Abstract
Background
While testicular cancer incidence rates have been widely reported in populations of Northern European ancestry, rates in other population have been less frequently examined. In a prior report, global testicular cancer incidence rates and trends for the years 1973–1997 were summarized. The current report extends these analyses with an additional 5 years of data from Cancer Incidence in Five Continents.
Methods
Age-standardized incidence rates over successive 5-year time periods were obtained for populations in the Americas, Asia, Europe, and Oceania.
Results
In general, testicular cancer incidence remained highest in Northern European populations (8.0–9.0 per 100,000) and lowest in Asian and African populations (<1 per 100,000). One notable exception to this pattern, however, was the very high rate reported by the Valdivia, Chile registry (8.8 per 100,000). In many populations, incidence rates rose between 1973 and 2002, although the increases were strongest and most consistent among populations of European ancestry. In some European populations, such as those of Denmark and of Geneva, Switzerland, some plateauing of rates was evident in recent years. There was little evidence of increase and possible evidence of modest decline in rates in east Asian populations. In general, the trends by histology (seminoma, nonseminoma) were similar to one another.
Conclusions
Risk of testicular cancer remains high in Northern European populations and low in Asian and African populations. Reasons for increasing rates among Northern Europeans and more stable or declining rates among East Asians are unexplained, supporting the need for future etiologic studies.
Keywords: testicular cancer, trends, seminoma, nonseminoma
Introduction
On a population basis, testicular cancer is relatively rare. In many countries, however, it is the most common cancer among men aged 15 to 40 years. The incidence of testicular cancer has been increasing over the last 30–40 years (1–2), although there are large variations in risk in different countries and among different racial and ethnic groups. Incidence rates among young, white men in Nordic countries are as high as 11.5 per 100,000 men, which is in stark contrast to rates of 1–2 per 100,000 among black and Asian men (2–3).
Germ cell tumors account for approximately 98% of testicular cancers. Histologically, testicular germ cell tumors are grouped into three main types; seminomas, nonseminomas and spermatocytic seminomas. In the United States, seminomas account for approximately 55% of germ cell tumors while nonseminomas, composed of embryonal carcinomas, teratomas, choriocarcinomas, yolk-sac tumors and mixed germ-cell tumors, comprise approximately 44%. The median age at diagnosis of seminoma is 35–39 years, whereas for nonseminoma, the median age at diagnosis is 10 years younger, at 25–29 years. Although seminomas and nonseminomas have different clinical characteristics, studies have revealed similar trends in incidence in the majority of countries, which may indicate that both types share common etiologic risk factors (4–5). The third, and least common, type of testicular germ cell tumor, spermatocytic seminoma, comprises approximately 1–2% of germ cell tumors in males (6). The incidence of spermatocytic seminoma peaks among men aged 50–54 years (6) and the tumor is thought to have an etiology distinct from that of either seminomas or nonseminomas.
Testicular cancer rates have been increasing in many populations over time and across successive birth cohorts, including those in the Nordic countries (5), the United Kingdom (5), Germany (7), France (8), the United States (4), and Australia (9). Although trends in testicular cancer incidence have been well-described in the United States and Europe, few studies have examined and compared international trends in incidence rates by histology. As a follow-up to our previous study (2), we examined international trends in testicular cancer incidence overall, and by histology, for the 30-year period 1973–2002.
Materials and Methods
Incidence rates of testicular cancer [ICD-8 (10) and ICD-9 (11) code 186, ICD-10 code C62 (12)], age-standardized to the world population, were obtained from Volumes 4–9 of Cancer Incidence in Five Continents (CI5) (13–14). For Volumes 4–8, the CI5 electronically updated data were used. Electronic updates are provided only for selected cancer registries that have been included in at least three consecutive volumes published in the CI5 series (http://www-dep.iarc.fr/). At the current time, Volume 9 data are yet to be electronically updated. The CI5 series includes incidence data from areas within the Americas, Europe, Africa, Asia, and Oceania that are covered by selected population-based registries, and for the five-year time periods 1973–1977, 1978–1982, 1983–1987, 1988–1992, 1993–1997, 1998–2002. Incidence rates by histology (seminoma, nonseminoma, and other/unspecified), were obtained from the CI5 ADDS software package (13) for Volumes 4–8 and directly from the website for Volume 9.
The registries selected for the current study were, to the greatest extent possible, identical to the registries included in our previous study (2). Inclusion criteria for the registries have been previously described (2). Briefly, only one registry from each country was chosen for trend analysis. However, if more than one registry within a country met the basic inclusion criteria, the registry that covered the largest population was included.
Data from the Puerto Rico registry were not included in the current report as the registry was absent from Volume 9. In place of data from the Puerto Rico registry, the current study included data from the registry in Costa Rica. Two registries changed population coverage or reporting format between Volumes 4 through 9. In Volumes 4–5, the New Zealand registry included incidence data stratified on ethnicity (Maori, non-Maori populations). In Volumes 6–9, however, stratified data were not reported. Through Volume 8, the South Thames, England registry covered a population of 6.8 million persons. In Volume 9, the former South Thames registry expanded to include the entire Thames region, covering more than 14 million persons. In addition, registries for Saarland, Germany and Mumbai, India were included in the current analysis. Incidence rates for the U. S. Surveillance Epidemiology and End Results (SEER) registries for whites and blacks, age-standardized to the world population, were obtained from the SEER*Stat package (15–16) as race-specific rates were not included in CI5 Volumes 4 and 5.
