Abstract
Normal cardiac function requires dynamic modulation of contraction. β1 adrenergic-induced protein kinase A (PKA) phosphorylation of cardiac myosin binding protein C (cMyBP-C) may regulate crossbridge kinetics to modulate contraction. We tested this idea with mechanical measurements and echocardiography in a mouse model lacking three PKA sites on cMyBP-C, i.e., cMyBP-C(t3SA). We developed the model by transgenic expression of mutant cMyBP-C with Ser to Ala mutations on the cMyBP-C knock-out (KO) background. Western blots, immunofluorescence, and in vitro phosphorylation combined to show that non-PKA-phosphorylatable cMyBP-C expressed at 74% compared to normal wild type (WT) and was correctly positioned in the sarcomeres. Similar expression of WT cMyBP-C at 72% served as control, i.e., cMyBP-C(tWT). Skinned myocardium responded to stretch with an immediate increase in force, followed by a transient relaxation of force, and finally a delayed development of force, i.e., stretch activation. The rate constants of relaxation, krel (s−1), and delayed force development, kdf (s−1), in the stretch activation response are indicators of crossbridge cycling kinetics. cMyBP-C(t3SA) myocardium had baseline krel and kdf similar to WT myocardium, but unlike WT, krel and kdf were not accelerated by PKA treatment. Reduced dobutamine augmentation of systolic function in cMyBP-C(t3SA) hearts during echocardiography corroborated the stretch activation findings. Furthermore, cMyBP-C(t3SA) hearts exhibited basal echocardiagraphic findings of systolic dysfunction, diastolic dysfunction, and hypertrophy. Conversely, cMyBP-C(tWT) hearts performed similar to WT. Thus, PKA phosphorylation of cMyBP-C accelerates crossbridge kinetics and loss of this regulation leads to cardiac dysfunction.
Keywords: cMyBP-C, phosphorylation, contraction kinetics
INTRODUCTION
The strength and kinetics of cardiac contraction vary on a beat-to-beat basis as a way to match cardiac output to the circulatory demands of the body. Reduced capacity to modulate contraction has long been recognized as a key feature of dysfunction in heart failure1 and more recently in hypertrophic cardiomyopathy (HCM).2 This study explores the possibility that phosphorylation of cardiac myosin binding protein C (cMyBP-C) modulates contraction in skinned and living myocardium.
Myosin binding protein C (MyBP-C) is a component of the thick filament in striated muscle,3 and is evident as 7-9 bands at 43 nm intervals4 within the center of each half-thick filament in the A-band. Its location at every third cross-bridge crown, i.e., every 42.9 nm5 suggests that cMyBP-C has a regulatory role with respect to thick filament activity. Unlike the skeletal muscle isoform, cMyBP-C is readily phosphorylated by protein kinase A (PKA),6, 7 calcium calmodulin kinase II (CaMKII),6, 7 and protein kinase C (PKC).8, 9 Phosphorylation of cMyBP-C may promote actin-myosin interactions by either relieving a structural constraint on myosin to allow closer proximity with actin10, 11 or reducing the binding of cMyBP-C to the S2 domain of myosin to allow greater flexibility of the rod that links the myosin head to the thick filament.12, 13 Thus, phosphorylation of cMyBP-C may affect cardiac function by regulating crossbridge kinetics.
Evidence suggests that cMyBP-C and its phosphorylation status are critical to regulation of cardiac function. For example, mutations of cMyBP-C comprise the most prevalent cause of familial hypertrophic cardiomyopathy.14, 15 Phosphorylation of cMyBP-C is reduced in human heart failure16 and atrial fibrillation.17 In animal models, increased cMyBP-C phosphorylation has been associated with reduced infarct size,18 while decreased phosphorylation is associated with dysfunction in failure19, and with either dysfunction20 or preservation21 of function in ischemia.
A previously developed cMyBP-C knock out (KO)22 mouse provided initial indications concerning the roles of cMyBP-C phosphorylation in the heart. For example, skinned myocardium from cMyBP-C KO hearts exhibits constitutively accelerated crossbridge kinetics similar to wild type (WT) hearts after treatment with PKA,23 suggesting that phosphorylation of cMyBP-C accelerates crossbridge kinetics in response to β1 adrenergic stimulation. Hearts from cMyBP-C KO mice exhibit hypertrophy, diastolic dysfunction, and systolic dysfunction.22 In contrast, PKA accelerates crossbridge kinetics in skinned myocardium from mouse hearts expressing mutant non-PKA-phosphorylatable cardiac troponin I (cTnI), i.e., cTnIala2.24 Unlike cMyBP-C KO hearts, cTnIala2 hearts exhibit normal function.24 These results narrow the mechanism of acceleration of crossbridge kinetics by PKA phosphorylation to cMyBP-C and suggest that cMyBP-C phosphorylation is important for normal cardiac function.
We hypothesized that PKA phosphorylation of cMyBP-C in response to β1 adrenergic stimulation accelerates crossbridge kinetics in vivo, while the loss of this regulatory mechanism leads to cardiac dysfunction. To test this idea, we transgenically expressed constitutively non-PKA-phosphorylatable cMyBP-C on the cMyBP-C KO22 background. We found that skinned myocardium from these hearts no longer exhibits acceleration of contraction kinetics in response to treatment with PKA. Furthermore, hearts with non-PKA-phosphorylatable cMyBP-C exhibit hypertrophy, diastolic dysfunction, systolic dysfunction and reduced contractile reserve.
