Abstract
Bilateral olfactory bulbectomy (OBX) in rodents produces behavioral and neurochemical changes associated clinically with depression and schizophrenia. Most notably, OBX induces hyperlocomotion in response to the stress of exposure to a novel environment. We examined the role of the endocannabinoid system in regulating this locomotor response in OBX and sham-operated rats. In our study, OBX-induced hyperactivity was restricted to the first 3 min of the open field test, demonstrating the presence of novelty (0–3 min) and habituation (3–30 min) phases of the open field locomotor response. Levels of the endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide were decreased in the ventral striatum, a brain region deafferented by OBX, whereas cannabinoid receptor densities were unaltered. In sham-operated rats, 2-AG levels in the ventral striatum were negatively correlated with distance traveled during the novelty phase. Thus, low levels of 2-AG are reflected in a hyperactive open field response. This correlation was not observed in OBX rats. Conversely, 2-AG levels in endocannabinoid-compromised OBX rats correlated with distance traveled during the habituation phase. In OBX rats, pharmacological blockade of cannabinoid CB1 receptors with either AM251 (1 mg kg−1 i.p.) or rimonabant (1 mg kg−1 i.p.) increased distance traveled during the habituation phase. Thus, blockade of endocannabinoid signaling impairs habituation of the hyperlocomotor response in OBX, but not sham-operated, rats. By contrast, in sham-operated rats, effects of CB1 antagonism were restricted to the novelty phase. These findings suggest that dysregulation in the endocannabinoid system, and 2-AG in particular, is implicated in the hyperactive locomotor response induced by OBX. Our studies suggest that drugs that enhance 2-AG signaling, such as 2-AG degradation inhibitors, might be useful in human brain disorders modeled by OBX.
Keywords: endocannabinoid, olfactory bulbectomy, novelty, 2-arachidonoylglycerol, 2-AG, locomotor response
1. Introduction
The endocannabinoid signaling system is an emerging target for drug discovery efforts aimed at identifying pharmacotherapies for psychiatric conditions [1]. The lipid mediators 2-arachidonoylglyerol (2-AG) and anandamide are the best characterized endocannabinoids isolated to date. Endocannabinoids are endogenous cannabis-like substances that bind to cannabinoid CB1 and CB2 receptors to produce physiological effects [1–3]. CB1 and CB2 receptors belong to a family of Gi/o-protein-coupled receptors and engage complex signaling mechanisms [3]. Activation of CB1 receptors suppresses neuronal excitability by inhibiting voltage-gated Ca++ channels and activating inwardly rectifying K+ channels [4–6]. Endocannabinoids are synthesized and released on demand upon receptor-stimulated or activity-dependent cleavage of membrane phospholipid precursors [7,8]. After release, endocannabinoids activate presynaptic CB1 receptors to modulate neurotransmission in a retrograde manner [9–11]. Endocannabinoid signaling at CB1 receptors is thus postulated to serve as a synaptic “circuit breaker” [12] within neuronal systems that modulate responses to stress, novelty and rewarding stimuli [13,14].
Consistent with a retrograde signaling mechanism, CB1 receptors are expressed presynaptically in the striatum [15–17]. Here, activation of cannabinoid CB1 receptors inhibits gamma-aminobutyric acid (GABA) release as well as K+-evoked and Ca++-dependent glutamate release. In striatum, cannabinoids also reduce basal glutamate outflow and uptake, although these phenomena are not necessarily CB1 receptor-dependent [18]. Thus, endocannabinoid signaling in the striatum modulates neural transmission at the interface between glutamatergic, GABA-ergic and dopaminergic transmission [18,19]. This circuitry is implicated in the regulation of emotion, motivation and locomotor behavior [13,14].
The olfactory bulb provides the primary glutamatergic innervation to the olfactory tubercle region of the ventral striatum. Olfactory bulbectomy (OBX) deafferents the ventral striatum, thereby altering dopaminergic regulation throughout the striatum. OBX increases striatal tyrosine hydroxylase and dopamine receptor levels, induces sprouting of dopaminergic axons [20,21] and enhances sensitivity to the catecholamine reuptake inhibitor cocaine [22]. Recent work in our laboratories documents that OBX increases striatal dopamine overflow [23] and increases locomotor responsivity to the indirect dopamine agonist amphetamine [24]. OBX thus induces changes in dopaminergic activity characterized by increased presynaptic tone and paradoxical receptor supersensitivity. Such dysregulation in ventral striatal function is reminiscent of neurochemical alterations observed in human depression and schizophrenia [25–27].
Most pertinent to the present studies, OBX induces hyperlocomotion in response to the stress of exposure to a novel open field [28,29]. This phenomenon is attributable to hyperdopaminergic transmission [20]. We recently reported that OBX rats exhibit insensitivity to acute and chronic effects of URB597, an inhibitor of the anandamide-degrading enzyme fatty-acid amide hydrolase (FAAH). Specifically, in sham-operated rats, URB597 produced a CB1-dependent suppression of novelty-induced locomotor activity following acute administration. Chronic administration of URB597 also produced a CB1-dependent suppression of amphetamine-induced locomotor sensitization in sham-operated rats. However, both the acute and chronic effects of URB597 were strikingly absent in OBX rats [24]. We, therefore, hypothesized that endocannabinoids, such as anandamide, dampen the locomotor response to novelty in intact rats, and that this regulation is impaired by OBX. Thus, OBX rats should exhibit decreases in endocannabinoid accumulation in brain regions deafferented by OBX, a differential sensitivity to CB1 receptor blockade, and a characteristic hyperlocomotor response to a novel open field. In the present study, we tested the hypothesis that dysregulation of the endocannabinoid signaling system contributes to the hyperlocomotor response induced by OBX using three complementary approaches. First, we compared the impact of CB1 receptor blockade on locomotor responses to novelty in OBX and sham-operated rats. Second, we quantified endocannabinoid levels in sensorimotor and limbic brain regions deafferented by OBX using liquid chromatography-mass spectrometry (LC/MS). Third, we compared cannabinoid receptor densities in brains of OBX and sham-operated rats using binding of the potent radioligand [3H]CP55,940 and quantitative autoradiography. These latter studies were performed in the same brains that were used to correlate open field locomotor activity with endocannabinoid content in both OBX and sham-operated rats. Our studies provide direct evidence that endocannabinoids are profoundly dysregulated in a rodent model of hyperdopaminergic symptomatology. Our findings further identify the endocannabinoid system as a possible pharmacological target for the treatment of psychiatric disorders such as depression and schizophrenia.