In order to display incidence rates from as wide a geographic range as possible, incidence rates from the latest time period were abstracted from 26 registries. To examine trends in testicular cancer incidence over the 30-year span, rates from 21 of these registries were included, and to examine trends in seminoma and nonseminoma, rates from 8 registries were included.
Percent change in incidence between the intervals 1973–1977 and 1998–2002 was calculated for each population. Trends in age-standardized (world standard population) incidence rates were assessed for the following 5-year periods: 1973–1977, 1978–1982, 1983–1987, 1988–1992, 1993–1997, 1998–2002. For certain registries, coverage was not complete over the entire earliest time period (1973–1977). Registries with non-continuous coverage were: Bas-Rhin, France (1975–1977); Cali, Colombia (1972–1976) and Hong Kong, China (1974–1977). For the 1978–1982 time period, Costa Rica only had data for 1977–1981, and Cali, Colombia had data for 1980–1982. For Zaragoza, Spain, data that correspond to Volumes 4–9 covered the periods 1973–1977, 1978–1982, 1983–1985, 1986–1990, 1991–1995, and 1996–2000, respectively. Trends in age-standardized incidence rates were plotted on graphs using a semi-log scale to facilitate the comparison of temporal trends and magnitude (17). Data were plotted at the midpoint of each 5-year time-interval.
Results
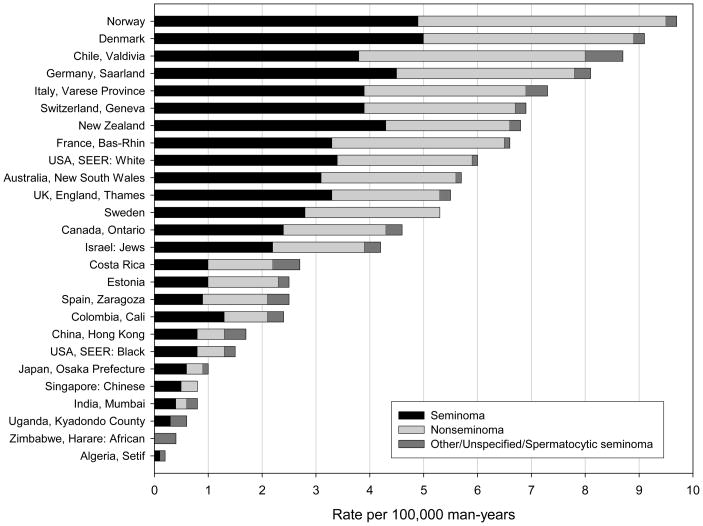
In the most recent time period (1998–2002), there was a greater than ten-fold difference in testicular cancer incidence between Norway (9.6 per 100,000 man-years), the registry with the highest rate, and Setif, Algeria (0.2/100,000), the registry with the lowest rate (Figure 1). Overall, the incidence was highest in Nordic registries and lowest in African and Asian registries and among U.S. black men. Among Asian registries, rates were notably lower in the east Asian registries (0.7–1.6/100,000) than they were in the sole west Asian registry, Israel (Jewish population) (4.1/100,000). Among the Central and South American registries, Valdivia, Chile reported rates strikingly higher (8.8/100,000) than the rates reported by Cali, Colombia (2.3/100,000) or Costa Rica (2.6/100,000). Among the Nordic registries, the discrepancy between the high-rate registries (Norway and Denmark) and the lower-rate registries (Sweden and Finland) was notable.
Figure 1.
Incidence rates of testicular cancer (per 100,000 man-years) age-standardized to the world population, 1998–2002.
During the most recent time period, seminomas comprised 50–60% of all testicular cancers in the majority of registries (Figure 1). The proportion of tumors that were seminomas, however, ranged from 36% in Zaragoza, Spain to 63% in New Zealand and in Singapore (Chinese population). There was no relationship between the proportion of tumors that were seminomas (or nonseminomas) and the overall rate of testicular cancer.
The incidence rates during the earliest (1973–1977) and latest (1998–2002) time periods are shown in Table 1. Between the two time periods, the registries with the largest proportional increases in incidence were those of Saarland, Germany (153%) and Finland (131%). Other registries that experienced increases of greater than 100% were Norway (116%) and Israel (Jewish population) (114%). In contrast, increases in incidence were modest in Hong Kong, China (19%) and Singapore (Chinese population) (8%). A drop in incidence (−24%) was seen in one registry; that of Mumbai, India.
Table 1.
