation device for tunnel vision in an extended wearing trial. Twelve patients with tunnel vision (5 to 22 degrees wide) due to retinitis pigmentosa or choroideremia participated in the 5-visit wearing trial. To expand the horizontal visual field, one spectacle lens was fitted with two apex-to-apex prisms that vertically bisected the pupil on primary gaze. This provides visual field expansion at the expense of visual confusion (two objects with the same visual direction). Patients were asked to wear these spectacles as much as possible for the duration of the wearing trial (median 8, range 6 to 60, weeks). Clinical success (continued wear, indicating perceived overall benefit), visual field expansion, perceived direction and perceived visual ability were measured. Of 12 patients, 9 chose to continue wearing the Trifield glasses at the end of the wearing trial. Of those 9 patients, at long-term follow-up (35 to 78 weeks), 3 reported still wearing the Trifield glasses. Visual field expansion (median 18, range 9 to 38, degrees) was demonstrated for all patients. No patient demonstrated adaptation to the change in visual direction produced by the Trifield glasses (prisms). For difficulty with obstacles, some differences between successful and non-successful wearers were found. Trifield glasses provided reported benefits in obstacle avoidance to 7 of the 12 patients completing the wearing trial. Crowded environments were particularly difficult for most wearers. Possible reasons for long-term discontinuation and lack of adaptation to perceived direction are discussed.
Keywords: tunnel vision, retinitis pigmentosa, choroideremia, low vision rehabilitation, Trifield glasses, mobility
Introduction
A common definition (e.g. Social Security Administration in the USA) of legal blindness is a constriction of the visual field to less than or equal to 20° diameter as measured by Goldmann perimetry using a III4e target size (tunnel vision). Tunnel vision is a debilitating symptom of retinitis pigmentosa and choroideremia. Retinitis pigmentosa, the most common cause of inherited blindness, has a prevalence of about 1 in 4000 people worldwide (Berson, 1993; Hartong et al., 2006).
Patients with tunnel vision have difficulties with navigating, avoiding obstacles, and performing visual search (Marron and Bailey, 1982; Lovie-Kitchin et al., 1990; Haymes et al., 1996; Black et al., 1997; Kuyk et al., 1998; Turano et al., 1999a; Turano et al., 2001; Broman et al., 2004; Luo and Peli, 2006; Apfelbaum et al., 2007; Fortenbaugh et al., 2007). The consequent loss of mobility with increased risk of falls (Felson et al., 1989; Lord et al., 1993; Tinetti and Williams, 1997; Lord and Dayhew, 2001; Biderman et al., 2002; Freeman et al., 2007) is detrimental to patients' independence and quality of life (Tinetti and Williams, 1997; Turano et al., 1999b; Shinkai et al., 2000; Biderman et al., 2002; Melzer et al., 2003; Cacciatore et al., 2004). Visual field extent is a significant predictor of the mobility performance (Marron and Bailey, 1982; Lovie-Kitchin et al., 1990; Haymes et al., 1996; Black et al., 1997; Kuyk et al., 1998; Turano et al., 1999a; Broman et al., 2004). Many people with tunnel vision receive orientation and mobility training, though the benefit of this training has not been proven (Soong, 2000; Soong et al., 2001; Kuyk et al., 2004; Virgili and Rubin, 2006).
Previously rehabilitation approaches for patients with tunnel vision included: training to scan (Cohen and Waiss, 1996); minifying devices (reversed telescopes, or hand held negative lenses) (Drasdo, 1976; Hoeft et al., 1985; Weiss, 1992; Szlyk et al., 1998) and various prism spectacle designs (Cohen, 1993; Onufryk, 1994). Most of these devices have not been commercially available and others have had limited clinical use due to their drawbacks. More recently augmented-vision head-mounted displays (Peli, 2001; Vargas-Martin and Peli, 2002) have shown some promise in early testing in visual search tasks (Luo and Peli, 2006) and judgements of potential collisions (Luo et al., 2009).
We are not aware of any publication explaining how to train patients with tunnel vision to modify their scanning eye movements or showing that patients actually increase their scanning following training. We believe that in most cases the training simply consisted of instructing patients that they should scan more. In recording eye movements of patients with tunnel vision while walking, it has been reported that the distribution of eye movements while walking was not larger than that of normally-sighted subjects (Vargas-Martin and Peli, 2006) and saccadic amplitudes and directions were very similar to those of normally-sighted subjects (Luo et al., 2008). Thus, contrary to clinical wisdom and the intent of training, it seems that patients with tunnel vision do not compensate for the restricted visual field by making larger scanning eye movements than patients with a full visual field.
Minifying devices shrink the view of the scene so that a wider section of the world is available within the patient's residual visual field but have poor clinical acceptance primarily due to the reduction in resolution (visual acuity). The Amorphic lens reversed telescope (Designs for Vision, Inc., Ronkonkoma, NY) minified only the horizontal meridian in order to reduce the impact on acuity (Hoeft et al., 1985) at a cost of image distortion. Despite some reports of success (Hoeft et al., 1985; Szlyk et al., 1998), they were discontinued recently.
The use of prisms in treating peripheral visual field defects has been controversial, as previously proposed designs had significant limitations (Cohen, 1993). The best known binocular sector prism design (Onufryk, 1994), the so-called Channel lens, was based on a field-shifting principle (rather than field expanding). These prisms, commercialized briefly in 1998 and 1999 as the InWave™ lenses (InWave Inc., Janesville, WI), had a central prism free channel (corresponding to the width of the residual visual field of the patient). As a result they had no effect in primary position of gaze. When the patient made eye movements towards the surrounding prismatic areas the effect was to shift (relocate) the image more centrally, rather than expand, the residual visual field. Further, the prism powers available laterally (12Δ ≈ 6° - nasal and temporal base) and below the channel (8Δ ≈ 4° - base down) were probably too small to have an impact on mobility. Also, the prism apex scotomata could interfere with their functionality (Giorgi et al., 2009; Ross et al., 2009). Somani et al. (2006) implemented this design using stick-on Fresnel prisms and claimed small increases in visual fields and activities of daily living.
Obstacle detection is likely to be best when the user does not need to make a specific action with a visual field expansion device to search for a potential obstacle. Rather, to be most effective the device should provide a view of the potential obstacle with natural use of the device, by providing visual field expansion all of the time. For potential obstacle detection, the Channel lens (prisms), the Amorphic lens in a bioptic configuration (Szlyk et al., 1998) and hand held negative lenses (Kozlowski et al., 1984) all require that the user initiate a search action by spotting through the device. As such, the use of these devices is unlike the use of bioptics for magnification. With a magnification bioptic the need for initiating device use is apparent to the user (i.e. cannot resolve object of interest that is detected without the device).
Trifield glasses have been proposed as a mobility aid (Peli, 2001). Trifield glasses expand the visual field without minification, or prism scotoma (Peli, 2001). Trifield glasses consist of two apex-to-apex prisms mounted in front of one eye, and a conventional spectacle lens (e.g. single vision with prescription) in front of the other eye (Fig. 1). The patient retains the original residual visual field of the non-prism eye, while the prism lens provides a laterally shifted visual field. The combination of the original visual field and the shifted visual field provides an expansion. The prism lens is fitted so that the prism apices vertically bisect the pupil when the prism eye is in primary gaze. As the gaze is shifted to the left, the prism eye sees a part of the scene that is laterally farther to the left compared to that seen by the eye with the conventional lens. Similarly, when the gaze is directed to the right, the prism eye sees objects farther to the right than the non-prism eye. The combined percept is double vision (specifically visual confusion: two objects having the same visual direction). As illustrated in Fig. 2, the direction of the lateral shift and expansion is dependent on the direction of gaze. As the patient looks right (Fig. 2B) or left (Fig. 2C), the prism eye looks through the right or left prism, respectively, and areas the patient would not otherwise see are shifted into their visual field from the right or the left, respectively. If the patient looks directly forward so that the prism junction is over the pupil, three parts of scene are visible: one through the conventional lens before the non-prism eye and two parts, laterally shifted from opposite directions, through the two prisms of the Trifield lens before the prism eye (Fig. 2D). Thus, “tri-field” glasses make three possible pieces of the visual field available to the wearer, two of which would not be seen without the glasses, and increasing the available visual field extent up to three-fold. While the part (or side) of the field of view that is expanded varies with gaze direction, the area of the visual field that is visible is about double at all times.
Figure 1.

