Abstract
Two experiments examined the effectiveness of the pupillary response as a measure of cognitive load in younger and older adults. Experiment 1 measured the change in pupil size of younger and older adults while listening to spoken digit lists that varied in length and retaining them briefly for recall. In Experiment 2 changes in relative pupil size were measured while younger and older adults listened to sentences for later recall that varied in syntactic complexity and sentence length. Both age groups’ pupil sizes were sensitive to the size of the memory set in Experiment 1 and sentence length in Experiment 2, with the older adults showing a larger effect of the memory load on a normalized measure of pupil size relative to the younger adults. By contrast, only the younger adults showed a difference in the pupillary response to a change in syntactic complexity, even with an adjustment for the reduced reactivity of the older pupil.
Over 40 years ago Hess and Polt (1964) reported a measurable change in the pupil size of the eye in response to mental activity. Two years later, Kahneman and Beatty (1966) elegantly demonstrated that pupil size increased incrementally as items of a to-be-recalled list of digits were heard and presumably stored in memory and then incrementally decreased as each item was recalled. Since these two early studies, the task-evoked pupillary response has been used as an assay of cognitive effort in a variety of perceptual and cognitive tasks (see Beatty, 1982, for a review). Such studies have demonstrated a relationship between pupil size and mental processing demands during digit-list recall (Granholm, Asarnow, Sarkin, & Dykes, 1996; Peavler, 1974), recall of lists in reverse order after visual or auditory presentation (Taylor, 1981), demands of attentional allocation (Verney, Granholm, & Marshall, 2004), and difficulty of a visual search task (Porter, Troscianko, & Gilchrist, 2007). In the domain of language processing, pupil size has been shown to be sensitive to the cognitive demands attendant to simultaneous interpretation or lexical translation of speech (Hyönä, Tommola, & Alaja, 1995), difficulty in processing ambiguous meanings within sentences (Ben-Nun, 1986), reading sentences that differed in syntactic complexity (Just & Carpenter, 1993), and effects of perceptual effort due to hearing loss (Kramer, Kapteyn, Festen, & Kuik, 1997).
The use of pupillometry as a measure of cognitive load in older adults in comparison with younger adults has been complicated by older adults’ generally smaller pupil size and more restricted range in pupil dilation. The concern is that because older adults have a restricted range of pupil dilation as measured by the pupillary response to a change in ambient light intensity (senile miosis), the older adult pupil might not be as responsive as the younger adult pupil to relatively small changes in cognitive effort (Van Gerven, Paas, Van Merriënboer, & Schmidt, 2004; see also Kim, Beversdorf, & Heilman, 2000). Even if observed, such pupil size measurements might systematically underestimate older adults’ degree of cognitive effort on a particular task relative to younger adults’.
Our question is whether the task-evoked pupillary response can serve as a useful measure of cognitive effort when younger and older adults are asked to listen to, and retain in memory, sentences that vary in their syntactic complexity. Numerous studies have shown that sentences which express semantic relationships with more complex syntactic structures, relative to those with simpler syntax, produce more errors in comprehension (Just & Carpenter, 1992) and recall (Fallon, Peelle, & Wingfield, 2006), as well as having shown increased patterns of neural activation in functional imaging studies (Cooke et al., 2002; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; Peelle, McMillan, Moore, Grossman, & Wingfield, 2004), with many of these effects magnified by adult aging (Fallon et al., 2006; Waters & Caplan, 2001; Wingfield & Grossman, 2006; Wingfield, Peelle, & Grossman, 2003).
Just and Carpenter (1993) measured on-line changes in pupil size as university undergraduates read sentences that varied in syntactic complexity. They found that reading sentences with greater syntactic complexity was accompanied by larger pupil sizes than when the same participants read sentences of equivalent length but with a less complex syntactic structure. The questions we ask in this present study are first, whether the task-evoked pupillary response is sensitive to differences in syntactic complexity of to-be-recalled auditorally presented sentences. Second, we wish to determine whether the pupillary response can be used as a measure of degrees of cognitive effort for older as well as for younger adults.
Before turning to these dual questions we conducted a preliminary experiment to determine the reliability of the task-evoked pupillary response as a measure of cognitive load in older adults in the well-studied domain of digit-list recall (e.g., Granholm et al., 1996; Peavler, 1974). For this purpose we compared the pupillary response of younger and older adults as they heard digit lists and briefly held them in memory in preparation for recall. As part of this initial experiment, we departed from the traditional methods of reporting absolute pupil size or pupil size relative to a pre-task baseline, measures that work well when all participants are younger adults (cf., Beatty & Lucero-Wagoner, 2000; E. H. Hess & Polt, 1964; Kahneman & Beatty, 1966; Porter et al., 2007; Taylor, 1981). To take into account potential age differences in pupil reactivity, we report younger and older adults’ pupil sizes relative to their pupillary change in response to a change in light intensity. We consider the utility of this procedure as a way of adjusting the pupillary response for a potential limit in the physiological reactivity of the older pupil.
Experiment 1
Method
Participants
Participants were 15 younger adults (5 men, 10 women) ranging in age from 19–27 years, (M = 22.6 years) and 15 older adults (7 men, 8 women) ranging in age from 67–83 years, (M = 74.1 years). The younger adults were university students and staff and the older participants were healthy community-dwelling volunteers. The two groups did not differ significantly in years of formal education (M younger = 16.5 years, SD = 2.5; M older = 15.7 years, SD = 3.1), t(28) <1.0), nor in verbal ability as assessed by the Shipley Vocabulary Test (Zachary, 1986) (M younger = 15.8, SD = 2.0; M older = 15.8, SD = 2.0), t(28) <1.0). All participants spoke English as their first language and all reported themselves to be in good health.
Because the stimulus lists were presented auditorally, audiometric screening was conducted to confirm that both the younger and older participants’ hearing acuity fell within a range considered to be clinically normal for speech (i.e., pure-tone thresholds, averaged over 500, 1000, and 2000 Hz, and speech reception thresholds, of less than 25 dB HL; (Hall & Mueller, 1997)). All participants’ normal or corrected visual acuity fell at or below 20/50 on the Snellen chart for visual acuity (Hetherington, 1954) and on the Rosenbaum Pocket Vision Screener for near vision (Rosenbaum, 1984).
Materials
The stimuli consisted of 36 recorded lists of non-repeating spoken digits drawn from the digits one through nine. Twelve of the lists were four digits in length, twelve were six digits in length, and twelve were eight digits in length. The lists were recorded by a female speaker of American English onto computer sound files (SoundEdit software; Macromedia, Inc., San Francisco, CA), adjusted to a rate of one digit per second, and normalized to insure an even loudness across all digits within and between lists.