Global testicular cancer incidence rates per 100,000 man-years, 1973–1977 to 1998–2002
| 1973–1977 |
1998–2002 |
||||||
|---|---|---|---|---|---|---|---|
| Population | Cases | Rate* | 95% Confidence Interval | Cases | Rate* | 95% Confidence Interval | Percent change |
| Europe, Nordic countries | |||||||
| Norway | 448 | 4.5 | 4 – 4.9 | 1171 | 9.6 | 9.0 – 10.2 | 116% |
| Denmark | 936 | 7.0 | 6.6 – 7.5 | 1361 | 9.2 | 8.7 – 9.7 | 31% |
| Sweden | 668 | 3.1 | 2.9 – 3.4 | 1214 | 5.3 | 5.0 – 5.6 | 70% |
| Finland | 205 | 1.6 | 1.4 – 1.8 | 469 | 3.7 | 3.3 – 4.1 | 131% |
| Europe, other countries | |||||||
| Germany, Saarland | 87 | 3.2 | 2.5 – 3.9 | 247 | 8.1 | 7.0 – 9.2 | 153% |
| Italy, Varese Province | - | 95 | 7.3 | 5.7 – 8.9 | - | ||
| Switzerland, Geneva | 48 | 5.0 | 3.6 – 6.5 | 82 | 6.9 | 5.4 – 8.4 | 37% |
| France, Bas-Rhin | 50 | 3.5 | 2.5 – 4.5 | 198 | 6.6 | 5.7 – 7.5 | 89% |
| UK, England, (South) Thames† | 541 | 3.3 | 3 – 3.6 | 2156 | 5.6 | 5.4 – 5.8 | 70% |
| Spain, Zaragoza | 33 | 1.7 | 1.1 – 2.3 | 57 | 2.5 | 1.8 – 3.2 | 47% |
| Estonia | 48 | 1.3 | 0.9 – 1.7 | 85 | 2.5 | 2 – 3 | 92% |
| Oceania | |||||||
| New Zealand | - | 696 | 6.8 | 6.3 – 7.3 | - | ||
| Australia, New South Wales | 386 | 3.0 | 2.7 – 3.3 | 1044 | 5.8 | 5.4 – 6.2 | 95% |
| Americas | |||||||
| USA: white population | 1625 | 3.8 | 3.6 – 4 | 3465 | 6.0 | 5.8 – 6.2 | 58% |
| Canada, Ontario | - | 1508 | 4.6 | 4.4 – 4.8 | - | ||
| Costa Rica | - | 276 | 2.6 | 2.3 – 2.9 | - | ||
| Colombia, Cali | 26 | 1.5 | 0.9 – 2.2 | 115 | 2.3 | 1.8 – 2.8 | 52% |
| USA: black population | 34 | 0.8 | 0.5 – 1.1 | 121 | 1.4 | 1.1 – 1.7 | 75% |
| Chile, Valdivia | - | 86 | 8.8 | 6.9 – 10.7 | - | ||
| Asia | |||||||
| Israel: Jewish population | 140 | 1.9 | 1.6 – 2.2 | 520 | 4.1 | 3.7 – 4.5 | 114% |
| China, Hong Kong | 114 | 1.3 | 1.1 – 1.6 | 292 | 1.6 | 1.4 – 1.8 | 19% |
| Japan, Osaka | 212 | 0.9 | 0.8 – 1 | 264 | 1.1 | 1.0 - 1.2 | 22% |
| Singapore: Chinese population | 32 | 0.8 | 0.5 – 1.1 | 64 | 0.9 | 0.7 – 1.1 | 8% |
| India, Mumbai | 92 | 0.9 | 0.6 – 1.2 | 256 | 0.7 | 0.6 – 0.8 | −24% |
| Africa | |||||||
| Algeria, Setif | - | 6 | 0.2 | 0 – 0.4 | - | ||
| Uganda, Kyandodo County | - | 7 | 0.6 | 0.1 – 1.1 | - | ||
| Zimbabwe, Harare: African population | - | 16 | 0.6 | 0.2 – 1.0 | - | ||
Rate is age-standardized to the world population, per 100,000 man-years
Through volume 8, the UK, England (South) Thames data included only the South Thames regions, covering ~6.8 million persons; in volume 9, this registry expanded to include the entire Thames region, expanding to >14 million persons
Prior to volume 6 (1983–1987), New Zealand rates were split into Maori and non-Maori populations
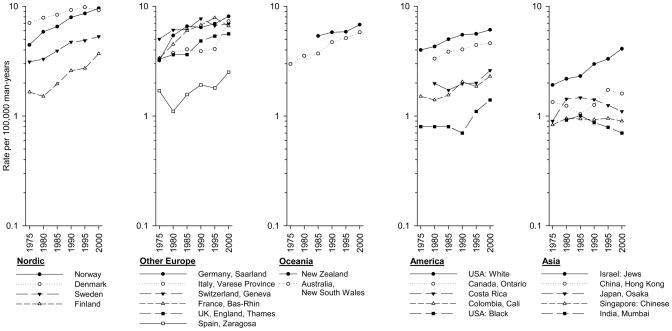
Trends in testicular cancer incidence from 1973–2002 to 1998–2002 are presented in Figure 2. Among the Nordic countries, the incidence in Norway surpassed that in Denmark (9.6 versus 9.2 per 100,000) in the most recent time period. While the rates in Denmark and Sweden appeared to be leveling, rates in Norway and Finland continued to increase. In other European registries, testicular cancer incidence increased in Saarland, Germany, while the incidence in the Thames, England and Geneva, Switzerland registries appeared to be plateauing. In contrast, the rate in Bas-Rhin, France declined in the final time period. The incidence in the Zaragoza, Spain registry, was lower that that of other European registries, but was continuing to increase during the most recent time interval. Rates in the Varese Province, Italy registry remained relatively stable until the 1993–1997 time period, but then increased from 4.1 per 100,000 to 7.3 per 100,000 between the final two time periods. Rates in New Zealand, New South Wales, Australia and the Americas all continued to increase. In Asian registries, other than that of Israel (Jewish population), incidence rates were low and either stable or modestly declining. By contrast, rates in the Israel (Jewish population) registry were in the moderate range (4.1) and increased more than 100% between the earliest and latest time periods.
