Abstract
The purpose of this study was to determine the pharmacokinetics of codeine and the active metabolites morphine and codeine-6-glucuronide after IV codeine administration and the pharmacokinetics of acetaminophen (APAP), codeine, morphine, and codeine-6-glucuronide after oral administration of combination product containing acetaminophen and codeine to dogs.
Six healthy Greyhound dogs were administered 0.734 mg/kg codeine IV and acetaminophen (10.46 mg/kg mean dose) with codeine (1.43 mg/kg mean dose) orally. Blood samples were obtained at predetermined time points for the determination of codeine, morphine, and codeine-6-glucuronide plasma concentrations by LC/MS and acetaminophen by HPLC with UV detection.
Codeine was rapidly eliminated after IV administration (T½ =1.22 hr; clearance=29.94 mL/min/kg; volume of distribution=3.17 L/kg) with negligible amounts of morphine present, but large amounts of codeine-6-glucuronide (CMAX=735.75 ng/mL) were detected. The oral bioavailability of codeine was 4%, morphine concentrations were negligible, but large amounts of codeine-6-glucuronide (CMAX=1952.86 ng/mL) were detected suggesting substantial first pass metabolism. Acetaminophen was rapidly absorbed (CMAX=6.74 μg/mL; TMAX=0.85 hr) and eliminated (T½=0.96 hr).
In conclusion, the pharmacokinetics of codeine were similar to other opioids in dogs with a short half-life, rapid clearance, large volume of distribution, and poor oral bioavailability. High concentrations of codeine-6-glucuronide were detected after IV and oral administration.
Keywords: pharmacokinetics, codeine, morphine, codeine-6-glucuronide, metabolite
Introduction
Codeine is a naturally occurring opioid with a complex and incompletely understood mechanism of action. Codeine is metabolized to numerous metabolites including morphine and codeine-6-glucuronide, which in addition to codeine appear to contribute to analgesic effects after codeine administration to humans (Lötsch, et al, 2006). Human extensive CYP2D6 metabolizers metabolize approximately 10% of codeine into morphine and morphine metabolites (Kirchheiner, et al, 2006). In contrast poor metabolizers convert approximately 0.3% of codeine into morphine. However poor metabolizers still produced a central miotic effect after codeine administration, which was considered an opiate mediated effect. The maximum plasma concentrations of codeine and codeine-6-glucuronide were 37 – 56 ng/mL and 626 – 841 ng/mL, respectively in the poor metabolizer group while the maximum morphine concentration never exceeded 1 ng/mL. In vitro receptor binding studies indicate the IC50 of codeine-6-glucuronide is 2100 ng/mL (Srinivasan, et al, 1997) however it is important to note that glucuronide conjugates are polar compounds and may not penetrate well into the CNS to bind with the opiate receptors. Peak plasma concentrations of codeine-6-glucuronide providing antinociceptive effects were 4176 ± 714 ng/mL in rats (Srinivasan, et al, 1997).
Codeine has been recommended as an orally administered analgesic in dogs (Gaynor, 2008), but no data are available to indicate it possesses efficacy in dogs. These recommendations however have to be interpreted cautiously as other opioids, such as morphine and oxycodone, are also recommended for oral administration (Gaynor, 2008), but data indicate these drugs are unlikely effective in dogs due to their poor oral bioavailability and lack of active metabolite formation in dogs (Weinstein & Gaylord, 1979; Yoshimura, et al, 1993; KuKanich, et al, 2005c).
Codeine administered 1 mg/kg SC to dogs resulted in significant antinociceptive effects in an electrical dental pulp stimulation model, but the duration of effect was short lived with values returning to baseline in less than 2 hours (Skingle & Tyers, 1980). Increasing doses (2 and 4 mg/kg SC) resulted in a greater duration of activity, but thresholds returned to baseline within 3 hours regardless of the dose. In comparison, morphine administered SC, 0.2 mg/kg, produced antinociceptive effects for 2–3 hours (Skingle & Tyers, 1980).
Little data are available on the pharmacokinetics of codeine in dogs. Two male Beagles administered 1.85 mg/kg codeine (base) PO resulted in an oral bioavailability of 6 and 7%, but these values are likely overestimated as the radioimmunoassay used had 19% cross-reactivity with codeine glucuronide which occurred at high concentrations, 600 –700 ng/mL (Findlay, et al, 1979). Morphine did not exceed 1 ng/mL in one of the Beagles, but the second Beagle peaked at ~ 2 ng/mL.
The pharmacokinetics of acetaminophen (APAP) in dogs have been previously reported (Savides, et al, 1984). The elimination half-life was 1.2 hours at doses of 100 and 200 mg/kg PO with a mean CMAX of ~45 μg/mL and ~90 μg/mL, respectively. No observable clinical toxicity was noted with the 4 dogs receiving 100 mg/kg PO whereas 3/4 dogs developed methemoglobinemia and one dog developed hematuria after 200 mg/kg PO.
The purpose of this study was to assess the pharmacokinetics of codeine, morphine, and codeine-6-glucuronide after IV administration of codeine and the pharmacokinetics of acetaminophen, codeine, morphine, and codeine-6-glucuronide after oral administration of a combination product containing acetaminophen and codeine.
Materials and methods
Animals
The Institutional Animal Care and Use committee at Kansas State University approved the study. Six healthy Greyhound dogs, 3 male and 3 female were used with an age range of 2 – 4 years and weight range of 27.4 – 39.3 kg.