2. Materials and Methods
2.1. Subjects and Surgical Procedures
Adult male Sprague-Dawley rats (n = 71; Harlan, Indianapolis, IN, USA) weighing 250 –300 g (8 – 10 weeks old) prior to surgery were used. The University of Georgia Animal Care and Use Committee approved all behavioral and surgical procedures. Rats were housed in groups of 2 to 5 per cage in a humidity- and temperature-controlled animal housing facility. Lights were on at 0600 and off at 1800. Behavioral testing took place during the light phase between 0830 and 1800. Rats were randomly assigned to either sham or OBX surgery and to either vehicle, rimonabant or AM251 drug treatment groups. To control for time-dependent variations in locomotor activity, the order in which rats were tested throughout the day was also random. For OBX surgery, rats (n = 34) were anesthetized with a combination of pentobarbital (65 mg kg−1 intraperitoneally (i.p.); Sigma, St. Louis, MO) and ketamine hydrochloride (100 mg kg−1 i.p.; Fort Dodge Laboratories, Fort Dodge, IA, USA) or isoflurane (Abbott Laboratories, North Chicago, IL, USA). Burr holes measuring 3 mm in diameter were bilaterally drilled approximately 5 mm anterior to bregma and 1 mm lateral to the midline. The dura mater was pierced and a curved plastic pipette tip was used to aspirate the olfactory bulbs. The resulting cavity was filled with Gelfoam (Upjohn, Kalamazoo, MI, USA). Rats receiving the sham surgery (n = 37) underwent the same procedure except that the olfactory bulbs were not aspirated. In all OBX rats, confirmation of olfactory bulb lesion was made by brain dissection at the end of the experiments. Lesions were considered complete if the bulbs were totally severed from the forebrain, the weight of the tissue dissected from the olfactory bulb cavity did not exceed 5 mg, and frontal lobes were not bilaterally damaged. Sham-operated rat brains were also examined to verify that olfactory bulbs were intact following sham surgery. Histological verifications were performed by an investigator blinded to the experimental conditions. Only the brains of drug-naïve rats were collected for post-mortem LC/MS analysis and receptor autoradiography.
2.2. Chemicals
[2H4]-Anandamide was prepared in the lab [30]. Rimonabant and [3H]CP55,940 were gifts from the National Institute on Drug Abuse. AM251 was purchased from Tocris Bioscience (Ellisville, MO, USA) or Cayman Chemical Company (Ann Arbor, MI, USA). AM251 was dissolved in a vehicle composed of dimethyl sulfoxide: cremophor: ethanol: saline vehicle (1:1:1:17 in volume). Rimonabant was dissolved in ethanol: emulphor: saline (1:1:8 in volume).
2.3. Locomotor Response to Open Field Exposure
Approximately two weeks following surgery, rats were placed individually in the center of a polycarbonate activity monitor chamber (Med Associates, St. Albans, VT, USA) measuring 44.5 × 44 × 34 cm housed in a darkened, quiet room and remained undisturbed for 30 min. A 25-watt bulb positioned one meter over the chamber provided illumination. Activity was automatically measured by computerized analysis of photobeam interrupts (Med Associates). Distance traveled in the arena was used for data analysis. Results are expressed as average distance traveled in each 3 min time bin. Locomotor responses upon exposure to the novel open field were compared in OBX (n = 10) and sham (n = 10) rats in the absence of pharmacological manipulations. The effect of CB1 receptor blockade on open field activity was also evaluated in OBX rats receiving AM251 (1 mg kg−1, n = 7), rimonabant (1 mg kg−1, n = 9), the 1:1:1:17 vehicle (n = 3) or the 1:1:8 vehicle (n = 5). Sham rats similarly received either AM251 (1 mg kg−1, n = 8), rimonabant (1 mg kg−1, n = 8), the 1:1:1:17 vehicle (n = 7) or the 1:1:8 vehicle (n = 4). All drug injections were administered i.p. in a volume of 1 mL kg−1 body weight. Antagonists were employed at low doses that fail to produce anxiogenic-like effects [31] or intrinsic effects on locomotor activity [32]. Drugs were administered 30 min prior to exposure to the novel open field. Animals remained in their home cages prior to assessment of open field activity.
2.4. Tissue Dissections
Drug-naive OBX and sham rats (n = 10 per group) were decapitated immediately following the exposure to the novel open field. Brains were rapidly dissected, divided into two hemispheres along the longitudinal axis and snap frozen in precooled isopentane (−30°C). Frozen brains were stored at low temperature (−30°C and −80°C) until use. One hemisphere was used to obtain tissue punches for endocannabinoid quantification and the other hemisphere was used to measure cannabinoid receptor density and distribution using [3H]CP55,940 binding and quantitative autoradiography.