Figure 2.
Trends in age-standardized testicular cancer incidence rates (per 100,000 man-years) by continent and area, 1973–1977 to 1998–2002.
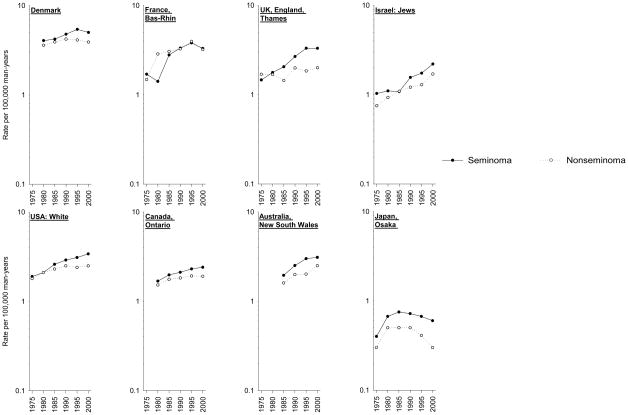
For the eight registries with histology data and sufficient numbers of cases, trends by histology are presented in Figure 3. In all but one registry, rates of seminoma consistently exceeded rates of nonseminoma, though the trends by histology were fairly similar within each population. Only in Bas-Rhin, France did the rates of seminoma and nonseminoma converge around 1985, and then begin to decline around 1995. In Denmark, declines in rates of both seminoma and nonseminoma occurred, though the decline in nonseminoma rates began earlier (1990) than the decline in seminoma rates (1995). In the Thames, England registry, nonseminoma rates were fairly stable over time, while seminoma rates increased until the final two time periods, at which point they appeared to plateau. In the registries of Israel (Jewish population) and New South Wales, Australia, increases in both histologic types were evident. In the USA white population and in Ontario, Canada, nonseminoma rates appeared to plateau around 1990, while increases in seminoma rates continued to the most recent time period. In Osaka, Japan rates of both seminomas and nonseminomas began to decline in the mid-1980s.
Figure 3.
Trends in age-standardized testicular cancer incidence rates (per 100,000 man-years) by histology (seminoma, nonseminoma), 1973–1977 to 1998–2002.
Because the trends in east Asian registries differed from those in other areas, several other registries from each country were also inspected. Only registries with longitudinal data could be examined. In China, registries other than Hong Kong with longitudinal data included those in Shanghai, Qidong City and Tianjin. As with the Hong Kong registry, rates were stable or modestly declining in all three (data not shown). In Japan, registries other than Osaka with longitudinal data included those in Hiroshima City, Nagasaki City, Miyagi Prefecture and Yamagata Prefecture. In contrast to the rates in Osaka, rates in the other registries tended to be rising than falling (data not shown). In India, two other registries could be examined; those of Bangalore and Chennai. As with the rates in Mumbai, rates in both Bangalore trended downward (data not shown).
Discussion
This analysis confirms the wide global discrepancy in testicular cancer incidence rates. Testicular cancer remains a disease predominantly of men descended from European populations; in particular, Northern European populations. Even among Northern European men, however, variability in incidence exists. Norway and Denmark have rates notably higher than those of Sweden and Finland. In general, developed countries have higher rates than developing countries, but the association is linked to the preponderance of European-descended populations in developed countries. In developed countries without European-descended populations, such as Japan, testicular cancer incidence tends to be low. Due to the poor understanding of testicular cancer etiopathogenesis, it is difficult to theorize concerning the possible causes of geographic variation in incidence. Propositions of causative factors have noted that seminomas and nonseminomas arise from carcinoma-in-situ cells. As testicular carcinoma-in-situ cells express markers in common with those expressed by embryonic stem cells and gonocytes, strong support for a very early life ontogenesis has been deduced (18–19). Epidemiologic observation of a strong birth-cohort effect when analyzed using age-period-cohort models has suggested that environmental factors are instrumental in determining risk (20–22). Proposed risk factors that may account for a birth-cohort effect include maternal smoking during pregnancy (23–24), lower age at puberty (25–27), marijuana use (28), increasing body mass index (29), exposure to endocrine disrupting chemicals (30–34), viral exposure (35–37), and occupational exposures (38–41). However, epidemiologic evidence in support of any of these exposures has been inconsistent (42–50). The inconsistencies may be explained, in part, by the relative rarity of testicular cancer, and the time lag between perinatal exposures and diagnosis of cancer, which, in turn, result in reduced statistical power, reduced capacity for replication, and potentially increased misclassification of epidemiologic and molecular data. Thus, the causes of geographic variability remain to be elucidated.