Trifield glasses correction for a patient with “tunnel vision” (severe bilateral visual field constriction). Inset. Top down view of the right lens showing the two prisms conjoined at each prism's apex (34 Δ temporal lens and 22 Δ nasal lens). The temporal prism is tinted red and the nasal prism is green in the hope that this would assist with determining the direction of objects. The fellow eye has a standard ophthalmic lens here shown with a bifocal.
Figure 2.
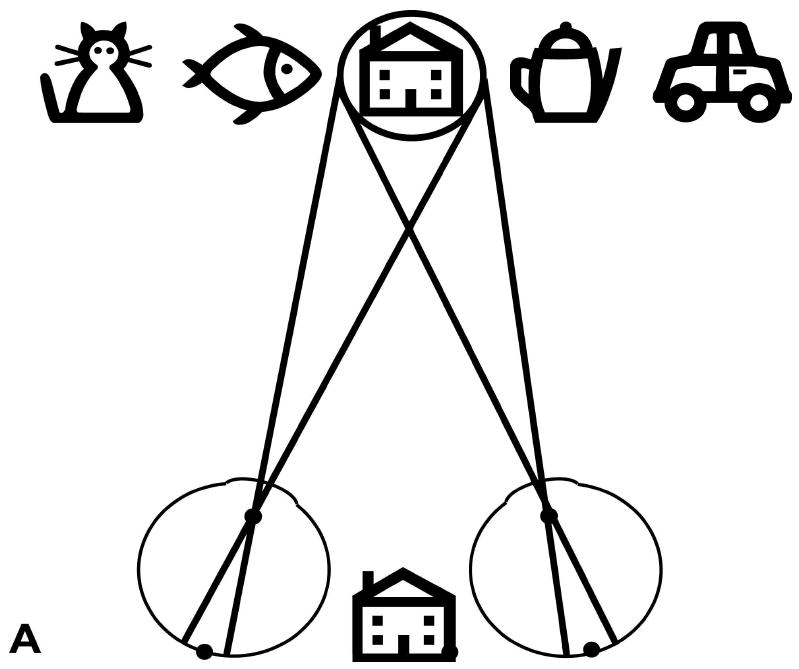
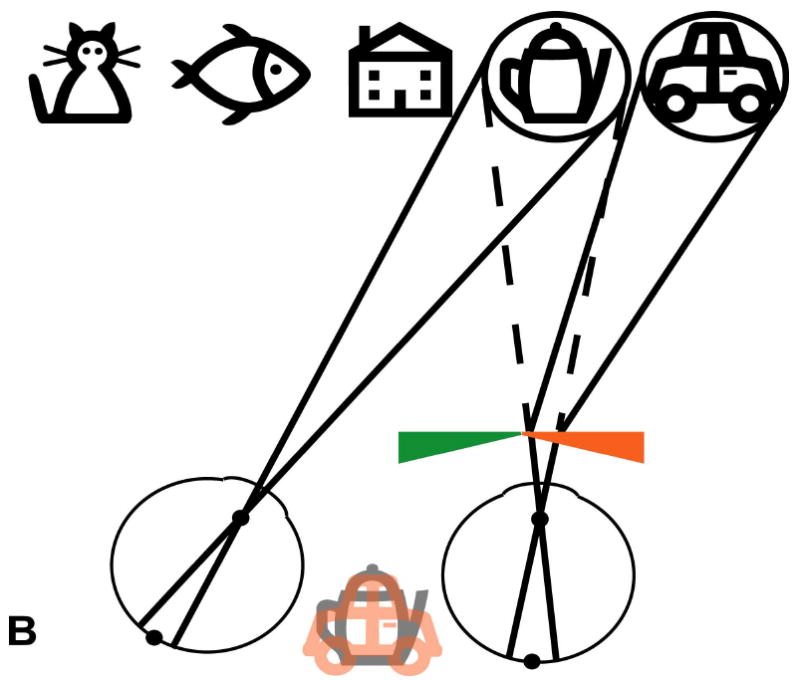
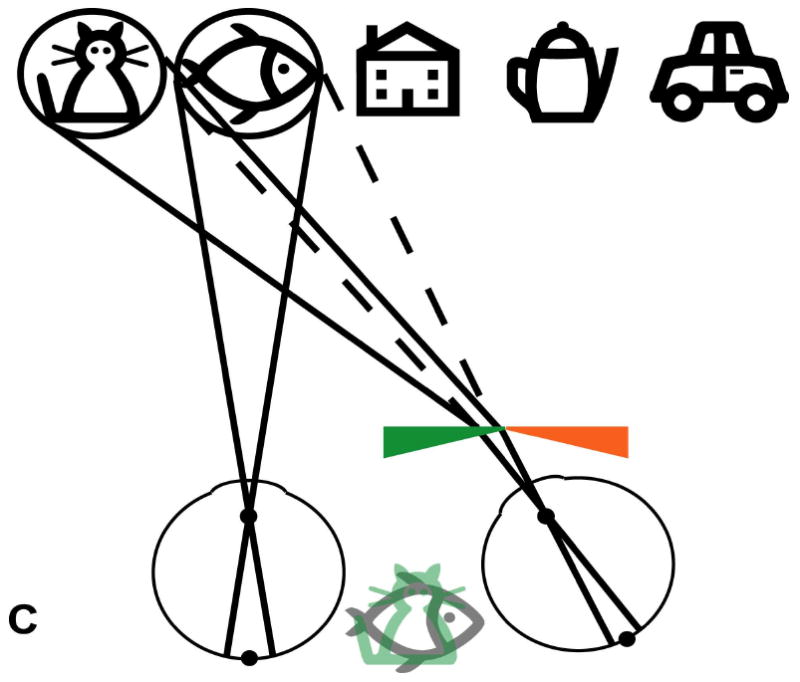
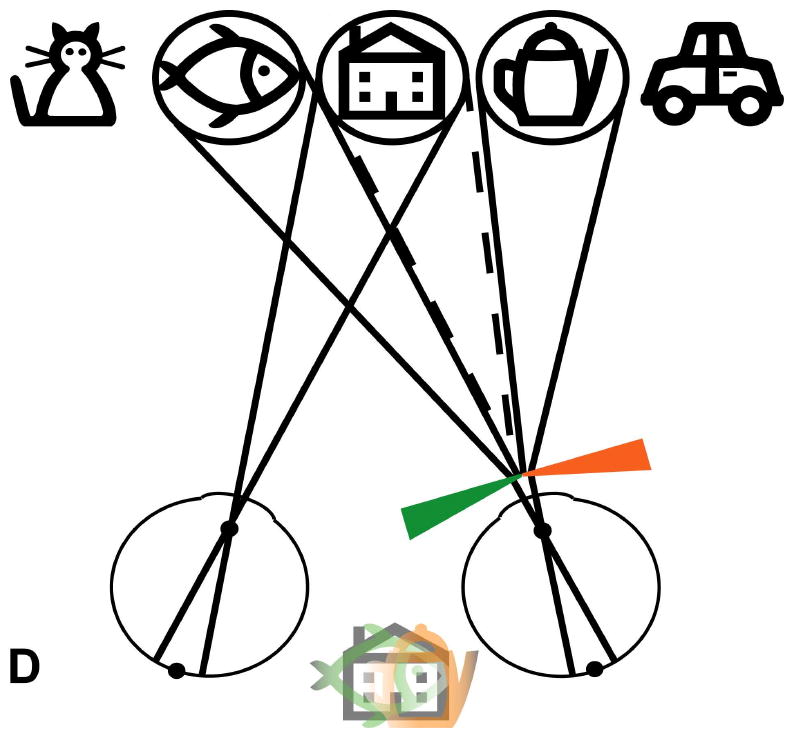
Schematic (not to scale) illustration of the visual field expansion effect of the Trifield glasses. (A) A person with tunnel vision is only able to see one of the objects at a time when viewing with both eyes simultaneously (in primary gaze, the house). (B) The Trifield lens is placed over the right eye and the wearer looks to the right, here fixating the coffee pot with the non-prism left eye. The right Trifield prism segment shifts the view of the prism eye to the right, here to the car. Thus, the car, which would not have been visible without the Trifield glasses, is now visible to the wearer. However, the binocular percept is that of visual confusion, with the pot and the car both seen in the same apparent visual direction (see illustration between eyes). (C) Similarly, when the wearer looks to the left, here viewing the fish with the non-prism left eye, the left Trifield prism segment shifts the view of the prism eye farther to the left so that the prism eye sees the cat. Without the Trifield glasses, the cat would not have been visible, illustrating the visual field expansion. Again, the binocular percept is that of visual confusion, with the fish and the cat perceived in the same visual direction. (D) When the wearer looks through the junction between the two Trifield prism segments, the view is more complicated, with part of the prism-eye view coming from each prism segment (due to the finite size of the pupil). As the gaze shifts across the junction, the view of the prism eye shifts from one side to the other (note the fading). Learning to interpret the double vision view induced by the prisms is the most challenging aspect of the Trifield glasses. Note that in this illustration, we assume for simplicity that the person has normal binocular vision and no phoria.