Study design
The study consisted of a non-randomized block in which all animals received IV codeine first and oral codeine second. At least 7 days were allowed between treatments. Codeine phosphate (15 mg/mL, Hospira, Inc, Lake Forest IL, USA) was administered at a dose of 1 mg/kg (equivalent to 0.734 mg/kg codeine base) IV through an aseptically placed cephalic catheter (Surflo, 20 g × 1.5 inch, Terumo Medical Corp, Elkton, MD, USA) followed by administration of 9 mL sterile 0.9% saline to flush the catheter. Blood samples were obtained from an aseptically placed jugular catheter (Venocath-16, Abbott Ireland, Sligo, Republic of Ireland) prior to drug administration and at 10, 20, 30, and 45 minutes and at 1, 2, 3, 4, 6, and 8 hours after drug administration and placed in tubes containing heparin. Plasma was separated by centrifugation at 2 000 × g for 10 minutes and stored frozen at −70 C until analysis. The jugular catheters were flushed with 3 mL sterile 0.9% saline after each collection to maintain patency.
Codeine tablets (Ranbaxy Inc, Princeton, NJ, USA) containing 300 mg acetaminophen and 60 mg codeine phosphate (equivalent to 45 mg base), 1 tablet per animal, were administered per os to 12-hr fasted animals. Blood samples were obtained from an aseptically placed jugular catheter prior to drug administration and at 10, 20, 30, and 45 minutes and at 1, 2, 3, 4, and 6 hours after drug administration and placed in tubes containing heparin. Plasma was separated by centrifugation at 2 000 × g for 10 minutes and stored frozen at −70 C until analysis. The jugular catheters were flushed with 3 mL sterile 0.9% saline after each collection to maintain patency.
High-pressure liquid chromatography
Acetaminophen concentrations were determined using HPLC with ultraviolet (UV) detection at 243 nm. The HPLC consisted of an autosampler, quaternary pump, degasser, and photodiode array detector (Finnigan Spectrasystem, Thermo Electron Corporation, Waltham, MA, USA). The mobile phase consisted of 90% trifluoroacetic acid (0.02%) and 10% acetonitrile at a flow rate of 1 mL/min with separation achieved with a 4.6 × 150 mm × 5μM column (Supelco, Discovery C18, Sigma-Aldrich, St. Louis, IL, USA). Standard curves were prepared daily by fortifying blank plasma with acetaminophen (Sigma-Aldrich, St. Louis, IL, USA) at concentrations of 0 and from 0.05 to 20 μg/mL and were accepted if predicted values were within 15% of actual values and the correlation coefficient was at least 0.99. Solid phase extraction cartridges (Bond Elut C18, Varian, Palo Alto, CA, USA) were first conditioned with methanol (1 mL), followed by deionized (di) water (1 mL), the plasma sample or standard was loaded (0.5 mL), the SPE were rinsed with 5% methanol (1 mL), and the drug was eluted with methanol (1 mL). The eluate was evaporated to dryness in a water bath (temperature = 40 C) under a stream of air for 25 minutes and reconstituted with 200 μL of 15% methanol. The injection volume was 50 μL. The standard curves were linear from 0.05 – 20 μg/mL, the accuracy of the assay was within 6 ± 4% of the actual concentration and the coefficient of variation of the assay was 7 ± 4% on replicates of 5 at 0.05, 1, and 10 μg/mL.
Liquid chromatography / mass spectrometry
Codeine, morphine, and codeine-6-glucuronide concentrations were determined using liquid chromatography (Shimadzu Prominence, Shimadzu Scientific Instruments, Columbia, MD, USA) with mass spectrometry (API 2000, Applied Biosystems, Foster City, CA, USA). Codeine (Cerilliant, Round Rock, TX, USA) m/z 300→152.2, was quantified with codeine d3 (Cerilliant, Round Rock, TX, USA) m/z 303→152.2, as the internal standard with the plasma standard curve linear from 1 – 500 ng/mL. Morphine (Cerilliant, Round Rock, TX, USA) m/z 286→152.1, was quantified with morphine d6 (Cerilliant, Round Rock, TX, USA) m/z 292.1→151.9, as the internal standard with the plasma standard curve linear from 1 – 500 ng/mL. Codeine-6-glucuronide (Lipomed, Cambridge, MA, USA) m/z 476→300.2, was quantified with morphine d6 as the internal standard with the plasma standard curve linear from 2.5 – 2000 ng/mL. Initially codeine d3 was tried as the internal standard for codeine-6-glucuronide, but morphine d6 produced more accurate standard curves as the internal standard.
The mobile phase consisted of A: acetonitrile and B: 0.1% formic acid at a flow rate of 0.4 mL/min. A mobile phase consisted of 100% B from 0 – 0.5 m with a linear gradient to 70% B at 6 minutes, which was held until 7 minutes followed by a linear gradient to 100% B at 9 minutes with a total run time of 10 minutes. Separation was achieved with a 3.0 × 150 mm × 5 μM column (Zorbax XDB Phenyl, Agilent Technologies, Wilmington, DE, USA) heated at 40 C.