The highest incidences of testicular cancer are found within Northern Europe and have long been of interest due to large differences in rates between countries within this geographical area; very high rates in Denmark and much lower rates in Finland. It is evident however, that changes are occurring in this region (51). As shown in Figure 1, Norway has now surpassed Denmark in having the highest incidence in the world. This change in position occurred due to the simultaneous plateauing of Denmark’s rates and continuing increase in Norway’s rates. Another notable trend is that Finland, noted for lower rates than any other Nordic country, had an increase in incidence of greater than 100% between 1973–1977 and 1998–2002. According to predictions calculated by the NORDPRED package (52) using data from the NORDCAN project (http://wwwdep.iarc.fr/NORDCAN/english/frame.asp) (53), rates in Finland will continue to increase until they eventually surpass rates in Sweden some time during the years 2023–2027. The causes of this predicted Finnish increase, however, are unclear, as are the reasons behind the current differences in rates among the Nordic countries. Though some differences in genetic backgrounds exist, particularly between Finnish and Scandinavian populations (54), migrant studies indicate that testicular cancer incidence rates move toward those of the adopted country within one generation of migrating (55–57). These findings imply that environmental exposures are playing a large role in determining risk, although genetic susceptibility to the effects of the exposure is almost certainly required to result in an increased incidence.
The current analysis provides evidence that testicular cancer rates may be reaching a plateau in the UK and Switzerland, and possibly starting a decline in France. In far eastern Asia, incidence rates also appear to be declining in Mumbai, India, Osaka, Japan, and recently in Hong Kong, China and among the Chinese population of Singapore. Japanese registries other than that of Osaka, however, seem to have increasing, rather than decreasing rates suggesting that the Osaka registry may not reflect trends in other areas of the country. The Osaka registry, however, is notably larger than the other Japanese registries examined, so may provide a better approximation of testicular cancer rates in the national population. Whatever is happening in Japan as a whole, these declines seen in Osaka, Mumbai and Hong Kong are notable as they argue against Westernization being a risk factor. Lack of genetic susceptibility to a putative risk factor may be affecting rates in eastern Asian populations, but declining rates over a thirty-year period argue that environmental influences are also at play.
In contrast to the low rates in eastern Asia, is the high rate reported by the Valdivia, Chile registry. Explanation of this high rate, however, is unclear, as other South American registries reported rates that were one-quarter the rate in Chile.
It has been perceived that seminoma and nonseminoma have a similar etiopathogenesis insofar that they arise from a common precursor lesion (58), have overlapping risk factors (59–65) and, for many countries, have fairly comparable incidence trends as demonstrated by this and prior studies (3, 66–69). However, while secular trends for each subtype are fairly comparable within each country, differences still exist in both the trend and magnitude of incidence. Based on specific registries, this analysis suggests that the discrepancy between the incidence of nonseminoma and seminoma is widening in all countries analyzed, except, perhaps, France, Israel (Jewish population), and Australia. Moreover, previous analyses have noted disparate trends by histology in East Anglia, UK (70), Vaud, Switzerland (71), USA (20), Sweden (69), Italy (5); and Germany (72). Age-period-cohort models also lend credence to the idea that there are dissimilarities between testicular histologic tumor types, specifically in analyses of data from Finland (69), U.S., (3), Canada (3, 73), Denmark (3), and Australia (3, 9). The magnitude of these differences may be smaller relative to other cancers that have been analyzed by histologic subtype, but this may be related to the relatively low incidence rate of testicular cancer. Differences between seminoma and nonseminoma remain evident and suggest that some of the unknown causative factors of testicular cancer may partially, or exclusively, affect risk of a single histologic group.
The current examination of testicular cancer rates had a number of strengths in that the data were abstracted from large, well-established registries throughout the world. In addition, for the first time, rates of particular histologic types of testicular cancer could be examined separately. The conclusions of the study, however, were limited by the lack of nationwide cancer registries in many countries. While every effort was made to select large, representative registries from each country, it remains possible that the registries included in the study do not accurately reflect patterns in their nations. Finally, as testicular cancer is a rare neoplasm even in countries which experience the highest rates, the trends reported in low-rate countries must be interpreted cautiously as they are based on small numbers which are prone to random variation.
In summary, this analysis of published testicular cancer incidence data suggests that incidence continued to increase in many populations worldwide between 1973–77 and 1998–2002. The increase, however, was most notable among some European-descended populations. Eastern Asian populations, in contrast, continued to have low rates that remained stable or declined. The increases in testicular cancer rates over a 30-year period argue that environmental risk factors are likely to be involved, although the great discrepancy in rates among persons of different racial and ethnic groups suggests that genetic susceptibility may also be an important determinant.
Acknowledgments
Source of funding: This work was supported by the Intramural Research Program of the NIH, National Cancer Institute.
