Abstract
Objective
Evaluate the pharmacokinetics (PK) of lamivudine (3TC), stavudine (d4T), and nevirapine (NVP) in HIV-infected Malawian children receiving quartered tablet multiples of Triomune 40™ (GT) compared to individual generic (GL) and trade (TL) liquids
Design
prospective, randomized, 3-way crossover study
Methods
Subjects [8-<12kg;18-<22kg; or 28-<32kg] taking Triomune 40™ were recruited and randomized to receive GT twice daily (¼, ½, or ¾ tablets using Malawi treatment guidelines); GL twice daily (in the equivalent dose of GT), or TL twice daily (dosed using weight and age from DHHS Pediatric treatment guidelines). After 10 days of one formulation, 6-hour PK sampling was performed, and subjects crossed over to subsequent formulations. C0h, AUC0-6h, Cmax, and Tmax were generated for each antiretroviral (ARV).
Results
7 male and 11 females (6 in each GT dosing group) with median (range) age of 7.2 (1.3-13.6) yrs, 19 (9-30.5) kg, and 109 (75-132) cm were recruited. Combining all subjects, no difference in pharmacokinetics was noted among the formulations for all drugs. However, subjects in the ¼ GT dosing group had lower 3TC exposures than with the GL or TL (3TC AUC0-6h = 1102, 1720, 2060 hr*ng/mL respectively; p<0.005) and had more subtherapeutic NVP C0h (10 of 13 occasions, versus ½ and ¾ tablet groups). Compared to western pediatric cohorts, Malawians had concentrations 30-40% lower for 3TC and d4T and 50% higher for NVP.
Conclusions
Quartered multiples of Triomune 40™ are appropriate for children 18-<22kg and 28-<32kg . However, alternative formulations are suggested in children 8-<12kg.
Introduction
As of December 2007, over 2.5 million children were infected with HIV worldwide, with an estimated 420,000 newly infected in 2007 alone [1]. The majority of infected adults and children live in resource-poor countries; two-thirds of those live in African countries such as Malawi. In 2005, out of a total population of 12.3 million, it was estimated that 700,000-1,120,000 Malawians were infected with HIV, and over 169,000 were in need of antiretroviral therapy [2].
The government in Malawi proactively addressed this issue in 2004 by providing the generic antiretroviral combination Triomune 40™, a one-pill product manufactured by Cipla Ltd. (India) containing lamivudine (3TC), stavudine (d4T), and nevirapine (NVP). The cost of this generic combination is substantially lower ($11 per month) than trade products ($170 per month). This product is used as whole tablets to treat adults—however, quartered multiples are being used to treat children. Although splitting unscored fixed dose combination tablets is a suboptimal method of drug administration, by the end of December 2007, over 141,449 patients had been placed on therapy, and 8% of these were children prescribed this dosing method [3].
Based on the established use of this product, characterizing the pharmacokinetic (PK) properties of these generic formulations is of vital importance to provide the appropriate care for these HIV-infected patients. Suboptimal exposure to antiretrovirals (ARVs) can lead to the emergence of resistance and treatment failure. Particularly in this setting of limited treatment options, durability of ARVs is of critical importance.
Data comparing Triomune to individual trade products show that the content of antiretrovirals in the generic and trade products are similar [4]. Additionally, after a single dose in healthy volunteers, Triomune 40™ is bioequivalent to trade formulations [5]. However, our group recently compared the pharmacokinetics of the generic tablet to individual trade products in HIV-infected adults in Malawi [6]. Triomune did not meet the strict definition of bioequivalence as compared to individual trade products. In particular, patients taking Triomune had higher stavudine Cmax compared to the trade product. Additionally, Malawian adults had higher nevirapine concentrations than those reported in Western patients. Since generic and trade formulations may result in different antiretroviral exposures, and using quartered table multiples may add additional pharmacokinetic variability, we designed this study to evaluate three antiretroviral formulations in three different pediatric dosing groups.
Methods
Study setting
The study subjects were recruited from both Kamuzu Central Hospital (KCH) and the Lighthouse Clinic in Lilongwe, Malawi where over 90,000 HIV-infected persons reside. KCH is a tertiary referral hospital for the central region of Malawi and houses outpatient clinics for HIV-infected patients. The Lighthouse clinic is on the campus of KCH and treats HIV-infected adults and children. Study visits were conducted at the UNC Project building which is located in proximity to and collaborates with both sites.
Study design
This was a prospective, non-blinded, randomized, 3-way crossover pharmacokinetic comparative bioavailability study of a generic fixed-dose combination tablet, generic liquids, and trade liquids formulations of d4T + 3TC + NVP in HIV-infected children taking Triomune 40™ (40mg d4T + 150mg 3TC + 200mg NVP) at the time of enrollment.