MATERIAL AND METHODS
Mouse Lines
N-terminus myc-tagged WT or non-PKA-phosphorylatable cMyBP-C was expressed on the cMyBP-C null background. Gautel et al.25 previously identified three PKA phosphorylation sites in human cMyBP-C at Ser275, Ser284, and Ser304; the homologous sites in mouse cMyBP-C are Ser273, Ser282, and Ser302. Here, all three residues were mutated to alanine (Figure 1A) and the α-myosin heavy chain promoter was used to promote expression of the mutant transgene in the FVB/N mouse strain. FVB/N mice expressing the transgene were bred with the previously generated cMyBP-C knockout mouse (E129X1 strain)22 to ensure expression of the exogenous transgene without competition from native cMyBP-C. WT cMyBP-C was also expressed in cMyBP-C KO mice to serve as a control. t3SA denotes transgenic expression of the Ser to Ala mutant cMyBP-C and tWT denotes transgenic expression of wild-type cMyBP-C. The protocols for care and use of animals were approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health.
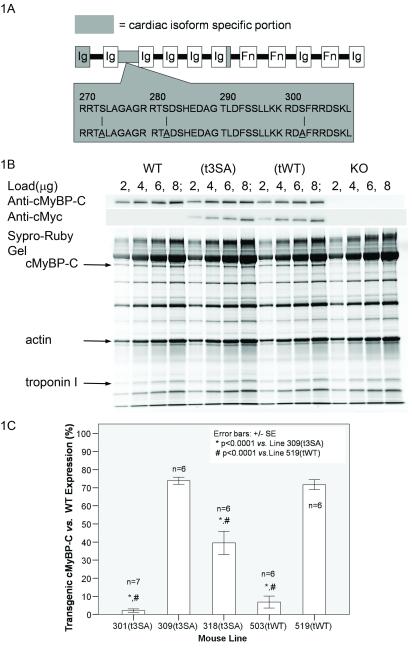
Figure 1.
WT and mutant cMyBP-C were expressed on cMyBP-C KO Background. (A) Mutant cMyBP-C(t3SA) consisted of Ala for Ser substitution at known PKA sites Ser273,Ser282,Ser302 in the cardiac specific linker region, (B) Positive anti-cMyBP-C showed that cMyBP-C was expressed in WT,t3SA, and tWT myocardium but not KO. Positive anti-cMyc in only t3SA and tWT confirmed transgenic expression of these proteins in these lines. Sypro-Ruby total protein staining also showed cMyBP-C expression in WT, t3SA, and tWT myocardium. (C) Percent expression (slope of transgenic anti-cMyBP-C staining vs. myofibril load / slope of WT anti-cMyBP-C staining vs. myofibril load) indicates that 309(t3SA) and 519(tWT) have similar levels of expression.
Myofibril Preparation
Myofibril homogenates were prepared from hearts for subsequent analysis using electrophoresis, Western blotting, immunofluorescence, and in vitro PKA phosphorylation (see data supplement “DS”).
Western Blots
Western blots were used to determine total expression of transgenes using a rabbit polyclonal anti-cMyBP-C antibody22 at a dilution of 1:10,000 and a mouse monoclonal anti-myc (Upstate® 05-419 clone 9E10) antibody at 1:250 dilution (see DS) .
Immunofluorescence
Immunofluorescence labeling of myofibrils was used to verify incorporation of transgenic cMyBP-C into the thick filament. Rabbit anti-cMyBP-C polyclonal antibody22 at 1:250 dilution was paired with AlexaFluor®647 (Molecular Probes®) goat anti-rabbit secondary antibody at 1:250. Mouse monoclonal Anti-myc (Upstate® 05-419 clone 9E10) antibody at 1:25 was paired with AlexaFluor®488 (Molecular Probes®) goat anti-mouse secondary antibody at 1:250. (See DS)
Protein Kinase A (PKA) Phosphorylation of Myofibrillar Proteins
Exogenous catalytic subunit of PKA (Sigma P2645) was used to phosphorylate cMyBP-C and cTnI in isolated myofibrils and in the preparations used for mechanical measurements (see DS). The ratio of enzyme to total myofibrillar protein (weight/weight) was 0.05 (1 unit of PKA/μl according to Sigma specifications for activity).
Comparison of Phosphorylation Levels
For each phosphorylation reaction, gel (10% polyacrylamide) lanes were loaded with different volumes of myofibrils at known concentrations. Pro-Q® Diamond (Molecular Probes, phosphoprotein stain) and Sypro-Ruby® (Molecular Probes, total protein stain) were used to compare phosphorylation levels of cMyBP-C and cTnI. Slopes of Pro-Q intensity versus protein load and slopes of Sypro-Ruby intensity versus protein load were calculated for each experiment. Double ratio “PL(experiment/WTbaseline)” of Pro-Q/protein slope to Sypro/Protein slope of an experiment in comparison to WT baseline provided a semi-quantitative assessment (see DS).
Stretch Activation and Force vs. pCa Measurements
Stretch activation was assessed in skinned myocardium23 ± exogenous PKA (Sigma P2645) to quantify the effects of PKA phosphorylation of regulatory proteins on crossbridge kinetics. This was done by setting pCa (pCa=−log10[Ca2+]) to achieve half-maximal force, setting sarcomere length at 2.1 μm, and applying a rapid stretch of 1% of fiber length (See DS). Force values were measured at various pCa to characterize force-pCa relationships.26
Heart and Body Weight Determinations
Heart weight to body weight ratios were determined as an indicator of cardiac hypertrophy. Once anesthetized, mice (2-6 months of age) were weighed to determine total body weight. Hearts were then removed, all major vessels and connective tissues were dissected away, and the heart including the atrial appendage was carefully blotted and weighed.
Echocardiography
Echocardiography was done using a Visualsonics Vevo 770 system with a 30 MHz probe to record intact in vivo cardiac structure and function using M-mode, B-mode, blood flow Doppler, tissue Doppler, and EKG gated reconstruction of left ventricle changes during a cardiac cycle (EKV™ mode). Dobutamine (10 μg/g) was administered by intraperitonial (IP) injection to elicit a maximum β1 response that was sustained for 15 minutes27 in order to maximize in vivo PKA phosphorylation. Post-dobutamine imaging was initiated 8 minutes after IP injection. All measured parameters were averages from a minimum of 3 cardiac cycles (See DS).