The lateral shift provided by the prisms is large enough to shift the extension outside the unchanged visual field of the non-prism eye, preventing the patient from experiencing diplopia (seeing one object twice in two different apparent directions). Diplopia occurs when the images of an object fall onto non-corresponding points of the two retinas. However, visual confusion does occur with the Trifield glasses. Visual confusion occurs when images of two different objects in the scene fall onto corresponding points of the two retinas and, therefore, appear to be in the same perceived direction. To help the patients differentiate the objects and their direction viewed through the prisms, the right prisms were tinted red and the left prisms were tinted green. While these cues are helpful, we expected that when wearers were first given the glasses, they would have difficulty interpreting what they saw. One of the two pieces of the field of view (the tinted one) brought into view by the prisms does not represent the objects in their true direction. However, we also expected that wearers would adapt to this shift and eventually be able to determine the true direction of objects viewed through the prisms (Kohler, 1964; Pick et al., 1969; Welch et al., 1993).
In this study, patients with severely restricted visual fields were fitted with the Trifield glasses for an extended period (a wearing trial of nominally six weeks) during which their benefit as a mobility aid was evaluated.
Methods
Extended Wearing Trial
The Trifield glasses were evaluated during a prospective five-visit extended wearing trial planned for six-weeks duration (Fig. 3). At the initial visit, the patient received a full eye examination by one of us (ELB) at the Massachusetts Eye and Ear Infirmary (MEEI) and was then further screened for study eligibility at Schepens Eye Research Institute (SERI). If eligible (described below), the patient was measured for Trifield glasses. Typically, the Trifield glasses took 6 to 10 weeks to manufacture. The Trifield glasses were dispensed at visit 2. Follow-up visits were performed nominally one (visit 3), three (visit 4) and six (visit 5) weeks after delivery of the Trifield glasses at visit 2. The benefits (or otherwise) of the Trifield glasses were evaluated through measurements of visual field expansion, change in perceived direction through the prisms, perceived visual ability and through clinical success; the willingness to continue wearing the Trifield glasses at the end the wearing trial. At approximately one year following the end of the wearing trial, a long-term follow-up telephone interview was conducted with those patients who chose to continue to wear the Trifield glasses at the last visit of the wearing trial (Fig. 3). There were large individual variations in the time between visits, and in the total duration of the wearing trial (Fig. 3) that resulted from difficulties scheduling the visits, including personal health, difficulties with travel (e.g. arranging a companion, short periods of daylight in the winter) and personal schedules.
Figure 3.
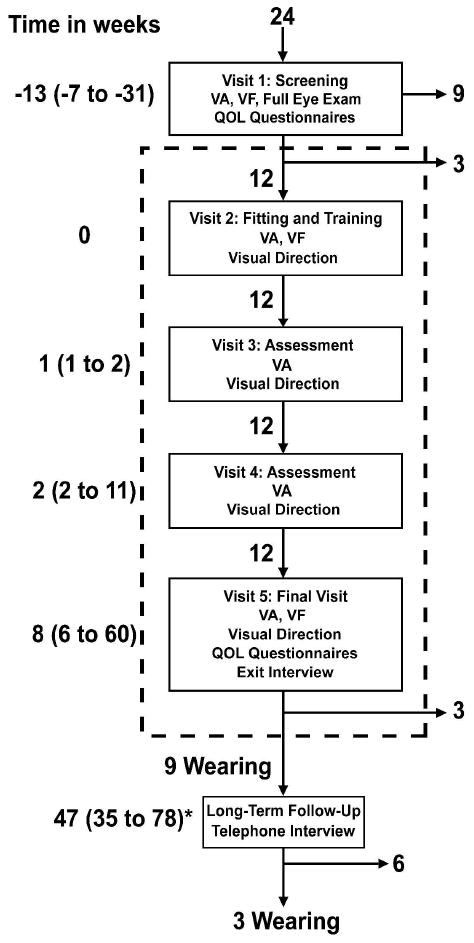
Study flow diagram showing the timing of visits, the main procedures at each visit and the number of patients attending each visit. Numbers on the left of the flow diagram are the median time in weeks (range in parentheses) relative to the Trifield glasses fitting and training visit (visit 2), except for the follow-up visit, which is relative to the study end (visit 5). Numbers next to the down pointing arrows within the dashed box indicate the number of patients moving to the next stage of the study. The numbers next to right pointing arrows outside of the dashed box indicate the number of patients who discontinued wear of the Trifield glasses at that stage. The formal visits of the study are enclosed by the dashed rectangle. VA = visual acuity; VF = visual field as measured by computer perimetry. The * indicates that the reported times are relative to visit 5.
Eligibility Criteria
Eligibility requirements included best-corrected single-letter visual acuity of 6/30 or better in the each eye, a stable condition that caused tunnel vision, ability to understand verbal instructions in English, and age 18 years or older. The primary visual field criterion was a residual central visual field horizontal diameter of 3° to 20° in each eye as measured by our custom computerized perimeter using a white (340 cd/m2), 12mm target on a grey (42 cd/m2) background viewed from 1 m. Patients with residual peripheral islands (as determined by Goldmann perimetry, target V4e) were considered for study inclusion if, based on patient report, the islands seemed to be of little functional value in mobility situations. Four patients enrolled in the study demonstrated such peripheral islands. Lastly, patients were excluded if they had disruption of binocular visual function that could lead to suppression of one eye (e.g. strabismus, amblyopia).
Patients
Patients were recruited from patient databases at the Berman-Gund Laboratory and SERI and were also referred by the Foundation Fighting Blindness and the Choroideremia Foundation. Twenty-four patients (18 male) with tunnel vision, deemed potentially eligible based on a telephone interview, were screened for participation in the study (visit 1). Of those 24 patients, 9 were ineligible due to inadequate visual acuity (n=5), visual fields that were too large (n=5) and large peripheral islands that were reported by the patient to be of use with mobility (n=1). Of the 15 eligible patients, 12 agreed to participate in the study and those 12 patients were enrolled in and completed the wearing trial (Fig. 3). As detailed in Table 1, for these 12 patients (7 male), the median age was 48 (range 39 to 59) years, median binocular visual acuity was 6/10 (range 6/6 to 6/30), all 12 had severely restricted visual fields (median 9.8°, range 5.0° to 21.5°, wide) due to either retinitis pigmentosa (n=10) or choroideremia (n=2), eight used a long cane to aid in mobility and seven had received orientation and mobility training.
Table 1.
Demographic information, clinical vision measurements with and without the Trifield glasses, wearing time and success at the end of the study (continued to wear after visit 5) for the twelve patients enrolled in the extended wearing trial.
| Pt. # | Age (y)/sex | Aetiology | Long cane user | O&M training | VA | VF width (degrees) | VF width with Prisms (degrees) | Prism Eye | Prisms Prescribed (N/T)(Δ) | Wearing time per day (hours) | Clinical success |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56/m | RP | Yes | Yes | 6/6 | 7.5 | 20 | Left | 15/17 | 0.61 | Yes |
| 2 | 49/m | RP | No | No | 6/9 | 9.5 | 28.5 | Right | 16/27 | 0.57 | Yes |
| 3 | 39/m | CHM | Yes | Yes | 6/9 | 16 | 29 | Right | 27/21 | 1.77 | Yes |
| 4 | 45/f | RP | Yes | Yes | 6/6 | 21.5 | 51 | Right | 44/34 | 1.18 | Yes |
| 5 | 41/m | CHM | No | No | 6/8 | 15 | 24 | Left | 32/19 | 1.27 | Yes |
| 6 | 45/f | RP | No | No | 6/13 | 8.5 | 34 | Right | 19/24 | 3.32 | Yes |
| 7 | 57/f | RP | No | No | 6/15 | 16.5 | 41 | Right | 25/34 | 0.57 | No |
| 8 | 58/m | RP | Yes | Yes | 6/14 | 7.5 | 18 | Left | 11/20 | 3.81 | Yes |
| 9 | 53/m | RP | Yes | Yes | 6/12 | 10 | 28 | Left | 13/28 | 0.57 | Yes |
| 10 | 43/f | RP | Yes | No | 6/30 | 17 | 55 | Left | 42/30 | 2.61 | No |
| 11 | 46/m | RP | Yes | Yes | 6/10 | 8 | 26 | Left | 16/16 | 1.38 | Yes |
| 12 | 59/f | RP | Yes | Yes | 6/11 | 5 | 23 | Left | 17/16 | 0.84 | No |
RP, retinitis pigmentosa; CHM, choroideremia; VA, binocular Snellen visual acuity; VF width, horizontal extent of visual field measured in degrees; N/T, prescription of nasal and temporal prisms.
