Abstract
Objective
To rely on the anatomical organization of the hippocampal formation to understand how late-life diseases such as diabetes and stroke contribute to age-related cognitive decline.
Methods
Magnetic resonance imaging (MRI) was used to document brain infarcts and to generate high-resolution functional maps of the hippocampal formation in 240 community-based non-demented elders (mean age=79.7) who received a comprehensive medical evaluation. Sixty participants had type 2 diabetes mellitus while 74 had MRI-documented brain infarcts, and the first analysis was designed to pinpoint hippocampal subregions differentially linked to each disorder. Then, guided by the results, additional fMRI studies in aging rhesus monkeys and mice were used to test proposed mechanisms of dysfunction.
Results
Although both diabetes and brain infarcts were associated with hippocampal dysfunction, each was linked to separate hippocampal subregions, suggesting distinct underlying mechanisms. The hippocampal subregion linked to diabetes implicated blood glucose as a pathogenic mechanism, a hypothesis confirmed by imaging aging rhesus monkeys and a mouse model of diabetes. The hippocampal subregion linked to infarcts suggested transient hypoperfusion as a pathogenic mechanism, a hypothesis provisionally confirmed by comparing anatomical patterns across subjects with infarcts in different vascular territories.
Interpretation
Taken together with previous findings, these results clarify how diseases of late-life differentially target the hippocampal formation, identify causes that contribute to age-related cognitive decline, and suggest specific interventions that can preserve cognitive health.
INTRODUCTION
With increasing longevity, decreasing morbidity, and as older individuals expect to live intellectually challenging lives, cognitive aging has emerged as a major societal problem. Aging does not cause diffuse brain dysfunction but rather targets select brain areas, in particular the frontal lobes and the hippocampal formation1–3. The hippocampal formation itself, a structure vital for memory 4, is made up of separate but interconnected subregions (Fig. 1), and because each subregion houses a molecularly-distinct population of neurons5 the subregions are differentially vulnerable to mechanisms of disease 2. The hippocampal subregion, therefore, is the basic anatomical unit of diseases targeting the hippocampal formation, and pinpointing subregions differentially linked to a particular disease can shed light on underlying mechanisms.
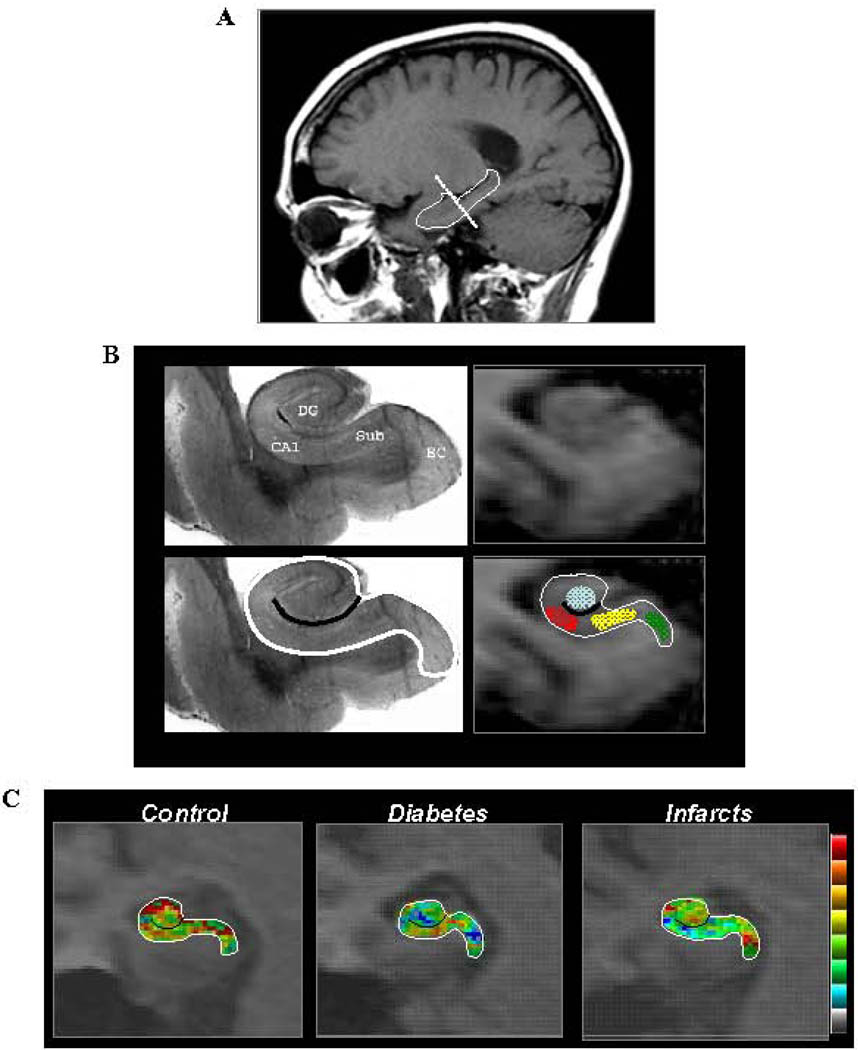
Figure 1. Functional maps of the hippocampal formation.
A. A sagital scout image was used to identify the long axis of the hippocampal formation (demarcated by the solid line). High-resolution T1-weighted images are acquired perpendicular to the hippocampal long axis (stippled line).