Study population
The population recruited were HIV-1 infected children who had been taking Triomune 40™ twice daily for at least 28 days for treatment of HIV and fell into three weight categories: 8-<12kg, 18-<22kg, or 28-<32kg. These weights corresponded to ages of approximately 1-15 years. Children with hemoglobin <8g/dL, AST or ALT > 1 times upper limit of normal, with concomitant drug interacting medications or who were unable to return to the clinic for study visits or were unable to have blood drawn were not included in the study.
Study procedures
Human experimentation guidelines of the US Department of Health and Human Services, the University of North Carolina at Chapel Hill, and the National Health Sciences Research Committee of Malawi were followed. After agreeing to participate, each eligible child and the child's guardian were presented with an informed consent/assent in either English or Chichewa (the national language for Malawi). A parental consent was signed by at least one parent or guardian and those children older than 7 years and literate were provided an assent form. Each subject was then randomized to one of six dosing sequences that included three dosing formulations in a cross-over fashion with no washout period using a random numbers generator (proc plan, SAS version 9.1). The dosing formulations were generic tablet (GT), generic liquid (GL), or trade liquid (TL) given twice daily. The GT formulation was Triomune 40™, containing 40mg d4T, 150mg 3TC, and 200mg NVP (Cipla, Ltd., India) [Batch numbers K40227 and 646374]. GL formulations were d4T (Stavir powder for oral solution 5mg/mL, GPO Thailand), 3TC (Lamivir oral solution 50mg/5mL, Cipla, Ltd., India), and NVP (Nevimune oral suspension 50mg/5mL Cipla, Ltd., India). TL formulations were d4T (Zerit powder for oral solution 1mg/mL, Bristol-Meyers Squibb, Princeton, New Jersey, USA), 3TC (Epivir oral solution 10mg/mL, GlaxoSmithKline, Research Triangle Park, North Carolina, USA), and NVP (Viramune oral suspension 50mg/5mL Boehringer Ingelheim, Columbus, Ohio, USA).
GT dosing was based on the Malawi pediatric dosing guidelines which use quartered tablet multiples of Triomune according to patients’ weight [14]. Since Triomune is a fixed dose combination, quartered multiple dosing was based on an approximation of a mg/kg daily dose of all three antiretrovirals. This approach lends to underdosing in some patients and overdosing in others when using quartered multiples of a fixed dose combination. The GL dose was an equivalent mg/day dose to the GT dosing while the TL dose was based on DHHS pediatric treatment guidelines from January 2004 (Table 1) [15]. Current recommendations for nevirapine dosing are based on BSA (mg/m2); however, at the time of study initiation (2003) weight based dosing (mg/kg) was the standard of care [15]. Each dosing formulation was given for 10-14 days with subsequent pharmacokinetic sampling over 6 hours within a dosing interval. A washout period was not implemented as these subjects required continuous antiretroviral therapy for treatment of HIV. Subjects and their guardians were given instructions on taking the medications and diaries to document dosing times. Pill counts and/or liquid weights were used to assess adherence at each visit. As overnight visits could not be accommodated, an abbreviated 6 hour PK visit followed a morning dose. Subjects reported to the UNC Project building in the morning prior to their dose and 10-11 hours after their previous evening dose. An intravenous catheter was placed and normal saline was administered to keep the line patent. A 5mL whole blood sample was drawn just prior to their morning dose of medications (t=0h). Subsequent samples were obtained at 1, 2, 4, and 6 hours after the witnessed morning doses. Subjects received breast milk and/or food within 1 hour after the dose and lunch after the 4 hour blood sample. Breakfast and lunch selections were similar for all subjects.
Table 1.
Dosing Table
| |
Group 1 (8-<12 kg) |
Group 2 (18-<22 kg) |
Group 3 (28-<32 kg) |
|---|---|---|---|
| Generic Tablet | 3TC: 75 mg/d | 3TC: 150 mg/d | 3TC: 225 mg/d |
| d4T: 20 mg/d | d4T: 40 mg/d | d4T: 60 mg/d | |
| |
NVP: 100 mg/d |
NVP: 200 mg/d |
NVP: 300 mg/d |
| Generic Liquid | 3TC: 75 mg/d | 3TC: 150 mg/d | 3TC: 225 mg/d |
| d4T: 20 mg/d | d4T: 40 mg/d | d4T: 60 mg/d | |
| |
NVP: 100 mg/d |
NVP: 200 mg/d |
NVP: 300 mg/d |
| Trade Liquid | 3TC: 8 mg/kg/d | 3TC: 8 mg/kg/d | 3TC: 8 mg/kg/d |
| D4T: 2 mg/kg/d | D4T: 2 mg/kg/d | D4T: 2 mg/kg/d | |
| NVP: <8 yo 14 mg/kg/d | NVP: < 8 yo 14 mg/kg/d | NVP: < 8 yo 14 mg/kg/d | |
| >8 yo 8 mg/kg/d | > 8 yo 8 mg/kg/d | > 8 yo 8 mg/kg/d |
All whole blood samples were collected in K3 containing Vacutainer tubes (Fisher Scientific, Hampton, New Hampshire, USA) and centrifuged at 1300x g at 4°C for 10 minutes. Plasma was removed and aliquoted to 2mL cryovials and stored at -70°C until shipment to UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry lab for analysis.