The internal standard solution (100 μL, containing 500 ng/mL codeine d3 and 500 ng/mL morphine d6) was added to 1 mL plasma and 1 mL 0.1 M borate buffer. Solid phase extraction cartridges (Bond Elut C18, Varian, Palo Alto, CA, USA) were first conditioned with methanol (1 mL), followed by deionized (di) water (1 mL), the plasma sample or standard mixture was loaded (2.1 mL), the SPE were rinsed with di water (1 mL), and the drug was eluted with methanol (1 mL). The eluate was evaporated to dryness in a water bath (temperature = 40 C) under a stream of air for 25 minutes and reconstituted with 200 μL of 25% methanol in 0.1% formic acid and filtered through a centrifugal filtration device (Costar, Spin-X, Corning Inc, Corning, NY, USA). The injection volume was 25 μL.
Pharmacokinetic Analysis
Pharmacokinetic analyses were performed with computer software (WinNonlin 5.2, Pharsight Corporation, Mountain View, CA, USA). The variables calculated included the area under the curve from time 0 to infinity (AUC0-inf) using the linear trapezoidal rule, area under the first moment curve from time 0 to infinity (AUMC0-inf), plasma clearance (Cl), plasma clearance per fraction of the dose absorbed (Cl/F), apparent volume of distribution at steady state (Vdss), apparent volume of distribution of the area during the elimination phase (Vz), apparent volume of distribution of the area during the elimination phase per fraction of the dose absorbed (Vz/F), first-order rate constant (λz), terminal half-life (T½λz), and mean residence time extrapolated to infinity (MRT). The maximum serum concentration (CMAX) and time to maximum serum concentration (TMAX) were determined directly from the data. The concentration at time 0 (C0) was calculated by log-linear regression using the first two time points after IV administration. The mean absorption time (MAT) was calculated by subtracting the IV MRT from the PO MRT. The fraction of the dose absorbed (F) for oral codeine was determined with equation 1: (AUC0-inf PO * Dose IV) / (AUC0-inf IV * Dose PO). The relative fraction of codeine-6-glucuronide formed (Frelative) for oral codeine was determined with equation 1.
Results
The treatments were well tolerated after both routes of administration. Mild sedation was present in some of the dogs after each route of administration. No vomiting occurred in either group. The mean (geometric) dose of codeine (base) administered PO was 1.43 mg/kg (range 1.13 – 1.62 mg/kg) while the mean dose of acetaminophen was 10.46 mg/kg (range 8.28 – 11.86 mg/kg).
Acetaminophen was rapidly absorbed with a mean CMAX of 6.74 μg/mL occurring at 0.85 hours (Table 1, Figure 1). Acetaminophen was also rapidly eliminated with a 0.96 hour terminal half-life.
Table 1.
Acetaminophen pharmacokinetic parameters after a single oral administration to 6 healthy Greyhound dogs.
| Geometric Mean | Min | Median | Max | ||
|---|---|---|---|---|---|
| Dose | mg/kg | 10.46 | 8.28 | 11.21 | 11.86 |
| AUCextrap | % | 2.04 | 1.68 | 1.88 | 2.04 |
| AUC0-inf | hr* μg /mL | 13.78 | 11.11 | 13.49 | 16.25 |
| AUMC0-inf | hr*hr* μg/mL | 27.12 | 20.03 | 27.29 | 37.38 |
| Cl/F | mL/min/kg | 12.65 | 10.55 | 12.11 | 17.70 |
| CMAX | μg/mL | 6.74 | 4.48 | 6.80 | 8.88 |
| T ½ λz | hr | 0.96 | 0.76 | 0.99 | 1.13 |
| λz | /hr | 0.73 | 0.61 | 0.70 | 0.92 |
| MRT | hr | 1.97 | 1.69 | 1.85 | 2.70 |
| TMAX | hr | 0.85 | 0.50 | 0.63 | 2.00 |
| Vz/F | L/kg | 1.05 | 0.86 | 1.04 | 1.57 |
Figure 1.
Plasma profile of acetaminophen after 10.6 ± 1.5 mg/kg PO to 6 healthy Greyhound dogs.