2.5. Lipid Extractions
Punches from single-hemisphere frozen brains of drug-naive rats were homogenized in methanol (0.3 mL) containing [2H4]-anandamide and [2H8]-2-AG (Cayman Chemicals, Ann Arbor, MI, USA) as internal standards. Protein concentration was determined in the homogenate to normalize samples using the bicinchinonic acid (BCA) protein assay (Pierce, Rockford IL). Tissue was punched according to distance from bregma using the rat brain atlas of Paxinos and Watson [33] as a guide. Tissue weights for punches derived from ventral striatum (1.7 mm anterior-posterior (AP), 2 mm medial-lateral (ML), −8 mm dorsal-ventral (DV)), hippocampus (−3.6 mm AP, 1 mm ML, −3.5 mm DV), amygdala (−3.6 mm AP, 4.5 mm ML, −9 mm DV) and cerebellum (−10.04 mm AP, 0 mm ML, −5 mm DV) ranged from 3–4 mg (2 mm × 1 mm punches). Tissue weights for the piriform cortex (−1.88 mm AP, 5.5 mm ML, −9 DV, relative to bregma) ranged from 1.5–2 mg (1 mm × 1 mm punches). Lipids were extracted with chloroform (2 vol), washed with water (1 vol), and fractionated by open-bed silica gel column chromatography [34]. Briefly, the lipids were reconstituted in chloroform and loaded onto small glass columns packed with Silica Gel G (60-Å 230–400 Mesh ASTM; Whatman, Clifton, NJ, USA). Anandamide and 2-AG were eluted from the columns with 9:1 chloroform/methanol (vol/vol). Eluates were dried under N2 and reconstituted in 50 µL of methanol for LC/MS analyses.
2.6. LC/MS Analyses
An 1100-LC system coupled to a 1946D-MS detector (Agilent Technologies, Inc., Palo Alto, CA, USA) equipped with an electrospray ionization interface was used to measure anandamide and 2-AG levels in select brain regions punched from a single hemisphere of each frozen sample. Lipids were separated using a XDB Eclipse C18 column (50 × 4.6 mm i.d., 1.8 mm, Zorbax), eluted with a gradient of methanol in water (from 75% to 85% in 2.5 min and then to 90% in 7.5 min) at a flow rate of 1.0 ml/min. Column temperature was kept at 40°C. MS detection was in the positive ionization mode, capillary voltage was set at 3 kV and fragmentor voltage was varied from 120V. N2 was used as drying gas at a flow rate of 13 liters/min and a temperature of 350 °C. Nebulizer pressure was set at 60 PSI. Quantifications were conducted using an isotope-dilution method, monitoring Na+ adducts of the molecular ions ([M+Na]+). The limits of quantitation were 0.08 pmol for anandamide and 0.4 pmol for 2-AG.
2.7. Receptor Binding and Autoradiography
Single hemisphere coronal brain tissue sections (14 µM thickness) were cryostat cut and mounted four sections per slide. Cannabinoid receptor binding was performed using [3H]CP55,940 (specific activity: 77.5 Ci/mmol; Research Triangle Institute, Research Triangle Park, NC, USA) as described previously [15,35,36]. Nonspecific binding was determined in the presence of 10 µM CP55,940. Briefly, binding was performed in cytomailers (3 h at 37 °C) in 50 mM Tris-HCl (pH 7.4) containing 5% bovine serum albumin and 5 nM [3H]CP55,940. Slides were washed (4 h at 0 °C) in the same buffer containing 1% bovine serum albumin, fixed in 0.5% formalin in 50 mM Tris-HCl (pH 7.4 at 25 °C) and blown dry. Sections were apposed to [3H]-sensitive film (Amersham Hyperfilm, GE Healthcare LifeSciences, Piscataway, NJ, USA) together with [3H] standards ([3H] microscales, Amersham, Arlington Heights, IL, USA) for 14 weeks. Images were captured using a Microtek ScanMaker 9800XL scanner.
2.8. Densitometry
Densitometry was performed using the public domain NIH Image software (U.S. National Institutes of Health, http://rsb.info.nih.gov/nih-image/) on a Macintosh computer (Macintosh, Cupertino, CA, USA). The mean densities for relevant brain regions of the scanned tissue images were calculated and converted to nCi/mg tissue wet weight based on a best-fit polynomial equation calibration formula that takes into account tissue equivalent values provided by Amersham. Brain areas were outlined using the rat brain atlas [33] as a guide. The nCi/mg values for each structure in each tissue section of each animal were calculated. Densitometric measurements were determined in 3–4 sections each for total and non-specific binding for each animal. Non-specific binding, determined in near adjacent sections, was subtracted from total binding values to obtain specific binding values used in data analysis. Receptor densities were calculated within surgery groups by averaging specific binding values across animals. Densitometric measurements were performed by an experimenter blinded to the experimental condition.
2.9. Statistical Analysis
For each animal, distance traveled was averaged for each three minute bin (over 30 min), generating ten time points that were used in the analyses. Data was analyzed using SPSS statistical software (version 15, Chicago, IL, USA). Because a hallmark feature of OBX is increased locomotor activity in response to a novel open field [24,28,29], planned comparisons (one-tailed Student’s t-tests) were used to compare distance traveled by drug-naive sham-operated and OBX animals during the first 3 min bin (novelty phase) following exposure to the novel open field with type of surgery as the between-subjects variable. Separate univariate analyses of variance (ANOVA) for each surgical group were employed to compare the effects of the between-subjects variable, drug treatment, on distance traveled during the novelty phase. Fisher’s Protected Least-Significant Difference (PLSD) post hoc tests were conducted in the case of a main effect of drug treatment. To investigate the effects of surgery (in drug-naive rats) or drug treatment on the habituation phase of the locomotor response to the open field (3–30 min), distance traveled during the first 3 min of exposure to the novel open field was used as the covariate in the analysis of covariance (ANCOVA) repeated measures analyses, with time serving as the repeated measure and drug treatment serving as the between-subjects variable; main effects in this study were further investigated by separate repeated measures ANCOVAs for each drug comparison. Significant interactions between time and drug treatment were corrected with the Huynh-Feldt factor and were further analyzed with Student’s t-tests at each time point. Based on previous data from our laboratory showing that OBX animals are insensitive to an inhibitor of the anandamide-hydrolyzing enzyme FAAH [24], anandamide levels in various brain regions were compared between surgical groups using planned comparisons (one-tailed Student’s t-tests). 2-AG levels were compared between surgical groups using two-tailed t-tests. Cannabinoid receptor densities in various brain regions were compared between surgical groups using Student’s t-tests (two-tailed). Relationships between variables were analyzed using two-tailed Pearson’s r correlation. P ≤ 0.05 was considered significant.