The visual status of the 12 patients completing the extended wearing trial remained stable during the wearing trial. Between visit 2 and visit 5, two patients had reductions in visual acuity of more than one line (0.1 logMAR) in visual acuity (-0.13 and -0.15 logMAR) and two patients had improvements of more than one line in visual acuity (+0.11 and +0.14 logMAR). The measurement error (95% confidence limits) of visual acuity in healthy eyes is about one line (Bailey et al., 1991; Arditi and Cagenello, 1993), but is greater when vision is impaired (Reeves et al., 1991; Woods, 1993; Kiser et al., 2005). No patient showed a change in the horizontal diameter of the monocular visual fields of more than 1° between visits 1 and visit 5.
The study protocol was approved by the Institutional Review Boards at MEEI and SERI. All patients read, or were read, the consent to participate forms, had any questions answered, and then signed the consent form.
Trifield Glasses Prescription, Fitting and Training
The Trifield glasses comprised a conventional single vision or bifocal lens placed over the dominant eye, and the prism lens over the fellow eye with both prism apices splitting the pupil (Fig. 1). The prism lens included any required distance optical correction. Ocular dominance was determined using polarized goggles and a mirror (Peli, 2002) If no dominance was noted with the polarized goggle, it was determined by comparison of the blurring effect of a +1.50 dioptre spherical trial lens placed over one or other eye (Benjamin and Borish, 1994; Siejas et al., 2007). If no ocular dominance was found with either test, the conventional lens was placed over the eye with the significantly better visual acuity or larger visual field. If there was no clinically significant difference in the monocular visual acuities or visual field extents, conventional lens placement was based on patient preference.
As illustrated in Fig. 2, the visual field shift of the prism eye provided field expansion at the expense of visual confusion (two objects seen in the same direction). The prism powers used were calculated to be large enough to avoid diplopia (a single object having two apparent visual directions), as would happen with a prism power causing a shift (in degrees) that was smaller than the residual visual field diameter. Since such separation eliminates all fusional clues, the eyes take up the phoria (resting) position with Trifield glasses. Phoria measurements taken at two intermediate distances (3 and 15 feet) were used to adjust the prism powers to account for the effect of phoria. As an example, for a patient wearing the prism lens over the right eye, the right (temporal) prism power must be sufficient to move the left edge of the right visual field to the right of the right edge of the left visual field, and thus avoiding overlap of the visual fields (Figure 2B). Thus, if those visual field extents were 2 and 4 degrees, respectively, a shift of at least 6 degrees would be required to avoid diplopia. If that patient were exophoric, the prism power could be reduced by the amount of the phoria. We measured phoria at two distances that we considered to be about the limits of the range of distances of obstacles that we expected would be usefully detected with Trifield glasses. In this example, if the phorias were 3 and 1Δ, the prism power could be reduced by the amount of the smaller phoria (here 1Δ ≈ 0.6 degrees). Finally, to minimize the risk of diplopia, we added 2 degrees of prism power, which was expected to ensure that the visual field margins did not overlap. The final right prism power in this example would have been 13Δ (7.4 degrees). The intent was to have at least 2 degrees between adjacent visual fields, but measured visual fields (e.g. Fig. 4b) often had inter-field gaps that were larger than intended. The difference between intended and measured inter-field gap was probably caused by between-session and between-condition variability in phorias and the reduction in the measured visual field of the prism eye due to the tint (reduced transmittance). Prescribed powers for the Trifield glasses used in this study ranged from 11 to 44Δ for the nasal prism and 16 to 34Δ for the temporal prism. The right prism was tinted red with a transmission of about 37% and the left was tinted green with a transmission of about 46%. Each patient was provided with a pair of clip-on lenses for use in bright conditions, with the non-prism-eye lens having a transmission of 15% and the prism-eye lens having a higher transmission, so that when combined with the tints in the prisms, the total transmission was about 15%.
Figure 4.
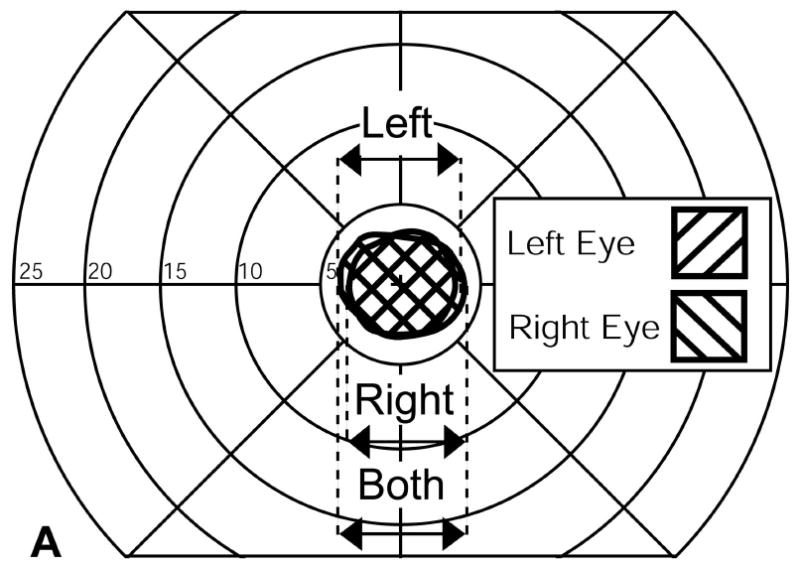
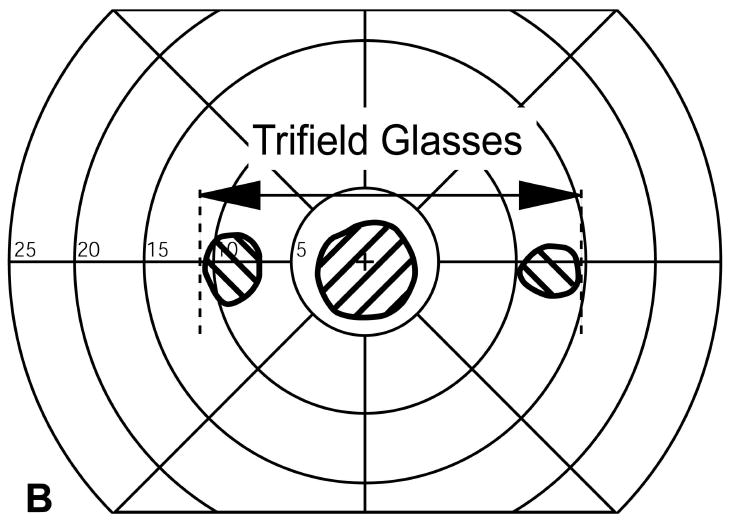
Visual field expansion measured using a computerized perimeter for one patient. (A) Left and right eye monocular visual fields. (B) The visual fields of the same patient when wearing Trifield glasses with the prisms over the right eye. Visual field expansion by the prisms is indicated by the two hatched areas to the left and right of fixation. Only one of these is available for the patient at any instant depending on the direction of gaze at that moment. The distance between the left most edge of the left expanded area and the rightmost edge of the right expanded area (shown by the arrow) was defined as the expanded visual field.
To familiarize a patient in the use of the Trifield glasses the patient was escorted for a walk through SERI corridors and into uncluttered rooms. The patient was instructed to pay particular attention to doorframes and other potential obstacles. The patient was then escorted both up and down a flight of stairs, and instructed to make use of handrails whenever using stairs.