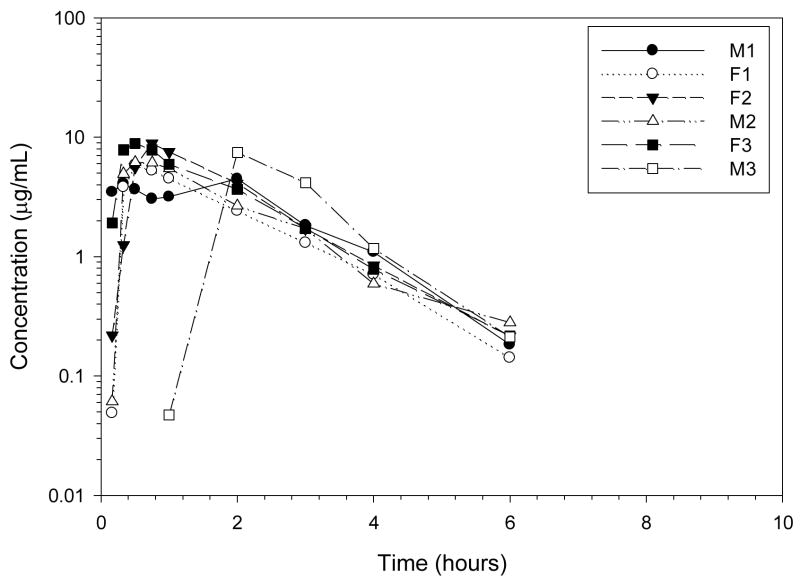
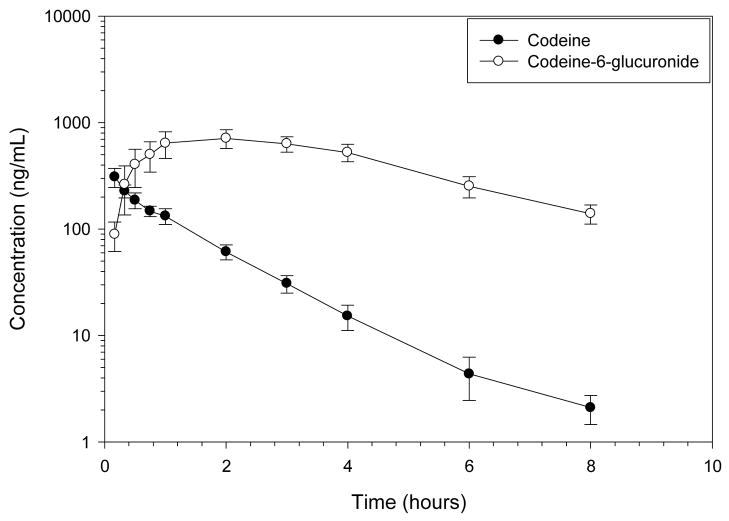
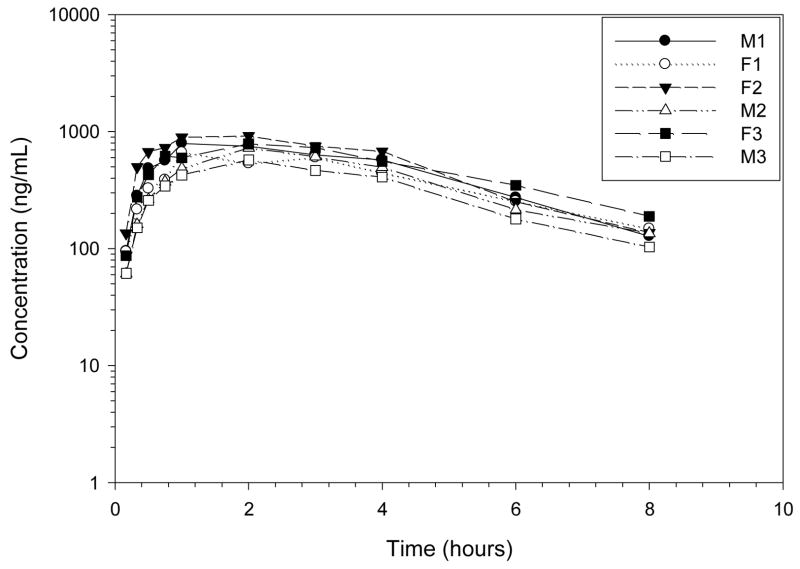
Codeine was rapidly eliminated after IV administration (Figures 2–4, Tables 2–3) with high concentrations of codeine-6-glucuronide formed. The terminal half-life of codeine was 1.22 hours, the clearance was rapid at 29.94 mL/min/kg, and the volume of distribution was large, 3.17 L/kg. The CMAX of codeine-6-glucuronide, 735.75 ng/mL, occurred at 1.59 hours after IV codeine administration. The terminal half-life of codeine-6-glucuronide was 2.25 hours after IV codeine administration.
Figure 2.
Plasma profile of codeine after codeine 0.734 mg/kg IV to 6 healthy Greyhound dogs.
Figure 4.
Mean ± SD plasma profile of codeine and codeine-6-glucuronide after codeine 0.734 mg/kg IV to 6 healthy Greyhound dogs. Morphine was not detected in any dog greater than 1 ng/mL.
Table 2.
Codeine pharmacokinetic parameters after codeine administration to 6 healthy Greyhound dogs.
| IV | Per Os | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean | Min | Median | Max | Geometric Mean | Min | Median | Max | ||
| Dose | mg/kg | 0.734 | 0.734 | 0.734 | 0.734 | 1.43 | 1.13 | 1.53 | 1.62 |
| AUCextrap | % | 0.91 | 0.71 | 0.89 | 1.28 | 9.56 | 3.02 | 10.40 | 21.57 |
| AUC0-inf | hr* ng/mL | 409.99 | 346.22 | 408.53 | 494.32 | 30.53 | 19.25 | 26.90 | 67.14 |
| AUMC0-inf | hr*hr*ng/mL | 550.29 | 465.34 | 513.64 | 756.81 | 91.18 | 75.33 | 85.84 | 130.87 |
| Cl | mL/min/kg | 29.94 | 24.84 | 30.05 | 35.46 | N/A | N/A | N/A | N/A |
| Cl/F | mL/min/kg | N/A | N/A | N/A | N/A | 1053.42 | 526.24 | 1045.05 | 1739.97 |
| C0 | ng/mL | 409.27 | 265.50 | 438.89 | 650.91 | N/A | N/A | N/A | N/A |
| CMAX | ng/mL | N/A | N/A | N/A | N/A | 10.01 | 3.82 | 8.34 | 39.90 |
| T ½ λz | hr | 1.22 | 0.89 | 1.28 | 1.37 | 1.60 | 1.12 | 1.57 | 2.04 |
| λz | /hr | 0.57 | 0.51 | 0.54 | 0.78 | 0.43 | 0.34 | 0.44 | 0.62 |
| MAT | hr | N/A | N/A | N/A | N/A | 1.50 | 0.42 | 1.67 | 2.99 |
| MRT | hr | 1.34 | 1.13 | 1.36 | 1.53 | 2.99 | 1.95 | 2.99 | 4.44 |
| TMAX | hr | N/A | N/A | N/A | N/A | 0.91 | 0.50 | 0.63 | 3.00 |
| Vz | L/kg | 3.17 | 2.55 | 3.20 | 3.84 | N/A | N/A | N/A | N/A |
| Vz/F | L/kg | N/A | N/A | N/A | N/A | 107.76 | 37.89 | 115.64 | 227.95 |
| F | % | N/A | N/A | N/A | N/A | 4 | 3 | 4 | 6 |
Table 3.