3. Results
3.1. Open Field Activity in Drug-naive Rats
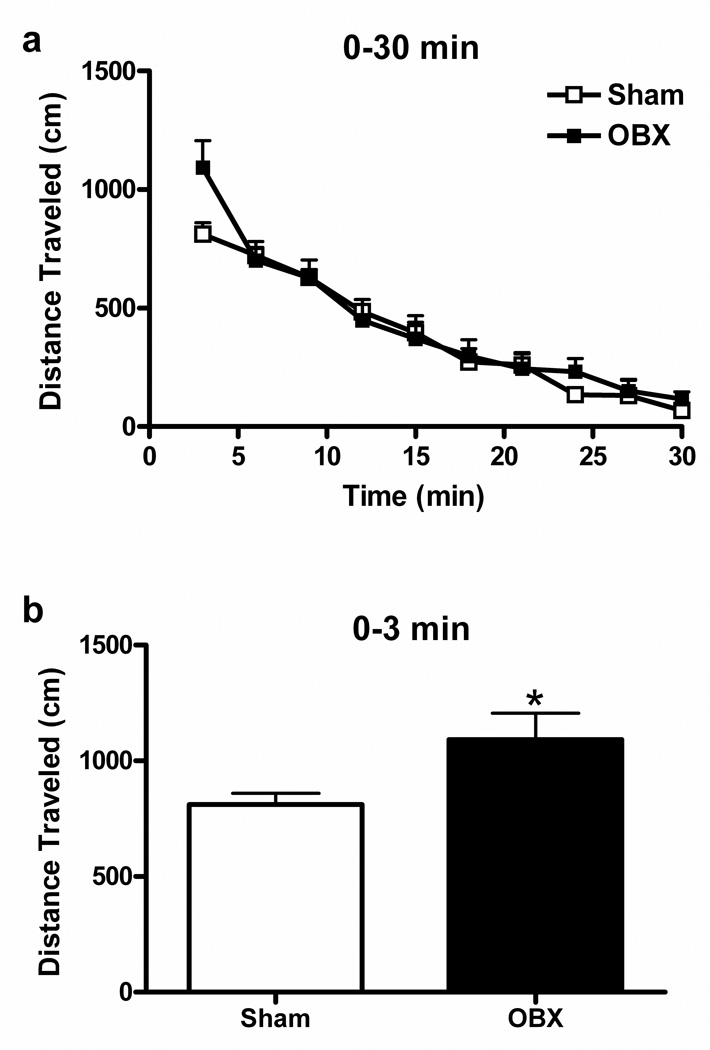
Exploratory behavior declined progressively over the 30 min observation interval following introduction of animals into the open field arena (F9, 162 = 61.84, P < 0.0001) (Figure 1a). OBX animals traveled greater cumulative distance than sham animals during the first three minutes of exposure to the novel open field (t18 = −2.28, P = 0.02, Student’s one-tailed t-test) (Figure 1b). By contrast, no difference in levels of exploratory behavior was observed between sham and OBX animals for the remainder of the observation period (3–30 min following introduction into the open field) (P = 0.847). Therefore, we divided the open field locomotor response into two phases for subsequent analyses: “novelty” (0–3 min post exposure to the novel open field) and “habituation” (3–30 min post exposure to the novel open field). Brains derived from these drug-naive animals were used to determine the impact of OBX on endocannabinoid levels and cannabinoid receptor densities in discrete brain regions.
Figure 1.
Locomotor responses elicited by exposure of drug-naive rats to a novel open field consist of novelty (0–3 min) and habituation (3–30 min) phases. (a) Distance traveled in sham-operated and OBX animals decreased over the 30 min exposure to a novel open field session. (b) OBX animals traveled greater distance than sham rats during the novelty phase (0–3 min) of the locomotor response to the open field (mean + SEM, n = 10 per group). *P < 0.05 compared to shams.
3.2. Control conditions
In each surgical group, distance traveled during the 30 min open field session was similar in drug-naive animals and animals that received vehicles. Therefore, these groups were pooled into a single “control” group for each surgical condition for subsequent analyses of drug effects.