Statistical Analysis
Statistical analyses were done using SPSS statistical analysis software with significance set at p < 0.05. ANOVA with post-hoc Tukey honestly significant difference (HSD) tests were used to determine significant differences between specific groups for comparisons involving more than 2 groups. Paired t-tests were used only to determine significant differences before and after treatment within a group. Unless otherwise noted, all data are presented as means ± standard error of mean (SEM).
RESULTS
cMyBP-C Transgene Expression
Double positive staining of anti-cMyBP-C and anti-myc Western blots verified expression of transgenes that were introduced into the KO mice (Figure 1B). WT myocardium showed positive staining for anti-cMyBP-C but not anti-myc, while KO showed no positive staining. The mouse lines 309 (t3SA) and 519 (tWT) chosen for these experiments had closely matched expression of transgenic cMyBP-C, i.e., 74%±2 and 72%±3 of control, respectively (Figure 1C).
Incorporation of cMyBP-C
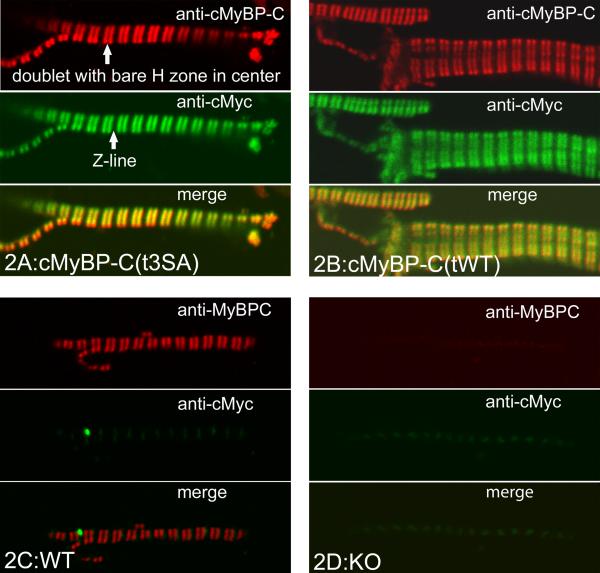
The expressed cMyBP-C appeared to be appropriately sited in both cMyBP-C(t3SA) and cMyBP-C(tWT) myocardium. Immunofluorescent labeling of myofibrils with anti-cMyBP-C/AlexaFluor®647 showed A-band doublets containing cMyBP-C in WT, cMyBP-C(tWT), and cMyBP-C(t3SA) myocardium but not in KO myocardium (Figures 2A-D). Anti-myc/AlexaFluor®488 showed the same doublets in cMyBP-C(tWT) and cMyBP-C(t3SA) but not in WT or KO myocardium (Figures 2A,2C,2D). Overlay of anti-cMyBP-C and anti-myc fluorescence images yielded matches for both cMyBP-C(tWT) and cMyBP-C(t3SA) myocardium (Figures 2C-D). Anti-myc/AlexaFluor®488 in WT myocardium showed only low-intensity non-specific fluorescence at the Z-line (Figure 2A). Anti-cMyBP-C/AlexaFluor®647 labeling of KO myofibrils exhibited only non-specific low-intensity background fluorescence at the same level as the secondary AlexaFluor®647 antibody alone (Figure 2B).
Figure 2.
Transgenic WT and mutant cMyBP-C are correctly incorporated into cMyBP-C KO myocardium. Red shows Anti-cMyBP-C with Alexafluor®647; green shows Anti-cMyc with Alexafluor®488; yellow shows match. (A) cMyBP-C(t3SA) myofibril shows matched red and green doublets (H zone in middle) to verify correctly positioned mutant cMyBP-C. (B) cMyBP-C(tWT) myocardium labeled the same as cMyBP-C(t3SA). (C) WT only had red doublets; the high concentration of anti-cMyc produced low level non-specific anti-cMyc binding at the Z-line, (D) KO only showed non-specific binding.
PKA Phosphorylation of cMyBP-C and cTnI
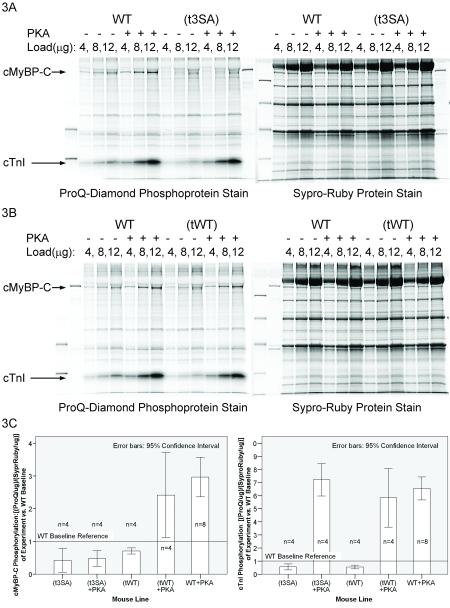
ProQ Diamond phosphoprotein staining showed that PKA did not phosphorylate the mutant cMyBP-C in cMyBP-C(t3SA) myocardium but phosphorylated cMyBP-C in both cMyBP-C(tWT) and WT myocardium (Figure 3 and Table 1). Conversely, PKA treatment increased phosphorylation of cTnI in all lines to essentially equal levels (Figure 3). The similar PKA-phosphorylation of cTnI in all mouse lines showed that PKA activity was similar between experiments; consequently, the lack of phosphorylation of mutant cMyBP-C could not be attributed to low PKA activity.
Figure 3.
PKA does not phosphorylate mutant cMyBP-C. Pro-Q Diamond stained phosphorylated proteins and Sypro-Ruby stained all proteins on the same gel. (A) PKA did not phosphorylate mutant cMyBP-C(t3SA), (B) PKA phosphorylated WT cMyBP-C in (tWT), (C) 95% confidence intervals (CI) were plotted to aid visualization of differences; non-overlapping CI indicates statistical significance. PKA phosphorylated cTnI in all lines to the same levels.