Codeine-6-glucuronide pharmacokinetic parameters after codeine administration to 6 healthy Greyhound dogs.
| IV | Per Os | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric Mean | Min | Median | Max | Geometric Mean | Min | Median | Max | ||
| Dose (codeine) | mg/kg | 0.734 | 0.734 | 0.734 | 0.734 | 1.43 | 1.13 | 1.53 | 1.62 |
| AUCextrap | % | 11.58 | 8.18 | 12.06 | 15.10 | 17.41 | 13.73 | 16.06 | 32.25 |
| AUC0-inf | hr* ng/mL | 3858.60 | 2889.75 | 3853.13 | 4691.82 | 8157.80 | 5929.60 | 7311.42 | 14842.15 |
| AUMC0-inf | hr*hr*ng/mL | 16184.41 | 12162.27 | 16175.49 | 21476.09 | 31612.94 | 22674.87 | 30850.58 | 49411.69 |
| Cl/F | mL/min/kg | 3.17 | 2.61 | 3.19 | 4.23 | 3.94 | 2.38 | 3.85 | 5.65 |
| CMAX | ng/mL | 735.75 | 572.00 | 756.00 | 919.00 | 1952.86 | 1160.00 | 1675.00 | 4280.00 |
| T – λz | hr | 2.25 | 1.94 | 2.28 | 2.55 | 1.94 | 1.70 | 1.92 | 2.49 |
| λz | /hr | 0.31 | 0.27 | 0.30 | 0.36 | 0.36 | 0.28 | 0.36 | 0.41 |
| MRT | hr | 4.19 | 3.66 | 4.25 | 4.66 | 3.88 | 3.33 | 3.68 | 5.53 |
| TMAX | hr | 1.59 | 1.00 | 2.00 | 2.00 | 1.62 | 1.00 | 1.50 | 3.00 |
| Vz/F | L/kg | 0.62 | 0.44 | 0.63 | 0.84 | 0.66 | 0.40 | 0.61 | 1.22 |
| Frelative | % | 109 | 90 | 106 | 150 | ||||
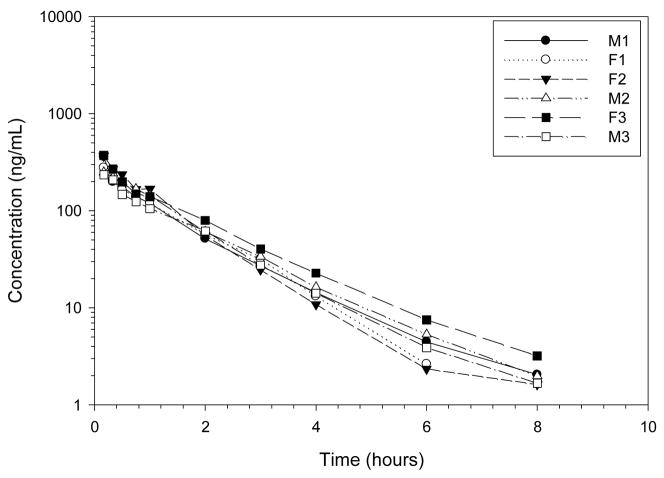
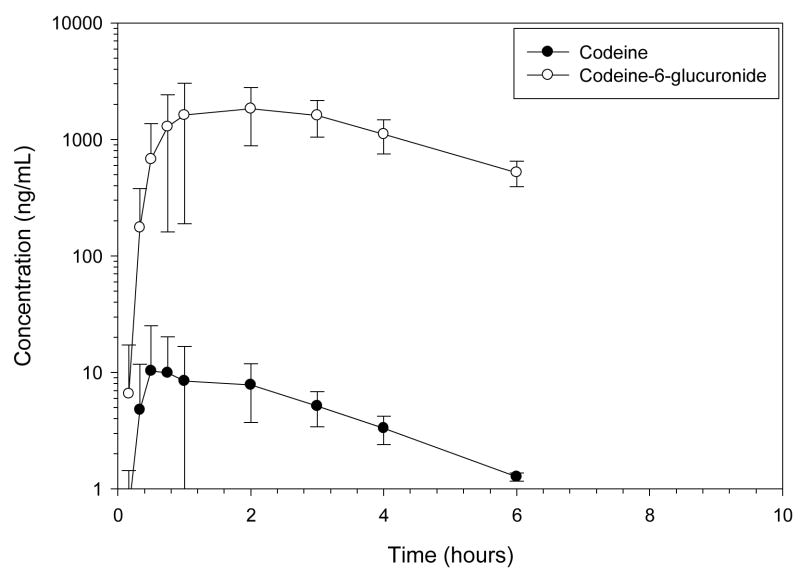
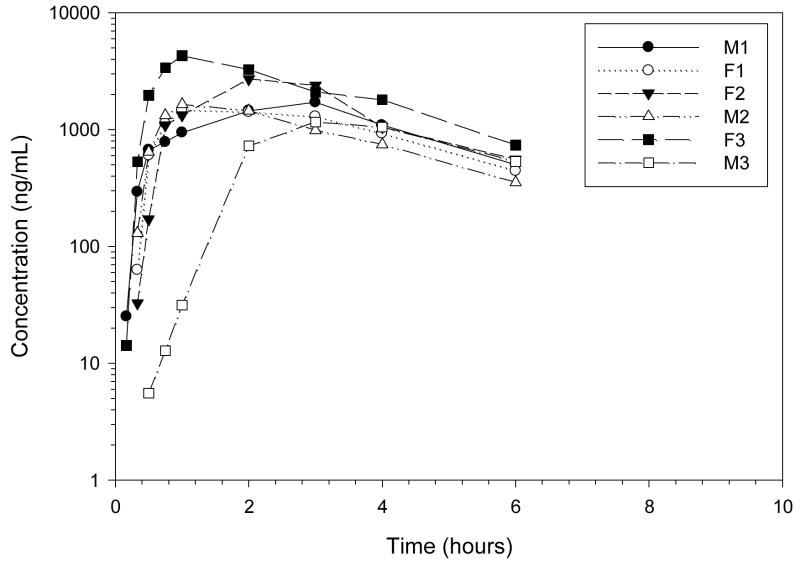
The fraction of codeine absorbed after oral codeine administration was low, 4%, with a mean CMAX, 10.01 ng/mL, occurring at 0.91 hours (Figures 5–7, Table 3). Codeine was rapidly eliminated with a 1.60 h terminal half-life after PO administration. Peak codeine-6-glucronide concentrations, 1952.86 ng/mL occurred at 1.62 hours after oral codeine administration. The MAT of codeine was 1.50 hours and the terminal half-life of codeine-6-glucuronide was 1.94 hours after oral codeine administration. The relative amount of codeine-6-glucuronide formed after oral codeine administration was 109% compared to dose normalized codeine-6-glucuronide formation after IV codeine administration.
Figure 5.
Plasma profile of codeine after codeine 1.43 mg/kg PO (mean dose) to 6 healthy Greyhound dogs.