In addition to this general instruction in the use of the Trifield glasses for obstacle detection a visual-direction training exercise was demonstrated at visit 2 and was prescribed for home practice in an effort to improve adaptation to the image shift caused by the prisms. The exercise was a manual reaching exercise based on Kohler's observation that adaptation to prism induced field shifts was hastened by tactile and proprioceptive cues (Kohler, 1964). While the patients looked through one of the two prisms, an experimenter presented a finger into the prism field, and patients were instructed to quickly reach and touch the experimenter's finger with their own. Initially, reaching was inaccurate due to the prism-induced change in visual direction, but as the response was closed loop (i.e. there was visual feedback on completion of each ballistic hand movement), reaching became accurate after a few trials, as is common in prism adaptation experiments (Welch et al., 1993). The reaching exercise was repeated with the other prism (monocularly) and then binocularly until the patient's hand movements were swift and accurate. The patient was asked to repeat the reach and touch exercises at home for objects detected through the prisms as often as possible; initially through the prism eye only (i.e. monocularly) and then with both eyes open when the patient felt proficient with the prism-eye only exercise. At subsequent visits, the patient was questioned about mobility with the Trifield glasses and the visual-direction training tasks and further instruction and training was provided if necessary.
Clinical Success
Clinical success was measured as the percent of patients who chose to continue to wear the Trifield glasses on conclusion of the wearing trial (visit 5). At visit 5 and long term follow-up (Fig. 3), the investigators recorded responses to questions covering use, benefits, and difficulties attributed to the glasses. At the visit 5 interview, a joint (patient and clinician) decision was made based upon the patient's perceived benefit from the Trifield glasses and the clinician's opinion, as to whether the patient should continue to wear them. If the patient continued wearing Trifield glasses, there was no cost to the patient, and no further clinical management was provided at SERI.
We asked patients to wear the Trifield glasses as much as possible during the course of the wearing trial. The Trifield glasses were designed as mobility devices, so they were only intended for use when walking, not when performing other tasks, such as reading, working at a desk or watching TV. Following fitting of the Trifield glasses (visit 2) patients were given a take-home wearing diary to record the number of hours per day that the glasses were worn. The form also had an open-ended comments section in which patients could record any difficulties or benefits experienced with the Trifield glasses. Patients were asked to return the diary to us at the subsequent visit and were given a new diary at visit 3 and visit 4 with the same instruction. During the interview at each visit, the investigator asked the patient for an estimate of the hours worn per day since the last visit.
Visual Field Expansion
Visual field extent was assessed with the 12mm stimuli on the computerized perimeter at visit 5. Extent of each monocular visual field was defined as the distance between the farthest lateral edges of the monocular visual field (Fig. 4a). The binocular visual field without the prism was defined as the width of the horizontal extent that would be covered by either eye (Fig 4a).
The visual field with Trifield glasses (Fig 4b) was measured in two steps. First, the patient fixated with the non-prism eye (left eye in Fig 4b) and tilted his head slightly to the right. That head shift brought the left prism in front of his right eye. The visual field of the left eye was measured under this condition (using standard kinetic perimetry) followed by the measurement of the visual field of the right eye that covered a field of view to the left of the fixation target. The patient then turned his head a little to the left to bring the right prism in front of his right eye. The visual field expansion to the right of fixation was then measured. While the patient had at any instant only one of the two peripheral extension areas, we defined the full extent of the visual field as the width covered with these three areas (double arrow in Fig 4b) as they represent potential visual field coverage at different times and available through scanning eye and head movements. As shown in Fig. 4b, sometimes this significantly overestimated the amount of visual field afforded by the Trifield glasses. This was especially true in the case illustrated where, apparently, the patient was more exophoric at the time of testing than during the fitting process (visit 1).
The enrolment criterion of no more than 20° diameter monocular central visual field was selected because the prism powers of much above 20° (36Δ) would impact visual acuity and are so thick that the prisms could impinge on the face and be uncomfortable or cause injury.
Perceived Direction
Initially the perceived direction of an object seen through a prism is not veridical (i.e. not in its true direction). People can adapt the perceived direction of objects seen through a prism (Kohler, 1964; Pick et al., 1969). Kohler (1964) reported a “dual” adaptation after full time wearing a binocular half-prism for 10 days. Initially, he reported adaptation to the directional change and visual distortions affected by the prisms. However, when looking through the clear portion of the spectacles, after-effects of adaptation to the prism were perceived (i.e., he perceived distortions and inversed directional change). After additional wearing time, these after-effects dissipated as well, and a “dual” adaptation was noted so that perception was correct both through the prism and through the prism-free segment of the lenses. We hypothesized that patients wearing the Trifield glasses may adapt to the directional change through the monocular prisms in a similar manner.
To measure adaptation to perceived direction we used a pointing task at visits 2, 3, 4 and 5. Patients were seated 1m from a 64° by 81° rear-projection screen. Large head movements were restricted by a chin rest and a tight fitting headband from a binocular indirect ophthalmoscope that was connected to the head rest. This experimental set-up is illustrated in Fig. 5 of Giorgi et al. (2009). Patients indicated the perceived lateral direction of stimuli on the screen using a large graphic bitpad tablet (90 by 120 cm) and a computer mouse held in the hand. Patients wore a brace to limit flexion of the elbow and were asked not to flex the elbow or the wrist, so that pointing was from the shoulder. Pointing was open-loop with no visual feedback, as a wooden box over the bitpad prevented a view of the arm, hand or mouse. Using small counter-rotations of the head, as used in visual field testing, the left and right extended areas were tested separately in consecutive sessions. In each session, stimuli were presented one at a time to the visual field of the non-prism eye or the prism eye. The fixation cross was always viewed with the non-prism eye.
A calibration step conducted without the prism glasses adjusted for individual differences in pointing responses. During calibration the patient turned his eyes (but not head) towards each approximately eye-level target. During perceived direction testing the non-prism eye fixated a central target.
The difference in the perceived visual direction for targets presented in the prism field of view and those presented in the visual field of the non-prism eye was used as the outcome measure. Adaptation of perceived visual direction would appear as no difference in these two directions. Lack of adaptation would appear as a persistent difference in reported direction, with the expected separation being the prism power. Figures 7 and 8 of Giorgi et al. (2009) show, for peripheral prisms for hemianopia, the effect of adaptation and the outcomes of similar measurements.
Perceived Visual Ability
Patients were administered two questionnaires during the first and last visits of the wearing trial. Both questionnaires, an early (1995) version of the National Eye Institute Visual Functioning Questionnaire-25 (NEI-VFQ: Mangione et al., 2001) and the Independent Mobility Questionnaire (IMQ: Turano et al., 1999a), contained items relating to mobility. The patients reported their perceived levels of difficulty for all items in each questionnaire. We performed planned analyses on responses to 27 mobility and obstacle avoidance items that we hypothesized would be affected by the use of Trifield glasses. The chosen items from the 1995 version of the NEI-VFQ were 19, 23, 31, 33, 34, 41, 43, 45 and 47 and from the IMQ were 2, 4, 6, 7, 8, 10, and 24 to 35. During interviews and in diaries, about half the patients reported increased difficulty with low-light situations when using the Trifield glasses. Though all 12 patients already had difficulties with low-light situations due to their condition and the prism lens reduced light transmission due to the tint, as the other eye had a non-tinted conventional spectacle lens, we had not expected increased problems with low-light situations. Post-hoc analyses were conducted on 6 lighting-related items from the two questionnaires: 22 from the NEI-VFQ and 19 to 23 from the IMQ (the text of all analysed items is reported in the Appendix).
Non-parametric tests were used in the data analysis. The Wilcoxon test was used for paired comparisons and the Mann-Whitney test for unpaired comparisons. We report test results as statistically significant when p ≤ 0.05. However, because of our small sample size, we have reported test results as “approaching” significance when 0.05 < p ≤ 0.10. As there were multiple comparisons (33 NEI-VFQ and IMQ items), if an experiment-wise error rate correction was applied the p (α-level) for significance would be p < 0.006, when the Bonferroni adjustment is corrected for the for correlation between items (average r=0.41) (Sankoh et al., 1997).
Results
Clinical Success
As shown in Fig. 3, of the fifteen patients who met the eligibility criteria at visit 1, three patients declined to participate, and twelve were dispensed a pair of Trifield glasses at visit 2. Unless otherwise noted, analyses of data on outcome measures were restricted to the twelve patients who completed the wearing trial.