Drug concentrations for stavudine, lamivudine, and nevirapine were analyzed using a validated HPLC-UV method that has been published previously [16]. Briefly, all three drugs and a hexobarbital internal standard were extracted from 500μL of plasma using solid-phase extraction columns (BOND ELUT-C18, Harbor City, California, USA) and 100% methanol as an eluent. Samples were dried under nitrogen steam and reconstituted in 100mL buffer. Each sample was injected onto an Agilent HP1100 system (Hewlett-Packard, Palo Alto, California, USA) and analytes eluted using reverse-phase chromatography with a Waters Atlantis dC C-18 (3.9mm × 150mm; 5μm particle size) analytical column (Waters, Milford, Massachusetts, USA). Peaks were detected using UV absorbance and data collected using Chemstation software (Agilent, Santa Clara, California, USA). The concentration range was 10-10,000 ng/mL, with intra-and interday precision of 0.5-5.1% and accuracy of 0.5-5.6% for all concentrations. The laboratory participates in two external proficiency testing programs: the AIDS Clinical Trials Group and the Dutch Association for Quality Assessment in TDM and Clinical Toxicology (KKGT International and Interlaboratory QC Program).
Non-compartmental methods were employed to calculate pharmacokinetic parameters using WinNonlin Pro 4.0.1 (Pharsight Corp, Mountain View, California, USA). C12hr was obtained using λz extrapolations. AUC0-6hr was calculated using the linear trapezoidal rule up to, and log trapezoidal rule after, Cmax. Cmax and tmax (time to Cmax) were both determined by directly observing the data. Medians and ranges were calculated for all demographic and baseline data. Geometric means (90% CI) and geometric mean ratios (90% CI) were calculated for comparisons between GT/GL, GT/TL, and GL/TL using SAS version 9.1 (Cary, North Carolina, USA). All comparisons to TL were dose adjusted [e.g, AUCGT/AUCTL)*(DoseTL/DoseGT)]. The trough concentrations were extrapolated using C0hr,ss = C/e-kt (C=predose concentration on day of PK visit; k=elimination rate; t=difference in time after previous dose and 12 hrs). Adherence rates were determined based on liquid measurements, pill counts, or unit dose powder counts (for stavudine) at each visit. Sample size was calculated using mean (SD) for both Cmax and AUC0-24hr for nevirapine. The most conservative sample size was determined to be 15 using a single-group repeated measures analysis of variance with a 0.05 significance level with 80% power to detect a 30% difference in means across the 3 levels of the repeated measures factor characterized by an effect size of 0.2570 (based on Cmax for NVP). A sufficient sample size of 18 subjects was chosen.
Results
From a total of 24 screened subjects, 18 HIV-infected children were enrolled and completed the study. Six subjects were enrolled into each weight-based dosing group. Demographics for each dosing group are presented in Table 2. Each child had been taking Triomune 40™ for a median (range) of 3.5 (1-26) months at the time of randomization. During the 30 days study period subjects had >98% adherence to study medications as assessed by diary cards, pill counts and liquid volume estimates.
Table 2.
