Abstract
Schizophrenia and ADHD are associated with similar deficits in working memory, attention, and inhibition. Both disorders also involve abnormalities of white matter integrity, possibly reflecting neural communication disruptions. There are likely some regional white matter abnormalities that underlie the common cognitive impairment, though also some regional abnormalities unique to each disorder. We used diffusion tensor imaging (DTI) to compare white matter integrity, as indicated by fractional anisotropy (FA), in adolescents with schizophrenia (n=15) or ADHD (n=14) and healthy controls (n=26). Schizophrenia patients had uniquely low FA, relative to the other two groups, in bilateral cerebral peduncles, anterior and posterior corpus callosum, right anterior corona radiata, and right superior longitudinal fasciculus. ADHD patients had uniquely high FA in left inferior and right superior frontal regions. Both clinical groups had lower FA than controls in left posterior fornix. The two disorders generally demonstrated distinct patterns of abnormal connectivity suggesting that common cognitive and behavioral deficits derive from distinct sources, though the posterior fornix may be involved in both disorders. Schizophrenia was associated with abnormally low FA in widespread circuitry indicative of general connectivity disruptions, whereas ADHD was associated with abnormally high FA in frontal networks that may indicate impaired branching of fibers.
Keywords: diffusion tensor imaging, white matter, cognition, connectivity
1. Introduction
Schizophrenia and Attention-Deficit/Hyperactivity Disorder (ADHD) are associated with similar cognitive and behavioral deficits, such as impairments in working memory, sustained attention, and response inhibition (Oie and Rund, 1999; Barr, 2001). There is also emerging neuroimaging evidence that disruptions of neural communication are involved in both ADHD (Durston, 2003; Casey et al., 2007; Makris et al., 2007; Castellanos et al., 2008) and schizophrenia (Friston and Frith, 1995; McGlashan and Hoffman, 2000; Ford et al., 2002; Douaud et al., 2007) and that measures of brain connectivity are related to cognitive skills in these groups (Nestor et al., 2004; Casey et al., 2007; Schlosser et al., 2007). It is plausible that some of these neural communication disruptions are associated with the shared cognitive and behavioral impairments and are therefore common to both disorders. However, it is also likely that there are regional connectivity abnormalities specific to each disorder that are associated with unique features, such as clinical symptoms.
Adolescence is a particularly critical time period for investigations of white matter due to the surge in myelination that occurs during this period of development (Giedd et al., 1999). Adolescence is also a time of emerging psychotic symptoms in schizophrenia and declining hyperactive/impulsive symptoms of ADHD, which may be related to maturation of frontal and frontostriatal circuitry (Durston, 2003; Woo and Crowell, 2005; Liston et al., 2006). Therefore, we have chosen to focus on adolescence to better characterize white matter abnormalities in these disorders during this critical time.
Previous studies of structural connectivity in clinical populations have used diffusion tensor imaging (DTI), and specifically fractional anisotropy (FA), to assess white matter integrity. In general, areas of densely packed, well-myelinated fibers that run in a consistent direction (i.e. “healthy” white matter) have higher FA than areas in which the fibers are sparse, poorly myelinated, or divergent (Beaulieu, 2002). Early DTI studies of adolescents with schizophrenia found lower FA in bilateral frontal regions (Kumra et al., 2004), left anterior cingulate (Kumra et al., 2005), and left posterior hippocampus (White et al., 2007). Two recent studies using white matter-specific registration, which provides better alignment of major tracts, have reported lower FA in adolescents (ages 13–19 years) with schizophrenia compared to healthy adolescents either limited to bilateral parietal and cerebellar regions (Kyriakopoulos et al., 2008) or encompassing several regions, including corticospinal/corticopontine tracts, superior thalamic radiations, left optic radiations, corpus callosum, left arcuate fasciculus, and the brainstem (Douaud et al., 2007). These results suggest that regions of abnormal FA reported in adolescents with schizophrenia are widespread, though they are similar to those reported in adult schizophrenia populations (White et al., 2008).