Figure 7.
Mean ± SD plasma profile of codeine and codeine-6-glucuronide after codeine 1.43 mg/kg PO (mean dose) to 6 healthy Greyhound dogs. Morphine was not detected in any dog greater than 1 ng/mL.
Morphine was occasionally detected after both routes of administration, but never exceeded the LOQ of the assay, 1 ng/mL. Morphine glucuronide conjugates were also detected, but again at low concentrations, indicating morphine is a minor codeine metabolite in dogs.
Discussion
Acetaminophen administered to humans at a dose of 1 gram every 6 hours resulted in an approximate mean CMAX of 16.6 μg/mL, CMIN of 3.5 μg/mL, and AUC per dosing interval of 47.1 hr*μg/mL at steady state (Gelotte, et al, 2007). Antinociception to an electrical stimulus in humans occurred at plasma acetaminophen concentrations approximately 4 μg/mL or greater, which coincides with the pharmacokinetic dosing interval recommended in humans (Pickering, et al, 2006). The amount of acetaminophen in codeine tablets however is less (300 mg), therefore it is expected a dose proportional AUC (14.1 hr*μg/mL) and CMAX (4.98 μg/mL) will occur in humans, which is similar to the values obtained in dogs, 13.78 hr*μg/mL and 6.74 μg/mL, administered 10.46 mg/kg PO in this study.
Very low concentrations of free morphine were produced in dogs after codeine administrations, similar to CYP2D6 poor metabolizer humans (Kirchheiner, et al, 2006). The AUC0-inf and CMAX were 0.5 hr*ng/mL and 0.05 ng/mL, respectively for poor metabolizer humans in comparison to 11 hr*ng/mL and 2.1 ng/mL in extensive metabolizer humans. The low concentrations of morphine in dogs may be due to the rapid conversion of morphine to morphine glucuronide conjugates, primarily morphine-3-glucuronide in dogs (Yoshimura, et al, 1993) and not due to lack of morphine formation. A similar scenario in dogs occurs with the conversion of dextromethorphan to the active metabolite, dextrorphan (CYP2D mediated, as is codeine to morphine metabolism), in which dextrorphan was not detected in the plasma, but dextrorphan glucuronide conjugates were present (KuKanich & Papich, 2004a).