3.3. CB1 Blockade with AM251and Rimonabant
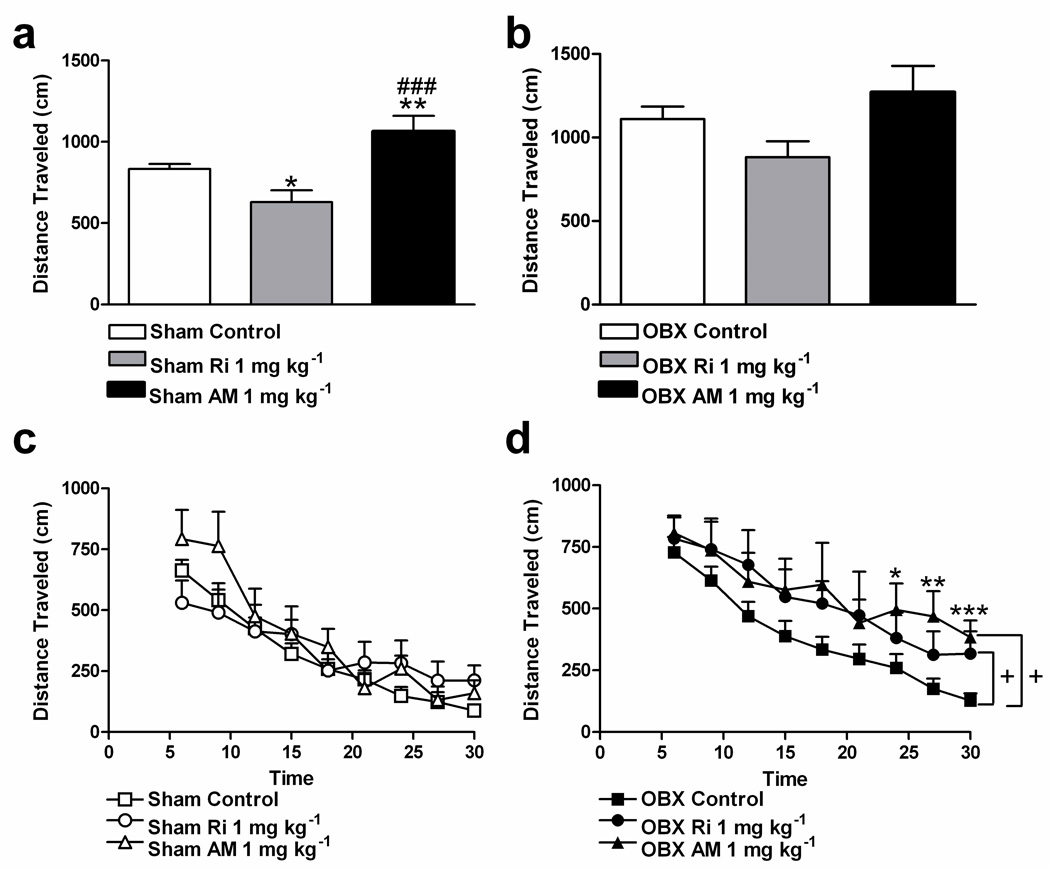
During the novelty phase of the open field locomotor response, OBX controls traveled a greater distance (1110.08 ± 75.39 cm) relative to sham controls (828.3 ± 34.77 cm) (t35 = −3.46, P = 0.001, Student’s one-tailed t-test). This represented a 34% increase in total distance traveled by OBX relative to sham-operated rats during the novelty phase. Drug effects were consequently analyzed in each surgical group separately. In sham rats, AM251 (1 mg kg−1 i.p.) increased distance traveled (F2, 34 = 10.52, P = 0.00027, P < 0.007 for post hoc comparison) relative to controls and rimonabant (1 mg kg−1 i.p.) during the novelty phase (Figure 2a). Also in sham animals, rimonabant (1 mg kg−1 i.p.) decreased distance traveled relative to control rats (P = 0.01 for post hoc comparison) (Figure 2a). Assessment of a subset of the activity meter data indicated that the unexpected rimonabant-induced decrease in distance traveled exhibited by shams during the novelty phase could not be attributed to a decrease in velocity; indeed, no difference in velocity was observed between sham animals in the rimonabant and control conditions (sham control: 31.95 ± 1.37 cm/s; sham Ri: 33.33 ± 1.84 cm/s, P = 0.55, two-tailed t-test, n = 7–9/group). By contrast, OBX rats were relatively insensitive to CB1 receptor blockade during this phase; neither AM251 nor rimonabant reliably altered distance traveled (P = 0.08) (Figure 2b).
Figure 2.
Blockade of CB1 receptors alters distance traveled during the novelty phase in sham rats and prevents habituation of the locomotor response to the open field in OBX rats. (a) In sham animals, AM251 (1 mg kg−1 i.p.) increased and rimonabant (1 mg kg−1 i.p.) decreased distance traveled during the novelty phase (0–3 min; ANOVA, Fisher’s PLSD post hoc applied to main effects). (b) In OBX rats, neither AM251 (1 mg kg−1 i.p.) nor rimonabant (1 mg kg−1 i.p.) reliably altered distance traveled during the novelty phase. (c) In sham animals, neither AM251 (1 mg kg−1 i.p.) nor rimonabant (1 mg kg−1 i.p.) affected distance traveled during the habituation phase (3–30 min) of the open field locomotor response. (d) In OBX rats, both antagonists increased distance traveled during the habituation phase of the open field locomotor response (separate ANCOVA repeated measures for rimonabant and AM251 versus control, Student’s two-tailed t-test post hoc comparisons applied to time x AM251 interaction with Huynh-Feldt correction). Results are the mean + SEM, n = 18–21 per group for sham and OBX controls, n = 7–9 per group for experimental groups; +, P < 0.05 relative to control, *P < 0.05, **P < 0.01, ***P < 0.001 relative to control, ###P < 0.001 versus rimonabant; Ri, rimonabant; AM, AM251.
The impact of CB1 receptor blockade on the habituation phase of the open field locomotor response (3–30 min) was analyzed in each surgical group separately. Distance traveled during the novelty phase (0–3 min) was used as a covariate in these analyses. In sham rats, neither AM251 nor rimonabant affected the habituation phase of the locomotor response to the open field (P = 0.36, Figure 2c). In OBX rats, CB1 blockade increased distance traveled relative to control conditions during the habituation phase (F2, 30 = 3.43, P = 0.046). Both AM251 (1 mg kg−1) (F1, 22 = 5.21, P = 0.03) and rimonabant (1 mg kg−1) (F1, 24 = 6.59, P = 0.02) increased distance traveled throughout the entire habituation phase in OBX rats. AM251 maximally increased distance traveled during the last 6 min of the habituation phase respectively (F8,176 = 2.22, Huynh-Feldt correction, P = 0.04; P < 0.05 for each post hoc comparison; Figure 2d).