Procedure
List presentations
Participants were tested individually in a light and sound controlled testing room and were comfortably seated approximately 60 cm from a computer screen bearing a centrally located fixation point. Participants were told that while looking at the fixation point they would hear lists of random digits, four, six, or eight digits in length, presented in a female voice at a rate of 1 digit per second. They were told that at the end of each list there would be a 3 s retention period followed by an audible tone signaling the participant to recall as many of the digits in the just-heard list as possible, in the correct order.
Each participant heard all thirty-six lists over binaural earphones individually adjusted to a comfortable listening level. Once selected, this sound level was maintained throughout the experiment. The 12 lists of each length were presented as a block, with the order of presentation of list-length blocks counterbalanced across participants such that, by the end of the experiment, each list-length block was presented an equal number of times in each order. So as not to disadvantage older adults who might be slower in giving their recall responses, no time pressure was placed on participants in completing their recall. Onsets of subsequent lists followed at variable intervals after completion of recall of the prior list.
Pupil size data collection
Pupil size was continuously recorded at a rate of 60 times per second using an infrared ASL eye tracker (Model 6 series, Applied Science Laboratories, Bedford, MA). Rays from an infrared camera aimed at the participant’s left eye were reflected by the retina, generating a high-resolution image of the pupil on a second computer screen along with a continuous recording of pupil diameter. The pupil diameter measurements were routed through the presentation software (GazeTracker, Applied Science Laboratories, Bedford, MA) to allow for pupil size measurements to be synchronized in time with the presentation of digits across each trial. Measures of pupil diameter were processed with software written with Matlab 7 (Mathworks, Natick, MA).
Eye blinks were filtered out on the basis of sudden drops in vertical pupil diameter. The criterion for detecting a drop was the ratio between the vertical and the horizontal pupil diameter. For an essentially circular pupil, the ratio would be approximately 1.0. During a blink or semi-blink the ratio quickly drops toward zero. All data with a ratio differing more than one standard deviation from the mean were eliminated.
Age differences in pupillary response range
Although the overall smaller size of the older adults’ pupil relative to younger adults’ can be accounted for by comparing task-evoked pupil sizes to individual participants’ non-task baselines, this method would not adjust for the previously noted more limited pupil size range in older adults. As a possible solution, we conducted a pre-test for each participant in which we took a range measurement of pupil size change in response to a change in light intensity, using these data to normalize the younger and older adults’ task-evoked pupillary responses. In this pre-test the pupillary response range was characterized by the difference in pupil diameter at two light intensities; the first while the participant focused for 10 s on a dark computer screen (.05 fL) and the second while the participant focused for 10 s on a light screen (30.0 fL). (Ambient light in the testing room was a constant 12.1 fL). This measured range was used to adjust pupil size measures for younger and older adults to take into account the narrower range of pupil size change associated with the older eye.
Results
Pupillary Response Range
The mean pupil size for the younger and older adults in response to the dark computer screen (.05 fL) was 2.58 mm (SD = .68 mm ) and 2.2 mm (SD = .47 mm), respectively. To the light computer screen (30.0 fL), younger and older adults’ responses were 6.45 mm (SD = 1.07 mm) and 4.71 mm (SD = 1.27 mm), respectively. Data represent peak diameters during the 10 s measurement period. These data were submitted to a 2(Age) × 2(Intensity) mixed design analysis of variance (ANOVA), with intensity as a within-participants variable and age as a between-participants variable. Both age groups showed a reduction in pupil size when going from a darker to a brighter screen, as confirmed by a main effect of light intensity, F(1,28) = 168.43, p <.001,ηp2 = .857. The impression of older adults showing a smaller pupil size in response to a given light intensity was confirmed by a main effect of age, F(1,28) = 23.53, p <.001, ηp2 = .457. Although both the younger and the older adults showed a reduction in pupil size to the change in light intensity, as might have been expected (e.g., Granholm et al., 1996; Kim, Beversdorf, & Heilman, 2000), the change in pupil diameter in response to the same intensity difference was differentially greater for the younger than for the older adults. This difference was confirmed by a significant Age × Intensity interaction, F(1,28) = 6.17, p <.05, ηp2 = .180.
Recall Accuracy
The greater difficulty of the longer lists was reflected in recall accuracy, with 98.9%, 90.6%, and 52.8% of the 4, 6, and 8 digit lists, respectively, correctly reported by the younger adults, and 97.7%, 69.4%, and 41.7% of the 4, 6, and 8 digit lists correctly reported by the older adults. A 2(Age) × 3(List-length) ANOVA conducted on these data showed a significant main effect of list length, F(2,56) = 59.39, p <.001. ηp2 = .680. Although there was a tendency for the older adults to show overall poorer recall than their younger counterparts, the main effect of age was not significant, F(1,28) = 2.80, n.s., ηp2 = .091, nor was there a significant Age × List-length interaction, F(2,56) = 2.22, n.s., ηp2 = .073. Only lists correctly reported were used for further analysis.
Pupil Size During List Acquisition
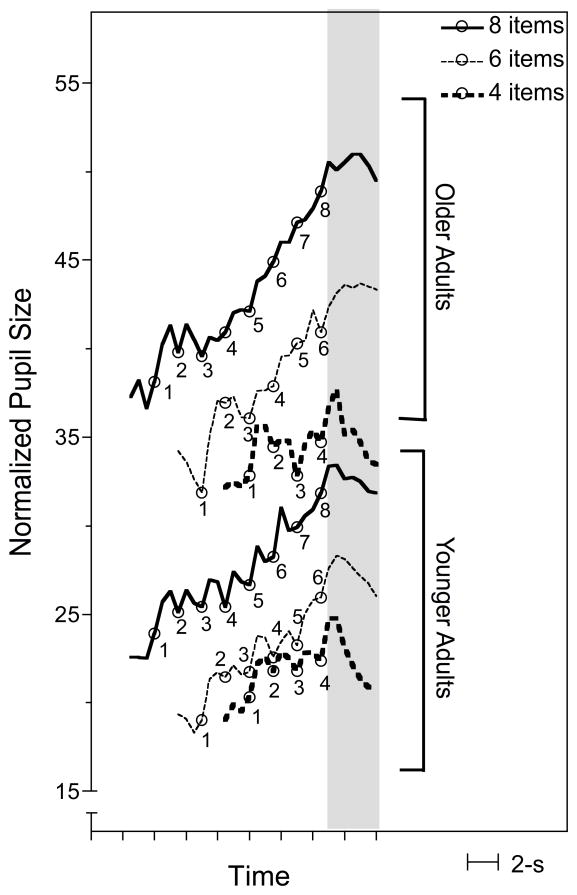
The six curves to the left of the shaded area in Figure 1 show the normalized pupil sizes (averaged over 500 ms bins) 1.5 s prior to the onset of the digit lists and while the 4, 6, and 8 digit lists were heard. The three lower curves and the three upper curves show these data for the younger participants and the older participants, respectively. Data are shown only for lists correctly recalled.