Demographics
| Parameter [Median (Range)] or proportion | All Groups (N = 18) | Group 1 (N = 6) (8-<12 kg | Group 2 (18-<22 kg) (N = 6) | Group 3 (28-<32 kg) (N = 6) |
|---|---|---|---|---|
| Age (years) | 7.5 (1.3-13.6) | 3.8 (1.3-7.8) | 7.0 (5.8-8.6) | 11.9 (1.2.-13.6) |
| Sex (M:F) | 7:11 | 3:3 | 2:4 | 2:4 |
| Weight (kg) | 19 (9-31) | 10.6 (9-11.5) | 19.0 (18-21) | 28.6 (28-30.5) |
| Height (cm) | 109 (75-132) | 79 (75-100) | 112 (100-116) | 131 (125-132) |
| CD4 count (cells/mm3) | 383 (13-1412) | 177 (13-1412) | 542 (448-693) | 237 (105-524) |
| % Undetectable at baseline | 59% | 6% | 29% | 24% |
| Time on Triomune Prior to Study Entry (months) | 3.5 (0.5-26) | 0.67 (0.5-1.9) | 4.1 (0.5-6.1) | 5.9 (2.4-26) |
All Dosing Groups
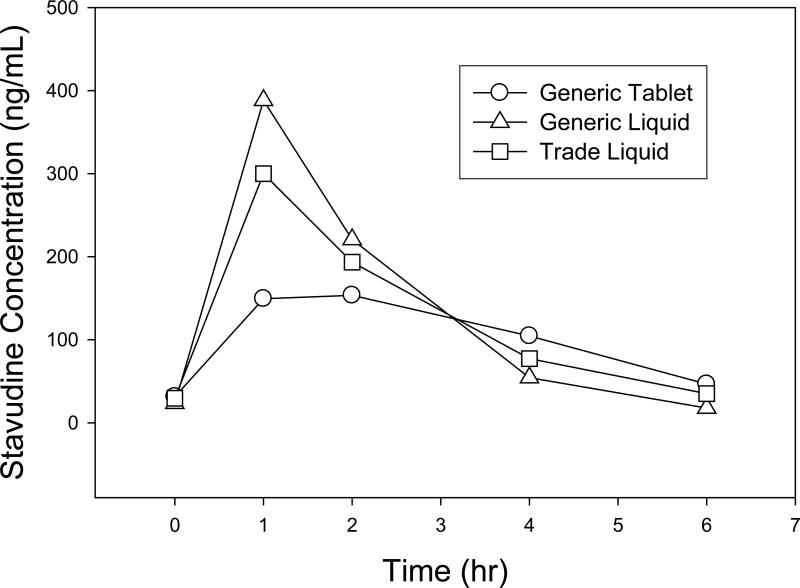
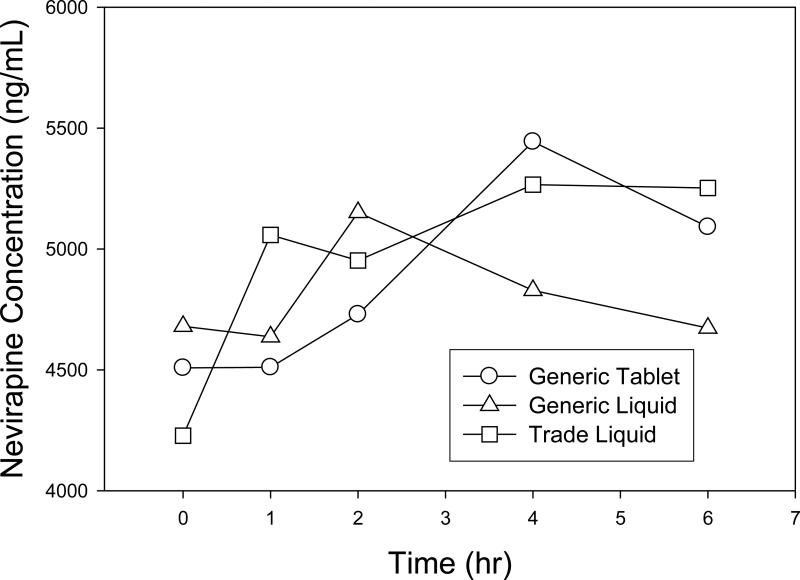
Descriptive statistics for the pharmacokinetic parameters of lamivudine, stavudine, and nevirapine for all 18 subjects are presented in Table 3. Figures 1a, 1b, and 1c are graphical representations of these data. Geometric mean ratios (90% CI) are presented for all formulation comparisons in table 4. Overall, for all dosing groups there were no significant differences in AUC0-6hr, Cmax, or C0hr,ss for d4T, 3TC, or NVP between formulations. Differences were only found for d4T and 3TC Tmax. Generally, the time to maximum concentration was delayed for d4T and 3TC for the GT formulation as compared to GL and TL, with the delay most significant in smaller children.
Table 3.
Pharmacokinetic Parameters-Geometric Means (90% CI) (N=18)*
| 3TC | PK Parameter | GT | GL | TL |
| All (n=18) | Cmax,ss | 660 (471, 923) | 764 (603, 968) | 872 (739, 1030) |
| AUC6hr,ss | 2278 (1676, 3098) | 2503 (1984, 3157) | 2845 (2385, 3394) | |
| d4T | PK Parameter | GT | GL | TL |
| All (n=18) | Cmax,ss | 380 (293, 493) | 442 (291, 670) | 433 (306, 614) |
| AUC6hr,ss | 949 (736, 1224) | 901 (637, 1276) | 989 (728, 1344) | |
| NVP | PK Parameter | GT | GL | TL |
| All (n=18) | Cmax,ss | 5810 (4771, 7075) | 5676 (4357, 7393) | 5998 (5028, 7154) |
| AUC6hr,ss | 29537 (24211, 36035) | 28952 (22363, 374783) | 29983 (25632, 35073) |
Cmax = ng/mL; AUC6hr,ss = hr*ng/mL
Figure 1a. Geometric Mean Concentrations vs Time (hr) (N=18).