3.4. LC/MS Analyses
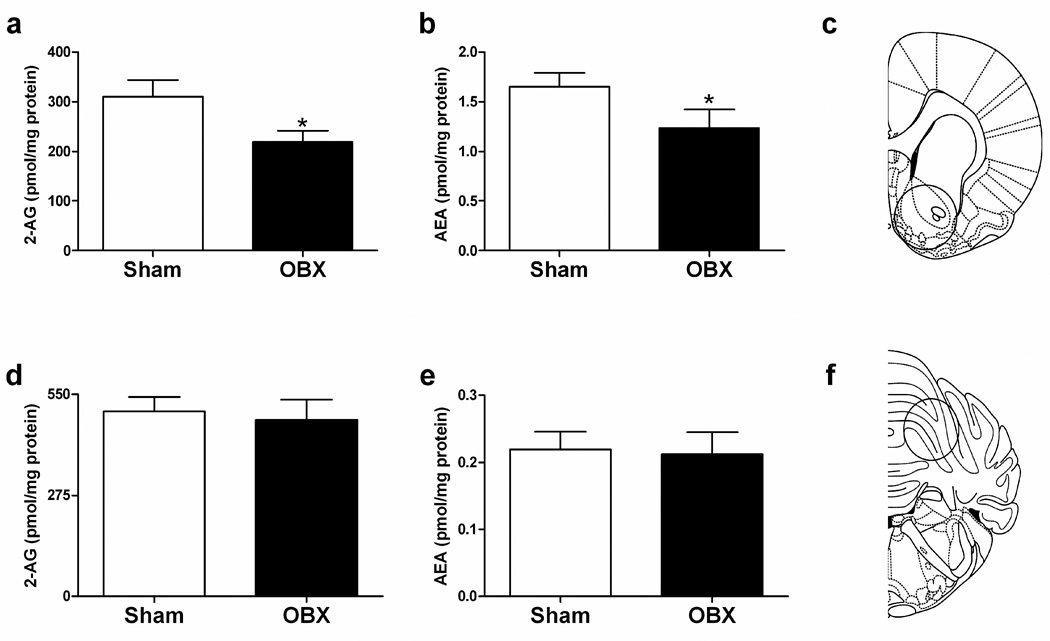
2-AG (t14 = 2.16, P = 0.048, two-tailed t-test) and anandamide (t14 = 1.81, P = 0.05, one-tailed t-test) levels were decreased in the ventral striatum of OBX relative to sham-operated groups (Figure 3a–c). A trend towards decreased anandamide (P = 0.09, one-tailed t-test) content in the amygdala was also observed in OBX relative to sham-operated rats (Table 1). Endocannabinoid levels in the cerebellum, piriform cortex, and hippocampus were not altered by olfactory bulbectomy (P > 0.23 for all comparisons) (Table 1, Figure 3d–f).
Figure 3.
Endocannabinoid content in sham-operated and OBX rats. (a) 2-AG and (b) anandamide levels are decreased in the ventral striatum of OBX rats relative to shams. (c) Approximate location of punch and relative punch size (circle) of ventral striatal tissue used in LC/MS analyses. (d) 2-AG and (e) anandamide levels were not altered by olfactory bulbectomy in the cerebellum. (f) Approximate location of punch and relative punch size (circle) of cerebellar tissue used in LC/MS analyses. Schematics adapted from [69]. Mean + SEM (n = 7–9 per group). *P < 0.05 relative to sham. AEA, anandamide.
Table 1.
Endocannabinoid content measured in brain regions of sham-operated and OBX rats
| Brain Area | 2-AG | AEA | ||
|---|---|---|---|---|
| Sham | OBX | Sham | OBX | |
| Hippocampus | 286.46 ± 31.99 | 312.69 ± 41.88 | 11.52 ± 1.31 | 9.49 ± 0.97 |
| Amygdala | 396.77 ± 32.96 | 324.82 ± 31.03 | 0.681 ± 0.09 | 0.532 ± 0.05 |
| Piriform Cortex | 545.00 ± 68.36 | 625.98 ± 99.95 | 2.17 ± 0.39 | 2.23 ± 0.24 |
Mean pmol/mg protein ± SEM shown (n = 6 −8 per group); AEA, anandamide.