Table 1.
Effects of PKA treatment on skinned myocardium
| WT | cMyBP-C(tWT) | cMyBP-C(t3SA) | KO | |
|---|---|---|---|---|
| Stretch Activation | ||||
| n | 11 | 11 | 11 | 10 |
| krel(s−1) | 230±12‡ | 232±19‡ | 222±15‡ | 338±20* |
| krel(s−1)+PKA | 330±12† | 303±19† | 220±21*‡ | 327±28 |
| kdf(s−1) | 18.2±1.7‡ | 21.2±1.1‡ | 22.5±1.6‡ | 29.9±1.7* |
| kdf(s−1)+PKA | 29.1±1.6† | 29.4±1.4† | 22.5±1.6*‡ | 29.6±1.6 |
| Force-pCa | ||||
| n | 8 | 8 | 8 | 8 |
| pCa50 | 5.76±0.02 | 5.75±0.02 | 5.76±0.02 | 5.73±0.02 |
| pCa50+PKA | 5.65±0.03† | 5.65±0.02† | 5.68±0.03† | 5.65±0.02† |
| cMyBP-C Phosphorylation | ||||
| n | 8 | 4 | 4 | 4 |
| PL (experiment/WTbaseline) | 1 | 0.71±0.03* | 0.42±0.11* | NA |
| PL ((experiment+PKA)/WTbaseline) | 2.97±0.25† | 2.42±0.40† | 0.48±0.08* | NA |
Data are means ± SEM. See DS for calculation of krel, kdf, and PL (experiment/WTbaseline). For force-pCa relationships, normalized force versus pCa data points were fitted to Hill’s equation where K50 is [Ca2+ at 50% force, N is the Hill coefficient, and pCa50=−log10[K50]. NA = not applicable.
Significantly different from WT with the same ± PKA treatment, p < 0.05
Significantly different from its own −PKA control, p < 0.05.
Significantly different from KO with the same ± PKA treatment, p<0.05
Stretch Activation of Myocardium
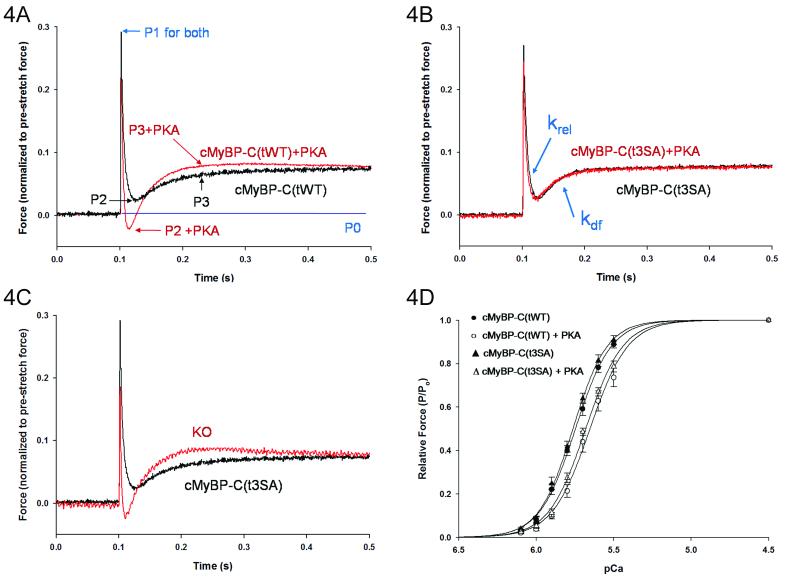
Stretch activation at 50% maximal force in cMyBP-C(t3SA) skinned myocardium differed from that in both WT and cMyBP-C(tWT) myocardium (Figures 4A-B and Table 1) but only after PKA treatment. Before PKA treatment, cMyBP-C(t3SA) myocardium exhibited similar rates of force decay (krel) and delayed force development (kdf) as in WT and cMyBP-C(tWT) myocardium (Table 1). Unlike WT and cMyBP-C(tWT), PKA treatment had no effect on either phase of the stretch activation response in cMyBP-C(t3SA) in that rate constants krel and kdf were unchanged after PKA treatment. Thus, the acceleration of contraction kinetics in WT myocardium requires phosphorylation of cMyBP-C but does not involve cTnI.
Figure 4.
PKA treatment did not affect stretch activation but decreased calcium sensitivity of force in cMyBP-C(t3SA) myocardium. (A) Forces were normalized to pre-stretch baseline P0. cMyBP-C(tWT) myocardium showed typical initial elastic response to stretch at P1, decay to P2, and delayed force development to P3 with PKA accelerating kinetics to shift P2 lower and P3 higher. (B) Stretch activation in cMyBP-C(t3SA) did not change with PKA. krel is the rate constant of force decay from P1 to P2 and kdf is the rate constant for delayed force development from P2 to P3, and (C) at baseline (no PKA treatement) cMyBP-C(t3SA) exhibited stretch activation responses similar to WT but slower than KO. (D) Normalized force-pCa plots were similar in cMyBP-C(t3SA) and cMyBP-C(tWT) myocardium at baseline and exhibited similar desensitization to calcium (right shift) after PKA treatment.
As shown in the recording in figure 4C, cMyBP-C(t3SA) myocardium exhibited slower rates of force decay and delayed force development in comparison to KO myocardium both before and after PKA treatment (Table 1). As previously reported23, contraction kinetics in KO were unaffected by PKA (Table 1).
cMyBP-C(t3SA) exhibited a small increase in β myosin heavy chain (βMHC) content, i.e., <10% of total MHC, which is significantly less than the 13-19% βMHC in KO myocardium (Online Figure I). All 4 lines of mice showed similar levels of titin phosphorylation (Online Figure II). Thus, neither βMHC expression nor titin phosphorylation can account for the observed differences in kinetics.