B. The general locale of 4 hippocampal subregions are shown in a post-mortem hippocampal slice (upper left panel)-- the entorhinal cortex (EC), dentate gyrus (DG), CA1 subfield (CA1), and the subiculum (Sub). A single MRI slice (upper right panel) contains all 4 hippocampal subregions and provides sufficient anatomical information to parse the hippocampal formation. Specifically, by identifying the external morphology of the hippocampal formation (as demarcated by the white line in lower left and right panels), and its internal architecture (as demarcated by the black line in the lower left and right panels), regions-of-interest can be drawn in the entorhinal cortex (green), dentate gyrus (blue), CA1 subfield (red), and the subiculum (yellow).
C. Cerebral blood volume (CBV) maps are shown for an individual control, and a subject with diabetes or infarcts. Maps are color-coded such that warmer colors indicate greater CBV.
Although the early stages of Alzheimer’s disease (AD) contribute to age-related hippocampal dysfunction, previous studies have suggested that the hippocampal formation is also vulnerable to other diseases that occur commonly in later life, in particular type 2 diabetes mellitus 6 and brain infarcts 7. Nevertheless, because of the interaction and co-existence of late-life diseases, understanding how each disorder affects the hippocampal formation is challenging. Not only are diabetes and infarcts typically co-morbid, but studies suggest that both interact with AD itself 8 9. Notably, the early stages of AD occur years before clinical detection10, and so hippocampal dysfunction observed in diabetes or infarcts might simply reflect an acceleration of AD pathophysiology. Because different disease mechanisms are predicted to target different subregions of the hippocampal formation, assessing the functional integrity of individual subregions can clarify how diseases of late-life target the aging brain.
With this goal in mind, we investigated a recently completed community-based study of non-demented elders, who together with a detailed medical assessment were evaluated with magnetic resonance imaging (MRI) to: A) document infarcts throughout the brain; and B) generate basal cerebral blood volume (CBV) maps of the hippocampal formation. As with other functional imaging variables, CBV is hemodynamically coupled to oxygen metabolism11, and is tightly correlated with other basal measures of brain function, such as cerebral blood flow as measured with MRI, or glucose uptake as measured with positron emission tomography12. Among all functional imaging approaches, measuring steady-state basal CBV with MRI provides the highest spatial resolution 13, a feature that is important for visualizing individual hippocampal subregions. An additional advantage of CBV mapping is that it can be now used to investigate the hippocampal formation of animal models, both in non-human primates14 and in rodents15, so that patterns observed in humans can be confirmed in experimental settings. Indeed, previous studies have used CBV mapping in humans and animal models to pinpoint dysfunction in select regions of the hippocampal formation in Alzheimer’s disease and normal aging 14 15.
By measuring both infarcts and type 2 diabetes in a large dataset we were able to first differentially link each disease to specific hippocampal subregions. Next, we tested hypotheses that emerged about underlying mechanisms by performing additional studies in non-human primates and a mouse model of diabetes, and by completing additional analyses on the human dataset. More than just linking diabetes and infarcts to hippocampal dysfunction, the series of reported findings clarify general mechanisms underlying cognitive aging and suggests specific interventions to preserve cognitive health.
METHODS
Subjects
Humans
Participants were part of a community-based study of elderly subjects (65 years and olde) who together with brain imaging16 received a detailed neuropsychological, neurological, and medical evaluation17. Within this cohort, hippocampal CBV maps were generated in 240 subjects. Relying on clinical criteria, all subjects were non-demented as determined by a consensus conference made up neurologists and neuropsychologists.
Type 2 diabetes mellitus was ascertained by self-report or by the use of diabetes medications. Brain infarcts were ascertained by MRI, relying on whole brain fluid attenuated inverse recovery (FLAIR) T2-weighted sequence (TR=11,000ms, TE=144ms, 3mm slice thickness) and T1-weighted high-resolution anatomical sequence (TR=20ms, TE=2.1ms, 1.3mm slice thickness) acquired in the axial orientation 18. Lesions greater than or equal to 3 mm were considered for classification as infarct.
Serum glucose and insulin levels were measured in in microIU/ml from serum collected within one month of the brain MRI and frozen at − 70° C. Glucose levels were measured on a Hitachi automated spectrophotometer (model 704, Hitachi Ltd, Tokyo, Japan) using commercial kits obtained from Wako Chemicals (Richmond, VA). Insulin levels were measured using a solid-phase chemiluminescent enzyme immunoassay (Immulite, Diagnostic Products Co, Los Angeles, CA). The intra-assay coefficient of variation was 4.7% and the inter-assay coefficient of variation was 8.2%. The normal insulin range for this assay is 6–27 microIU/ml. The study was approved by the appropriate institutional ethics review committee and all subjects gave written informed consent to participate.
Non-human primates
Rhesus monkeys (Macaca mulatta) 7–31 years of age were used as subjects in this experiment. The animal colony rooms were on a 12/12 hour light/dark (6am to 6pm light) schedule, maintained at 26 °C, and all animals were trained to deliver their arms so that morning fasting blood samples could be obtained. The arm was shaved, wiped with alcohol and ~1cc of blood was drawn from a vein in the arm. The blood was centrifuged for 10 min at 3500 rpm, cooled, and glucose levels determined using a standard serum chemistry panel in the Veterinary Medicine Teaching Hospital’s clinical laboratory at the Primate Center in Davis. The test was performed using a Hitachi 917 (Roche Biomedical, Indianapolis, IN) instrument. All protocols were approved by the UC Davis Animal Care and Use Committee.