To our knowledge, there have only been three studies comparing FA between individuals with a primary diagnosis of ADHD and healthy controls. In children (ages 7–11 years), ADHD was associated with lower FA in right premotor, right striatum, right cerebral peduncle, left cerebellar peduncle and cerebellum, and left parieto-occipital regions (Ashtari et al., 2005). In somewhat older children, ADHD was associated with lower FA in corticospinal and superior longitudinal fasciculus regions of interest (Hamilton et al., 2008). In adults (ages 37–46 years) who had ADHD as children, lower FA relative to control adults was reported in right cingulum and superior longitudinal fasciculus regions of interest, which are believed to underlie the attention and executive function abnormalities in ADHD (Makris et al., 2007). Comparison of these regions to those reported in adolescent schizophrenia populations reveals potentially common abnormalities in parietal tracts, the cerebral peduncles, and cerebellum. In contrast, abnormal FA in the corpus callosum, anterior cingulate, and hippocampal regions may be specific to schizophrenia.
Because adolescence is such a critical period in white matter development and in the clinical course of both ADHD and schizophrenia, the paucity of information about white matter integrity in adolescents with ADHD or schizophrenia, and specifically the lack of studies directly comparing these groups, limits our understanding of the role connectivity disruptions play in the cognitive, behavioral, and clinical features of these disorders. One goal of the current study was to determine whether there are areas in which schizophrenia and ADHD patients demonstrate common FA abnormalities, as these areas may reflect common neural communication disruptions that underlie cognitive or behavioral deficits. Based on previous studies, we expected these to be located in the brainstem and parietal regions. The other goal was to identify areas in which one of the clinical groups demonstrated unique FA abnormalities, which may be associated with cognitive or clinical features unique to that group. We expected schizophrenia to be associated with abnormal FA in the corpus callosum and limbic regions and ADHD to be associated with abnormal FA in frontal and frontostriatal tracts.
2. Methods
2.1 Participants
Participants consisted of 55 children and adolescents (age range 10–20 years) with either youth-onset schizophrenia (n=15), ADHD (n=14), or healthy volunteers (n=26). Table 1 summarizes demographic and clinical characteristics of these groups. In addition to the neuroimaging protocol described in this report, most of these participants were also administered computerized cognitive tasks. Results of these analyses are reported in other manuscripts (11 healthy and 9 ADHD participants were included in Karatekin, 2006; 20 healthy participants were included in Karatekin et al., 2007; and 14 healthy and 11 psychosis participants were included in White et al., 2007).
Table 1.
Demographic and Clinical Characteristics of the Participants
| Control | Schizophrenia | ADHD | Statistical Tests | |
|---|---|---|---|---|
| N | 26 | 15 | 14 | |
| M:F | 16:12 | 8:7 | 12:2 | ns |
| Age in years (SD) | 14.8 (2.41) | 15.2 (2.42) | 15.0 (2.34) | ns |
| Range | 11–20 | 10–19 | 12–18 | |
| Estimated IQ (SD)* | 114.2 (10.4) | 93.2 (14.4) | 113.1 (15.7) | F2,46 = 10.13, p < 0.001; (CTRL = ADHD) > SCHZ |
| Parental SES (SD)** | 51.0 (9.65) | 38.4 (14.61) | 54.4 (8.32) | F2,48 = 8.65, p < 0.001; (CTRL = ADHD) > SCHZ |
Notes. ns = not significant; CTRL = controls; SCHZ = schizophrenia patients
Based on 25 controls, 10 schizophrenia patients, and 14 ADHD patients
Based on 23 controls, 15 schizophrenia patients, and 13 ADHD patients
Participants with schizophrenia were recruited from child and adolescent psychiatric inpatient and outpatient clinics at the University of Minnesota, mental health professionals in the community, and flyers distributed at regional mental health conferences. ADHD participants were recruited from advertisements in the local community or from support groups for ADHD. Healthy volunteers were recruited through flyers posted in the community, through schools, via word of mouth from others who participated, or via advertisements in local newspapers.