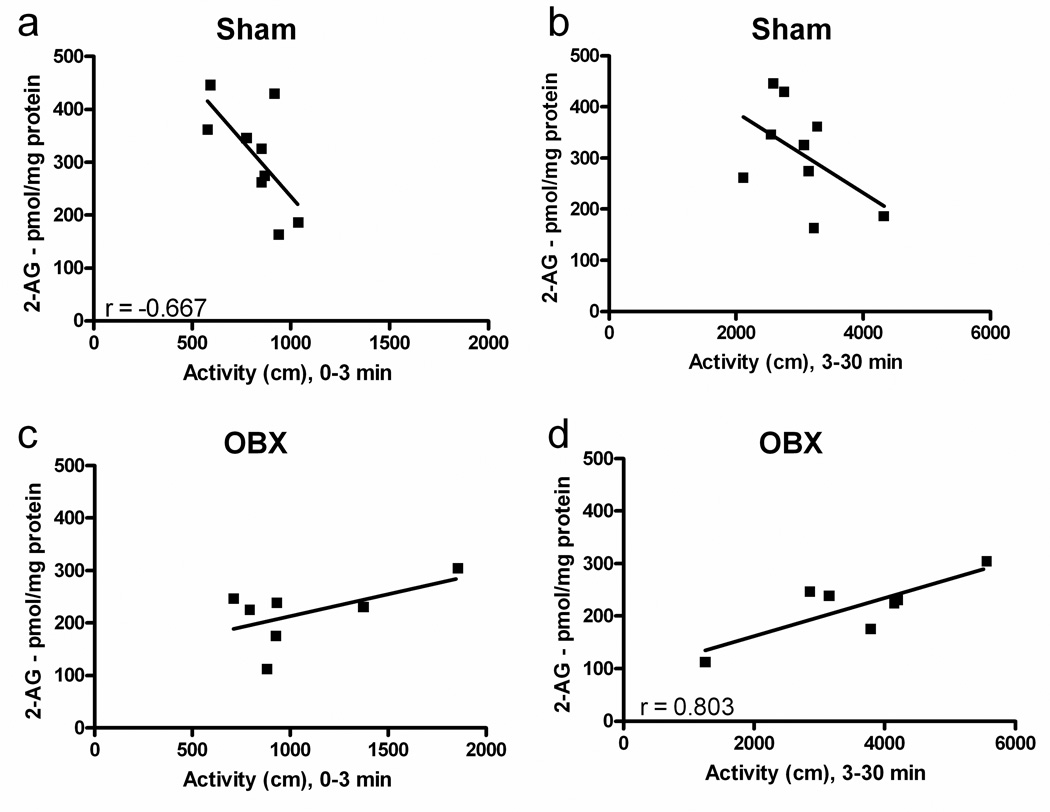
In sham-operated rats, distance traveled during the novelty phase (0–3 min) of the open field locomotor response was negatively correlated with 2-AG levels in the ventral striatum (r92 = 0.45, P = 0.05) (Figure 4a). Endocannabinoid levels did not correlate (P = 0.84 for 2-AG and P = 0.974 for anandamide) with locomotor activity observed during the novelty phase in OBX rats (Figure 4c). In OBX rats, 2-AG levels were positively correlated with distance traveled during the habituation phase (3–30 min after exposure to novelty) (r72 = 0.64, P = 0.03) (Figure 4d). Endocannabinoid levels in sham-operated rats did not correlate (P = 0.17 for 2-AG and P = 0.12 for anandamide) with locomotor activation during the habituation phase (Figure 4b). Moreover, reliable correlations between anandamide and locomotor activity were not observed in either sham-operated or OBX rats at these behaviorally relevant time points (P > 0.12 for all analyses; data not shown).
Figure 4.
Endocannabinoid content is differentially correlated with distance traveled in sham-operated and OBX rats. In sham animals, 2-AG levels in the ventral striatum were (a) negatively correlated with distance traveled over the novelty phase (0–3 min) of the open field locomotor response (P = 0.05) but not in (c) OBX animals during this same time period. (b) In sham animals, endocannabinoid levels were not correlated with distance traveled over the habituation phase (3–30 min) but, in OBX animals, 2-AG content was (d) positively correlated with distance traveled during this same time period (P = 0.03). n = 7–9 per group. AEA, anandamide.
3.5. Receptor Binding and Autoradiography
OBX did not reliably alter cannabinoid receptor densities in any brain region analyzed (Table 2).
Table 2.
Cannabinoid receptor densities in sham-operated and OBX rats
| Brain region | Surgical Group |
|
|---|---|---|
| Sham | OBX | |
| Basal ganglia | ||
| Dorsolateral striatum | 9.69 ± 0.66 | 9.22 ± 1.54 |
| Dorsomedial striatum | 6.40 ± 0.36 | 5.70 ± 0.78 |
| Ventrolateral striatum | 8.36 ± 0.54 | 6.71 ± 1.00 |
| Ventromedial striatum | 6.50 ± 0.41 | 5.19 ± 0.73 |
| Nucleus accumbens | 5.93 ± 0.38 | 5.33 ± 0.72 |
| Olfactory tubercle | 4.14 ± 0.33 | 4.2 ± 0.51 |
| Caudate putamen | 3.84 ± 0.19 | 3.58 ± 0.26 |
| Lateral globus pallidus | 4.37 ± 0.41 | 3.87 ± 0.28 |
| Entopeduncular nucleus | 3.77 ± 0.61 | 3.42 ± 0.76 |
| Substantia nigra | 5.46 ± 0.32 | 5.72 ± 0.4 |
| Ventral tegmental area | 1.85 ± 0.22 | 1.51 ± 0.25 |
| Cerebral cortex | ||
| Rostral cingulate cortex | 5.48 ± 0.33 | 5.92 ± 0.42 |
| Anterior cingulate cortex | 2.66 ± 0.25 | 2.80 ± 0.11 |
| Posterior cingulate cortex | 2.39 ± 0.12 | 2.70 ± 0.17 |
| Piriform cortex | 1.86 ± 0.07 | 1.93 ± 0.16 |
| Entorhinal cortex | 2.93 ± 0.30 | 3.46 ± 0.20 |
| Amygdala | ||
| Central n. amygdala | 1.91 ± 0.12 | 1.89 ± 0.20 |
| Amygdaloid nucleus | 3.64 ± 0.36 | 3.09 ± 0.16 |
| Hippocampus | ||
| Dorsal formation | 3.21 ± 0.10 | 3.26 ± 0.16 |
| Dorsal CA1 | 4.29 ± 0.25 | 4.22 ± 0.22 |
| Dorsal CA2 | 5.13 ± 0.46 | 4.79 ± 0.33 |
| Dorsal CA3 | 5.02 ± 0.38 | 5.2 ± 0.27 |
| Dorsal dentate gyrus | 4.25 ± 0.36 | 3.73 ± 0.28 |
| Ventral formation | 3.67 ± 0.28 | 3.63 ± 0.20 |
| Ventral CA1 | 6.08 ± 0.49 | 6.00 ± 0.60 |
| Ventral CA2 | 5.65 ± 0.39 | 5.33 ± 0.26 |
| Ventral CA3 | 5.58 ± 0.58 | 4.66 ± 0.24 |
| Ventral dentate gyrus | 4.53 ± 0.30 | 4.34 ± 0.18 |
| Ventral subiculum | 4.41 ± 0.27 | 4.29 ± 0.19 |
| Diencephalon | ||
| Ventromedial hypothalamus | 2.13 ± 0.15 | 2.06 ± 0.14 |
| Arcuate nucleus | 1.40 ± 0.17 | 1.81 ± 0.23 |
| Brain stem | ||
| Periaqueductal gray | 2.82 ± 0.16 | 2.51 ± 0.28 |
| Superior colliculus | 2.49 ± 0.24 | 2.41 ± 0.21 |
Mean nCi/mg tissue wet weight ± SEM shown (Sham, n = 7 −10; OBX, n = 6–10 per structure)
4. Discussion
4.1. Summary of Study
OBX rats exhibit increased locomotor activity in response to a novel open field, a behavior attributed to hyperdopaminergic activity [20,23,37,38]. The OBX model has consequently been described as a model of dopaminergic dysfunction [20,21,23,26,39]. The modulatory role of endocannabinoids at the interface between glutamatergic, GABAergic and dopaminergic function was, therefore, investigated by comparing locomotor behavior, endocannabinoid levels and cannabinoid receptor densities in OBX and sham-operated rats. Our study is the first to demonstrate that OBX induces a selective depletion of endocannabinoid accumulation in the ventral striatum, a brain region deafferented by OBX. Moreover, a dysregulation of endocannabinoid signaling is specifically implicated in the hyperlocomotor response exhibited by OBX animals exposed to a novel open field.