Lamivudine concentration (ng/mL) vs time (hr)
Figure 1b. Geometric Mean Concentrations vs Time (hr) (N=18).
Stavudine concentration (ng/mL) vs time (hr)
Figure 1c. Geometric Mean Concentrations vs Time (hr) (N=18).
Nevirapine concentration (ng/mL) vs time (hr)
Individual Dosing Groups
Lamivudine
In examining the weight-based dosing groups separately, significant differences in PK parameters were found between the formulations. For Group 1, GT AUC0-6hr and Cmax were approximately 36-58% lower than GL and TL exposures. Conversely for group 2, GT AUC0-6hr and Cmax were 36-46% higher compared to GL and TL. Additionally, the time to maximum concentration was delayed by 33-70% for the GT formulation compared to GL and TL for both groups 1 and 2. No clinically significant differences between the formulations were seen in group 3.
Stavudine
For Group 1, AUC0-6hr and Cmax were up to 61% higher for GT compared to GL and TL. For Group 3, d4T exposure only was 29-45% lower with the GT compared to GL and TL. Tmax was 0.15-2.68 hrs longer for GT compared to both GL and TL for all groups while GL was 0.20-0.83 hrs shorter than TL for all groups. No clinically significant differences between the formulations were seen in group 2.
Nevirapine
The least amount of variability in drug exposure between the formulations was seen for NVP. For group 1, GT and GL AUC0-6hr and Cmax were 28-36% lower compared to TL. Ten of thirteen (77%) episodes of subtherapeutic (<3000ng/mL) concentrations were seen in this group, with 80% occurring during the GT and GL formulation phases. The current recommendation for nevirapine dosing is 300 mg/m2. Calculated this way, the median (range) dose of nevirapine in the GT/GL, and TL groups was 251 (177-304) and 285 (204-356) mg/m2 with 2/18 (11%) subjects in the GT/GL groups and 7/18 (39%) in the TL group having mg/m2 dosing >300 mg/m2.
Overall, all formulations were well tolerated. Ten percent of the children commented on the enjoyable taste of the liquid formulations. Over the study period, all subjects either had a stable or declining HIV RNA [median (range) <400 (261-2,837,779) at baseline and <400 (49-38,546) copies/mL at end of study].
Discussion
Antiretrovirals that are safe, effective, and less expensive are critical to the treatment of HIV in resource-poor countries such as Malawi where >12% of the population is infected, ~140,000 are receiving ARVs and the annual GNP is $190USD [3, 17]. Of those infected, approximately 10% are children <15 years old [18]. Ten percent of infected children are taking antiretroviral therapy; however, this constitutes only one-half of the children in need of treatment, who mostly rely on the government for their care. [17]. The Malawi Ministry of Health guidelines, which was developed in 2003, provided for the use of quartered multiples of generic fixed dose combinations for children, and provided children with access to these medications. Although splitting unscored fixed dose combination tablets is not an optimal method of drug delivery, the guidelines for dosing Malawian children were thoughtfully developed based on the availability of resources. This study provides PK data to support this practice for some, but not all, children.
One study examining split dose GPO-VIR S30 (fixed dose combination of stavudine 30mg, lamivudine 150mg, and nevirapine 200mg) in Thai children found only one of 34 children to have suboptimal nevirapine concentrations, with 12 of 13 children using this as their first line ARV regimen having undetectable viral loads [19]. However, previous pharmacokinetic evaluations by Ellis, et al in African children using split tablets of Triomune have demonstrated that some do not achieve adequate NVP concentrations [7]. Direct comparison of these studies cannot be performed, as two ethnically different populations were given two different generic fixed dose formulations. The low nevirapine exposures in the Ellis study do, however, further support the suboptimal nevirapine exposures seen in 50% of our subjects. Additionally, in both investigations in African children, the majority of patients with low nevirapine exposures were younger children. No studies to date have performed intra-patient comparisons for Triomune quartered tablets, individual generic and individual trade liquid products. Therefore, this study aimed to evaluate the pharmacokinetics of 3TC, d4T, and NVP in HIV-infected children receiving quartered multiples of Triomune 40™ as compared to the equivalent doses of the generic liquid formulations and the NIH recommended dose for children (at the time the study was conducted) using the FDA-approved trade products. Since little is known about the population differences in drug disposition, the latter comparison allowed us to evaluate exposures of drug from trade product in the Malawian population to Western cohorts.
The current investigation demonstrated that, for most children, the use of a split generic fixed dose combination provided similar exposures to both generic and trade liquids. This occurred despite the visible differences in size of each quarter tablet split with a commercial tablet splitter. Unpublished data from our lab determined that although Triomune 40™ had similar nevirapine content to Viramune tablets, it took longer to dissolve in an aqueous solution (25% less drug available at 6 hours).