Of the 12 patients who completed the wearing trial, 9 were wearing the Trifield glasses when interviewed at visit 5, and all 9 (75% of those completing the wearing trial) chose to continue to wear following the wearing trial (visit 5). Male patients were more likely to be successful (Fisher exact test, p=0.045). Success was not related to the eye fitted with the prisms, long cane use or having received orientation and mobility training (Fisher exact test, p>0.52). There was no significant difference between successful and non-successful patients for age, visual acuity, stereoacuity (Titmus stereotest), visual field width or the number of hours that the Trifield glasses were worn (Mann-Whitney test, z11<1.1, p>0.37), while non-successful patients had a tendency to have wider visual fields with the Trifield glasses (Mann-Whitney test, z11=1.94, p=0.064). Patients not wishing to continue to wear the Trifield glasses returned their glasses at the final visit. At the long-term telephone follow-up (median = 47 weeks, range 35 to 78), 3 of the 9 patients reported still wearing Trifield glasses.
At the visit 5 interview, 6 of 9 (67%) successful wearers reported benefits of the Trifield glasses as useful in avoiding collision with objects and passersby, as did one of the three non-successful wearers (7 of all 12 patients: 58%). At the same time, 5 of the 9 (56%) successful wearers reported some difficulties associated with the Trifield glasses in “street crossing”, 6 of 9 with “crowds”, with one patient describing crowded environments as “visual noise”. All 3 non-successful patients cited “crowds” as a difficulty, and these 3 patients reported insufficient observed benefit using the Trifield glasses as the reason to discontinue use.
During interviews and in diaries, patients made comments about perceived benefits and difficulties with Trifield glasses. All 12 patients reported improved detection of objects to the side, including corridors, footpaths (sidewalks) and street crossing. Five patients reported benefits in supermarkets, with navigation, obstacle avoidance and with locating items on shelves (visual search) and one patient even reported an improved ability to follow games of ice hockey (fast action). Difficulties that were reported included double vision (n=6), steps and stairs (n=7), vertigo (n=3) and low-light situations (n=6).
Visual Field Expansion
Visual field expansion was recorded for all patients completing the wearing trial (Table 1), as illustrated in Fig. 4. The median absolute difference between the expanded visual field and the normal visual field was 18° (range 9 to 38) for the 12 patients completing the wearing trial. The median ratio of visual field with-to-without the Trifield glasses was 2.6 (range 1.6 to 4.0).
Perceived Direction
No patient in the study demonstrated adaptation to the prismatic image displacement. Possible reasons for the lack of adaptation are addressed in the discussion.
Perceived Visual Ability
We hypothesized that Trifield glasses would affect the perceived visual ability of the patients, either benefiting or hindering them in their daily mobility situations. Analyses were performed for all 12 patients. One of those patients did not complete the visit 5 questionnaires for health reasons. Planned analyses were performed on the 27 items considered pertinent to mobility (Appendix). Comparing wearing trial start (visit 1) and end (visit 5), wearing Trifield glasses may have increased the perceived difficulty of visiting with people in their homes, at parties, or in restaurants (Wilcoxon signed ranks z10=2.24, p=0.025) and the successful patients may have been more likely to report that they stay at home most of the time because of my eyesight (Wilcoxon signed ranks z8=1.63, p=0.10). Comparing successful and non-successful patients, for NEI-VFQ items, at the start of the wearing trial successful patients may have been less likely to report that they stay at home most of the time because of my eyesight (Mann-Whitney Z11 = 1.90, p = 0.10) than non-successful patients, while there were no significant differences at wearing trial end. For the IMQ items, at wearing trial start, successful-wearers may have reported greater difficulty in moving about at work (Mann-Whitney Z8 = 2.16, p = 0.06), avoiding bumping into walls (Mann-Whitney Z11 = 1.79, p = 0.10) and avoiding shoulder height objects (Mann-Whitney Z11 = 2.06, p = 0.06) than non-successful patients. At wearing trial end, non-successful patients may have reported more difficulty walking in unfamiliar areas (Mann-Whitney Z10 = 2.08, p = 0.07), being aware of another person's presence (Mann-Whitney Z10 = 2.13, p = 0.04) and avoiding bumping into knee-high objects (Mann-Whitney Z10 = 1.97, p = 0.07) than successful patients.
Post-hoc analyses were performed on the six lighting-related items (Appendix). Comparing wearing trial start (visit 1) and end (visit 5), there were no significant changes in responses for all 12 patients. However, the nine successful patients may have reported less difficulty adjusting to lighting changes during the day outdoor to indoor (Wilcoxon signed ranks z7=1.90, p=0.06) and going down steps, stairs, or curbs in dim light or at night (Wilcoxon signed ranks z8=2.45, p=0.01) at the end of the wearing trial, after use of Trifield glasses, compared to visit 1. Thus, despite patients making comments about low-light situations with the Trifield glasses, the perceived level of difficulty in some low-light situations may have improved.
Discussion
Initial success with the Trifield glasses appeared to be very high as 75% (9 of 12 patients who completed the wearing trial) felt that they benefited from the Trifield glasses and wanted to continue to wear the Trifield glasses following about 2 months of experience. However, after about a year only 25% (3 of the 12 who completed the 6-week wearing trial) continued to wear the Trifield glasses. Even that reduced success rate (25%) may be considered high in view of the lack of effective visual aids for patients with tunnel vision. Yet, it is relatively low when compared to about 47% (Bowers et al., 2008) and 42% (Giorgi et al., 2009) found for long term retention of the prismatic correction for hemianopia. The decline in use with time suggests that the perceived benefits were either eliminated or were overcome by the perceived limitations and difficulties of the Trifield glasses. We believe that it is the latter. While patients with tunnel vision have greater impairment of mobility than those with hemianopia and thus would be expected to benefit more from a visual aid, the Trifield glasses are more difficult to use and may present more difficulties than the peripheral prism correction for hemianopia. The peripheral prisms do not interfere with central vision and thus do not affect visual acuity. Trifield glasses affect central vision and, at least in one eye, the relatively high power prisms may reduce visual acuity and cause spatial distortions. While the peripheral prism glasses use higher-power, lower-image-quality Fresnel prisms, their placement out of central vision makes these limitations less apparent and easier to use. However, it appears that the greatest difficulty in adapting to the Trifield glasses was the central double vision experienced as visual confusion. Double vision was reported by six patients in patient diaries or during interviews. The visual confusion occurs because objects (potential obstacles) that would not be detected without the prism lens can be detected, but they appear to be in a different (prism shifted) direction, superimposed on another object in the environment seen by the non-prism eye. This double vision is known to be uncomfortable, annoying and disturbing in central vision. Peripheral double vision as occurs with physiological diplopia (Peli, 2000) is common and thus easier to accept.
Based on the findings of Kohler (1964) we hypothesized that at least some patients would obtain veridical perceived direction of objects seen through the prism segments. This did not happen. The reason for the lack of adaptation of the type described by Kohler may include the short and intermittent wearing times (0.6 to 3.8 hours/day even for successful patients) as compared to full time wear by Kohler and his subjects, and that our Trifield prisms were fitted monocularly and not binocularly as did Kohler, and the higher power of the prisms we fitted, at least for some of the patients.
Patients with tunnel vision at the level enrolled in our study have had many years of slow progression of their visual field loss. It appears that many of them have slowly adapted to the situation by gradually changing their life style to reduce the challenges of pedestrian mobility as much as possible. As a result, these patients have reduced opportunities to walk and to gain experience with the Trifield glasses. Most of these patients also suffer from night blindness and that reduces their mobility further. This is particularly true in a place like Boston when the light hours after work hours are limited for most of the year (Bowers et al., 2003). Thus, our patients had fewer opportunities to walk with the Trifield glasses and to learn to benefit from them. Our patients wore the glasses for a median of 1.2 hours per day (range 0.6 to 3.8), whereas, patients with hemianopia in comparable studies wore their peripheral prism glasses for a median of 3.0 hours per day (range 1.0 to 13.4: Giorgi et al., 2009) to 8.0 hours per day (range 1.0 to 16: Bowers et al., 2008).
Using the Trifield glasses when reading may be possible, but is certainly uncomfortable due to the visual confusion. Thus, the user has to remove the glasses or shut his prism eye even for spot reading (e.g. reading labels or price tags in stores) or reading distance text such as street signs. This additional limitation may have contributed to the eventual rejection of the Trifield glasses by some patients. In comparison the peripheral prisms for hemianopia may be used with small bifocal segments that permit spot reading with little difficulty.
Long-term discontinuation may have been related to a lack of continued clinical care by the prescriber (us) following the end of the 5-visit wearing trial. At visit 5 patients were advised to continue receiving normal clinical care. However, that clinical care would not have provided advice on this experimental device. It is possible that follow up care from the prescriber, with further instruction on the use of Trifield glasses, might have resulted in a higher long-term retention rate.