Mice
Male C57/Bl6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine) at 4 weeks of age. At 5 weeks of age, diabetes was induced by 5 daily injections (i.p.) of streptozotocin (55 mg/kg BW) freshly dissolved in 0.2M citrate buffer, pH 4.5. Control animals received an equal volume of diluent. Blood glucose was analyzed in tail blood with a glucometer at 5 days after the last injection and diabetes was confirmed by a blood glucose value ≥250 mg/dl. All protocols were approved by the Columbia University Animal Care and Use Committee.
Functional Imaging
Humans
The technical and analytic details of how CBV maps of the human hippocampal formation were generated has been previously described15. Briefly, a 1.5 Tesla scanner (Philips Intera) was used to acquire oblique coronal 3D T1-weighted images (TR=20ms; TE=6ms; flip angle=25 degrees; in plane resolution=0.86mm × 0.86mm; slice thickness=3mm), perpendicular to the hippocampal long axis (Fig. 1A), before and 4-minutes after IV administration of gadolinium-pentate (Omniscan, 0.1mmol/kg). Then, the post-contrast images were subtracted from pre-contrast images, and the difference in the superior sagittal sinus, which serves as an estimate of the image intensity change of 100% blood, was recorded13, 15. Finally, the subtracted image was divided by the difference in the top 4 pixels measured from the sagittal sinus and multiplied by 100 yielding percent CBV.
In all cases, a single ideal slice was identified, anterior to the lateral geniculate nucleus and posterior to the uncus, that contains all hippocampal subregions and provides sufficient anatomical information to parse the subregions15 (Fig. 1B). Because spatial co-registration across subjects is problematic when evaluating small regions in clinical populations, strict anatomical criteria15 were used to identify the following regions-of-interest within the hippocampal formation: The entorhinal cortex, dentate gyrus, CA1 subfield, and the subiculum (Fig. 1B). On an individual basis, mean CBV values were measured for each hippocampal ROI and used for group data analysis.
Non-human primates
As previously described14, CBV maps of the monkey hippocampal formation were generated in a 1.5 Tesla scanner using a gadolinium-based approach nearly identical to the method used in humans. The main difference was that T1-weighted images (TR = 50 ms; TE = 5 ms; flip angle = 35 degrees; in plane resolution = 0.62 mm × 0.62 mm; slice thickness 2 mm) and hippocampal parsing were optimized for the monkey brain.
Mice
The technical and analytic details of how CBV maps of the mouse hippocampal formation were generated has been previously described 15 Briefly, a 9.4 Tesla scanner (Bruker, AVANCBV 400WB) was used to acquire axial T2-weighted images (TR/TE=2000ms/70ms; FOV=19.6mm; acquisition matrix=256 × 256; 8 slices; slice thickness=0.6mm), perpendicular to the hippocampal long axis, before and 45-minutes after IP administration of gadolinium-pentate (Omniscan, 013mmol/kg). Isofluorane was chosen as the anesthetic agent because of its minimal effects on hemodynamic coupling, and heart rate, respiratory rate, and SaO2 were monitored throughout. Relative CBV maps were generated according to formula CBV ∞ ΔR2 = ln (Spre/Spost) / TE; where TE = effective echo-time; Spre = signal before contrast; Spost = signal contrast agent reaches steady-state. The derived image was then divided by the maximum 4 pixels signal value of the posterior cerebral vein to control for differences in levels of contrast yielding relative CBV.
As in humans and monkeys, an ideal single slice, acquired through the body of hippocampal formation, was identified that contains all hippocampal subregions and has sufficient anatomical information to parse the subregions 15. Strict anatomical criteria 15 were used to identify the following regions-of-interest within the hippocampal formation: The entorhinal cortex, dentate gyrus, CA1 subfield, and subiculum. On an individual basis, mean CBV values were measured for each hippocampal ROI and used for group data analysis.
RESULTS
Diabetes and brain infarcts are differentially linked to hippocampal dysfunction
Among the 240 human subjects who participated in this study, 60 individuals had type 2 diabetes. Two hundred and sixteen subjects were also evaluated for vascular disease and 74 subjects had documented brain infarcts. When comparing demographics (Table 1), the diabetes and infarct groups were similar in age (F (3, 216) = 2.221, p = 0.087), sex distribution (X2(3) = 1.606, p = 0.658), education (F (3, 214) = 0.430, p = 0.732), and ethnicity (X2(9) = 13.158, p = 0.156), although those with diabetes were more likely to be African American.
Table 1.