Figure 1.
Mean normalized pupil size for the younger adults (three lower curves) and the older adults (three upper curves) over the course of list presentations for 4, 6, and 8 item digit lists and for the 1.5 s prior to list presentation. Small circles along curves indicate digit arrival times. Shaded area indicates a 3 s retention interval prior to a recall signal.
Because of the above-noted difference between younger and older adults’ average pupil sizes and the more limited responsiveness of the pupil of the older eye, we have plotted the measured pupil diameter during the task (dM), relative to the difference in the individual participant’s range in pupil diameter in response to the darker .05 fL screen (dmax) versus the brighter 30.0 fL screen (dmin), with the value shown on the ordinate calculated as (dM − dmin) / (dmax − dmin) × 100. (Plotting these data based on absolute pupil size yielded the same shaped curves, but showed the younger participants as having larger pupil sizes at each point on the x-axis relative to the older participants.)
The small circles along the curves indicate the 1 s per item arrival times of each of the list items in the 4, 6, and 8 digit lists. The shaded region at the apex of the curves indicates normalized pupil sizes during the 3 s retention interval for each list length. (Pupil sizes beyond this point showed a progressive return to the original pre-list pupil size baseline, similar to that observed by Kahneman and Beatty (1966), who required participants to deliver their recall at a metronome-timed 1 s per item rate designed to mirror the input rate. To avoid complicating the task for the older adults we did not force the output timing for either the younger or older participants, such that output rates were very variable, with some participants spacing their output and others giving their full response in a short time burst following the recall signal.)
As has been found previously for younger (e.g., Kahneman & Beatty, 1966; Granholm et al., 1996; Peavler, 1974) one sees for both younger and older adults an increase in adjusted pupil size with the incremental arrival of list items as the memory load progressively builds, at least for the 6 and 8 item lists. The 4 digit list did not show the same degree of challenge; a much shallower slope of adjusted pupil size was observed across this shorter digit-list than for the longer digit lists. Slope constants for the best fitting linear functions were calculated for each participant from the onset of the first digit of the spoken list through the final digit. These slope constants were submitted to a 3(List-length) × 2(Age) mixed design ANOVA, with list-length as a within-participants variable. This analysis revealed a significant main effect of list-length on the slopes of the acquisition curves, F(2,48) = 8.96, p <.001, ηp2 = .272, with this effect appearing primarily in the difference between rates of increase in pupil size for the 6 and 8 digit lists versus the 4 digit list. This increase in the steepness of the slopes for the longer lists was similar for both the younger and older adults, reflected in the absence of a main effect of age on the slopes, F(1,24) <1.0, ηp2 = .004, nor was there a significant List-length × Age interaction, F(2,48) = 1.84, n.s., ηp2 = .071.
Pupil Size During the Retention Interval
It could be argued that pupil size during this post-list retention interval would be the best reflection of the cumulative memory load for the lists of each length. For this reason, in addition to examining the slopes of the pupil size increases as list items were being heard, we also examined the mean normalized pupil sizes during the retention interval (the shaded area in Figure 1). An ANOVA conducted on these data showed a significant main effect of list-length, F(2,56) = 46.56, p <.001, ηp2 = .624, supporting the appearance of larger pupil sizes for longer list-lengths. A significant main effect of age also appeared, supporting the appearance in Figure 1 of the older adults having overall larger normalized pupil sizes than the younger adults, F(1,28) = 6.15, p <.05, ηp2 = .180. The relative effect of list-length on pupil size during the retention interval was similar for both age groups, consistent with the absence of a significant Age × List-length interaction, F(2,56) = 1.10, n.s., ηp2 = .038.
Discussion
Experiment 1 represents a basic replication of Kahneman and Beatty’s (1966) finding for younger adults of an increase in pupil size as items in a memory set are sequentially heard. In the present study we find that both younger and older adults exhibit a similar progressive increase in pupil size as the to-be-recalled digit-list unfolded in time. However, when normalized for their more limited range, the older adults’ pupil sizes are larger both during list acquisition and during the retention interval prior to the signal to recall the list. The larger normalized pupil sizes for the older relative to the younger adults is consistent with the notion that the older adults’ success came at the cost of greater effort relative to the younger adults’.
As part of our analysis we also plotted the data for each age group in terms of unadjusted pupil size and pupil size at each point as a difference from their pre-stimulus onset baseline, the methods typically employed in younger adult studies (e.g., Beatty & Lucero-Wagoner, 2000; E. H. Hess & Polt, 1964; Kahneman & Beatty, 1966; Porter et al., 2007; Taylor, 1981). Using either metric shows similar within age group patterns in terms of a progressive increase in pupil size as list items arrived and larger pupil sizes during the retention interval for longer relative to shorter lists. Most noticeably, however, the mean pupil sizes using either metric show larger pupil sizes at each point for the younger adults relative to the older adults.
To the extent that a change in pupil size serves as a measure of cognitive effort, this latter finding would appear to suggest the counterintuitive notion that the younger adults require more cognitive effort to encode and recall the digit lists than the older adults. Such a finding would be inconsistent with an extensive literature affirming older adults’ greater difficulty with tasks that involve rote memory for unstructured materials relative to younger adults (Kausler, 1994; Wingfield & Kahana, 2002). Plotting pupil sizes as difference scores from each age group’s pre-stimulus onset baseline would adjust for the smaller pupil size of older adults. It would not, however, take into account the more restricted range of pupil dilation of the older eye. We thus suggest that an apparent finding of larger pupil sizes for younger adults during acquisition and retention would be an artifact of a failure to take into account both the smaller average pupil size, and the overall reduced reactivity (response range) of the older pupil.
Whether one plots these data in terms of unadjusted pupil size or using our recommended normalization procedure, one sees an interesting effect also found in Kahneman and Beatty’s (1966) original study. This is what one might refer to as an anticipation effect prior to the onset of the first stimulus digit. Using a design blocked by list-length, as is done in the present experiment, Kahneman and Beatty (1966) observed a trend toward a larger pupil diameter prior to the onset of longer lists than the shorter ones. We observe the same anticipatory pupil enlargement for 8 digit lists relative to 4 and 6 digit lists prior to the actual list presentations, an effect that holds for both the younger and the older participants. One would not expect to see such an anticipatory effect of list difficulty if the lists of various length had not been presented in a blocked design. As will be seen, in Experiment 2 the stimuli representing presumed differences in processing difficulty were intermixed in presentation order.
Experiment 1 confirms that the task-evoked pupillary response is sensitive to the size of a short-term memory load for both younger and older adults. It does not tell us, however, whether the pupillary response would be sensitive to potentially more subtle processing differences associated with the syntactic complexity of well-structured meaningful sentences. In Experiment 2, we test the sensitivity of the task-evoked pupillary response in younger and older adults when asked to encode in memory such sentential material that varied in processing challenge.