Potential participants were excluded if they were not fluent in English, were color blind, had been premature by more than four weeks, had a history of significant neurological conditions, or had an IQ lower than 70. Potential participants were excluded from the ADHD and control groups if they had been adopted or had first-degree biological relatives with schizophrenia. Potential participants were excluded from the ADHD group if they were taking psychoactive medications other than psychostimulants, their parents were not willing to discontinue psychostimulants for cognitive testing, they had been diagnosed with or suspected of having a pervasive developmental disorder, or had never met criteria for the Combined subtype. Potential controls were excluded if they had ever taken psychoactive medications, had been diagnosed with a major psychiatric disorder or met criteria for a current disorder, had attention problems for which they had sought help, or had first-degree biological relatives with ADHD or schizophrenia. All participants were screened for the presence of implanted metal, medical devices, and other contraindications to MRI before entry into the study, and this screening was repeated immediately before the scan.
Diagnoses were made using DSM-IV criteria (American Psychiatric Association, 1994) and were based on semi-structured interviews conducted separately with participants and parents (Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version; Kaufman et al., 1996). Diagnoses of participants in the ADHD group were also based on developmental and medical history questionnaires completed by the parents, and ratings of general behavioral symptoms completed by the parents and teachers [Child Behavior Checklist (CBCL) and Achenbach Teacher Report Form (Achenbach, 1991a; Achenbach, 1991b; Achenbach and Rescorla, 2001); Swanson, Nolan, and Pelham (SNAP-IV) Teacher and Parent Rating Scale (Swanson, 1992)] to ensure that the diagnoses were based on reports from multiple informants familiar with the participants' behavior in different settings. All ADHD participants had a history of treatment with stimulant medication, though three had been unmedicated for at least 2 weeks at the time of the scan.
All individuals in the ADHD group met criteria for the Combined subtype at the time of participation except one participant who met criteria for the Inattentive subtype but who met full criteria for the Combined type in the past. Of the 15 individuals in the psychosis group, 12 had a primary diagnosis of schizophrenia of the paranoid (n=5), undifferentiated (n=5), or disorganized (n=2) subtypes. Two participants were given a diagnosis of schizoaffective disorder, and one was given a diagnosis of psychosis NOS but later converted to schizophrenia. The mean age of onset for the 11 schizophrenia patients for whom this information was available was 12.5 years (SD=2.16).
To estimate general intellectual ability, participants were administered the Vocabulary and Block Design subtests from the Wechsler Intelligence Scale, 3rd ed. (WISC-III; Wechsler, 1991), the WISC-IV (Wechsler, 2003), or the Wechsler Adult Intelligence Scale, 3rd ed. (Wechsler, 1997). IQs are reported in Table 1.
Families were provided with monetary compensation for their participation. Families with children in the clinical groups, and some families of controls, were provided with diagnostic reports. The study was approved by the University of Minnesota Institutional Review Board, and after complete description of the study to participants and their parents, written informed consent and assent were obtained.
2.2 Imaging data collection
Images were acquired on a 3T Siemens Trio (Erlangen, Germany) scanner using a standard birdcage coil. Head movement was minimized by placing cushions around the participant's head. A 3-plane localizer was used for orientation and prescription of 3D scans. DTI data were collected with a single-shot spin echo planar diffusion sequence of 64 axial slices covering the entire cerebrum and as much of the cerebellum as possible. Thirteen images were collected at each slice location, twelve of which had a diffusion gradient applied in one of 12 non-collinear directions with b=1000s/mm2 and one had no diffusion gradient (b=0). Additional acquisition parameters for the diffusion sequence were TR/TE=11000/104msec, 128×128 matrix, 256mm FOV, 2mm thickness, 0 skip, GRAPPA=2. Acquisition voxel size was 2×2×2 mm3.
2.3 Image processing
The diffusion-weighted images were corrected for eddy current distortions, and fractional anisotropy (FA) values were computed at each brain voxel using FSL software (Smith et al., 2004). The FA image from a control participant of median age (15 years) who had a full set of 64 images with good image quality was registered to the MNI (Montreal Neurologic Institute) standard reference brain with a 12-parameter affine transformation to create a template to which all other images were registered using a nonlinear algorithm (Rueckert et al., 1999). Following registration, all images were smoothed with a 4mm full-width-at-half-maximum Gaussian kernel to reduce measurement noise (Ashburner et al, 2000; White et al., 2001; Jones et al., 2005).
2.4 Statistical analysis
Demographic measures were compared across groups with one-way analyses of variance (ANOVAs) except for gender composition, which was compared with a chi-square test. Significant effects of ANOVAs were followed up using Tukey post-hoc tests.