Subject demographics
| No infarct or diabetes |
Infarct only |
diabetes only |
Both infarct and diabetes |
Total Sample |
||
|---|---|---|---|---|---|---|
| Number of subject | 110 | 53 | 32 | 21 | 240 | |
| Age (mean years ± SD) | 80.3 ± 5.6 | 80.0 ± 5.8 | 78.9 ± 4.1 | 77.3 ± 3.7 | 79.8 ± 5.3 | |
| Sex (% women) | 59 | 58 | 69 | 52 | 58 | |
| Education (mean years ± SD) |
11.2 ± 5.0 | 10.5 ± 4.6 | 10.3 ± 4.9 | 10.4 ± 4.1 | 10.8 ± 4.8 | |
| Ethnicity | % White | 31.8 | 32.1 | 6.3 | 14.3 | 26.4 |
| % African American |
34.5 | 32.1 | 46.9 | 47.6 | 37.0 | |
| % Hispanic | 30.9 | 35.8 | 43.8 | 33.3 | 34.3 | |
| % Other | 1.4 | 0.0 | 3.1 | 4.8 | 2.3 |
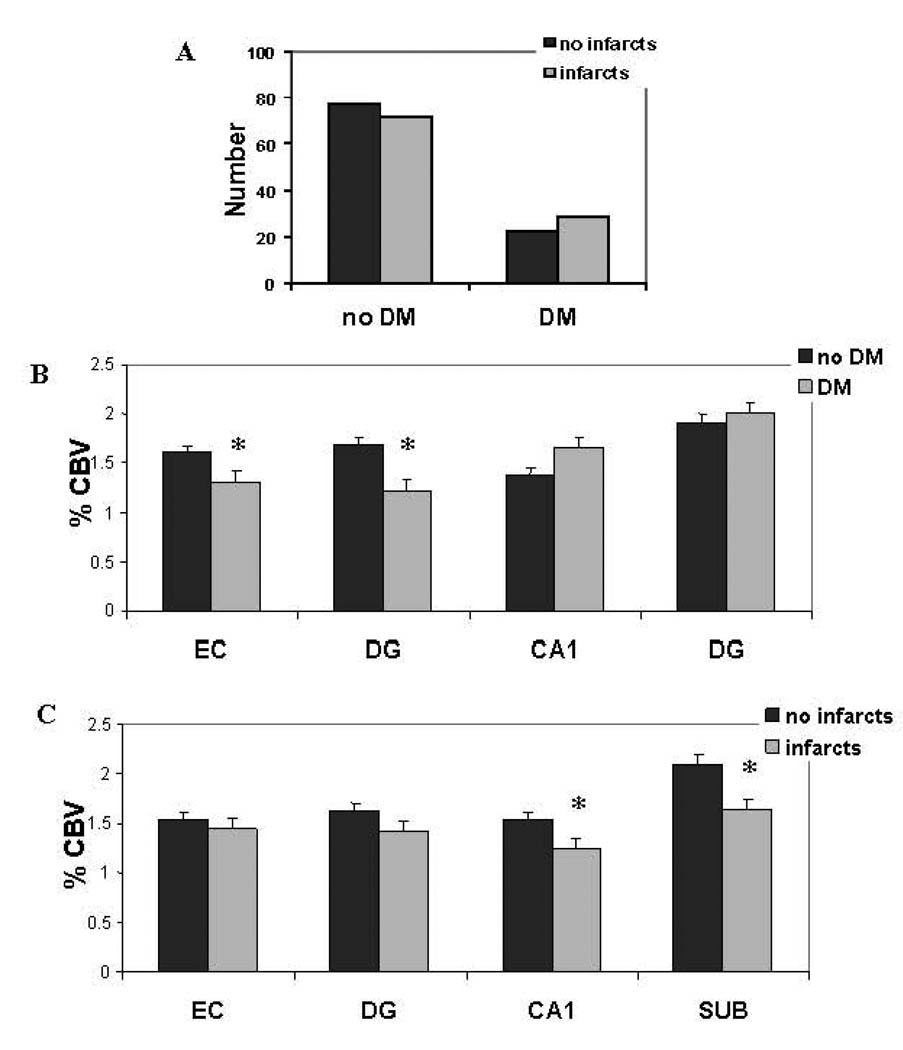
Although subjects with diabetes had relatively more infarcts than those without diabetes, this difference was small and not statistically significant (chi-square=0.34, p=0.40; Fig 2A). Therefore, in our first series of analyses, we independently investigated the relationship between hippocampal dysfunction to diabetes and to infarcts. To identify hippocampal subregions linked to each disorder an ANOVA model was constructed in which CBV measured in the hippocampal subregions was included as the dependent variables, group (disease vs. no-disease) was included as the fixed factor, and age, sex, ethnicity and education were included as covariates. Results revealed that diabetes was associated with global hippocampal dysfunction, as indicated by the model’s multivariate test (F=5.88, p=0.0002), and univariate tests showed that this effect was selectively driven by dysfunction in the entorhinal cortex (F=4.4, p=0.036) and the dentate gyrus (F=10.7, p=0.001) (Figs. 1C & 2B). In contrast, while infarcts was also associated with global hippocampal dysfunction (F=2.92, p=0.022), the effect was selectively driven by dysfunction in the CA1 subfield (F=3.97, p=0.047) and the subiculum (F=7.38, p=0.007) (Figs. 1C & 2C).
Figure 2. Diabetes and infarcts are differentially linked to separate hippocampal subregions.
A. The distribution of subjects with and without type 2 diabetes mellitus (diabetes), with and without infarcts.
B. Mean cerebral blood volume (CBV) of subjects with (gray bars) and without (black bars) diabetes across the different hippocampal subregions—the entorhinal cortex (EC), dentate gyrus (DG), CA1 subfield (CA1), and the subiculum (SUB). Compared to subjects without diabetes (no diabetes), those with diabetes (diabetes) have selective reductions in entorhinal cortex and dentate gyrus CBV (as indicated by asterisks).
C. Mean cerebral blood volume (CBV) of subjects with (gray bars) and without (black bars) infarcts across the different hippocampal subregions. Compared to subjects without infarcts, those with infarcts have selective reductions in CA1 and subiculum CBV (as indicated by asterisks).