Experiment 2
In this experiment we employed sentence materials that allowed us to contrast potential effects on the pupillary response when hearing sentences embodying two independent sources of processing load. The first of these was the syntactic complexity of the sentences. The second was an orthogonal manipulation of an additional short-term memory load, the feature that had been the sole focus of Experiment 1.
Syntactic Complexity
To manipulate syntactic complexity we employed two types of sentences of the form used by Just and Carpenter (1993) in their reading study. Both sentence types contained centrally-embedded clauses. The more canonical of the two were subject-relative sentences in which the noun phrase (NP) precedes one or more action verbs and is the agent or source of the actions. For example, in the subject-relative sentence, “The gambler who signaled the dealer revealed the card,” the gambler is the agent of the action verbs, “signaled” and “revealed.” The contrasting sentence structure employed for this experiment was represented by less canonical object-relative sentences, such as, “The gambler who the dealer signaled revealed the card.” In this case the first NP that precedes the verb is not the expected agent of the first action but instead is the recipient; it is the second NP, “the dealer” who is the agent of the action “signaled.” The first NP, “the gambler”, however, remains the agent of the second action “revealed.”
Models of sentence processing suggest a number of reasons why object-relative sentences would be expected to impose a greater information processing load than subject-relative sentences. In object-relative sentences, which are less frequently encountered in everyday listening (Fanselow, Kliegl, & Schlesewsky, 1999) determining the appropriate thematic roles of the two noun phrases is computationally more difficult than with subject-relative sentences (King & Just, 1991), with successful disambiguation of the thematic roles requiring the listener to keep the subject of the sentence in mind for a longer time period than in subject-relative sentences (Cooke et al., 2002). As a result, object-relative sentences are known to produce more errors in comprehension and recall than subject-relative sentences for both younger and older adults (Cooke et al., 2002; Just & Carpenter, 1992; Wingfield et al., 2003).
Short-Term Memory Load
As can be seen above, the processing challenge attendant to complex syntax is of a different character than the short-term memory demands for recall of unrelated digit lists as represented in Experiment 1. An advantage of using subject-relative and object-relative sentences of the sort given in our examples is the ability to add modifiers to the nouns in the sentences as a means of increasing the memory demands for accurate sentence recall without affecting the subject-relative or object-relative nature of the sentence structures. The addition of modifiers to the basic sentences (e.g., “The professional gambler that signaled the suspicious dealer revealed the perfect card.”) would have two memory-related effects on the processing challenge. Interpolating the modifiers adds to potential memory demands by increasing the distance between constituents that must be integrated for successful comprehension (Cooke et al., 2002). In addition, the specific modifiers themselves, in this example, professional, suspicious, and perfect, add to the number of words to be retained for recall. Our question in Experiment 2 was whether the processing load imposed by grammatical complexity and the added short-term memory load imposed by the need to recall the noun modifiers operate to an equivalent degree on the pupillary response in younger adults and whether the pattern obtained for younger adults would hold in a similar way for older adults.
Method
Participants
The participants were 18 younger adults (6 men, 12 women) ranging in age from 18–27 years (M = 20.6 years) and 18 older adults (9 men, 9 women) ranging in age from 65–79 years (M = 71.9 years). The younger participants were university students and staff and the older participants were healthy community-dwelling volunteers. The older adults had at time of testing an average of 2.6 more years of formal education than the younger adults (M younger = 14.7 years, SD = 2.3; M older = 17.3 years, SD = 1.9; t(34) = 5.55, p < .001). The older participants in this experiment also had higher Shipley vocabulary scores (Zachary, 1986) than the younger group (M younger = 15.5, SD = 2.0; M older = 17.3, SD = 2.1; t(34) = 2.45, p <.05). All participants spoke English as their first language and all reported themselves to be in good health. Audiometric and visual acuity measures for the two age groups were similar to those reported in Experiment 1.
Materials
Preparation of the stimuli began with the construction of 32 nine word subject-relative sentences, each consisting of an agent, an action, a receiver, and an additional outcome. From each of these subject-relative sentences we constructed an object-relative counterpart, for a total of 64 sentences. The sentences were constructed such that the agent of the action and the receiver of the action could be plausibly interchanged. Two forms of each subject-relative and object-relative sentence were created: a basic 9 word sentence and an extended version in which three plausible modifiers were added to produce an additional 12 word version of each sentence. This procedure resulted in a total of 128 sentences available for stimuli. Table 1 gives an example of a subject-relative sentence, its object-relative counterpart, and their shorter and longer versions.
Table 1.
Example Sentences for Experiment 2
| Sentence Type | Without Modifiers | With Modifiers |
|---|---|---|
| Subject-relative | The gambler that signaled the dealer revealed the card. | The professional gambler that signaled the suspicious dealer revealed the perfect card. |
| Object-relative | The gambler that the dealer signaled revealed the card. | The professional gambler that the suspicious dealer signaled revealed the perfect card. |
An additional set of 16 filler sentences was also created. Eight of these were 9 words in length and eight were 12 words in length. The filler sentences also had actions, agents and recipients of an action, but presented with an active-conjoined structure that differed from the embedded clause structures of the subject-relative and object-relative test sentences. All sentences were recorded onto computer sound files (SoundEdit software; Macromedia, Inc., San Francisco, CA) by a female speaker of American English in normal intonation at an average speaking rate of 124 words per minute.
Procedure
Each participant heard a total of 48 sentences: eight short (9 word) subject-relative sentences, eight long (12 word) subject-relative sentences, eight short (9 word) object-relative sentences, and eight long (12 word) object-relative sentences. Also heard were eight short (9 word) filler sentences and eight long (12 word) filler sentences. No participant heard the same core sentence more than once, with the particular core sentence and its syntactic and length conditions varied between participants. The particular sentence and condition combinations were counterbalanced across participants such that, by the end of the experiment, each core sentence was heard in each of its four syntactic structure and sentence-length conditions an equal number of times. It is known that individual word factors such as their affective content can have an effect on pupil size (Partala & Surakka, 2003). To control for this or other possible word effects, the particular noun modifiers used to lengthen the subject-relative and object-relative sentences were counterbalanced across sentences and across participants such that, by the end of the experiment, each noun modifier was heard an equal number of times in the subject-relative and object-relative sentences.
The sentences were heard over binaural earphones individually adjusted for each participant to a comfortable listening level prior to the beginning the experiment. Once selected, this sound level remained constant throughout the experiment. The sentence types (long and short subject-relative sentences, long and short object-relative sentences, and filler sentences) were presented in a mixed list design, with the order of sentences randomized between participants.