References
- 1.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. J Urol. 2003;170:5–11. doi: 10.1097/01.ju.0000053866.68623.da. [DOI] [PubMed] [Google Scholar]
- 2.Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. Int J Cancer. 2005;115:822–7. doi: 10.1002/ijc.20931. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Devesa SS, McGlynn KA, Moller H. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol. 2006;3:532–43. doi: 10.1038/ncpuro0606. [DOI] [PubMed] [Google Scholar]
- 4.Shah MN, Devesa SS, Zhu K, McGlynn KA. Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl. 2007;30:206–13. doi: 10.1111/j.1365-2605.2007.00795.x. discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Richiardi L, Ekbom A, et al. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiol Biomarkers Prev. 2006;15:652–8. doi: 10.1158/1055-9965.EPI-05-0565. [DOI] [PubMed] [Google Scholar]
- 6.Carriere P, Baade P, Fritschi L. Population based incidence and age distribution of spermatocytic seminoma. J Urol. 2007;178:125–8. doi: 10.1016/j.juro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Stang A, Rusner C, Eisinger B, Stegmaier C, Kaatsch P. Subtype-specific incidence of testicular cancer in Germany: a pooled analysis of nine population-based cancer registries. Int J Androl. 2007 doi: 10.1111/j.1365-2605.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 8.Walschaerts M, Huyghe E, Muller A, Bachaud JM, Bujan L, Thonneau P. Doubling of testicular cancer incidence rate over the last 20 years in southern France. Cancer Causes Control. 2008;19:155–61. doi: 10.1007/s10552-007-9081-x. [DOI] [PubMed] [Google Scholar]
- 9.Baade P, Carriere P, Fritschi L. Trends in testicular germ cell cancer incidence in Australia. Cancer Causes Control. 2008;19:1043–9. doi: 10.1007/s10552-008-9168-z. [DOI] [PubMed] [Google Scholar]
- 10.WHO. International Classification of Diseases, 1965 Revision. Geneva: World Health Organization; 1967. [Google Scholar]
- 11.WHO. International Classification of Diseases, 1975 Revision. Geneva: World Health Organization; 1977. [Google Scholar]
- 12.WHO. International Classification of Diseases, 1992 Revision. Geneva: World Health Organization; 1992. [Google Scholar]
- 13.Parkin DM, Whelan SL, Ferlay J, Storm H. IARC CancerBase No. 7. I to VIII. Lyon: 2005. Cancer incidence in five continents. [Google Scholar]
- 14.Curado MP, Edwards B, Shin HR, et al. IARC Scientific Publications No. 160. IX. Lyon: 2007. Cancer Incidence in Five Continents. [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties. ( www.seer.cancer.gov) released April 2008, based on the November 2007 submission. [Google Scholar]
- 16.Surveillance Research Program. National Cancer Institute SEER*Stat software. seer.cancer.gov/seerstatversion 6.4.4.
- 17.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 18.Rajpert-De Meyts E, Jorgensen N, Brondum-Nielsen K, Muller J, Skakkebaek NE. Developmental arrest of germ cells in the pathogenesis of germ cell neoplasia. APMIS. 1998;106:198–204. doi: 10.1111/j.1699-0463.1998.tb01336.x. discussion-6. [DOI] [PubMed] [Google Scholar]
- 19.Honecker F, Stoop H, de Krijger RR, Chris Lau YF, Bokemeyer C, Looijenga LH. Pathobiological implications of the expression of markers of testicular carcinoma in situ by fetal germ cells. J Pathol. 2004;203:849–57. doi: 10.1002/path.1587. [DOI] [PubMed] [Google Scholar]
- 20.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 21.Ekbom A, Akre O. Increasing incidence of testicular cancer--birth cohort effects. APMIS. 1998;106:225–9. doi: 10.1111/j.1699-0463.1998.tb01340.x. discussion 9–31. [DOI] [PubMed] [Google Scholar]
- 22.Bergstrom R, Adami HO, Mohner M, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst. 1996;88:727–33. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- 23.Pettersson A, Kaijser M, Richiardi L, Askling J, Ekbom A, Akre O. Women smoking and testicular cancer: one epidemic causing another? Int J Cancer. 2004;109:941–4. doi: 10.1002/ijc.20088. [DOI] [PubMed] [Google Scholar]
- 24.Clemmesen J. Is smoking during pregnancy a cause of testicular cancer? Ugeskr Laeger. 1997;159:6815–9. [PubMed] [Google Scholar]
- 25.Moller H, Jorgensen N, Forman D. Trends in incidence of testicular cancer in boys and adolescent men. Int J Cancer. 1995;61:761–4. doi: 10.1002/ijc.2910610604. [DOI] [PubMed] [Google Scholar]
- 26.Weir HK, Kreiger N, Marrett LD. Age at puberty and risk of testicular germ cell cancer (Ontario, Canada) Cancer Causes Control. 1998;9:253–8. doi: 10.1023/a:1008864902104. [DOI] [PubMed] [Google Scholar]