The Trifield lenses expand the visual field only laterally, which may be important in avoiding collisions with other pedestrians and some obstacles. However, the lower visual field is known to be of more importance for mobility (Lovie-Kitchin et al., 1990) and was not expanded at all by the Trifield glasses. An earlier design included a lower base-down prism segment for that purpose. This was eliminated when it became apparent that the prism powers practical in our design provided a lower visual field expansion that was too small to be meaningful. This aspect needs to be reconsidered in future designs of devices for tunnel vision. The use of tinted prism lenses was based on feedback during pilot testing, in which the patients with tunnel vision reported difficulty determining the direction of a detected object, particularly which side (i.e. from which prism). As all the patients in our study had difficulty in low light conditions (night vision, dark adaptation), there was the risk that the tints would have a negative impact on object detection. Two patients reported that the red lens was better and two patients reported that the green lens was better. It is not clear whether these differences were related to tint or to other issues. Six patients reported that the tint seemed to cause a (greater) problem with dark adaptation or they were difficult to use in low light conditions, however, that is not consistent with the perceived visual ability questionnaire responses, for which there was some modest improvement. We did not assess whether the tints provided a real benefit, and patients did not report being able to easily make use of the different colour appearances from the two prisms, perhaps due to colour adaptation. In the future, the tints might be used only during a training period and be removed once the patient became familiar with the mobility device.
We have developed (Vargas-Martin and Peli, 2002) and tested (Vargas-Martin and Peli, 2001; Peli et al., 2007; Luo et al., 2009) an augmented-vision system using a head-mounted display which can be used by patients with one or two eyes. The electronic system is much more complicated to maintain and probably much more expensive and thus, although it may be an attractive option, we still consider the Trifield glasses as a potentially useful design perhaps with some modifications of the design, the fitting procedures and training in its use.
Acknowledgments
Supported in part by a grant from the Joint SERI-MEEI Clinical Research Center, by NIH grant #EY12890 (EP), and by the Foundation Fighting Blindness (ELB). Dan Stringer and Jenn Segawa helped with data collection. Bob Goldstein developed the software for measurement of the perceived direction. Karen Keeney (Chadwick Optical, White River Junction, VT) was instrumental in the development of techniques to make the Trifield glasses.
Appendix
The text of items in the two perceived visual ability questionnaires that we hypothesised would be affected by the Trifield glasses. Patients rated all of the following items in terms of degree of perceived difficulty on a scale of 1 to 5. For the 1995 version of the NEI-VFQ (Mangione et al., 2001) the scale for items 19 to 34 was described as going from “No difficulty at all” (1) to “Stopped doing this because of your eyesight” (5) and for items 41 to 47 was described as going from “Definitely true” (1) to ” Definitely false” (5). For the IMQ (Turano et al., 1999a) the scale was described as going from “represents no difficulty” (1) to “represents extreme difficulty” (5).
National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25) (1995 version) (Mangione et al., 2001)
Because of your eyesight, how much difficulty do you have finding something on a crowded shelf?
Because of your eyesight, how much difficulty do you have going down steps, stairs, or curbs in dim light or at night?
Because of your eyesight, how much difficulty do you have noticing objects off to the side?
Because of your eyesight, how much difficulty do you have visiting with people in their homes, at parties, or in restaurants?
Because of your eyesight, how much difficulty do you have taking part in active sports or other outdoor activities that you enjoy (like golf, bowling, jogging, or walking)?
Because of your eyesight, how much difficulty do you have going out to see movies, plays or sports events?
I stay at home most of the time because of my eyesight.
I have much less control over what I do, because of my eyesight.
I don't go out of my home alone, because of my eyesight.
I need a lot of help from others because of my eyesight.
Independent Mobility Questionnaire (IMQ) (Turano et al., 1999a)
Walking in unfamiliar areas?
Moving about at work?
Moving about in stores?
Moving about outdoors?
Moving about in crowded situations?
Moving about using public transportation?
Adjusting to lighting changes during the day - indoor to outdoor?
Adjusting to lighting changes during the day - outdoor to indoor?
Adjusting to lighting changes during at night - indoor to outdoor?
Adjusting to lighting changes during at night - outdoor to indoor?
Walking in dimly lit indoor areas?
Being aware of another person's presence?
Avoiding bumping into people?
Avoiding bumping into walls?
Avoiding bumping into head height objects?
Avoiding bumping into shoulder height objects?
Avoiding bumping into waist height objects?
Avoiding bumping into knee height objects
Avoiding bumping into low lying objects?
Avoiding tripping over uneven travel surfaces?
Moving around in social gatherings?
Finding restrooms in public places?
Seeing cars at intersections?
References
- Apfelbaum H, Pelah A, Peli E. Heading assessment by “tunnel vision” patients and control subjects standing or walking in a virtual reality environment. ACM Trans Appl Percept. 2007;4 doi: 10.1145/1227134.1227142. article 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditi A, Cagenello R. On the statistical reliability of letter-chart visual acuity measurements. Invest Ophthalmol Vis Sci. 1993;34:120–129. [PubMed] [Google Scholar]
- Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–432. [PubMed] [Google Scholar]
- Benjamin WJ, Borish IM. Presbyopia and the influence of aging on prescription of contact lenses. In: Rubin M, Guillon M, editors. Contact Lens Practice. Chapman & Hall Medical; London: 1994. pp. 800–802. [Google Scholar]
- Berson EL. Retinitis Pigmentosa: The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- Biderman A, Cwikel J, Fried AV, Galinsky D. Depression and falls among community dwelling elderly people: A search for common risk factors. J Epidemiol Community Health. 2002;56:631–636. doi: 10.1136/jech.56.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black A, Lovie-Kitchin JE, Woods RL, Arnold N, Byrnes J, Murrish J. Mobility performance in retinitis pigmentosa. Clin Exp Optom. 1997;80:1–12. [Google Scholar]
- Bowers AR, Keeney K, Peli E. Community-based trial of peripheral prism visual expansion device for hemianopia. Arch Ophthalmol. 2008;126:657–664. doi: 10.1001/archopht.126.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AR, Luo G, Peli E. Functionally relevant illumination levels for evaluation of a new night vision device (abstract) Invest Ophthalmol Vis Sci. 2003;44:2772. [Google Scholar]
- Broman AT, West SK, Munoz B, Bandeen-Roche K, Rubin GS, Turano KA. Divided visual attention as a predictor of bumping while walking: The Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 2004;45:2955–2960. doi: 10.1167/iovs.04-0219. [DOI] [PubMed] [Google Scholar]
- Cacciatore F, Abete P, Maggi S, Luchetti G, Calabrese C, Viati L, Leosco D, Ferrara N, Vitale DF, Rengo F. Disability and 6-year mortality in elderly population. Role of visual impairment. Aging Clin Exp Res. 2004;16:382–388. doi: 10.1007/BF03324568. [DOI] [PubMed] [Google Scholar]
- Cohen JM. An overview of enhancement techniques for peripheral field loss. J Am Optom Assoc. 1993;64:60–70. [PubMed] [Google Scholar]
- Cohen JM, Waiss B. Visual field remediation. In: Rosenthal BP, editor. Remediation and Management of Low Vision. Mosby; St. Louis: 1996. pp. 1–25. [Google Scholar]
- Drasdo N. Visual field expanders. Am J Optom Physiol Opt. 1976;53:464–467. doi: 10.1097/00006324-197609010-00005. [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Hannan MT, Milton RC, Wilson PWF, Kiel DP. Impaired vision and hip fracture. The Framingham study. J Am Geriatr Soc. 1989;37:495–500. doi: 10.1111/j.1532-5415.1989.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Fortenbaugh FC, Chaudhury S, Hicks JC, Hao L, Turano KA. Gender differences in cue preference during path integration in virtual environments. ACM Trans Appl Percept. 2007;4 article 6. [Google Scholar]