Force-pCa Relationships
Myocardium from all four lines exhibited similar steady state force responses to variations in activating calcium, both at baseline and following treatment with PKA (Table 1). Figure 4D shows force-pCa relationships for cMyBP-C(t3SA) and cMyBP-C(tWT) myocardium. PKA treatment decreased pCa50 within each line; however, there were no differences between the lines either at baseline or following PKA treatment (see Online Table II). Thus, the PKA-induced decrease in Ca2+-sensitivity of force does not involve phosphorylation of cMyBP-C but instead is due to phosphorylation of cTnI.28
Heart weight/body weight ratio
Both cMyBP-C(t3SA) and cMyBP-C(tWT) mice exhibited a generally normal physical appearance and no evidence of early mortality. However, cMyBP-C(t3SA) and KO mice had significantly increased heart weight to body weight ratios compared to ratios for WT and cMyBP-C(tWT) mice, indicating cardiac hypertrophy (Table 2).
Table 2.
Functional and Physical Measurements of Hearts
| WT | cMyBP-C (tWT) |
cMyBP-C (t3SA) |
KO | |
|---|---|---|---|---|
| n | 6 | 5 | 5 | 6 |
| HR(beats/min) | 412±25 | 424±57 | 427±45 | 453±59 |
|
HR (beats/min)
w/Dobutamine |
615±31† | 673±35† | 651±30† | 652±23† |
| LVIDd (mm) | 3.7±0.1 | 4.0±0.1 | 4.0±0.1 | 4.2±0.2 |
| LVIDs (mm) | 2.6±0.2‡ | 3.0±0.2 | 3.2±0.1 | 3.5±0.2* |
| PWd (mm) | 0.8±0.1‡ | 1.0±0.1‡ | 1.5±0.1* | 1.3±0.1* |
| PWs (mm) | 1.0±0.1‡ | 1.2±0.1‡ | 1.6±0.1* | 1.5±0.1* |
| IVRT (msec) | 15.3±1.3‡ | 16.0±1.0‡ | 22.5±1.6* | 24.8±1.4* |
| E/e’ | 10.7±1.7‡ | 18.1±2.1‡ | 30.1±3.9* | 30.7±2.8* |
| FS (%) | 30.3±2.8‡ | 24.6±2.5 | 20.5±1.3* | 17.2±1.2* |
| FS (%) w/Dobutamine | 55.3±5.0‡,† | 47.3±3.5‡,† | 38.1±1.7*,‡,† | 24.1±2.2*,† |
| EF (%) | 49.8±3.2‡ | 46.1±3.0 | 38.6±2.7 | 35.2±3.0* |
| EF(%) w/Dobutamine | 91.0±1.0‡,† | 88.6±1.8‡,† | 74.7±3.0*,‡,† | 55.8±5.7*,† |
| n | 10 | 11 | 12 | 5 |
| Heart/Body (mg/g) | 5.3±0.1‡ | 5.8±0.2‡ | 7.3±0.1* | 7.2±0.3* |
HR=heart rate, LVIDd=left ventricular inner diameter in diastole, LVIDs=LVID in systole, PWd=posterior wall thickness in diastole, PWs=PW in systole, FS=fractional shortening, EF=ejection fraction, IVRT=isovolumetric relaxation time, E/e’=ratio of peak blood in-flow Doppler at mitral valve to peak tissue movement Doppler at mitral valve annulus.
denotes p<0.05 vs. WT
denotes p<0.05 vs KO
denotes p<0.05 after dobutamine treatment in comparison to baseline.
Echocardiography
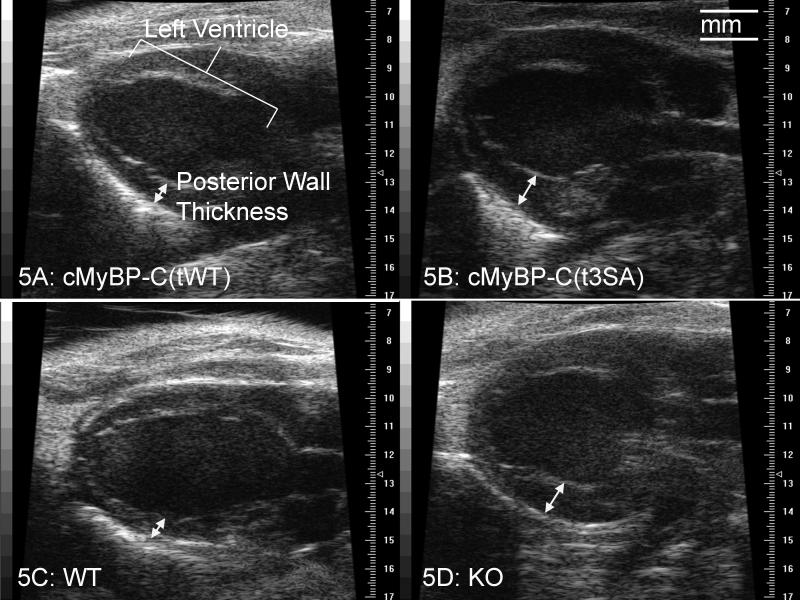
Echocardiography demonstrated similar hypertrophy and severe global dysfunction in cMyBP-C(t3SA) and KO hearts (Table 2 and Figure 5). Dramatically increased posterior wall thickness during diastole with normal LV inner diameter indicated hypertrophy. Increased IVRT indicates a longer time for LV relaxation before the start of filling during diastole, implying impaired relaxation. The ratio of the blood flow Doppler peak (E) at mitral inflow to the tissue movement Doppler peak (e’) at the lateral mitral annulus (E/e’)29 provided another index of diastolic function. The increased E/e’ ratio indicated that the rates of myocardial relaxation were slowed, ie., there was diastolic dysfunction in these hearts. Reduced fractional shortening (FS) under basal conditions indicated systolic dysfunction in both cMyBP-C(t3SA) and KO hearts.
