Abstract
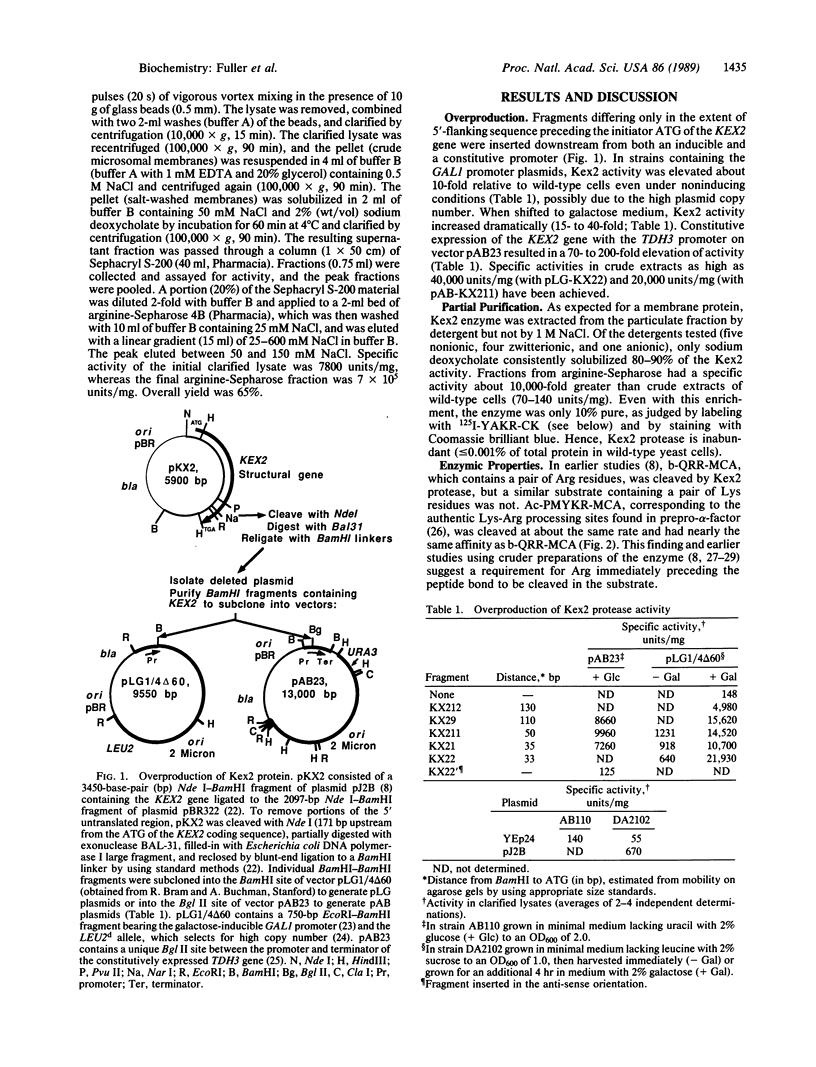
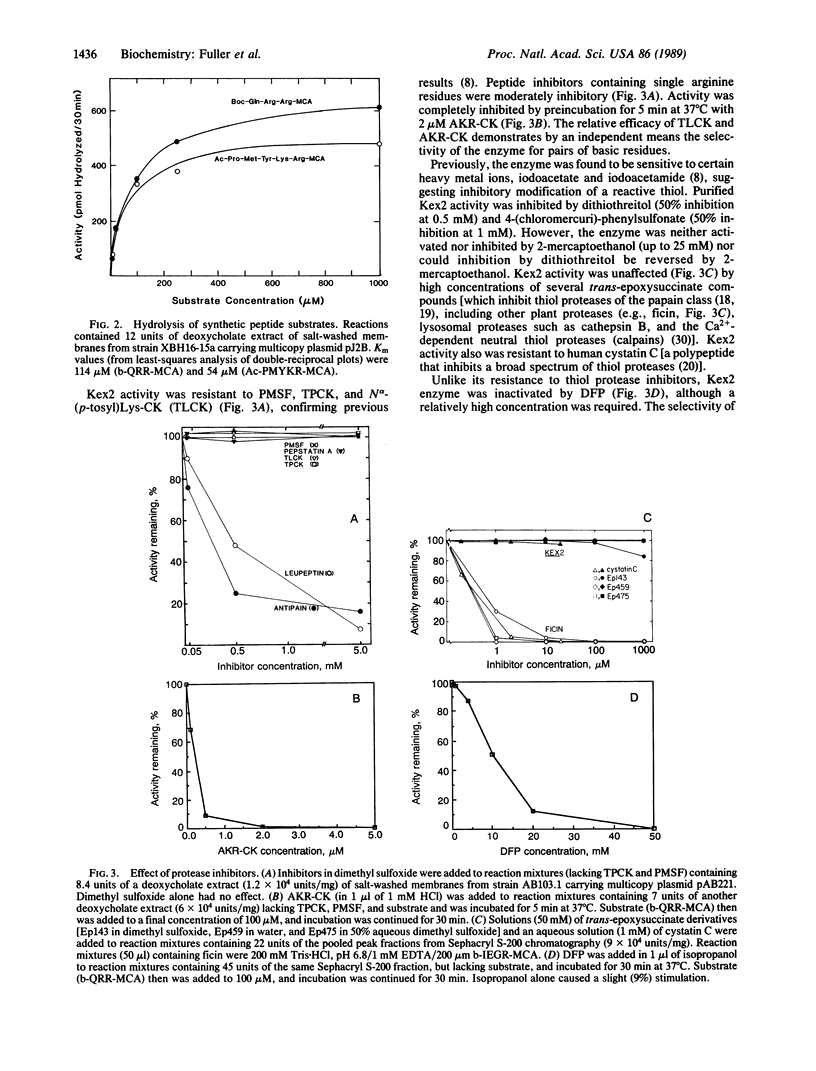
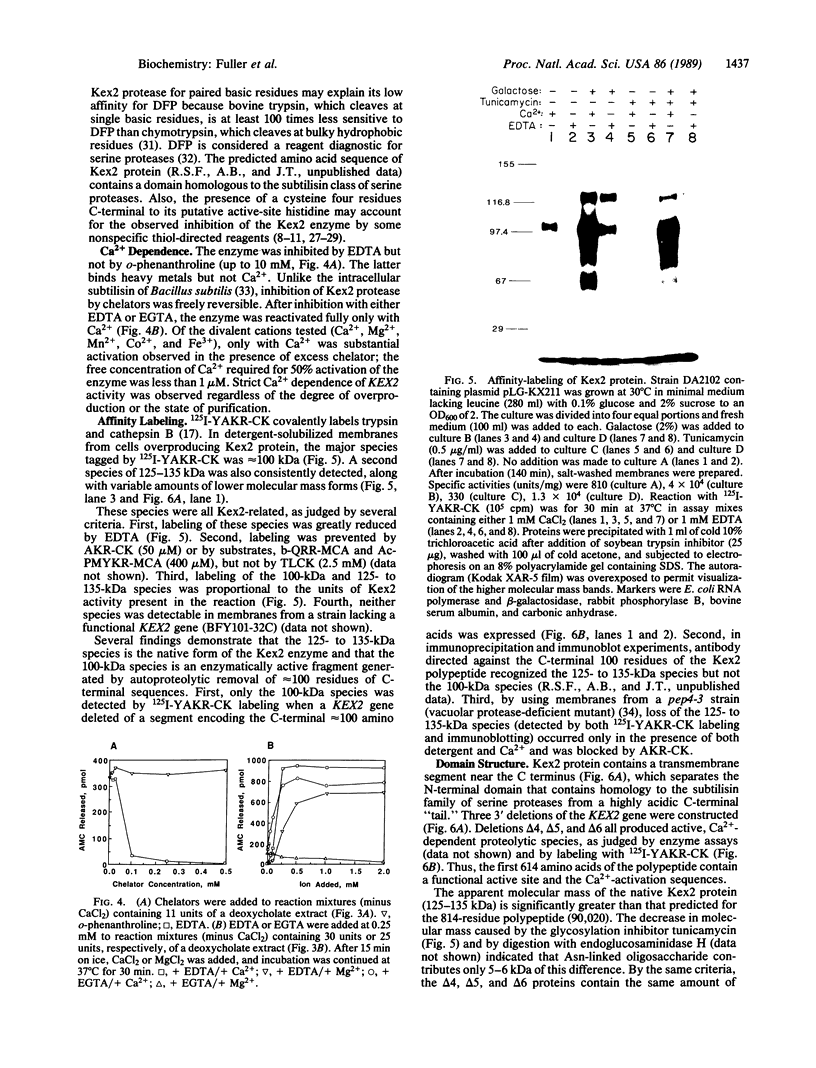
The KEX2-encoded endoprotease was overproduced in yeast several hundred-fold and further purified to achieve a 10,000-fold enrichment in specific activity. The enzyme was (i) membrane-bound, but solubilized by detergents; (ii) able to cleave peptide substrates at both Lys-Arg and Arg-Arg sites; (iii) inhibited by EDTA and EGTA (but not o-phenanthroline), but fully reactivated by Ca2+; (iv) unaffected by 5-10 mM phenylmethylsulfonyl fluoride, N alpha-(ptosyl)lysine chloromethyl ketone, or L-1-tosylamido-2-phenylethyl chloromethyl ketone, but inactivated by 1-2 microM Ala-Lys-Arg-chloromethyl ketone; (v) labeled specifically by 125I-labeled Tyr-Ala-Lys-Arg-chloromethyl ketone; and (vi) resistant to trans-epoxysuccinate compounds (which inactivate thiol proteases), but inactivated by diisopropyl fluorophosphate (a diagnostic serine protease inhibitor). Mutant enzyme molecules lacking as many as 200 C-terminal residues still retained Ca2+-dependent protease activity and were labeled by 125I-labeled Tyr-Ala-Lys-Arg-chloromethyl ketone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Hormone processing and membrane-bound proteinases in yeast. EMBO J. 1985 Jan;4(1):173–177. doi: 10.1002/j.1460-2075.1985.