A one-way ANOVA was conducted on a voxelwise basis to test for the effect of group after covarying for age. Only voxels that had FA > 0.25 in all subjects were included to ensure that analyses were conducted only at locations that were likely to include white matter in all subjects. Clusters were identified in which at least one voxel was significant at P<0.005, all voxels were significant at P<0.05, and the volume was greater than 200mm3. Regions were labeled according to a standard white matter atlas (Mori et al., 2005). To better examine how the groups differed at each significant location, the average value across all voxels within each cluster was calculated for each subject, and these cluster averages were imported into statistical software to test for group effects using one-way ANOVA. Tukey post-hoc tests were used to characterize significant group effects.
3. Results
3.1 Demographic results
As can be seen in Table 1, groups did not differ significantly on age. Although the ADHD group tended to have fewer females, differences in gender composition across groups did not reach statistical significance. The psychosis group had lower estimated IQ and parental SES than the ADHD and control groups, who did not differ from each other.
3.2 Imaging results
Table 2 summarizes the regions in which the groups differed on FA according to the initial voxelwise ANOVA and post hoc comparisons. Briefly, the schizophrenia patients had lower FA than the other two groups in the bilateral cerebral peduncles, genu and splenium of the corpus callosum, right anterior corona radiata, and right superior longitudinal fasciculus. The ADHD group demonstrated higher FA than the other two groups in left inferior and right superior prefrontal regions, which were considered to be in the anterior corona radiata. Both clinical groups had lower FA than the control group in the left posterior fornix. In the remaining clusters (left anterior limb of the internal capsule, left retrolenticular part of the internal capsule, and forceps minor), one group failed to differ from either of the other two.
Table 2.
Regional Fractional Anisotropy Differences across Groups.
| Location | Volume mm3 (voxels) | Center (x, y, z) | F(2,52) | P | Tukey | Schiz | ADHD | Control |
|---|---|---|---|---|---|---|---|---|
| L cerebral peduncle | 1480 (185) | (−14, −18, −8) | 13.27 | <0.001 | S<A=C | 0.4651 | 0.5083 | 0.5124 |
| R cerebral peduncle | 416 (52) | (22, −12, −8) | 7.78 | 0.001 | S<A=C | 0.4459 | 0.4830 | 0.4892 |
| L anterior corona radiata | 632 (79) | (−22, 28, −4) | 5.45 | 0.007 | A>S=C | 0.4456 | 0.4841 | 0.4455 |
| L posterior fornix | 240 (30) | (−30, −34, −2) | 6.98 | 0.002 | S=A<C | 0.4151 | 0.4144 | 0.4553 |
| Anterior corpus callosum | 272 (34) | (0, 24, 4) | 5.63 | 0.006 | S<A=C | 0.5384 | 0.6030 | 0.5871 |
| L anterior limb of the internal capsule | 496 (62) | (−22, 8, 4) | 7.28 | 0.002 | A>C | 0.4829 | 0.5072 | 0.4657 |
| R anterior corona radiata | 208 (26) | (32, 34, 8) | 5.21 | 0.009 | S<A=C | 0.3476 | 0.3788 | 0.3820 |
| L retrolenticular part of the internal capsule | 336 (42) | (−28, −34, 14) | 6.74 | 0.002 | S>C | 0.5336 | 0.5093 | 0.4928 |
| L splenium of the corpus callosum | 280 (35) | (−6, −36, 18) | 5.32 | 0.008 | S<A=C | 0.5426 | 0.5897 | 0.5922 |
| R superior longitudinal fasciculus | 224 (28) | (36, −50, 20) | 6.57 | 0.003 | S<A=C | 0.4377 | 0.4777 | 0.4690 |
| L forceps minor | 200 (25) | (−14, 42, 22) | 8.07 | 0.001 | S>A | 0.4939 | 0.4324 | 0.4604 |
| R anterior corona radiata | 424 (53) | (24, 24, 26) | 6.05 | 0.004 | A>S=C | 0.3835 | 0.4165 | 0.3761 |
L = left; R = right; A = ADHD group; C = control group; S = schizophrenia group
Clusters are ordered from inferior to superior based on the center, which is given in MNI coordinates.