Next, to test for a potential relationship between the two diseases, the ANOVA was repeated, but both diabetes and infarcts were included as covariates in a single model. Replicating the first series of studies, each disease was selectively associated with global hippocampal dysfunction (diabetes: F=5.41, p=0.0004; infarcts: F=2.85, p=0.025). However, while in the case of infarcts the effect was driven by the same hippocampal subregions (CA1 subfield; F=4.48, p=0.036; subiculum: F=7.24, p=0.008), in the case of diabetes the effect was driven selectively by the dentate gyrus (F=14.27, p=0.0002) not the entorhinal cortex (F=0.79, p=0.38).
Finally, in post-hoc analyses, we further examined hippocampal effects by comparing subjects with infarcts only versus those without diabetes or infarcts; and, we examined hippocampal effects by comparing subjects with diabetes only versus those without diabetes or infarcts. The results again show that subjects with infarcts have greatest dysfunction in the CA1 subfield (F=3.70, p=0.042) and the subiculum (F=6.41, p=0.012), while subjects with diabetes have selective dysfunction in the dentate gyrus (F=10.59, p=0.001).
These results suggest that the dentate gyrus is selectively and differentially linked to diabetes, that the CA1 subfield and the subiculum are selectively and differentially linked to infarcts, and that there is complex interplay linking the entorhinal cortex to diabetes and infarcts. In the next sections each of these three observations are further investigated separately.
The relationship between diabetes and the aging hippocampal formation
Type 2 diabetes is associated with elevations in blood insulin and glucose, and impaired glucose regulation in particular has been found to affect hippocampal function19, 20. To determine whether blood glucose or insulin might underlie dentate gyrus dysfunction we investigated their relationship with hippocampal CBV. Levels of blood glucose and insulin were measured in a subset of 181 subjects. A partial correlation analysis was performed between these measurements and hippocampal CBV, covarying for age, sex, ethnicity, and education. Importantly, because some subjects had fasting blood measurements while others did not, this factor was also included as a covariate. Results showed that among all hippocampal subregions blood glucose levels were inversely and selectively correlated with dentate gyrus CBV (beta=−0.163, p=0.032) (table 2). Additionally, blood glucose levels were found to selectively and inversely correlate with total recall on the Selective Reminding Test (beta=−0.180, p=0.018), a cognitive measure of hippocampal function21. Using the same statistical model, blood insulin levels did not correlate with hippocampal CBV, although a trend for an inverse relationship was observed with the entorhinal cortex (beta=−0.128, p=0.08) (table 2).
Table 2.
The relationship between blood glucose or blood insulin and hippocampal CBV
| EC | DG | CA1 | SUB | ||
|---|---|---|---|---|---|
| Glucose | Correlation | −0.09 | −0.16 | 0.08 | −0.02 |
| p value | 0.22 | *0.03 | 0.32 | 0.76 | |
| Insulin | Correlation | −0.13 | −0.6 | 0.10 | −0.03 |
| p value | 0.08 | .17 | 0.76 | 0.72 |
These findings suggest that elevations of blood glucose might differentially target the dentate gyrus. Nevertheless, these results are limited by the fact that only some subjects had fasting glucose measurements, and by the general limitation of human-based studies in which it is difficult to control for all the factors that can potentially affect CBV. To test the hypothesis that elevations in blood glucose is differentially linked to the dentate gyrus in a more controlled experimental setting we turned to animal models. We returned to a previous study in which we mapped hippocampal CBV in aging rhesus monkeys 14. Among the 14 subjects imaged, 11 had blood glucose measured within a few months of the imaging study. A correlation analysis revealed that among the hippocampal subregions, blood glucose levels were selectively and inversely correlated with dentate gyrus CBV (beta= −0.64, p=0.033) (Fig. 3A).
Figure 3. Blood glucose selectively targets the dentate gyrus.
A. Blood glucose levels are inversely correlated with dentate gyrus (DG) CBV in rhesus monkeys.
B. Compared to control mice (black bars), streptazosin-treated mice (gray bars) have dominant reductions in dentate gyrus (DG) CBV.
Although the findings in rhesus monkeys support the interpretation that blood glucose differentially targets the dentate gyrus, they too have limitations. First, insulin levels were not measured and so we cannot exclude that it might have contributed to the finding. Second, and more importantly, the findings in monkeys were only correlational.
To address both limitations, we turned to a streptozosin mouse model of diabetes. By killing insulin-producing cells of the pancreas, administrating streptozosin causes an abnormal elevation in blood glucose by reducing insulin. Streptozosin was administered to 3 month old mice and at 6 months of age CBV maps of the hippocampal formation were generated in 4 treated mice and 4 non-treated mice. An ANOVA was performed, in which CBV measured from the entorhinal cortex, dentate gyrus, CA1, and the subiculum were included as the dependent variables and group (treated vs. non-treated) was included as the fixed factor. Results revealed that the treated mice had it greatest affect on dentate gyrus CBV (F=6.62, p=0.042) (Fig. 3B).
The relationship between brain infarcts and the aging hippocampal formation
None of the subjects had infarcts in the hippocampal formation, according the criteria described in the methods section. Nevertheless, an ANOVA, covarying for age, sex, ethnicity, and education, showed that compared to subjects without infarcts, those with infarcts in regions other than the hippocampal formation, had poorer performance on the Selective Reminding Test (F=9.59, p=0.002).