Participants were instructed to listen carefully to each sentence in preparation for a recall of the sentence. They were told that the end of the sentence would by followed by a two second retention interval, after which a tone would be heard as a signal to recall the just-heard sentence as completely and as accurately as possible. Pupil size measures were continuously recorded for the duration of each trial using the same eye-tracking apparatus as described in Experiment 1. The main experiment was again preceded by a measure of each participant’s pupillary response range using the same procedures as used in Experiment 1. Individual participants’ pupillary response range would again be used to normalize pupil sizes for the two age groups.
Results
Pupillary Response Range
The mean pupil size for the younger and older adults in response to the dark (.05 fL) computer screen was 2.51 mm (SD = .8 mm ) and 2.15 mm (SD = .65 mm), respectively. To the light (30.0 fL) computer screen, younger and older adults’ responses were 6.37 mm (SD = 1.04 mm) and 4.98 mm (SD = 1.35 mm), respectively, following the same conditions and procedures as in Experiment 1.
As was found in Experiment 1, an ANOVA on these data showed a main effect of intensity, F(1,34) = 208.03, p <.001, ηp2 = .860 and a main effect of age, F(1,34) = 13.5, p <.001, ηp2 = .284. As seen in Experiment 1, the reduction in pupil size in response to a change in light intensity was differentially smaller for the older adults than for the younger adults, as reflected by a significant Age × Intensity interaction, F(1,34) = 4.99, p <.05, ηp2 = .128. These data would again be used to normalize task-evoked changes in pupil sizes for individual differences in pupil reactivity.
Recall Accuracy
Recall accuracy for the sentences was excellent for both age groups. For the younger adults, an average of 99.8% and 95.6% of the words were recalled correctly for the 9 word subject-relative and object-relative sentences, respectively, and 99.9% and 96.5% for the 12 word subject-relative and object-relative sentences, respectively. Corresponding values for the older adults were 99.7% and 95.0% words correctly reported for the 9 word subject-relative and object-relative sentence, and 99.9% and 94.4% words correct for the 12 word subject-relative and object-relative sentences. Although these differences were small and near ceiling, an ANOVA conducted on these data confirmed a significant recall advantage for the shorter sentences relative to the longer sentences, F(1, 34) = 76.33, p <.001, ηp2 = .692. The two age groups did not differ significantly in recall accuracy F(1,26) = 1.81, n.s., ηp2 = .051, nor was there a significant Age × Sentence-length interaction, F(1, 26) = 1.61, n.s., ηp2 = .045. Neither the main effect of syntactic type, nor any of the remaining interactions (Age × Syntactic type, Age × Sentence-length, Sentence-length × Syntactic type, or Age × Syntactic type × Sentence-length) were significant.
Pupil Size During Sentence Acquisition
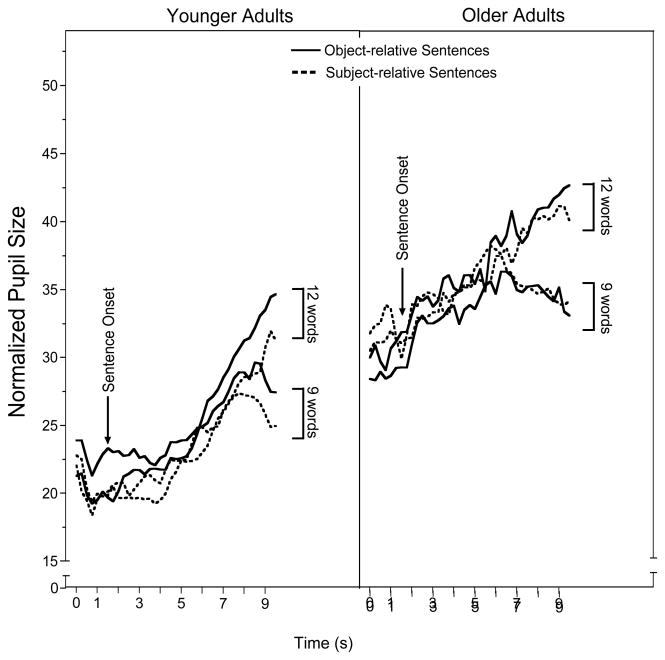
Figure 2 shows the mean normalized pupil sizes for the shorter (9 word) and longer (12 word) subject-relative and object-relative sentences for trials on which the sentences were recalled with perfect accuracy (averaged over 250 ms bins to gain additional pupillary measurements). Pupil sizes were normalized for age differences in pupil reactivity, with the values shown on the ordinate again calculated as (dM − dmin) / (dmax − dmin) × 100. The younger adults’ data are shown in the left panel of Figure 2 and the older adults’ data are shown in the right panel. The vertical arrow on each curve indicates the time of the onset of the sentence. The end of each sentence is marked by the left edge of the vertical shaded bar which represents the duration of the 2 s retention interval. (As was the case in Experiment 1, pupil sizes beyond this point showed a progressive return to the original pre-list pupil size baseline, in this case, as the words of the sentences were recalled aloud. Similar to digit recall in Experiment 1, participants’ rate of output was variable, with some participants spacing their output and others giving their full response in a short time-burst following the recall signal.)
Figure 2.
Mean normalized pupil size for younger adults (left panel) and older adults (right panel) over the course of listening to short (9 word) and long (12 word) subject-relative and object-relative sentences for later recall. Vertical arrows show the time of onset of the sentences. Shaded area indicates a 2 s retention interval prior to a recall signal.
As can be seen in Figure 2, both age groups show considerable variability in pupil size prior to the sentence onsets. Unlike in Experiment 1, in which list lengths were presented in a blocked design, in this experiment participants did not know the nature of the sentences they would hear, such that this variability is unrelated to either the length or syntactic complexity of the stimuli. Once beyond this point, all conditions for both participant groups yielded a generally progressive increase in pupil size as more and more of the sentence was heard. An exception is seen for the younger adults, who show a J-shaped pupillometry curve reflecting a minimal change in pupil dilation during the first two seconds of the sentences followed by a progressive increase in pupil size as the sentences continue to unfold in time. For the younger adults the slopes of the best fitting functions for the linear portions of the curves (2 s after sentence onset, through the final word), were calculated for each participant. These slope constants were submitted to a 2(Syntax) × 2(Sentence-length) ANOVA, with sentence-length and syntax as within participant variables. This analysis supported the appearance of a steeper rate of increase in pupil size for the longer than for the shorter sentences, with a main effect of sentence-length, F(1,17) = 18.82, p <.001, ηp2 = .525. Although, as will be described in the next section, syntax had a significant effect on pupil size during the retention interval, the slopes of the increase of pupil size across the subject-relative and object-relative sentences were similar. Consistent with this appearance, the main effect of syntax on the younger adults’ slopes did not reach significance, F(1,17) = 3.38, n.s., ηp2 = .166, nor was there a Sentence-length × Syntax interaction, F(1,17) < 1, ηp2 = .000.