4. Discussion
In a study of adolescents with ADHD or schizophrenia, we used DTI to identify areas of white matter integrity abnormalities that were common to both disorders or unique to one disorder. The primary strength of this study is the direct comparison of two clinical syndromes, which provides information about the specificity of effects. An initial voxelwise ANOVA identified 12 areas in which FA differed among the three groups. According to post-hoc tests, six of these were regions in which schizophrenia patients had uniquely low FA, two were regions in which ADHD patients had uniquely high FA, and one was a region of abnormally low FA common to both clinical groups. In the remaining areas, one group failed to differ from the other two, meaning any difference in FA was neither unique to one clinical group nor common to both.
Despite the similarities of cognitive impairment between ADHD and schizophrenia and emerging evidence that these impairments involve disruptions of communication among brain regions, only one cluster was identified in which both clinical groups had lower FA than controls. That cluster was located in the left posterior fornix, which is similar to the area of abnormally low FA identified by our group in the same set of adolescent schizophrenia patients compared to healthy adolescents (White et al., 2007). In that earlier report, we suggested that the observed effect could be a generalized finding associated with lower IQ; however, the observation of lower FA in ADHD subjects, whose average IQ was almost identical to that of the controls, reduces this possibility. Furthermore, the detection of this effect in both studies of these patients, despite using a higher quality non-linear registration and different analysis techniques, suggests that the finding is not due to an artifact or anomaly of image processing. The implicated region likely contains output fibers of the hippocampus in which disrupted communication may have widespread effects on cognitive processing through connections with prefrontal regions (Goldman-Rakic et al., 1984).
Areas of abnormal FA that were unique to schizophrenia are similar to those reported in previous studies of adolescent-onset schizophrenia using the same registration technique (Douaud et al., 2007; Kyriakopoulos et al., 2008). Lower FA in the cerebral peduncles, which includes the corticospinal/corticopontine tracts, may represent disruption of sensorimotor circuitry (Douaud et al., 2007) and further implicates dysfunction of the cortico-cerebellar-thalamo-cortical circuit, which has been suggested to be a primary trait of schizophrenia (Andreasen et al., 1996). The areas of abnormally low FA in the anterior and posterior portions of the corpus callosum are indicative of impaired interhemispheric communication in frontal and parietal regions, which is consistent with behavioral and electrophysiological evidence of increased functional lateralization in schizophrenia (Endrass et al., 2002). Abnormally low FA in areas identified as the right anterior corona radiata and superior longitudinal fasciculus further implicates disrupted cortical communication in frontal and parietal tracts. Together, these regions implicate a general deficit in intra- and interhemispheric communication among frontal, parietal, and brainstem regions. As mentioned in earlier work from our group (White et al., 2007), the possibility that these deficits are related to lower IQ or parental SES, rather than the disorder itself, cannot be ruled out with the current data.
Areas of abnormal FA that were unique to ADHD were remarkable in that they represented higher FA relative to the other groups, which has generally been interpreted as consistent with healthier white matter, including fibers that are more numerous, of greater density, more myelinated, and/or run in a more consistent direction (Beaulieu, 2002). However, a recent study of individuals with Williams syndrome found that higher FA in the superior longitudinal fasciculus was associated with higher cognitive impairment, possibly indicating that increased FA in this region is due to decreased dendritic branching (Hoeft et al., 2007). Both regions of abnormally high FA detected in the current study were located in the anterior corona radiata, which is composed of heavily branched frontostriatal connections (Mori et al., 2005). Although this may be further evidence that both higher and lower FA can be indicative of pathology (Tuch et al., 2005; Hoeft et al., 2007), it is also possible that these areas of increased FA represent compensatory mechanisms.