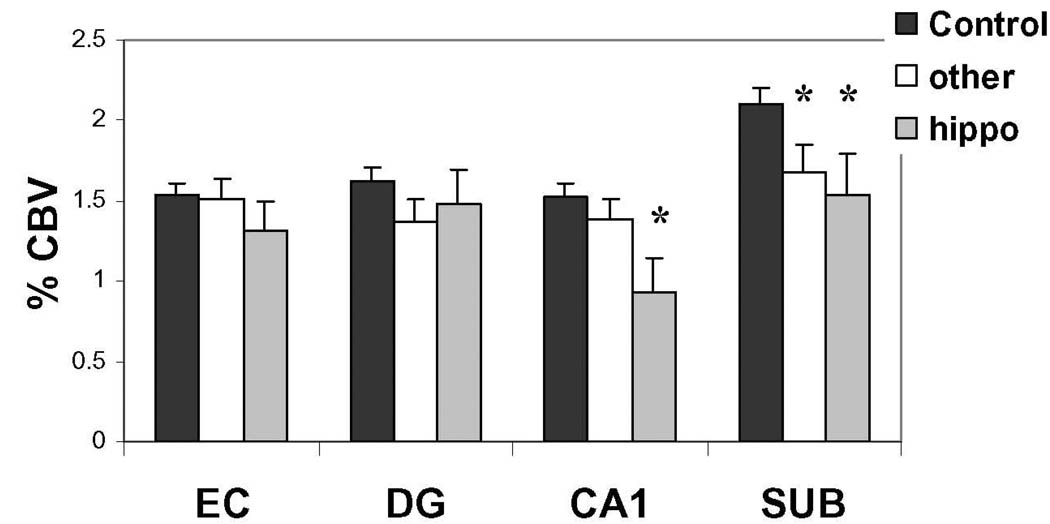
The observation that CA1 dysfunction was linked to infarcts, as documented above, suggested a possible mechanism for how strokes in extra-hippocampal sites might cause hippocampal dysfunction. Specifically, previous studies have shown that the CA1 subfield is differentially vulnerable to transient hypoperfusion7. Furthermore, studies have suggested that during the evolution of an infarct in a particular brain region, transient hypoperfusion occurs throughout the vascular territory supplying that region 22. Taken together, we postulated that CA1 dysfunction might occur only when infarcts occurs in a vascular territory that also supplies the hippocampal formation, namely the anterior choroidal and posterior cortical arteries. To test this hypothesis, we subdivided the 74 subjects with stroke, into three groups: Twenty two subjects who had infarcts in a vascular territory that supplies the hippocampal formation (the basal ganglia, thalamus, internal capsule, and occipital cortex), 52 subjects who had infarcts in other vascular territories, and 142 subjects who were infarct free. Confirming the hypothesis, CA1 dysfunction was observed only in subjects with infarcts in the hippocampal vascular territory (F=7.41, p=0.007) (Fig. 4).
Figure 4. Infarcts in the hippocampal vascular territory are linked to the CA1 subfield.
A reduction in CA1 CBV was found selectively among subjects with infarcts in the hippocampal vascular territory (“hippo”). In contrast, a reduction in subiculum CBV was found in subjects with infarcts in the hippocampal vascular territory and infarcts in other vascular territories (“other”).
Compared to subjects without infarcts, subiculum dysfunction was observed in subjects with infarcts in the hippocampal territory (F=6.56, p=0.035), and subiculum dysfunction was observed in subjects with infarcts in other territories (F=4.11, p=0.044) (Fig. 4). This suggests that stroke-associated dysfunction in the subiculum is linked to other mechanisms.
The inter-relationship between diabetes and brain infarcts
As reported above, the link between diabetes and entorhinal cortex CBV was found to be stroke-dependent, and furthermore, there was a suggestion that entorhinal cortex CBV might be correlated with insulin levels. We postulated that the entorhinal cortex might be selectively associated with insulin levels only among subjects with infarcts in the hippocampal vascular territory.
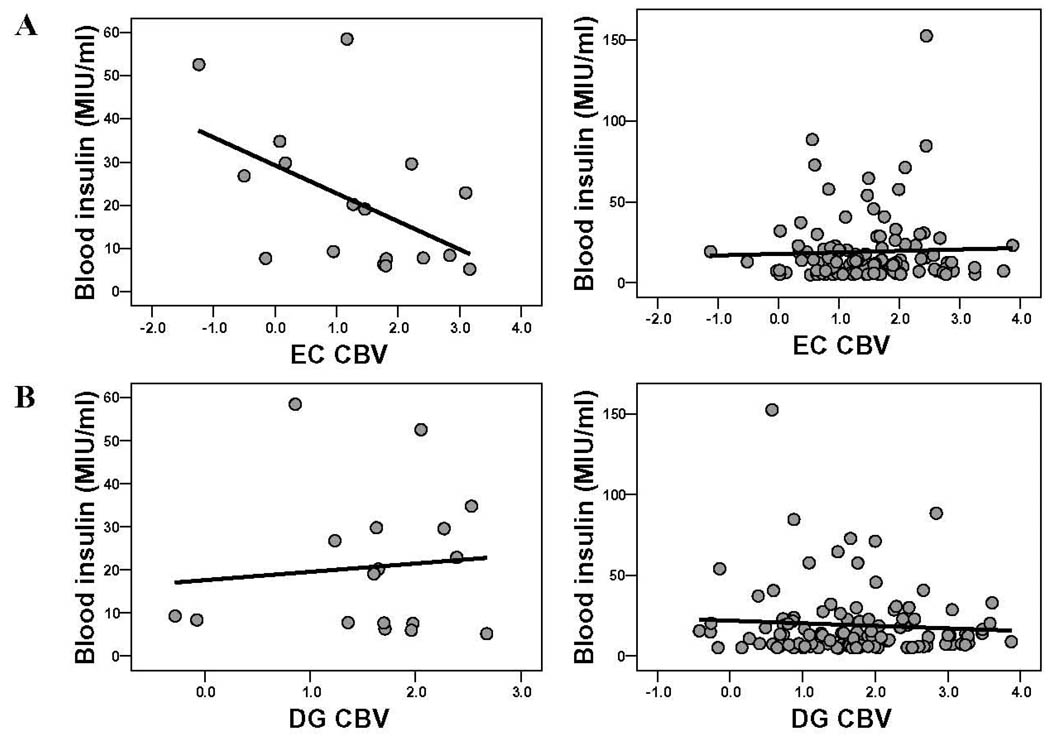
To test this hypothesis, we repeated the correlation analysis among the three stroke subgroups and found an inverse relationship between entorhinal cortex CBV and blood levels of insulin only among the subjects with infarcts in the hippocampal vascular territory (beta=−0.58, p=0.027) (Fig 5). No correlations was observed among subjects with infarct in other territories (beta=−0.06, p=0.78), or among subjects without infarct (beta=−0.04, p=0.69). Of note, only 1 of the subjects with stroke in the hippocampal vascular territory carried a diagnosis of diabetes.