The older adults’ pupil sizes (right panel) showed considerable variability prior to the sentence onset, but then an approximately linear increase from the onset to the end of the sentences for the longer subject-relative and object-relative sentences. By contrast, one sees a tendency for pupil size for the shorter subject-relative and object-relative sentences to begin to decrease over the final 1 s of the sentence, representing on average the duration of the final word of the sentence. Slopes of the best-fitting linear functions for the 12 word sentences and for the linear portions of the 9 word sentences prior to the end-of-sentence declines, showed similar rates of increase in pupil size as the sentences unfolded in time, with no effect of syntax, F(1,17) < 1, ηp2 = .002, sentence-length, F(1,17) < 1, ηp2 = .002, or a Sentence-length × Syntax interaction, F(1,17) < 1, ηp2 = .003.
Pupil Size During the Retention Interval
As in Experiment 1, of primary interest was pupil size during the retention interval (the shaded areas in Figure 2), as this region appears to be the most sensitive reflection of cumulative memory load, in this case, for the sentences of different lengths. A two-way ANOVA conducted on mean adjusted pupil size during the retention interval for the younger adults revealed a significant main effect of sentence-length, F(1,17) = 22.96, p < .001, ηp2 = .575, reflecting a larger mean pupil size during the retention interval for longer sentences than shorter sentences. There was also a significant main effect of syntax, F(1,17) = 6.92, p < .025, ηp2 = .289, reflecting a larger mean pupil size during the retention interval for the object-relative than the subject-relative sentences. There was no Sentence-length × Syntax interaction, F(1,17) < 1, ηp2 = .003, although one sees an interesting tendency for pupil size to continue to increase over the 2 s retention interval for both longer subject-relative and object-relative sentences, while the shorter subject-relative and object-relative sentences begin to show a decline.
Like the younger adults, the mean pupil size during the retention interval for the older adults showed a significant effect of sentence-length, F(1,17) = 37.23, p < .001, ηp2 = .687, reflecting a larger mean pupil size for the 12 word sentences than the 9 word sentences. Unlike the younger adults, however, there was no effect of syntax, F(1,17) <1, ηp2 = .007. The suggestion in Figure 2 that object-relative sentences produced a larger pupil size than the subject-relative sentences for the longer sentences was not supported by a significant Sentence-length × Syntax interaction, F(1,17) = 1.46, n.s., ηp2 = .012. (An omnibus ANOVA conducted on the data for both age groups confirmed the appearance in Figure 2 of the older adults showing larger normalized pupil sizes during the retention interval than the younger adults.)
General Discussion
The finding that cognitive effort is accompanied by dilation of the pupil of the eye has been observed in a variety of perceptual and cognitive tasks (e.g., Beatty, 1982; Ben-Nun, 1986; Hyönä et al., 1995; Just & Carpenter, 1993; Porter et al., 2007; Steinhauer, Siegle, Condray, & Pless, 2004; Taylor, 1981; Van Gerven, Paas, Van Merriënboer, & Schmidt, 2004). Specific to our present interests has been the finding that the size of the pupil progressively increases as short-term memory demands are increased, at least until the point of memory overload (Granholm et al., 1996; Kahneman & Beatty, 1966; Peavler, 1974). Experiment 1 shows this effect to operate in older adults as well as younger adults, with both age groups showing a similar increase in pupil size with an increase in the size of a digit memory set during list acquisition and during the retention interval prior to the signal to begin recall. When adjusted for older adults’ more limited range in pupil dilation, however, older adults show a larger pupil size at each set-size level than their younger adult counterparts. To the extent that a task-evoked pupillary enlargement reflects the engagement of cognitive effort, this would imply that the older adults attain their level of success with more effort than the younger adults.
Experiment 2 was designed to determine whether the similarity between younger and older adults in the pattern of pupil size changes in response to the increase in processing load imposed by longer digit lists, would also hold for meaningful sentences that differed in syntactic complexity. There is good reason to separate conceptually the nature of memory processing for meaningful sentences versus the recall of unrelated words. Unlike unrelated digit and word lists, recall of meaningful sentences is facilitated by grammatical structure and the semantic relations among the lexical elements. This is evidenced in the research literature both by the recall advantage for meaningful sentences relative to unstructured verbal materials (Kausler, 1994) and by the nature of the omissions and intrusions one observes in sentence recall that tend to preserve syntactic coherence and sentence meaning (Wingfield, Tun, & Rosen, 1995).
We argue that although the processing and recall of the 9 words of the core subject-relative and object-relative sentences in Experiment 2 were facilitated by the meaning and structure of the sentences, the additional noun modifiers that added length to the sentences are not so constrained. Although the noun modifiers are in all cases a plausible semantic fit within the sentence contexts, they do not affect the word ordering of major syntactic elements that makes determination of thematic roles in object-relative sentences more difficult than in sentences with a subject-relative structure. It is the case that the added modifiers increase the temporal distance between syntactic elements of the sentences, which might, in turn, add to this difficulty. There is also reason to believe that such an effect might differentially impact older adults, although such effects appear primarily when the distances between syntactic elements are large (e.g., Light, Capps, Singh, & Albertson Owens, 1994; Zurif, Swinney, Prather, Wingfield, & Brownell, 1995). As can be seen in the example in Table 1, however, the added modifiers in this experiment increase the distance between the critical syntactic elements by only a single word.
Results for the younger adults in Experiment 2 are clear: one sees significantly larger pupil sizes during the retention interval for the object-relative sentences, whose syntactic relations are more difficult to determine, than for the syntactically less complex subject-relative sentences. The younger participants also show an additional “add on” of pupil size in the retention interval when modifiers are added to increase sentence length without changing the syntactic structure of the sentence itself. In contrast with the younger adults, where pupil diameter responds to effects of both sentence-length and syntactic structure, the older adults’ pupil diameter responds only to a difference in sentence-length.
The absence of a combined effect of both syntactic complexity and sentence length for the older adults during the retention interval does not seem to be an artifact of the older adults’ pupil size being constrained by an upper limit on potential pupil enlargement. There are two reasons to discount this likelihood. The first is that even for the most demanding condition, the 12 word object-relative sentences, the older adults’ mean pupil size averaged less than 73% of their maximum pupil size as the pre-test measure of pupillary response range shows. Even more direct evidence is the absence of an effect of syntactic complexity for the shorter, 9 word sentences, where the older adults’ mean pupil size during the retention interval for both syntactic forms is smaller than that observed for the 12 word sentences. The absence of an effect of syntactic complexity for these shorter sentences cannot be due to an upper limit on pupil size constraining a potential pupil enlargement in response to the more syntactically complex sentences. These two findings support the reality of the observation in the present study of an age-specific dissociation of memory load versus syntactic complexity effects on the task-evoked pupillary response.