- 27.Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. United Kingdom Testicular Cancer Study Group. BMJ. 1994;308:1393–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Daling JR, Doody DR, Sun X, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115:1215–23. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieckmann KP, Hartmann JT, Classen J, Diederichs M, Pichlmeier U. Is increased body mass index associated with the incidence of testicular germ cell cancer? J Cancer Res Clin Oncol. 2009;135:731–8. doi: 10.1007/s00432-008-0504-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993;341:1392–5. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- 31.McGlynn KA, Quraishi SM, Graubard BI, Weber J-P, Rubertone MV, Erickson RL. Persistent Organochlorine Pesticides and Risk of Testicular Germ Cell Tumors. J Natl Cancer Inst. 2008;100:663–71. doi: 10.1093/jnci/djn101. [DOI] [PubMed] [Google Scholar]
- 32.Hardell L, Van Bavel B, Lindstrom G, et al. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int J Androl. 2004;27:282–90. doi: 10.1111/j.1365-2605.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 33.Hardell L, Bavel B, Lindstrom G, Eriksson M, Carlberg M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int J Androl. 2006;29:228–34. doi: 10.1111/j.1365-2605.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 34.Hardell L, van Bavel B, Lindstrom G, et al. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ Health Perspect. 2003;111:930–4. doi: 10.1289/ehp.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holl K, Surcel HM, Koskela P, et al. Maternal Epstein-Barr virus and cytomegalovirus infections and risk of testicular cancer in the offspring: a nested case-control study. APMIS. 2008;116:816–22. doi: 10.1111/j.1600-0463.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 36.Mueller N, Hinkula J, Wahren B. Elevated antibody titers against cytomegalovirus among patients with testicular cancer. Int J Cancer. 1988;41:399–403. doi: 10.1002/ijc.2910410314. [DOI] [PubMed] [Google Scholar]
- 37.Algood CB, Newell GR, Johnson DE. Viral etiology of testicular tumors. J Urol. 1988;139:308–10. doi: 10.1016/s0022-5347(17)42394-9. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen P, Andersen A, Irgens LM, Bye AS, Vagstad N. Testicular cancer and parental use of fertilizers in agriculture. Cancer Epidemiol Biomarkers Prev. 1996;5:3–9. [PubMed] [Google Scholar]
- 39.Knight JA, Marrett LD. Parental occupational exposure and the risk of testicular cancer in Ontario. J Occup Environ Med. 1997;39:333–8. doi: 10.1097/00043764-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Hardell L, Malmqvist N, Ohlson CG, Westberg H, Eriksson M. Testicular cancer and occupational exposure to polyvinyl chloride plastics: a case-control study. Int J Cancer. 2004;109:425–9. doi: 10.1002/ijc.11709. [DOI] [PubMed] [Google Scholar]
- 41.Swerdlow AJ, Skeet RG. Occupational associations of testicular cancer in south east England. Br J Ind Med. 1988;45:225–30. doi: 10.1136/oem.45.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersson A, Akre O, Richiardi L, Ekbom A, Kaijser M. Maternal smoking and the epidemic of testicular cancer--a nested case-control study. Int J Cancer. 2007;120:2044–6. doi: 10.1002/ijc.22523. [DOI] [PubMed] [Google Scholar]
- 43.McGlynn KA, Zhang Y, Sakoda LC, Rubertone MV, Erickson RL, Graubard BI. Maternal smoking and testicular germ cell tumors. Cancer Epidemiol Biomarkers Prev. 2006;15:1820–4. doi: 10.1158/1055-9965.EPI-06-0389. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow AJ, De Stavola BL, Swanwick MA, Mangtani P, Maconochie NE. Risk factors for testicular cancer: a case-control study in twins. Br J Cancer. 1999;80:1098–102. doi: 10.1038/sj.bjc.6690470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGlynn KA, Sakoda LC, Rubertone MV, et al. Body size, dairy consumption, puberty, and risk of testicular germ cell tumors. Am J Epidemiol. 2007;165:355–63. doi: 10.1093/aje/kwk019. [DOI] [PubMed] [Google Scholar]
- 46.Bjorge T, Tretli S, Lie AK, Engeland A. The impact of height and body mass index on the risk of testicular cancer in 600,000 Norwegian men. Cancer Causes Control. 2006;17:983–7. doi: 10.1007/s10552-006-0032-8. [DOI] [PubMed] [Google Scholar]
- 47.McGlynn KA, Quraishi SM, Graubard BI, Weber J-P, Rubertone MV, Erickson RL. Polychlorinated Biphenyls and Risk of Testicular Germ Cell Tumors. Cancer Res. 2009;69:1901–9. doi: 10.1158/0008-5472.CAN-08-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinzer H, Dieckmann KP, Huland E. Virus-related serology and in situ hybridization for the detection of virus DNA among patients with testicular cancer. Eur Urol. 1993;24:271–6. doi: 10.1159/000474308. [DOI] [PubMed] [Google Scholar]
- 49.Rajpert-De Meyts E, Hording U, Nielsen HW, Skakkebaek NE. Human papillomavirus and Epstein-Barr virus in the etiology of testicular germ cell tumours. APMIS. 1994;102:38–42. doi: 10.1111/j.1699-0463.1994.tb04842.x. [DOI] [PubMed] [Google Scholar]
- 50.Akre O, Lipworth L, Tretli S, et al. Epstein-Barr virus and cytomegalovirus in relation to testicular-cancer risk: a nested case-control study. Int J Cancer. 1999;82:1–5. doi: 10.1002/(sici)1097-0215(19990702)82:1<1::aid-ijc1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen R, Moller H, Thoresen SO, Pukkala E, Kjaer SK, Johansen C. Trends in testicular cancer incidence in the Nordic countries, focusing on the recent decrease in Denmark. Int J Androl. 2006;29:199–204. doi: 10.1111/j.1365-2605.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 52.Moller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22:2751–66. doi: 10.1002/sim.1481. [DOI] [PubMed] [Google Scholar]