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Davies M. E., Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984 Apr 30;120(2):631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- Clamagirand C., Creminon C., Fahy C., Boussetta H., Cohen P. Partial purification and functional properties of an endoprotease from bovine neurosecretory granules cleaving proocytocin/neurophysin peptides at the basic amino acid doublet. Biochemistry. 1987 Sep 22;26(19):6018–6023. doi: 10.1021/bi00393a011. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Peshavaria M., Hutton J. C. Proteolytic conversion of proinsulin into insulin. Identification of a Ca2+-dependent acidic endopeptidase in isolated insulin-secretory granules. Biochem J. 1987 Sep 1;246(2):279–286. doi: 10.1042/bj2460279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Gluschankof P., Gomez S., Morel A., Cohen P. Enzymes that process somatostatin precursors. A novel endoprotease that cleaves before the arginine-lysine doublet is involved in somatostatin-28 convertase activity of rat brain cortex. J Biol Chem. 1987 Jul 15;262(20):9615–9620. [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kettner C., Shaw E. Inactivation of trypsin-like enzymes with peptides of arginine chloromethyl ketone. Methods Enzymol. 1981;80(Pt 100):826–842. doi: 10.1016/s0076-6879(81)80065-1. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Isolation and characterization of yeast strains carrying mutations in the glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1985 Dec 5;260(28):15013–15018. [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Takada K., Sakakibara S., Matsuo H. A membrane-bound, calcium-dependent protease in yeast alpha-cell cleaving on the carboxyl side of paired basic residues. Biochem Biophys Res Commun. 