A strength of this study is that we have attempted to equate groups on factors that are unrelated to the disorders but may affect FA, such as age. Factors that are related to the disorders, such as clinical variables (e.g. symptom severity, duration of illness, and medication exposure), could not be equated, and continued investigation is required to determine whether FA abnormalities in schizophrenia and ADHD are directly related to the presence of the disorder itself or are instead related to intrinsic features of the disorder, such as individual symptoms. Based on studies of adults with schizophrenia, neuroleptic medication exposure has not been shown to have a substantial effect on FA (Christensen et al., 2004) and there is currently no information about the effects of psychostimulants on FA in ADHD patients. There is potentially a recruitment bias created by using clinical sources for the schizophrenia patients but a community-based approach for the other two groups. However, the very low prevalence of adolescent-onset schizophrenia makes community-based recruitment difficult. Similarly, the overrepresentation of males in the ADHD group and lower IQ and parental SES in the schizophrenia group may also limit the results of this study, though it is unlikely that the groups are unrepresentative of their respective populations because these are factors often associated with these disorders and to equalize groups on these factors may also limit the generalizability of results. However, future studies that are able to recruit larger groups may benefit from determining whether the results change when SES is matched or when only males are included in analyses. Another potential limitation is the assumption, inherent to using age as a covariate, that age-related changes during adolescence are similar across these three groups. This limitation may be confounded by the wide age range used in this study that was required to obtain a sufficient sample size. Future studies of FA in ADHD and schizophrenia during adolescence are necessary to better characterize the appropriateness of this assumption. Finally, a limitation present for all voxel-based studies is that of the potential for systematic registration error to confound comparisons of FA. This concern is reduced by the use of a custom template, though we also visually inspected the overlay of group averages to verify that there were no obvious registration differences across groups. In addition, studies that have attempted to control for this confound have found that it accounts for a minority of effects if any at all (Ardekani et al. 2003; Burns et al. 2003; Vangberg et al., 2006; Seok et al., 2007)
In summary, both clinical groups had abnormally low FA in the left posterior fornix, which may be associated with the common cognitive and/or behavioral impairments. The schizophrenia patients had widespread areas of uniquely low FA that suggest deficits of neural communication in multiple networks. The unique abnormalities seen in the ADHD patients were of higher FA in bilateral frontostriatal tracts. The majority of areas represented FA abnormalities unique to one of the clinical groups, suggesting that the cognitive and behavioral deficits shared by the two disorders likely arise from different sources.
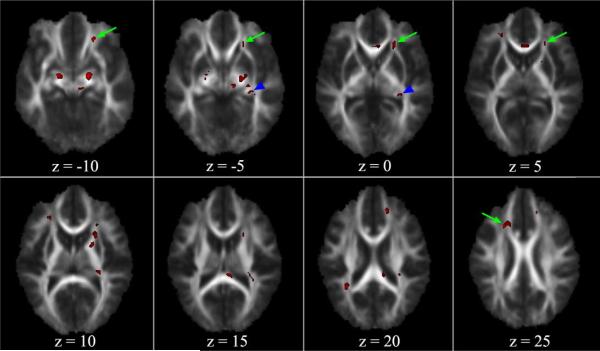
Figure 1.
Regional Fractional Anisotropy Differences across Groups. Significant clusters identified by voxelwise ANOVA are overlaid on the average FA map. The left side of the brain is represented on the right side of the image. Both clinical groups had lower FA in the left posterior fornix (arrowheads in B and C). ADHD patients had uniquely high FA in the left (arrows in A–D) and right (arrow in H) anterior corona radiata. Schizophrenia patients had uniquely low FA in bilateral cerebral peduncles (visible on A and B), anterior (C, D) and posterior (F, G) corpus callosum, right anterior corona radiata (D, E), and right superior longitudinal fasciculus (G).