- 53.Engholm G, Ferlay J, Christensen N, et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Prediction in the Nordic Countries, Version 3.5. Association of the Nordic Cancer Registries. Danish Cancer Society. 2009 ( http://www.ancr.nu)
- 54.Salmela E, Lappalainen T, Fransson I, et al. Genome-wide analysis of single nucleotide polymorphisms uncovers population structure in Northern Europe. PLoS One. 2008;3:e3519. doi: 10.1371/journal.pone.0003519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemminki K, Li X. Cancer risks in second-generation immigrants to Sweden. Int J Cancer. 2002;99:229–37. doi: 10.1002/ijc.10323. [DOI] [PubMed] [Google Scholar]
- 56.Hemminki K, Li X, Czene K. Cancer risks in first-generation immigrants to Sweden. Int J Cancer. 2002;99:218–28. doi: 10.1002/ijc.10322. [DOI] [PubMed] [Google Scholar]
- 57.Myrup C, Wohlfahrt J, Oudin A, Schnack T, Melbye M. Risk of testicular cancer according to birthplace and birth cohort in Denmark. Int J Cancer. 2010;126:217–23. doi: 10.1002/ijc.24736. [DOI] [PubMed] [Google Scholar]
- 58.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–22. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 59.Hardell L, Nasman A, Ohlson CG, Fredrikson M. Case-control study on risk factors for testicular cancer. Int J Oncol. 1998;13:1299–303. doi: 10.3892/ijo.13.6.1299. [DOI] [PubMed] [Google Scholar]
- 60.Moller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: case-control studies in Denmark. Cancer Causes Control. 1997;8:904–12. doi: 10.1023/a:1018472530653. [DOI] [PubMed] [Google Scholar]
- 61.Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V. Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol. 1986;124:39–52. doi: 10.1093/oxfordjournals.aje.a114369. [DOI] [PubMed] [Google Scholar]
- 62.Prener A, Hsieh CC, Engholm G, Trichopoulos D, Jensen OM. Birth order and risk of testicular cancer. Cancer Causes Control. 1992;3:265–72. doi: 10.1007/BF00124260. [DOI] [PubMed] [Google Scholar]
- 63.Weir HK, Marrett LD, Kreiger N, Darlington GA, Sugar L. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. Int J Cancer. 2000;87:438–43. doi: 10.1002/1097-0215(20000801)87:3<438::aid-ijc20>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 64.Coupland CA, Chilvers CE, Davey G, Pike MC, Oliver RT, Forman D. Risk factors for testicular germ cell tumours by histological tumour type. United Kingdom Testicular Cancer Study Group. Br J Cancer. 1999;80:1859–63. doi: 10.1038/sj.bjc.6690611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabroe S, Olsen J. Perinatal correlates of specific histological types of testicular cancer in patients below 35 years of age: a case-cohort study based on midwives’ records in Denmark. Int J Cancer. 1998;78:140–3. doi: 10.1002/(sici)1097-0215(19981005)78:2<140::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 66.Wanderas EH, Tretli S, Fossa SD. Trends in incidence of testicular cancer in Norway 1955–1992. Eur J Cancer. 1995;31A:2044–8. doi: 10.1016/0959-8049(95)00321-5. [DOI] [PubMed] [Google Scholar]
- 67.dos Santos Silva I, Swerdlow AJ, Stiller CA, Reid A. Incidence of testicular germ-cell malignancies in England and Wales: trends in children compared with adults. Int J Cancer. 1999;83:630–4. doi: 10.1002/(sici)1097-0215(19991126)83:5<630::aid-ijc11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 68.Weir HK, Marrett LD, Moravan V. Trends in the incidence of testicular germ cell cancer in Ontario by histologic subgroup, 1964–1996. CMAJ. 1999;160:201–5. [PMC free article] [PubMed] [Google Scholar]
- 69.Richiardi L, Bellocco R, Adami HO, et al. Testicular cancer incidence in eight northern European countries: secular and recent trends. Cancer Epidemiol Biomarkers Prev. 2004;13:2157–66. [PubMed] [Google Scholar]
- 70.Nethersell AB, Drake LK, Sikora K. The increasing incidence of testicular cancer in East Anglia. Br J Cancer. 1984;50:377–80. doi: 10.1038/bjc.1984.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levi F, Te VC, Randimbison L, La Vecchia C. Trends in testicular cancer incidence in Vaud, Switzerland. Eur J Cancer Prev. 2003;12:347–9. doi: 10.1097/00008469-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 72.Stang A, Rusner C, Eisinger B, Stegmaier C, Kaatsch P. Subtype-specific incidence of testicular cancer in Germany: a pooled analysis of nine population-based cancer registries. Int J Androl. 2009;32:306–16. doi: 10.1111/j.1365-2605.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 73.Liu S, Semenciw R, Waters C, Wen SW, Mery LS, Mao Y. Clues to the aetiological heterogeneity of testicular seminomas and non-seminomas: time trends and age-period-cohort effects. Int J Epidemiol. 2000;29:826–31. doi: 10.1093/ije/29.5.826. [DOI] [PubMed] [Google Scholar]