Generally, 3TC exposures were lower for generic products as compared to trade formulations. This difference was driven by the consistently lower concentrations of 3TC in Group 1 subjects (8-<12kg). Due to trade liquid dosing being 11% higher than the generic dosing, this suggests underdosing of this group for 3TC. Stavudine results were highly variable, with subjects in Group 3 (28-<32kg) having the lowest concentrations when given the generic tablet formulation. Due to the median mg/kg dosing for all groups being similar (1.9, 2.1, and 2.1mg/kg for Groups 1, 2, and 3, respectively), this does not appear to be a phenomenon of underdosing.
Perhaps most concerning are the NVP data, where 10 of the 13 episodes of subtherapeutic NVP concentrations (<3000ng/mL) occurred in Group 1. This difference is likely due to a suboptimal mg/kg dosing strategy . Group 1 subjects were given lower doses compared to Groups 2 and 3. Comparing current mg/m2 dosing guidelines, children in group 1 were given a median (range) dose of 217 (177,312) mg/m2 compared to children in groups 2 and 3 who were given nevirapine at 271 (204,356) mg/m2 and 286 (226,304) mg/m2, respectively.
Overall, product source also appeared to affect drug exposure. Although the GL dosing strategy was 13% lower than the TL dosing strategy, exposures with this generic formulation were approximately 30% lower than with the trade formulation. We did not perform any testing on these formulations to determine whether differences in drug content could explain this finding.
This investigation also found some differences in the trade liquid formulation exposures in Malawian children as compared to Western cohorts [20-22]. Lamivudine and stavudine Cmax exposures with trade liquid were both 39% lower in Malawians, while nevirapine exposures were 100% higher than that reported in Western cohorts [20-22]. Although this comparison was not an objective in this investigation, it does warrant discussion of the potential pharmacokinetic differences in these populations with consideration for safety and efficacy.
Since 3TC and d4T are readily absorbed, eliminated renally and have no liver metabolism, the reason for these differences in exposure is unclear. This may be due to differences in age, weight, nutritional status, or maturation of the gastrointestinal tract and/or renal system between the populations. The differences in NVP have been seen previously in an adult population, and may be due to drug metabolism enzyme or transporter differences [6]. Studies are currently underway to assess what contribution P-glycoprotein and CYP450 polymorphisms may have on differences in NVP exposure in the Malawian population.
These data provide some support for the use of Triomune 40™ using these doses for children in Groups 2 and 3 [18-<22kg and 28-<32kg]. Based on the low concentrations of 3TC and NVP in children in Group 1 [8-<12kg], the investigators do not feel that dosing Triomune 40™ ¼ tablet twice daily will provide adequate concentrations. Based on these data and other investigations, the Malawi Ministry of Health changed the guidelines for treating HIV-infected children in 2006. Currently, it is recommended to use Triomune 30™ (30mgd4T/150mg3TC/200mgNVP) in lieu of the Triomune 40™ formulation [23]. Overall these new guidelines provide higher daily doses of each antiretroviral in children 8->12kg and avoid the underdosing that was found in this PK analysis. Additional generic pediatric formulations, such as Triomune Baby (6mg d4T/30mg 3TC/50mg NVP) and Triomune Junior (12mg d4T/60mg 3TC/100mg NVP), allow whole tablets to be administered to children [24]. The 3rd Edition of these guidelines, currently under evaluation in Malawi, may recommend use of these newer products and be the best alternative to liquid formulations. However, in children 18-<22 and 28-<32kg, split dose Triomune is an appropriate alternative.
Table 4a. Pharmacokinetic Parameters-Geometric Mean Ratios (90% CI) (N=18).
Lamivudine (3TC) Plasma PK Parameter Ratios
| 3TC | PK Parameter | GT/GL | GT/TL | GL/TL |
|---|---|---|---|---|
| All (n=18) | Cmax,ss | 0.86 (0.72, 1.04) | 0.76 (0.58, 0.99) | 0.88 (0.75, 1.02) |
| AUC6hr,ss | 0.91 (0.77, 1.08) | 0.80 (0.64, 1.01) | 0.88 (0.77, 1.00) | |
| Group 1 (n=6) | Cmax,ss | 0.57 (0.37, 0.69)* | 0.42 (0.27, 0.65)* | 0.73 (0.51, 1.05) |
| AUC6hr,ss | 0.64 (0.51, 0.81)* | 0.54 (0.38, 0.75)* | 0.84 (0.61, 1.14) | |
| Group 2 (n=6) | Cmax,ss | 1.36 (1.07, 1.72)* | 1.41 (1.11, 1.80)* | 1.04 (0.85, 1.27) |
| AUC6hr,ss | 1.39 (1.15, 1.68)* | 1.46 (1.21, 1.77)* | 1.06 (0.94, 1.18) | |
| Group 3 (n=6) | Cmax,ss | 0.83 (0.68, 1.01) | 0.73 (0.58, 0.93)* | 0.88 (0.77, 1.01) |
| AUC6hr,ss | 0.85 (0.74, 0.97)* | 0.66 (0.51, 0.85)* | 0.77 (0.67, 0.88)* |
p< 0.005
Table 4b. Pharmacokinetic Parameters-Geometric Mean Ratios (90% CI) (N=18).