These results resonate with a study by Van Gerven et al. (2004), who compared pupillary responses of younger and older adults in a memory search task patterned after Sternberg (1966). Consistent with the results of Kahneman and Beatty (1966), and the results of the present Experiment 1, both younger and older adults’ pupil size increases when the number of items to be remembered increases. By contrast, in the memory search phase, in which participants were required to indicate whether a probe item had been present in the memory set, only the younger adults’ pupil size was sensitive to the size of the memory set to be searched. Van Gerven et al. (2004) suggested that the pupillary response of older adults may not be sensitive to small changes in cognitive load, in their case represented by the memory search phase as distinct from the initial process of memory encoding.
An analogous account might hold for the age dissociation observed in the present study in the domain of language. Unlike the previously cited deficits in episodic memory, which are almost a hallmark of adult aging (Kausler, 1994; Wingfield & Kahana, 2002), linguistic knowledge and the procedural rules for its application, are well preserved in adult aging (Wingfield & Stine-Morrow, 2000). It might thus be that the older adults’ lifelong experience with language comprehension minimized the effort-related pupillary response to object-relative versus subject-relative sentences that is observed for the younger adults. Such an effect of language-processing expertise would not be expected to extend to the memory-load challenge represented by the addition of the syntactically neutral modifiers that added to the sentence length. The presence of a significantly larger pupillary response to the longer sentences of each syntactic type by the older adults is consistent with this argument.
This interpretation holds an apparent paradox, in that older adults often show greater difficulty with syntactically complex sentences than their younger counterparts, whether measured in terms of accuracy (Fallon et al., 2006; Kemper, 1987), processing speed (Wingfield et al., 2003), or neural activation (Wingfield & Grossman, 2006). It may be the case, however, that these effects of syntactic complexity on older adults’ performance are the result of age differences in what Caplan and Waters (1999) refer to as post-interpretive processes, rather than in the on-line interpretive processing of sentences that might require fewer processing resources. We do not wish to argue that on-line syntactic processing is resource free, as the younger adults in this study show significant effects of syntactic complexity along with effects of sentence length. Rather, in keeping with Van Gerven et al.’s (2004) suggestion, it may be that, for the older adults with their greater linguistic experience, the cognitive effort required for dealing with on-line interpretive processing does not exceed a threshold necessary to yield a measurable change in pupil size.
Good progress has been made in differentiating the roles of the sympathetic and parasympathetic branches of the autonomic nervous system as they pertain to pupil dilation in response to affective and cognitive factors (Bradley, Miccoli, Escrig, & Lang, 2008; Steinhauer et al., 2004). In this regard it is important to recognize that the present data cannot isolate potential contributions of cognitive effort versus non-cognitive factors in participants’ pupillary responses. That is, as the memory load is increased by adding digits to the lists in Experiment 1 and by adding noun modifiers to the sentences in Experiment 2, it remains possible that this increase in memory challenge is accompanied by anxiety associated with task difficulty (cf., Carver, 1971; Johnson, 1971). This might be especially so in the case of older adults, who often express concern about their memory performance, especially in tasks that involve rote memory (Erber, Prager, Williams, & Caiola, 1996; T. M. Hess, Auman, Colcombe, & Rahhal, 2003). Although this group of high-performing older adults does not overtly express such concern, it does not rule out this possibility.
Taken together, the pupillometry data of Experiments 1 and 2 confirm that both age groups show a general increase in pupil size in response to a larger short-term memory load, whether these are digit lists as in Experiment 1 or extraneous noun modifiers as in Experiment 2. In contrast to unstructured materials, the pupillometry data in Experiment 2 implies that the older adults do not find greater cognitive challenge, or perceived challenge, in response to an increase in syntactic complexity. This finding is consistent with the literature suggesting that linguistic knowledge is generally well maintained in older adulthood, as is the ability to use this knowledge to deal effectively with comprehension and recall of meaningful speech (see the review in Wingfield & Stine-Morrow, 2000).
At a methodological level, the results of Experiments 1 and 2 support the utility of pupillometry for the study of auditory sentence processing in younger adults, as Just and Carpenter (1993) have shown in reading studies. Comparing the results of younger and older results, especially for spoken input, requires additional cautions. Because hearing acuity often declines in adult aging (Morrell, Gordon-Salant, Pearson, Brant, & Fozard, 1996), it is important that audiometric screening be conducted on all participants, as we have done here. We adopted this procedure because of the possibility that perceptual effort associated with reduced hearing acuity might also be reflected in a pupillary responses (e.g., Kramer et al., 1997).
The second caution arises in regard to conducting performance contrasts between age groups. This is so because of the smaller base pupil size of older relative to younger adults, as well as the reduced reactivity of the older pupil that limits the potential range of pupil size change that may be expected (Kim et al., 2000; Van Gerven et al., 2004). Adjusting for age differences in baseline pupil size can be accomplished by expressing measured pupil sizes relative to each groups’ base pupil size prior to the stimulus onset. As we have argued, however, this method does not take into account the reduced reactivity of the older pupil, which we estimate in the present study by comparing pupil size change in response to a change in light intensity. Our method of normalizing measured pupil size for age differences in overall pupil size and reactivity yields results that were conceptually reasonable in regard to our understanding of age differences in response to short term memory load and age similarities in comprehension and recall of meaningful sentences, at least when speech rates do not exceed older adults’ limits on processing speed (Wingfield et al., 2003).
It should be noted that our method of normalizing pupil size by taking into account the estimated potential response range of the younger and older pupil carries an implicit assumption of equivalent linearity in the degree of potential change as the limits of pupil dilation are approached. Although a difference in this regard could affect the relative height of the pupillary response curves on the y-axis as measures of cognitive effort for the two age groups, it is not expected to reverse or eliminate the effects of stimulus conditions within an age group that is a major focus of the present studies.
The relative immediacy of the pupillary response has a distinct advantage in the search for an objective measure of cognitive effort as individuals are confronted by mental tasks that vary in difficulty. Confirmation of these data with other physiological measures currently available (e.g., Rohleder & Nater, 2009) is an important direction for further research. In terms of our present interests, we believe that such work can expand our understanding of areas of preservation and limitation, in the important domain of older adults’ comprehension and recall of spoken language.
Acknowledgments
The authors acknowledge support from NIH Grant AG019714 from the National Institute on Aging. We also gratefully acknowledge support from the W.M. Keck Foundation.