- Freeman EE, Munoz B, Rubin GS, West SK. Visual field loss increases the risk of falls in older adults: The Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 2007;48:4445–4450. doi: 10.1167/iovs.07-0326. [DOI] [PubMed] [Google Scholar]
- Giorgi RG, Woods RL, Peli E. Clinical and experimental evaluation of peripheral prism glasses for hemianopia. Optom Vis Sci. 2009;86:492–502. doi: 10.1097/OPX.0b013e31819f9e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Haymes S, Guest D, Heyes A, Johnston A. Mobility of people with retinitis pigmentosa as a function of vision and psychological variables. Optom Vis Sci. 1996;73:621–637. doi: 10.1097/00006324-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Hoeft WW, Feinbloom W, Brilliant R, Gordon R, Hollander C, Newman J, Novak E. Amorphic lenses: A mobility aid for patients with retinitis pigmentosa. Am J Optom Physiol Opt. 1985;62:142–148. [PubMed] [Google Scholar]
- Kiser AK, Mladenovich D, Eshraghi F, Bourdeau D, Dagnelie G. Reliability and consistency of visual acuity and contrast sensitivity measures in advanced eye disease. Optom Vis Sci. 2005;82:946–954. doi: 10.1097/01.opx.0000187863.12609.7b. [DOI] [PubMed] [Google Scholar]
- Kohler I. The formation and transformation of the visual world. Psychol Issues. 1964;3:14–173. [Google Scholar]
- Kozlowski JM, Mainster MA, Avila MP. Negative-lens field expander for patients with concentric field constriction. Arch Ophthalmol. 1984;102:1182–1184. doi: 10.1001/archopht.1984.01040030960025. [DOI] [PubMed] [Google Scholar]
- Kuyk T, Elliott J, Fuhr PS. Visual correlates of obstacle avoidance in adults with low vision. Optom Vis Sci. 1998;75:174–182. doi: 10.1097/00006324-199803000-00022. [DOI] [PubMed] [Google Scholar]
- Kuyk T, Elliott JL, Wesley J, Scilley K, McIntosh E, Mitchell S, Owsley C. Mobility function in older veterans improves after blind rehabilitation. J Rehabil Res Dev. 2004;41:337–346. doi: 10.1682/jrrd.2003.03.0038. [DOI] [PubMed] [Google Scholar]
- Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001;49:508–515. doi: 10.1046/j.1532-5415.2001.49107.x. [DOI] [PubMed] [Google Scholar]
- Lord SR, Ward JA, Williams P, Anstey KJ. An epidemiological study of falls in older community-dwelling women: The Randwick falls and fractures study. Aust J Public Health. 1993;17:240–245. doi: 10.1111/j.1753-6405.1993.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Lovie-Kitchin J, Mainstone J, Robinson J, Brown B. What areas of the visual field are important for mobility in low vision patients? Clin Vision Sci. 1990;5:249–263. [Google Scholar]
- Luo G, Peli E. Use of an augmented-vision device for visual search by patients with tunnel vision. Invest Ophthalmol Vis Sci. 2006;47:4152–4159. doi: 10.1167/iovs.05-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Vargas Martin F, Peli E. The role of peripheral vision in saccade planning: Learning from people with tunnel vision. J Vis. 2008;8:1–8. doi: 10.1167/8.14.25. article 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Woods RL, Peli E. Collision judgement when using an augmented-vision head-mounted display device. Invest Ophthalmol Vis Sci. 2009;50:4509–4515. doi: 10.1167/iovs.08-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-Item National Eye Institute Visual Function Questionanaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- Marron JA, Bailey IL. Visual factors and orientation-mobility performance. Am J Optom Physiol Opt. 1982;59:413–426. doi: 10.1097/00006324-198205000-00009. [DOI] [PubMed] [Google Scholar]
- Melzer D, Lan TY, Guralnik JM. The predictive validity for mortality of the index of mobility-related limitation - results from the EPESE study. Age Ageing. 2003;32:619–625. doi: 10.1093/ageing/afg107. [DOI] [PubMed] [Google Scholar]
- Onufryk M. Ophthalmic prismatic image relocating eye glasses for persons having retinitis pigmentosa and hemianopia and method for making same. USA Patent #5,323,190 1994
- Peli E. Field expansion for homonymous hemianopia by optically-induced peripheral exotropia. Optom Vis Sci. 2000;77:453–64. doi: 10.1097/00006324-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Peli E. Vision multiplexing: An engineering approach to vision rehabilitation device development. Optom Vis Sci. 2001;78:304–315. doi: 10.1097/00006324-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Peli E. Treating with spectacle lenses: A novel idea!? Optom Vis Sci. 2002;79:569–580. doi: 10.1097/00006324-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Peli E, Luo G, Bowers A, Rensing N. Applications of augmented vision head-mounted systems in vision rehabilitation. J Soc Inf Disp. 2007;15:1037–1045. doi: 10.1889/1.2825088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick HL, Hay JC, Martin R. Adaptation to split-field wedge prism spectacles. J Exp Psychol. 1969;80:125–132. doi: 10.1037/h0027111. [DOI] [PubMed] [Google Scholar]
- Reeves BC, Wood JM, Hill AR. Vistech VCTS 6500 charts - within- and between-session reliability. Optom Vis Sci. 1991;68:728–737. doi: 10.1097/00006324-199109000-00010. [DOI] [PubMed] [Google Scholar]
- Ross NC, Bowers AR, Peli E. Consideration of optical scotomas in designing visual field expansion devices (abstract) Invest Ophthalmol Vis Sci. 2009;50:E-4734. [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statist Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Shinkai S, Watanabe S, Kumagai S, Fujiwara Y, Amano H, Yoshida H, Ishizaki T, Yukawa H, Suzuki T, Shibata H. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing. 2000;29:441–446. doi: 10.1093/ageing/29.5.441. [DOI] [PubMed] [Google Scholar]
- Siejas O, Gomez De Liano P, Gomez De Liano R, Roberts CJ, Piedrahita E, Diaz E. Ocular dominance diagnosis and its influence in monovision. Am J Ophthalmol. 2007;144:209–216. doi: 10.1016/j.ajo.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Somani S, Brent MH, Markowitz SN. Visual field expansion in patients with retinitis pigmentosa. Can J Ophthalmol. 2006;41:27–33. doi: 10.1016/S0008-4182(06)80062-1. [DOI] [PubMed] [Google Scholar]
- Soong GP. Unpublished PhD Thesis. Queensland University of Technology; Brisbane, Australia: 2000. The Effect of Orientation and Mobility Training on Vision and Mobility Performance in Visually Impaired Adults. [Google Scholar]
- Soong GP, Lovie-Kitchin JE, Brown B. Does mobility performance of visually impaired adults improve immediately after orientation and mobility training? Optom Vis Sci. 2001;78:657–666. doi: 10.1097/00006324-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Szlyk JP, Seiple W, Laderman DJ, Kelsch R, Ho K, McMahon T. Use of bioptic amorphic lenses to expand the visual field in patients with peripheral loss. Optom Vis Sci. 1998;75:518–524. doi: 10.1097/00006324-199807000-00021. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- Turano KA, Geruschat DR, Baker FH, Stahl JW, Shapiro MD. Direction of gaze while walking a simple route: Persons with normal vision and persons with retinitis pigmentosa. Optom Vis Sci. 2001;78:667–675. doi: 10.1097/00006324-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Turano KA, Geruschat DR, Stahl JW, Massof RW. Perceived visual ability for independent mobility in persons with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999a;40:865–877. [PubMed] [Google Scholar]
- Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999b;40:2803–2809. [PubMed] [Google Scholar]
- Vargas-Martin F, Peli E. Digest of Technical Papers SID 01 XXXII. Society for Information Display; San Jose, CA: 2001. Augmented view for tunnel vision: device testing by patients in real environments; pp. 602–605. [Google Scholar]
- Vargas-Martin F, Peli E. Augmented-view for restricted visual field: Multiple device implementations. Optom Vis Sci. 2002;79:715–723. doi: 10.1097/00006324-200211000-00009. [DOI] [PubMed] [Google Scholar]
- Vargas-Martin F, Peli E. Eye movements of patients with tunnel vision while walking. Invest Ophthalmol Vis Sci. 2006;47:5295–5302. doi: 10.1167/iovs.05-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili G, Rubin G. Orientation and mobility training for adults with low vision. Cochrane Database Syst Rev. 2006;3:CD003925. doi: 10.1002/14651858.CD003925.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss NJ. Remediation of peripheral visual field defects in low vision patients. Probl Optom. 1992;4:34–54. [Google Scholar]
- Welch RB, Bridgeman B, Anand S, Browman K. Alternating prism exposure causes dual adaptation and generalization to a novel displacement. Percept Psychophys. 1993;54:195–204. doi: 10.3758/bf03211756. [DOI] [PubMed] [Google Scholar]
- Woods RL. Reliability of visual performance measurement under optical degradation. Ophthalmic Physiol Opt. 1993;13:143–150. doi: 10.1111/j.1475-1313.1993.tb00443.x. [DOI] [PubMed] [Google Scholar]