Stavudine (d4T) Plasma PK Parameter Ratios
| d4T | PK Parameter | GT/GL | GT/TL | GL/TL |
|---|---|---|---|---|
| All (n=18) | Cmax,ss | 0.86 (0.63, 1.18) | 0.88 (0.60, 1.28) | 1.02 (0.69, 1.50) |
| AUC6hr,ss | 1.05 (0.81, 1.36) | 0.96 (0.69, 1.34) | 0.91 (0.71, 1.16) | |
| Group 1 (n=6) | Cmax,ss | 1.29 (0.71, 2.36) | 0.98 (0.42, 2.25) | 0.76 (0.32, 1.78) |
| AUC6hr,ss | 1.61 (1.02, 2.54) | 1.16 (0.51, 2.61) | 0.72 (0.41, 1.26) | |
| Group 2 (n=6) | Cmax,ss | 0.83 (0.46, 1.47) | 1.27 (0.83, 1.93) | 1.53 (0.94, 2.48) |
| AUC6hr,ss | 1.02 (0.66, 1.57) | 1.24 (0.93, 1.64) | 1.21 (0.89, 1.65) | |
| Group 3 (n=6) | Cmax,ss | 0.60 (0.46, 0.78)* | 0.55 (0.34, 0.88)* | 0.92 (0.56, 1.49) |
| AUC6hr,ss | 0.71 (0.57, 0.89)* | 0.62 (0.44, 0.86)* | 0.87 (0.68, 1.11) |
p<0.04
Table 4c. Pharmacokinetic Parameters-Geometric Mean Ratios (90% CI) (N=18).
Nevirapine (NVP) Plasma PK Parameter Ratios
| NVP | PK Parameter | GT/GL | GT/TL | GL/TL |
|---|---|---|---|---|
| All (n=18) | Cmax,ss | 1.02 (0.88, 1.18) | 0.97 (0.82, 1.15) | 0.95 (0.78, 1.16) |
| AUC6hr,ss | 1.02 (0.88, 1.18) | 0.99 (0.84, 1.15) | 0.97 (0.81, 1.16) | |
| Group 1 (n=6) | Cmax,ss | 1.09 (0.76, 1.55) | 0.72 (0.62, 0.82)* | 0.66 (0.43, 1.00) |
| AUC6hr,ss | 1.13 (0.80, 1.60) | 0.72 (0.64, 0.82)* | 0.64 (0.46, 0.89)* | |
| Group 2 (n=6) | Cmax,ss | 1.02 (0.94, 1.11) | 1.07 (0.86, 1.34) | 1.05 (0.89, 1.25) |
| AUC6hr,ss | 1.01 (0.94, 1.08) | 1.07 (0.89, 1.29) | 1.06 (0.89, 1.27) | |
| Group 3 (n=6) | Cmax,ss | 0.97 (0.75, 1.24) | 1.18 (0.82, 1.71) | 1.22 (0.96, 1.57) |
| AUC6hr,ss | 0.93 (0.74, 1.17) | 1.23 (0.91, 1.67) | 1.32 (1.12, 1.56)* |
p<0.03
Acknowledgements
The authors wish to thank all the investigators that worked on this study and the patients and guardians of patients that participated.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization (WHO) AIDS Epidemic Update 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 2.World Health Organization Malawi summary country profile for HIV/AIDS treatment scale-up 2005. www.who.int/hiv.
- 3.Malawi Ministry of Health ART in the public and private sectors in Malawi: Results up to 30th December 2007.