Figure 5.
Echocardiography revealed hypertrophy in cMyBP-C(t3SA) and KO hearts. 2-D echocardiography in the parasternal long axis allowed qualitative assessment of the entire LV. In diastole, the LV posterior walls of cMyBP-c(t3SA) and KO myocardium exhibited increased thickness. All hearts showed similar LV inner diameter in diastole. Higher resolution m-mode measurements at the level of the papillary muscle corroborated findings of these 2-D views (Table 2).
Dobutamine treatment showed that cMyBP-C(t3SA) and KO hearts have different levels of reduced contractile reserve (Table 2). Dobutamine increased FS and EF in WT and cMyBP-C(tWT) hearts to the same maximal level. However, dobutamine increased FS and EF of cMyBP-C(t3SA) hearts to lower levels than either WT or cMyBP-C(tWT) hearts, which indicates reduced contractile reserve. KO hearts exhibited smaller increases in both EF and FS than did cMyBP-C(t3SA) hearts, indicating that KO hearts had the lowest contractile reserve of all the lines, ie., the rank order of contractile reserve was WT = cMyBP-C(tWT) > cMyBP-C(t3SA) > KO.
DISCUSSION
We have transgenically expressed non-PKA-phosphorylatable cMyBP-C on a cMyBP-C KO background to study the functional effects of PKA phosphorylation of cMyBP-C in living myocardium. Stretch activation studies on skinned myocardium from cMyBP-C(t3SA) mice demonstrated the loss of PKA mediated acceleration of contraction kinetics. In vivo echocardiography showed hypertrophy, diastolic dysfunction, systolic dysfunction, and reduced dobutamine augmentation of systolic function in cMyBP-C(t3SA) hearts. Thus, our data indicated that PKA phosphorylation of cMyBP-C in response to β1 adrenergic stimulation accelerates crossbridge kinetics, and loss of this regulatory mechanism contributes to cardiac dysfunction.
Stretch activation measurements showed that PKA phosphorylation of cMyBP-C accelerated crossbridge kinetics, a phenomenon that does not involve cTnI. After an initial elastic response to stretch, the concurrent processes of crossbridge detachment, crossbridge attachment, and reversal of power stroke all contribute to the phases of force decay and delayed force development, with detachment and attachment comprising the dominant contributions.30-32 The rate constants of force decay (krel) and delayed force development (kdf) manifest crossbridge turnover kinetics. WT and cMyBP-C(tWT) myocardium showed increased krel and kdf following PKA treatment, with corresponding increases in phosphorylation of cMyBP-C. In contrast, cMyBP-C(t3SA) myocardium did not show any changes in krel or kdf in response to PKA treatment, due to the failure of PKA to phosphorylate the mutant cMyBP-C.
Previous work by Stelzer, et al.24 showed that the PKA acceleration of kdf was absent in cMyBP-C null myocardium, but baseline kinetics in null myocardium were accelerated to levels observed in WT myocardium following PKA treatment. This left open the possibility that cross-bridge cycling kinetics were saturated in null myocardium, such that if cTnI phosphorylation has an effect on kinetics, it would not be observed in this preparation. The present results eliminate this possibility since the baseline (pre-PKA) kinetics of cMyBP-C(t3SA) myocardium were similar to WT and cMyBP-C(tWT) myocardium, and yet, cMyBP-C(t3SA) myocardium exhibited no change in kdf upon treatment with PKA.
Echocardiography during dobutamine augmentation provided evidence for enhancement of global function due to PKA phosphorylation of cMyBP-C. Dobutamine stimulated β1 adrenergic activity in vivo to cause PKA phosphorylation of contractile proteins including cMyBP-C. Dobutamine also increases the amplitude and accelerates the kinetics of calcium transients.33 In this study, dobutamine increased EF and FS of cMyBP-C(t3SA) hearts to lesser extents than in WT or cMyBP-C(tWT) hearts. Dobutamine augmentation of systolic function in cMyBP-C(t3SA) hearts can be attributed to enhanced calcium transients, while the lesser effects of dobutamine on cMyBP-C(t3SA) can be attributed to the absence of acceleration of crossbridge kinetics due to lack of PKA phosphorylation of cMyBP-C.
Considerable evidence supports the idea that the global cardiac dysfunction observed in cMyBP-C(t3SA) hearts is related to the inability to modulate crossbridge kinetics as a means to vary cardiac performance. For example, the non-selective β-blocker propranolol reduces phosphorylation of cMyBP-C in rats34 and depresses the positive force frequency response of intact papillary muscle from mice.35 In cMyBP-C(t3SA) myocardium, the inability to accelerate crossbridge kinetics with β1 adrenergic stimulation could provide the stimulus for compensatory hypertrophy and further dysfunction. Work by Perrino et al.36 showing that either chronic or intermittent pressure overload can cause hypertrophy and dysfunction in mice is consistent with this hypothesis. Increased LV thickness with normal LV inner cavity dimension seen on echocardiography of cMyBP-C(t3SA) mice and the increased heart weight to body weight ratios provided evidence for hypertrophy. Increased IVRT and E/e’ ratio indicated diastolic dysfunction while reduced FS indicated systolic dysfunction. Thus, cMyBP-C(t3SA) hearts showed global dysfunction that is attributed directly or via compensatory hypertrophy to the inability to accelerate crossbridge kinetics in response to β adrenergic agonists.
PKA also phosphorylates cTnI in response to β1 stimulation and previous studies suggest that phosphorylation of cTnI plays a role in PKA acceleration of crossbridge kinetics;37 however, our results cannot be attributed to PKA phosphorylation of cTnI, since PKA treatment phosphorylated cTnI in cMyBP-C(t3SA) and WT myocardium to similar levels. Our observation that PKA treatment caused a similar decrease in calcium sensitivity of force in both cMyBP-C(t3SA) and WT myocardium provided functional verification that PKA similarly phosphorylated cTnI in the two mouse lines. In a separate study, PKA treatment accelerated crossbridge kinetics in myocardium expressing native cMyBP-C and exclusively non-PKA-phosphorylatable cTnI; however, PKA did not accelerate cross-bridge kinetics in cMyBP-C null myocardium, despite similar increases in the level of phosphorylation of TnI.24 Thus, in the present study, the acceleration of crossbridge kinetics due to PKA treatment of skinned myocardium is mediated by phosphorylation of cMyBP-C while the decrease in Ca2+ sensitivity of force is due to phosphorylation of cTnI.