The pharmacokinetics of codeine in dogs are similar to the pharmacokinetics of other opioids in dogs including morphine (Barnhart, et al, 2000; KuKanich, et al, 2005b; KuKanich & Borum, 2008; Henao-Guerrero, et al, 2009), hydromorphone (KuKanich, et al, 2008a; Guedes, et al, 2008), oxymorphone (KuKanich, et al, 2008b), methadone (Garret, et al, 1985; KuKanich, et al 2005a), and tramadol (KuKanich & Papich, 2004b; McMillan, et al, 2008; Vettorato, et al, 2009). The plasma clearance of codeine was rapid, 29.94 mL/min/kg, similar to previously reported values of hepatic blood flow in Greyhounds, 25 mL/min/min (Mills, 2003). High hepatic clearance drugs are expected to have a large first pass metabolism and a subsequent low fraction of the oral dose absorbed. As expected, only 4% of oral codeine was absorbed systemically as codeine. The low oral bioavailability of codeine is similar to that seen with morphine (KuKanich, et al, 2005c), oxycodone (Weinstein & Gaylord, 1979), and methadone (KuKanich, et al, 2005a) in dogs.
The AUC0-inf of codeine in CYP2D6 poor metabolizer humans administered 30 mg PO was 180 hr*ng/mL as compared to 30.53 hr*ng/mL in dogs (Kirchheiner, et al, 2006). The CMAX of codeine in humans was 45 ng/mL whereas in dogs it was 10.01 ng/mL.
The large relative fraction of codeine-6-glucuronide formed after oral codeine administration, 109%, and the low oral bioavailability (4%) indicate a substantial amount of first pass metabolism of codeine occurs as little reaches systemic circulation as the parent compound, codeine. In humans, CYP2D6 poor metabolizers produce large amounts of codeine-6-glcuronide with typical doses of codeine (30 mg PO) with an AUC0-inf approximately 4000 hr*ng/mL (Kirchheiner, et al, 2006) which is approximately half of that produced in dogs (8157 hr*ng/mL) administered codeine phosphate approximately 2 mg/kg PO. The CMAX of codeine-6-glucuronide in humans was 628 ng/mL as compared to 1952.86 ng/mL in dogs.
In conclusion, the purpose of this study was to assess the plasma profiles and pharmacokinetics of acetaminophen, codeine, morphine, and codeine-6-glucuronide in dogs. The pharmacokinetics of codeine in dogs are most similar to that seen in humans that are CYP2D6 poor metabolizers, however differences still occurred. Dogs administered approximately 2 mg/kg codeine phosphate PO had similar acetaminophen and morphine pharmacokinetics, produced higher concentrations of codeine-6-glucuronide, and lower concentrations of codeine compared to CYP2D6 poor metabolizer humans. The results of this study are similar to a previous study in which 2 dogs were administered codeine with plasma concentrations determined with a nonspecific RIA (Findlay, et al, 1979). Future studies are needed to assess the antinociceptive effects of oral codeine in dogs to determine if it can be effectively used.
Figure 3.
Plasma profile of codeine-6-glucuronide after codeine 0.734 mg/kg IV to 6 healthy Greyhound dogs.
Figure 6.
Plasma profile of codeine-6-glucuronide after codeine 1.43 mg/kg PO (mean dose) to 6 healthy Greyhound dogs.
Acknowledgments
The authors would like to thank Dr. Stacey Borum Smith and Michelle Hubin for their technical contributions and Dr. Mark G. Papich for his contributions to the study design. The authors would also like to thank the Benjamin Kurz Fund at Kansas State University, the Veterinary Research Scholars Program at Kansas State University (funded by NIH NCRR 5T35RR007064-10), the Merck-Merial Veterinary Scholars Program, the Department of Anatomy and Physiology, and the Analytical Pharmacology Laboratory at Kansas State University for providing financial support.
References
- 1.Barnhart MD, Hubbell JA, Muir WW, Sams RA, Bednarski RM. Pharmacokinetics, pharmacodynamics, and analgesic effects of morphine after rectal, intramuscular, and intravenous administration in dogs. American Journal of Veterinary Research. 2000;61:24–8. doi: 10.2460/ajvr.2000.61.24. [DOI] [PubMed] [Google Scholar]
- 2.Findlay JW, Jones EC, Welch RM. Radioimmunoassay determination of the absolute oral bioavailabilities and O-demethylation of codeine and hydrocodone in the dog. Drug Metabolism and Disposition. 1979;7:310–4. [PubMed] [Google Scholar]
- 3.Garrett ER, Derendorf H, Mattha AG. Pharmacokinetics of morphine and its surrogates. VII: High-performance liquid chromatographic analyses and pharmacokinetics of methadone and its derived metabolites in dogs. Journal of Pharmaceutical Sciences. 1985;74:1203–14. doi: 10.1002/jps.2600741114. [DOI] [PubMed] [Google Scholar]
- 4.Gaynor JS. Control of cancer pain in veterinary patients. Veterinary Clinics of North America Small Animal Practice. 2008;38:1429–48. doi: 10.1016/j.cvsm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Gelotte CK, Auiler JF, Lynch JM, Temple AR, Slattery JT. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clinical Pharmacology and Therapeutics. 2007;81:840–8. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- 6.Guedes AG, Papich MG, Rude EP, Rider MA. Pharmacokinetics and physiological effects of intravenous hydromorphone in conscious dogs. Journal of Veterinary Pharmacology and Therapeutics. 2008;31:334–43. doi: 10.1111/j.1365-2885.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 7.Henao-Guerrero PN, McMurphy R, Kukanich B, Hodgson DS. Effect of morphine on the bispectral index during isoflurane anesthesia in dogs. Veterinary Anaesthesia and Analgesia. 2009;36:133–43. doi: 10.1111/j.1467-2995.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lötsch J, Roots I, Brockmöller J. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. The Pharmacogenomics Journal. 2006;7:257–65. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- 9.KuKanich B, Borum SL. Effects of ketoconazole on the pharmacokinetics and pharmacodynamics of morphine in healthy Greyhounds. American Journal of Veterinary Research. 2008;69:664–9. doi: 10.2460/ajvr.69.5.664. [DOI] [PubMed] [Google Scholar]