References
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91(2):276–292. [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Hillsdale, NJ: Cambridge University Press; 2000. pp. 142–162. [Google Scholar]
- Ben-Nun Y. The use of pupillometry in the study of on-line verbal processing: evidence for depths of processing. Brain and Language. 1986;28(1):1–11. doi: 10.1016/0093-934x(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters GS. Verbal working memory and sentence comprehension. The Behavioral and Brain Sciences. 1999;22(1):77–94. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Carver RP. Pupil dilation and its relationship to information processing during reading and listening. Journal of Applied Psychology. 1971;55(2):126–134. doi: 10.1037/h0030664. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, et al. Neural basis for sentence comprehension: grammatical and short-term memory components. Human Brain Mapping. 2002;15(2):80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber JT, Prager IG, Williams M, Caiola MA. Age and forgetfulness: confidence in ability and attribution for memory failures. Psychology and Aging. 1996;11(2):310–315. doi: 10.1037//0882-7974.11.2.310. [DOI] [PubMed] [Google Scholar]
- Fallon M, Peelle JE, Wingfield A. Spoken sentence processing in young and older adults modulated by task demands: evidence from self-paced listening. The Journals of Gerontology: Psychological Sciences and Social Sciences. 2006;61(1):10–17. doi: 10.1093/geronb/61.1.p10. [DOI] [PubMed] [Google Scholar]
- Fanselow G, Kliegl R, Schlesewsky M. Processing Difficulty and Principles of Grammar. In: Kemper S, Kliegl R, editors. Constraints on Language: Aging, Grammar, and Memory. Boston: Kluwer Academic Publishers; 1999. pp. 171–201. [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33(4):457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Hall J, Mueller G. Audiologist desk reference. San Diego, CA: Singular Publishing; 1997. [Google Scholar]
- Hess EH, Polt JM. Pupil size in relation to mental activity during simple problem-solving. Science. 1964;143(3611):1190–1192. doi: 10.1126/science.143.3611.1190. [DOI] [PubMed] [Google Scholar]
- Hess TM, Auman C, Colcombe SJ, Rahhal TA. The impact of stereotype threat on age differences in memory performance. The Journals of Gerontology: Psychological Sciences and Social Sciences. 2003;58(1):3–11. doi: 10.1093/geronb/58.1.p3. [DOI] [PubMed] [Google Scholar]
- Hetherington R. The Snellen Chart as a test of visual acuity. Psychologische Forschung. 1954;24:349–357. doi: 10.1007/BF00422033. [DOI] [PubMed] [Google Scholar]
- Hyönä J, Tommola J, Alaja AM. Pupil dilation as a measure of processing load in simultaneous interpretation and other language tasks. The Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology. 1995;48(3):598–612. doi: 10.1080/14640749508401407. [DOI] [PubMed] [Google Scholar]
- Johnson DA. Pupillary responses during a short-term memory task: cognitive processing, arousal, or both? Journal of Experimental Psychology. 1971;90(2):311–318. doi: 10.1037/h0031562. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99(1):122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. The intensity dimension of thought: pupillometric indices of sentence processing. Canadian Journal of Experimental Psychology. 1993;47(2):310–339. doi: 10.1037/h0078820. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274(5284):114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154(3756):1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kausler DM. Learning and Memory in Normal Aging. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Kemper S. Syntactic complexity and elderly adults’ prose recall. Experimental Aging Research. 1987;13(1):47–52. doi: 10.1080/03610738708259299. [DOI] [PubMed] [Google Scholar]
- Kim M, Beversdorf DQ, Heilman KM. Arousal response with aging: pupillographic study. Journal of the International Neuropsychological Society. 2000;6(3):348–350. doi: 10.1017/s135561770000309x. [DOI] [PubMed] [Google Scholar]
- King J, Just MA. Individual differences in syntactic processing: The role of working memory. Journal of Memory and Language. 1991;30(5):580–602. [Google Scholar]
- Kramer SE, Kapteyn TS, Festen JM, Kuik DJ. Assessing aspects of auditory handicap by means of pupil dilatation. Audiology. 1997;36(3):155–164. doi: 10.3109/00206099709071969. [DOI] [PubMed] [Google Scholar]
- Light LL, Capps JL, Singh A, Albertson Owens SA. Comprehension and Use of Anaphoric Devices in Young and Older Adults. Discourse processes. 1994;18(1):77–103. [Google Scholar]
- Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. The Journal of the Acoustical Society of America. 1996;100(4):1949–1967. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- Partala T, Surakka V. Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies. 2003;59(1):185–198. [Google Scholar]
- Peavler WS. Pupil Size, Information Overload, and Performance Differences. Psychophysiology. 1974;11(5):559–566. doi: 10.1111/j.1469-8986.1974.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain and Language. 2004;91(3):315–325. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Porter G, Troscianko T, Gilchrist ID. Effort during visual search and counting: insights from pupillometry. The Quarterly Journal of Experimental Psychology. 2007;60(2):211–229. doi: 10.1080/17470210600673818. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34(4):469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JG. The biggest reward for my invention isn’t money. Medical Economics. 1984;61:152–163. [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52(1):77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Taylor JS. Pupillary response to auditory versus visual mental loading: a pilot study using super 8-mm photography. Perceptual and Motor Skills. 1981;52(2):425–426. doi: 10.2466/pms.1981.52.2.425. [DOI] [PubMed] [Google Scholar]
- Van Gerven PWM, Paas F, Van Merriënboer JJG, Schmidt HG. Memory load and the cognitive pupillary response in aging. Psychophysiology. 2004;41(2):167–174. doi: 10.1111/j.1469-8986.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- Verney SP, Granholm E, Marshall SP. Pupillary responses on the visual backward masking task reflect general cognitive ability. International Journal of Psychophysiology. 2004;52(1):23–36. doi: 10.1016/j.ijpsycho.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Waters GS, Caplan D. Age, working memory, and on-line syntactic processing in sentence comprehension. Psychology and Aging. 2001;16(1):128–144. doi: 10.1037/0882-7974.16.1.128. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology. 2006;96(6):2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Kahana MJ. The dynamics of memory retrieval in older adulthood. Canadian Journal of Experimental Psychology. 2002;56(3):187–199. doi: 10.1037/h0087396. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Peelle JE, Grossman M. Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. Aging, Neuropsychology, and Cognition. 2003;10(4):310–322. [Google Scholar]
- Wingfield A, Stine-Morrow EAL. Language and Speech (Second Edition) In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Mahwah, NJ: Erlbaum; 2000. pp. 359–416. [Google Scholar]
- Wingfield A, Tun PA, Rosen MJ. Age differences in veridical and reconstructive recall of syntactically and randomly segmented speech. The Journals of Gerontology: Psychological Sciences and Social Sciences. 1995;50(5):257–266. doi: 10.1093/geronb/50b.5.p257. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Zurif E, Swinney D, Prather P, Wingfield A, Brownell H. The allocation of memory resources during sentence comprehension: evidence from the elderly. Journal of Psycholinguistic Research. 1995;24(3):165–182. doi: 10.1007/BF02145354. [DOI] [PubMed] [Google Scholar]