Figure 5. Blood insulin selectively targets the entorhinal cortex in a stroke-dependent manner.
A. Blood insulin levels are inversely correlated with entorhinal cortex (EC) CBV in subjects with infarcts in the hippocampal vascular territory (left scatter plot) but not in subjects without infarcts (right scatter plot).
B. Blood insulin levels are not correlated with dentate gyrus (DG) CBV in subjects with infarcts in the hippocampal vascular territory (left scatter plot) or in subjects without infarcts (right scatter plot).
DISCUSSION
By investigating the aging hippocampal formation in a large clinically-characterized cohort, our results clarify how type 2 diabetes and brain infarcts, two common diseases of late-life, interact with the hippocampal formation. Moreover, taken together with previous studies, our results suggest mechanisms underlying cognitive aging and point to therapeutic strategies.
The results highlight the importance of evaluating the hippocampal formation in a manner compatible with its anatomical and molecular complexity. As indicated by the multivariate results, both diabetes and infarcts were associated with global hippocampal dysfunction; it was only by examining the univariate results subregion-by-subregion that differences between the diseases were observed. Showing that each disease is differentially linked to separate subregions immediately suggests distinct mechanisms of pathogenesis. Moreover, by pinpointing the individual subregions linked to each disease specific hypotheses could be generated and tested.
In the case of diabetes, our primary human findings suggested that the dentate gyrus is differentially vulnerable to blood glucose levels. Illustrating the utility of cross-species CBV mapping, we were then able to test this hypothesis, first in aging rhesus monkeys in whom there is greater experimental control, and then, more definitively, in a mouse model of hyperglycemia. Although each study had it own limitations, as a composite the human, monkey, and rodent findings establish that blood glucose differentially targets the dentate gyrus.
In the case of vascular disease, our results suggested that infarcts outside of the hippocampal formation are differentially linked to dysfunction in the CA1 subfield and the subiculum. Guided by previous studies7, 22, we postulated that the link between CA1 dysfunction and infarcts might reflect transient hippocampal hypoperfusion. Non-human primates do not naturally develop infarcts, and experimentally causing an infarct selectively in the hippocampal vascular territory is difficult. Therefore, unable to test this hypothesis in animal models we returned to the human dataset. By showing that CA1 dysfunction was differentially observed only in subjects with infarcts in the hippocampal vascular territory, this proposed mechanism was provisionally confirmed. More direct confirmation will be provided by generating hippocampal CBV maps during the acute phases of stroke in different vascular territories.
In contrast to the CA1 subfield, we also found stroke-related dysfunction in the subiculum, and by showing that this effect occurs independent of vascular territory our results suggest that there are additional mechanisms that link infarcts to hippocampal dysfunction. Nevertheless, strokes in the hippocampal vascular territory are common, and the proposed mechanism for CA1 dysfunction has potential therapeutic implications. Specifically, because excitotoxicity is thought be mediate CA1 dysfunction, our results suggest that as in the case of bilateral carotid occlusion23, glutamate blocking agents such as memantine might be beneficial in patients who suffer an acute focal stroke in the hippocampal vascular territory.
A range of previous studies have established that the entorhinal cortex is differentially vulnerable to the early stages of AD, which has been captured by hippocampal CBV imaging15. Because diabetes and infarcts might interact with AD pathology, it is theoretically possible that hippocampal dysfunction observed in stroke and diabetes is simply a reflection of these processes accelerating underlying AD pathology. By targeting other hippocampal subregions, our results suggest that both diabetes and infarcts can cause hippocampal dysfunction independent of AD pathophysiology. At the same time, our last analysis, showing that the entorhinal cortex is differentially sensitive to insulin in a stroke-dependent manner, provides an interesting anatomical site of convergence linking AD, diabetes, and infarcts. Because this final analysis was based on a relatively low number of subjects, however, further studies are required to better understand this complex relationship.