- 4.Penzak SR, Acosta EP, Tavel JA, Masur H. Analysis of generic nevirapine products in developing countries. JAMA. 2003;289:2648–49. doi: 10.1001/jama.289.20.2648-c. [DOI] [PubMed] [Google Scholar]
- 5.Narang VS, Lulla A, Malhotra G, Purandare S. A combined-formulation tablet of lamivudine/nevirapine/stavudine: bioequivalence compared with concurrent administration of lamivudine, nevirapine, and stavudine in healthy Indian subjects. J Clin Pharmacol. 2005;45:265–274. doi: 10.1177/0091270004273343. [DOI] [PubMed] [Google Scholar]
- 6.Hosseinipour MC, Kanyama C, Nkhalamba T, Phiri S, Weigel R, Fusani C, et al. Safety and efficacy of d4T/3TC/NVP among HIV positive adults in Lilongwe, Malawi.. 2004 World AIDS Conference; Bankok, Thailand. Abstract TuPeB4522. [Google Scholar]
- 7.Ellis JC, L'homme RF, Ewings FM, Mulenga V, Bell F, Chileshe R, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12(2):253–260. [PubMed] [Google Scholar]
- 8.Laurent C, Kouanfack C, Koulla-Shiro S, Nkoue N, Bourgeois A, Calmy A, et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364:29–34. doi: 10.1016/S0140-6736(04)16586-0. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois A, Laurent C, Mougnutou R, Nkoue N, Lactuock B, Ciaffi L, et al. Field assessment of generic antiretroviral drugs: a prospective cohort study in Cameroon. Antivir Ther. 2005;19(2):335–41. [PubMed] [Google Scholar]
- 10.Kumarasamy N, Solomon S, Chaguturu SK, Mahajan AP, Flanigan TP, Balakrishnan P, et al. The safety, tolerability and effectiveness of generic antiretroviral drug regimens for HIV-infected patients in south Indian. AIDS. 2003;17:2265–2271. doi: 10.1097/00002030-200310170-00019. [DOI] [PubMed] [Google Scholar]
- 11.Pujari SN, Patel AK, Naik E, Patel KK, Dravid A, Patel JK, et al. Effectiveness of generic fixed-dose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. J Acquir Immune Defic Syndr. 2004;37:1566–1569. doi: 10.1097/00126334-200412150-00005. [DOI] [PubMed] [Google Scholar]
- 12.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferrandini L. Generic fixed-dose combination antiretroviral treatment in resource poor settings: multicentric observational cohort. AIDS. 2006;20:1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinipour MC, Corbett AH, Kanyama C, Mshali I, Phakati S, Rezk NL, van der Horst C, Kashuba ADM. Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine, and nevirapine in HIV-infected Malawian adults. AIDS. 2007;21:59–54. doi: 10.1097/QAD.0b013e3280117ca0. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health . Treatment of AIDS: guidelines for the use of antiretroviral therapy in Malawi. first ed. Ministry of Health; Lilongwe, Malawi: 2003. [Google Scholar]
- 15.Working Group on Antiretroviral Therapy and Medical Management in HIV-infected Children convened by the National Resource Center at the Francois-Xavier Bagnoud Center (UMDNJ), Health Resource and Services (HRSA) and the National Institutes of Health (NIH) Guidelines for the use of antiretroviral agents in pediatric HIV infection. January 20, 2004. www.aidsinfo.nih.gov.
- 16.Rezk NL, Tidwell RR, Kashuba ADM. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography and ultraviolet absorbance detection. J Chromatogr B. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 17.Office of the President and Cabinet Republic of Malawi. Malawi HIV and AIDS monitoring report 2007. http://data.unaids.org/pub/Report/2008/malawi_2008_country_progress_report_en.pdf.
- 18.Epidemiological fact sheet on HIV and AIDS. Malawi. 2008 Update. http://www.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_MW.pdf.
- 19.Chokephaibulkit K, Pilpat N, Cressey TR, Frederix K, Phongsamart W, Capparelli E, et al. Pharmacokinetics of nevirapine in HIV-infected children receiving an adult fixed-dose combination of stavudine, lamivudine, and nevirapine. AIDS. 2005;19:1495–1499. doi: 10.1097/01.aids.0000183625.97170.59. [DOI] [PubMed] [Google Scholar]
- 20.Kline MW, Dunkle LM, Church JA, Goldsmith JC, Harris AT, Federici ME, et al. A phase I/II evaluation of stavudine (d4T) in children with human immunodeficiency virus infection. Pediatrics. 1995;96:247–52. [PubMed] [Google Scholar]
- 21.Sokal EM, Roberts EA, Mieli-Vergani G, McPhillips P, Johnson M, Barber J, et al. A dose ranging study of the pharmacokinetics, safety, and preliminary efficacy of lamivudine in children and adolescents with chronic hepatitis B. Antimicrob Agents Chemother. 2000;44(3):590–97. doi: 10.1128/aac.44.3.590-597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luzuriaga K, Bryson Y, McSherry G, Robinson J, Stechenberg B, Scott G, et al. Pharmacokinetics, safety, and activity of nevirapine in human immunodeficiency virus type1-infected children. J Infect Dis. 1996;174:713–21. doi: 10.1093/infdis/174.4.713. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health . Treatment of AIDS: guidelines for the use of antiretroviral therapy in Malawi. second ed. Ministry of Health; Lilongwe, Malawi: 2006. [Google Scholar]
- 24.L'Homme RFA, Kabamba D, Ewings FM, Mulenga V, Kankasa C, Thomason MJ, et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008;22:557–565. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]