Our results do not eliminate the possibility that cMyBP-C phosphorylation would affect Ca2+ sensitivity of force at a sarcomere length different from that used here, i.e., 2.1 μm, since Cazorla, et al.38 reported length-dependent differences in the effects of PKA on Ca2+ sensitivity in wild-type and null myocardium. While both types of myocardium exhibited a decrease in Ca2+ sensitivity at an SL of 1.9 μm,38 only WT exhibited this decrease at 2.3 μm,38 leaving open the possibility that cMyBP-C phosphorylation modulates length-dependence of contraction.
Alterations in myofilament responsiveness to calcium during the cardiac cycle may explain why different changes in kinetics in cMyBP-C(t3SA) and KO myocardium led to similar cardiac dysfunction. Initial calcium binding to troponin converts the thin filament from a “blocked” state to a “closed” state in which crossbridges are more likely to bind.39 Subsequent crossbridge attachment with the ensuing transition from weakly to strongly bound states further activates the thin filament to an “open” state and cooperatively promotes further crossbridge attachment.39-41 Cooperative binding of strongly bound crossbridges maintains the thin filaments in the open state, which allows continued crossbridge attachment and results in a continued increase in force during the initial decay in calcium.35, 42, 43 The constitutively slower kinetics of cMyBP-C(t3SA) myocardium would reduce the number of crossbridges that bind during a twitch in response to the rise in calcium and due to cooperative recruitment. In contrast, the constitutively accelerated kinetics of KO myocardium would reduce the dwell time of strongly bound crossbridges which would reduce the cooperative recruitment of cross-bridges during the initial decline in Ca2+ during the twitch. The shortened ejection time in KO hearts is consistent with this idea.44 Thus, the slowed kinetics of cross-bridge turnover in cMyBPC(t3SA) myocardium would reduce force generated per unit calcium change during a twitch while the faster kinetics of KO myocardium would abbreviate force generation once Ca2+ begins to decline, both of which would decrease peak myocardial force and lead to pump dysfunction. Conversely, KO myocytes demonstrated prolonged calcium transient decay;45 thus, slowed calcium handling likely caused impaired relaxation that was seen by echocardiography of KO hearts.
There are other potential phosphorylation sites on cMyBP-C, but these were not changed by the mutations and did not affect our findings. Yuan et al.21 recently reported additional phosphorylation sites in canine cMyBP-C with murine homologs at Ser284 and Ser307. Basal phosphorylation of these additional sites in vivo could explain the weakly positive ProQ-Diamond staining of the mutant cMyBP-C in cMyBP-C(t3SA) myofibrils. However, the lack of increase in phosphorylation after PKA treatment suggests that these sites are not phosphorylated by PKA.
In an earlier study, Sadayappan et al.19 developed a mouse expressing non-PKA-phosphorylatable cMyBP-C (MyBP-CAllP-:(t/t)) on an essentially null background due to the constitutive expression of a truncated variant of cMyBP-C. They observed that LV chamber diameter was dilated at diastole, septal thickness was increased, and systolic function was depressed. In the present work we show that systolic dysfunction in the presence of a non-PKA-phosphorylatable cMyBP-C is due to slowing of cross-bridge cycling kinetics. Furthermore, we find that expression of a non-PKA-phosphorylatable cMyBP-C results in significant diastolic dysfunction in the absence of adrenergic stimulation, presumably due to slower cross-bridge cycling in the absence of baseline phosphorylation of cMyBP-C. Finally, our mutant mouse exhibits substantially blunted contractile reserve in response to adrenergic stimulation as a consequence of the inability to phosphorylate cMyBP-C. There are some differences between our mouse model and Sadayappan’s in terms of background genetics (KO22 vs. truncation46), mutations (MyBP-CAllP-:(t/t) has additional Thr to Ala mutations on Thr272 and Thr28119), and expression level of mutant cMyBP-C (74% vs. 40%19). Not surprisingly, echocardiography revealed some phenotypic differences between the two models, i.e., in contrast to MyBP-CAllP-:(t/t) hearts, our cMyBP-C(t3SA) hearts showed profoundly increased LV posterior wall thickness but no LV inner chamber dilation in comparison to WT.
In conclusion, our results show that PKA phosphorylation of cMyBP-C accelerates crossbridge kinetics in myocardium and the loss of this regulatory mechanism leads to hypertrophy, diastolic dysfunction, systolic dysfunction, and reduced contractile reserve.
Supplementary Material
ACKNOWLEDGEMENT
The authors are grateful to Dr. Hector Valdivia for help with immunofluorescence, Dr. Timothy Hacker for echocardiography, Dr. J. Carter Ralphe and Peter Chen for help with data supplement.
SOURCES OF FUNDING Supported by NIH grant R37-HL82900 to RM.
Footnotes
DISCLOSURES: None.