1987 Apr 29;144(2):807–814. doi: 10.1016/s0006-291x(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Mullenbach G. T., Tabrizi A., Blacher R. W., Steimer K. S. Chemical synthesis and expression in yeast of a gene encoding connective tissue activating peptide-III. A novel approach for the facile assembly of a gene encoding a human platelet-derived mitogen. J Biol Chem. 1986 Jan 15;261(2):719–722. [PubMed] [Google Scholar]
- Rose M., Grisafi P., Botstein D. Structure and function of the yeast URA3 gene: expression in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):113–124. doi: 10.1016/0378-1119(84)90172-0. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Ermakova L. M., Baratova L. A., Belyanova L. P., Stepanov V. M. Intracellular serine protease of Bacillus subtilis: sequence homology with extracellular subtilisins. J Bacteriol. 1978 Mar;133(3):1401–1411. doi: 10.1128/jb.133.3.1401-1411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S. Effect of Ca2+ on the inhibition of calcium-activated neutral protease by leupeptin, antipain and epoxysuccinate derivatives. FEBS Lett. 1981 Dec 21;136(1):119–122. doi: 10.1016/0014-5793(81)81227-6. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J. Processing and metabolism of neuropeptides. Essays Biochem. 1986;22:69–119. [PubMed] [Google Scholar]
- Wagner J. C., Escher C., Wolf D. H. Some characteristics of hormone (pheromone) processing enzymes in yeast. FEBS Lett. 1987 Jun 22;218(1):31–34. doi: 10.1016/0014-5793(87)81012-8. [DOI] [PubMed] [Google Scholar]