- 10.KuKanich B, Hogan BK, Krugner-Higby LA, Smith LJ. Pharmacokinetics of hydromorphone hydrochloride in healthy dogs. Veterinary Anaesthesia and Analgesia. 2008a;35:256–64. doi: 10.1111/j.1467-2995.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 11.KuKanich B, Lascelles BD, Aman AM, Mealey KL, Papich MG. The effects of inhibiting cytochrome P450 3A, p-glycoprotein, and gastric acid secretion on the oral bioavailability of methadone in dogs. Journal of Veterinary Pharmacology and Therapeutics. 2005a;28:461–6. doi: 10.1111/j.1365-2885.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 12.KuKanich B, Lascelles BD, Papich MG. Use of a von Frey device for evaluation of pharmacokinetics and pharmacodynamics of morphine after intravenous administration as an infusion or multiple doses in dogs. American Journal of Veterinary Research. 2005b;66:1968–74. doi: 10.2460/ajvr.2005.66.1968. [DOI] [PubMed] [Google Scholar]
- 13.KuKanich B, Lascelles BD, Papich MG. Pharmacokinetics of morphine and plasma concentrations of morphine-6-glucuronide following morphine administration to dogs. Journal of Veterinary Pharmacology and Therapeutics. 2005c;28:371–6. doi: 10.1111/j.1365-2885.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- 14.KuKanich B, Papich MG. Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs. Journal of Veterinary Pharmacology and Therapeutics. 2004a;27:337–41. doi: 10.1111/j.1365-2885.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 15.KuKanich B, Papich MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. Journal of Veterinary Pharmacology and Therapeutics. 2004b;27:239–46. doi: 10.1111/j.1365-2885.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- 16.KuKanich B, Schmidt BK, Krugner-Higby LA, Toerber S, Smith LJ. Pharmacokinetics and behavioral effects of oxymorphone after intravenous and subcutaneous administration to healthy dogs. Journal of Veterinary Pharmacology and Therapeutics. 2008b;31:580–3. doi: 10.1111/j.1365-2885.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 17.Lötsch J, Skarke C, Schmidt H, Rohrbacher M, Hofmann U, Schwab M, Geisslinger G. Evidence for morphine-independent central nervous opioid effects after administration of codeine: contribution of other codeine metabolites. Clinical Pharmacology and Therapeutics. 2006;79:35–48. doi: 10.1016/j.clpt.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.McMillan CJ, Livingston A, Clark CR, Dowling PM, Taylor SM, Duke T, Terlinden R. Pharmacokinetics of intravenous tramadol in dogs. Canadian Journal of Veterinary Research. 2008;72:325–31. [PMC free article] [PubMed] [Google Scholar]
- 19.Mills PC. A model to investigate hepatic extraction of oxygen during anaesthesia in the dog. Research in Veterinary Science. 2003;75:179–83. doi: 10.1016/s0034-5288(03)00140-1. [DOI] [PubMed] [Google Scholar]
- 20.Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Analgesic effect of acetaminophen in humans: first evidence of a central serotonergic mechanism. Clinical Pharmacology and Therapeutics. 2006;79:371–8. doi: 10.1016/j.clpt.2005.12.307. [DOI] [PubMed] [Google Scholar]
- 21.Savides MC, Oehme FW, Nash SL, Leipold HW. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicology and Applied Pharmacology, 15. 1984;74:26–34. doi: 10.1016/0041-008x(84)90266-7. [DOI] [PubMed] [Google Scholar]
- 22.Skingle M, Tyers MB. Further studies on opiate receptors that mediate antinoception: tooth pulp stimulation in the dog. British Journal of Pharmacology. 1980;70:323–7. doi: 10.1111/j.1476-5381.1980.tb07939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan V, Wielbo D, Tebbett IR. Analgesic effects of codeine-6-glucuronide after intravenous administration. European Journal of Pain. 1997;1:185–90. doi: 10.1016/s1090-3801(97)90103-8. [DOI] [PubMed] [Google Scholar]
- 24.Vettorato E, Zonca A, Isola M, Villa R, Gallo M, Ravasio G, Beccaglia M, Montesissa C, Cagnardi P. Pharmacokinetics and efficacy of intravenous and extradural tramadol in dogs. The Veterinary Journal. 2009 doi: 10.1016/j.tvjl.2008.11.002. (E PUB, in press) [DOI] [PubMed] [Google Scholar]
- 25.Weinstein SH, Gaylord JC. Determination of oxycodone in plasma and identification of a major metabolite. Journal of Pharmaceutical Sciences. 1979;68:527–8. doi: 10.1002/jps.2600680441. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimura K, Horiuchi M, Konishi M, Yamamoto K. Physical dependence on morphine induced in dogs via the use of miniosmotic pumps. Journal of Pharmacological Toxicological Methods. 1993;30:85–95. doi: 10.1016/1056-8719(93)90011-3. [DOI] [PubMed] [Google Scholar]