Acknowledgements
This study was funded through awards from the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD), NIH MH-060662, K08 MH06540, R03 MH063150-01A2, and by the Mental Illness and Neuroscience Discovery (MIND) Institute and the Center for Neurobehavioral Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no competing interests.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991a. [Google Scholar]
- Achenbach TM. Manual for the Teacher's Report Form and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington, VT: 1991b. [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. ASEBA; Burlington, VT: 2001. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Author; Washington, DC: 1994. [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LLB, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: A positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. NeuroReport. 2003;14(16):2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry – The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-Deficit/Hyperactivity Disorder: A preliminary diffusion tensor imaging study. Biological Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Barr WB. Schizophrenia and Attention Deficit Disorder: Two complex disorders of attention. Annals of the New York Academy of Science. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, MacGillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: A diffusion tensor magnetic resonance imaging study. British Journal of Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Casey BJ, Epstein JN, Buhle J, Liston C, Davidson MC, Tonev ST, Spicer J, Niogi S, Millner AJ, Reiss A, Garrett A, Hinshaw SP, Greenhill LL, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Glover G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164(11):1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, DiMartino A, Biswal B, Sonuga-Barke EJS, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Research: Neuroimaging. 2004;130:71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Durston S. A review of the biological bases of ADHD: What have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Endrass T, Mohr B, Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: Evidence from event-related potentials. Neuroscience Letters. 2002;320:57–60. doi: 10.1016/s0304-3940(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clinical Neuroscience. 1995;3:89–97. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O'Neill J, Alger JR, Luders E, Phillips OR, Caplan R, Toga AW, McCracken J, Narr KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;00:000–000. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, Korenberg J, Bellugi U, Galaburda A, Reiss AL. More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. The Journal of Neuroscience. 2007;27(44):11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercignani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Improving antisaccade performance in adolescents with Attention-Deficit/Hyperactivity Disorder (ADHD) Experimental Brain Research. 2006;174:324–341. doi: 10.1007/s00221-006-0467-x. [DOI] [PubMed] [Google Scholar]
- Karatekin C, Marcus DJ, White TJ. Oculomotor and manual indices of incidental and intentional spatial sequence learning in middle childhood and adolescence. Journal of Experimental Child Psychology. 2007;96:107–130. doi: 10.1016/j.jecp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia -- Present and Lifetime Version (K-SADS-PL) University of Pittsburgh School of Medicine; Pittsburgh, PA: 1996. [Google Scholar]
- Kumra S, Ashtari M, Cervellione KL, Henderson I, Kester H, Roofeh D, Wu J, Clarke T, Thaden E, Kane JM, Rhinewine J, Lencz T, Diamond A, Ardekani BA, Szeszko PR. White matter abnormalities in early-onset schizophrenia: A voxel-based diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(9):934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- Kumra S, Ashtari M, McMeniman M, Vogel J, Augustin R, Becker DE, Nakayama E, Gyato K, Kane JM, Lim K, Szeszko P. Reduced frontal white matter integrity in early-onset schizophrenia: A preliminary study. Biological Psychiatry. 2004;55:1138–1145. doi: 10.1016/j.biopsych.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biological Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME. Neurosychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18(4):629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie M, Rund BR. Neuropsychological deficits in adolescent-onset schizophrenia compared with attention deficit hyperactivity disorder. American Journal of Psychiatry. 1999;156(8):1216–1222. doi: 10.1176/ajp.156.8.1216. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonada LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Non-rigid registration using free-form deformation: Applications to breast MR images. IEEE Transactions on Medical Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schlosser RGM, Nenadic I, Wagner G, Gullmar D, von Consbruch K, Kohler S, Schultz CC, Koch K, Fitzek C, Matthews PM, Reichenbach JR, Sauer H. White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophrenia Research. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Seok J-H, Park H-J, Chun J-W, Lee S-K, Cho HS, Kwon JS, Kim J-J. White matter abnormalities associated with auditory hallucinations in schizophrenia: A combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Research: Neuroimaging. 2007;156:93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Swanson J. School based assessments and interventions for ADD students. KC Publishing; Irvine, CA: 1992. [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. PNAS. 2005;102(34):12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk A-M, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- White T, Kendi ATK, Lehericy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO. Disruption of hippocampal connectivity in children and adolescents with schizophrenia -- A voxel-based diffusion tensor imaging study. Schizophrenia Research. 2007;90:302–307. doi: 10.1016/j.schres.2006.09.032. [DOI] [PubMed] [Google Scholar]
- White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in Magnetic Resonance Imaging. 2008;19(2):97–10. doi: 10.1097/RMR.0b013e3181809f1e. [DOI] [PubMed] [Google Scholar]
- White T, O'Leary D, Magnotta V, Arndt S, Flaum M, Andreasen NC. Anatomic and functional variability: The effects of filter size in group fMRI data analysis. NeuroImage. 2001;13:577–588. doi: 10.1006/nimg.2000.0716. [DOI] [PubMed] [Google Scholar]
- Woo T-UW, Crowell AL. Targeting synapses and myelin in the prevention of schizophrenia. Schizophrenia Research. 2005;73:193–207. doi: 10.1016/j.schres.2004.07.022. [DOI] [PubMed] [Google Scholar]