REFERENCES
- 1.Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s Heart Disease: a textbook of cardiovascular medicine. 7th Edition Elsevier Saunders; Philadelphia: 2005. [Google Scholar]
- 2.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 4.Craig R, Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976;192:451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- 5.Huxley HE, Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967;30:383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- 6.Hartzell HC, Glass DB. Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases. J Biol Chem. 1984;259:15587–15596. [PubMed] [Google Scholar]
- 7.Schlender KK, Bean LJ. Phosphorylation of chicken cardiac C-protein by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1991;266:2811–2817. [PubMed] [Google Scholar]
- 8.Venema RC, Kuo JF. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem. 1993;268:2705–2711. [PubMed] [Google Scholar]
- 9.Xiao L, Zhao Q, Du Y, Yuan C, Solaro RJ, Buttrick PM. PKCepsilon increases phosphorylation of the cardiac myosin binding protein C at serine 302 both in vitro and in vivo. Biochemistry. 2007;46:7054–7061. doi: 10.1021/bi700467k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci U S A. 1996;93:8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisberg A, Winegrad S. Relation between crossbridge structure and actomyosin ATPase activity in rat heart. Circ Res. 1998;83:60–72. doi: 10.1161/01.res.83.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Gruen M, Gautel M. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286:933–949. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- 13.Gruen M, Prinz H, Gautel M. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett. 1999;453:254–259. doi: 10.1016/s0014-5793(99)00727-9. [DOI] [PubMed] [Google Scholar]
- 14.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 15.Van Driest SL, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Sarcomeric genotyping in hypertrophic cardiomyopathy. Mayo Clin Proc. 2005;80:463–469. doi: 10.1016/S0025-6196(11)63196-0. [DOI] [PubMed] [Google Scholar]
- 16.El-Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 18.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein c phosphorylation is cardioprotective. Proc Natl Acad Sci U S A. 2006;103:16918–16923. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97:1156–1163. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, Klocke FJ, Winegrad S. Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation. 2005;111:906–912. doi: 10.1161/01.CIR.0000155609.95618.75. [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts-- evidence for novel phosphorylation sites. Proteomics. 2006;6:4176–4186. doi: 10.1002/pmic.200500894. [DOI] [PubMed] [Google Scholar]
- 22.Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, Powers PA, Moss RL. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90:594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 23.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 24.Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–511. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 25.Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: a modulator of cardiac contraction? Embo J. 1995;14:1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J. 2006;90:4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincelette J, Martin-McNulty B, Vergona R, Sullivan ME, Wang YX. Reduced cardiac functional reserve in apolipoprotein E knockout mice. Transl Res. 2006;148:30–36. doi: 10.1016/j.lab.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Wattanapermpool J, Guo X, Solaro RJ. The unique amino-terminal peptide of cardiac troponin I regulates myofibrillar activity only when it is phosphorylated. J Mol Cell Cardiol. 1995;27:1383–1391. doi: 10.1006/jmcc.1995.0131. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM, Jacobsen SJ, Burnett JC, Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi V, Piazzesi G, Ferenczi MA, Thirlwell H, Dobbie I, Irving M. Elastic distortion of myosin heads and repriming of the working stroke in muscle. Nature. 1995;374:553–555. doi: 10.1038/374553a0. [DOI] [PubMed] [Google Scholar]
- 31.Piazzesi G, Linari M, Reconditi M, Vanzi F, Lombardi V. Cross-bridge detachment and attachment following a step stretch imposed on active single frog muscle fibres. J Physiol. 1997;498(Pt 1):3–15. doi: 10.1113/jphysiol.1997.sp021837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tregear RT, Edwards RJ, Irving TC, Poole KJ, Reedy MC, Schmitz H, Towns-Andrews E, Reedy MK. X-ray diffraction indicates that active cross-bridges bind to actin target zones in insect flight muscle. Biophys J. 1998;74:1439–1451. doi: 10.1016/S0006-3495(98)77856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buser PT, Wu SY, Parmley WW, Jasmin G, Wikman-Coffelt J. Distinct modulation of myocardial performance, energy metabolism, and [Ca2+]i transients by positive inotropic drugs in normal and severely failing hamster hearts. Cardiovasc Drugs Ther. 1995;9:151–157. doi: 10.1007/BF00877756. [DOI] [PubMed] [Google Scholar]
- 34.Herron TJ, Korte FS, McDonald KS. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes. Circ Res. 2001;89:1184–1190. doi: 10.1161/hh2401.101908. [DOI] [PubMed] [Google Scholar]
- 35.Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol. 2004;558(Pt 3):927–941. doi: 10.1113/jphysiol.2004.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrino C, Prasad SV Naga, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 38.Cazorla O, Szilagyi S, Vignier N, Salazar G, Kramer E, Vassort G, Carrier L, Lacampagne A. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res. 2006;69:370–380. doi: 10.1016/j.cardiores.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J. 1994;67:273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss RL, Razumova M, Fitzsimons DP. Myosin crossbridge activation of cardiac thin filaments: implications for myocardial function in health and disease. Circ Res. 2004;94:1290–1300. doi: 10.1161/01.RES.0000127125.61647.4F. [DOI] [PubMed] [Google Scholar]
- 41.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 42.Fitzsimons DP, Patel JR, Moss RL. Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J Physiol. 2001;530(Pt 2):263–272. doi: 10.1111/j.1469-7793.2001.0263l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong CW, Kolomenskii A, Lioubimov VA, Schuessler HA, Trache A, Granger HJ, Muthuchamy M. Measurements of the cross-bridge attachment/detachment process within intact sarcomeres by surface plasmon resonance. Biochemistry. 2001;40:13915–13924. doi: 10.1021/bi0101648. [DOI] [PubMed] [Google Scholar]
- 44.Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res. 2004;94:1249–1255. doi: 10.1161/01.RES.0000126898.95550.31. [DOI] [PubMed] [Google Scholar]
- 45.Brickson S, Fitzsimons DP, Pereira L, Hacker T, Valdivia H, Moss RL. In vivo left ventricular functional capacity is compromised in cMyBP-C null mice. Am J Physiol Heart Circ Physiol. 2007;292:H1747–1754. doi: 10.1152/ajpheart.01037.2006. [DOI] [PubMed] [Google Scholar]
- 46.McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, Turnbull DH, Georgakopoulos D, Kass D, Bond M, Niimura H, Schoen FJ, Conner D, Fischman DA, Seidman CE, Seidman JG. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104:1235–1244. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.