Showing that blood glucose selectively targets the dentate gyrus is not only our most conclusive finding, but it is the one most important for ‘normal’ aging—i.e., hippocampal dysfunction that occurs in the absence of disease states, such as AD, infarcts, and diabetes. Indeed, cognitive studies have established that normal age-related hippocampal dysfunction begins quite early24, typically during the 4th decade of life, before the onset of age-related diseases. Furthermore, age-related hippocampal dysfunction occurs in all non-human mammals25, who do not typically develop AD, stroke, or diabetes. Consistent with this, our cross-species findings document that the detrimental affects of glucose on the hippocampus19, 20 occurs independent of AD and infarcts, and our monkey findings in particular suggest that it occurs independent of overt diabetes.
With increasing longevity and decreasing morbidity age-related hippocampal dysfunction has emerged as a cognitive epidemic. Nevertheless, underlying causes of age-related hippocampal dysfunction have remained unknown. Importantly, converging evidence in humans26 27, non-human primates14 28, and rodents14, 15 have suggested that the dentate gyrus is differentially vulnerable to normal aging29. At the same time, an independent series of cross-species studies has shown that glucose regulation worsens with advancing age30, 31. Taken together, our findings suggest that glucose dysregulation is as at least one systemic etiology underlying age-related hippocampal dysfunction.
Beyond the obvious conclusion that preventing late-life disease would benefit the aging hippocampal formation, our findings suggest that maintaining glucose control, even in the absence of disease, should be strongly recommended to preserve cognitive health. More specifically, our findings predict that any intervention that causes a decrease in blood glucose should increase dentate gyrus function and would therefore be cognitively beneficial. In fact, separate studies examining the effects of physical exercise support this prediction. Imaging studies in humans and mice have documented that among all hippocampal subregions physical exercise causes a differential improvement in dentate gyrus function 32. By improving glucose metabolism, physical exercise also reduces blood glucose33. It is possible, therefore, that the cognitive enhancing effects of physical exercise are mediated by the beneficial effect of lower glucose on the dentate gyrus. Whether through physical exercise or other behavioral or pharmacological interventions, our results suggest that improving glucose metabolism is a clinically tractable approach for ameliorating the cognitive slide that occurs in all of us as we age.
Acknowledgments
AG008702 (SAS), AG025161 (SAS) AG07232 (SAS, CD, RM), American Diabetes Association 1-05-RA-139 (SV), AGOO3376 and the Evelyn F McKnight Brain Research Foundation (CB)
REFERENCES
- 1.Rapp PR, Heindel WC. Memory systems in normal and pathological aging. Current opinion in neurology. 1994;7:294–298. doi: 10.1097/00019052-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Small SA. Age-related memory decline; current concepts and future directions. Archives of Neurology. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- 3.Gazzaley A, Sheridan MA, Cooney JW, D'Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21:532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Lein ES, He A, et al. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- 6.Gold SM, Dziobek I, Sweat V, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–719. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Kastner R, Freund TF. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 8.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 10.Mayeux R, Small SA. Finding the beginning or predicting the future? Arch Neurol. 2000;57:783–784. doi: 10.1001/archneur.57.6.783. [editorial; comment] [DOI] [PubMed] [Google Scholar]
- 11.Belliveau JW, Kennedy DN, Jr, McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez RG, Fischman AJ, Guimaraes AR, et al. Functional MR in the evaluation of dementia: correlation of abnormal dynamic cerebral blood volume measurements with changes in cerebral metabolism on positron emission tomography with fludeoxyglucose F 18. AJNR Am J Neuroradiol. 1995;16:1763–1770. [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Celik A, Paczynski RP. Regional cerebral blood volume: a comparison of the dynamic imaging and the steady state methods. J Magn Reson Imaging. 1999;9:44–52. doi: 10.1002/(sici)1522-2586(199901)9:1<44::aid-jmri6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Small SA, Chawla MK, Buonocore M, et al. From The Cover: Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno H, Wu WE, Lee T, et al. Imaging the abeta-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- 16.Brickman A, Schupf N, Manly JJ, et al. Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from Northern Manhattan. Arch Neurol. 2008 doi: 10.1001/archneur.65.8.1053. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Awad N, Gagnon M, Desrochers A, et al. Impact of peripheral glucoregulation on memory. Behav Neurosci. 2002;116:691–702. doi: 10.1037/0735-7044.116.4.691. [DOI] [PubMed] [Google Scholar]
- 20.Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci U S A. 2003;100:2019–2022. doi: 10.1073/pnas.0336073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- 22.Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- 23.Simon RP, Swan JH, Griffiths T, Meldrum BS. Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science. 1984;226:850–852. doi: 10.1126/science.6093256. [DOI] [PubMed] [Google Scholar]
- 24.Albert MS. The ageing brain: normal and abnormal memory. Philosophical transactions of the Royal Society of London. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 26.Small SA, Tsai WY, DeLaPaz R, et al. Imaging hippocampal function across the human life span: is memory decline normal or not? Ann Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 27.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 28.Gazzaley AH, Siegel SJ, Kordower JH, et al. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci U S A. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla MK, Barnes CA. Hippocampal granule cells in normal aging: insights from electrophysiological and functional imaging experiments. Progress in brain research. 2007;163:661–678. doi: 10.1016/S0079-6123(07)63036-2. [DOI] [PubMed] [Google Scholar]
- 30.Shimokata H, Muller DC, Fleg JL, et al. Age as independent determinant of glucose tolerance. Diabetes. 1991;40:44–51. doi: 10.2337/diab.40.1.44. [DOI] [PubMed] [Google Scholar]
- 31.Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. American journal of physiology. 2001;281:E757–E765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 32.Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annual review of medicine. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]