4.2. Novelty and Habituation Phases of Exposure to a Novel Environment
In agreement with previous studies [28,29], OBX rats exhibited increased locomotor activity relative to sham rats following exposure to a novel open field. We verified and extended these observations by documenting that OBX rats exhibit this characteristic hyperlocomotor activity, under our experimental conditions, during the initial phase (0–3 min) of the open field locomotor response. OBX rats exhibit locomotor activity similar to that of shams during the remainder of the open field session (3–30 min). Thus, the locomotor response elicited by a 30-min exposure to a novel open field can be divided into a “novelty” phase (0–3 min), during which OBX rats exhibit hyperlocomotion relative to sham animals, and a “habituation” phase (3–30 min), during which OBX animals show activity levels comparable to those of sham-operated rats that decline steadily over time. These findings are in agreement with prior studies documenting that OBX-induced hyperlocomotor activity, similar in magnitude to that observed in our study, is restricted to the first few minutes of exposure to the open field [40,41]. The duration and magnitude of the hyperlocomotor response to novelty may vary due to open field conditions such as luminance and length of time that activity is monitored. In particular, Mar et al. [41] found that OBX rats habituated more quickly to the open field than sham-operated rats under low-luminance (40 W) conditions. By contrast, under high-luminance (100 W) conditions, OBX rats exhibited initially high locomotor reactivity but habituated similarly to sham-operated rats over a 5 min period. Interestingly, under our open field conditions, including luminance of 25 W, OBX rats exhibited a pattern of locomotor reactivity to the open field similar to the high luminance condition in the Mar et al. [41] study over 30 min. Thus, differences in the magnitude and duration of novelty-induced reactivity in OBX rats may be accounted for by differences in the testing apparatus, lighting conditions, length of time that activity is monitored and the method used to assess locomotor activity (i.e. distance traveled versus squares crossed). Regardless of differing experimental conditions, the hyperactive locomotor response to a novel open field is a hallmark symptom of the OBX rat (see [25] for review). Our results further support the conclusion that OBX-induced hyperactivity is a stress-related phenomenon that closely resembles psychomotor agitation, which is a prevalent symptom of schizophrenia and depression [26,42]. Thus, it is noteworthy that dysregulation in endocannabinoid signaling has been postulated to contribute to depression-like symptoms in the OBX rat and other animal models such as the forced swim-test, chronic mild and/or unpredictable stress and CB1-receptor knockout mice [43–48].
4.3. Olfactory Bulbectomy Induces Ventral Striatum-specific Changes in Endocannabinoid Function
Our studies provide direct evidence that endocannabinoid content is decreased in a brain region deafferented by OBX, the ventral striatum. OBX decreased 2-AG and anandamide content in the ventral striatum, but not in other brain regions (piriform cortex, amygdala) that are known to be deafferented by OBX. This observation may reflect OBX-induced glutamatergic deafferentation of the ventral striatum and consequent loss of 2-AG biosynthesis [49] and endocannabinoid release [50]. 2-AG biosynthesis is stimulated in postsynaptic neurons through activation of postsynaptic metabotropic glutamate receptor subtype 5 in corticostriatal brain slice cultures [49]. In the striatum, activation of group I metabotropic glutamate receptors stimulates the endocannabinoid system to produce both short- and long-term changes in synaptic strength [51]. A threshold level of neuronal firing is required for postsynaptic endocannabinoid release and subsequent long-term depression of glutamatergic and GABAergic synaptic activity in the striatum [50]. Modulation of endocannabinoid content by dopamine may be secondary to changes in glutamatergic transmission [19]. The hyperdopaminergic tone induced by OBX may, therefore, lead to the observed decrease in ventral striatal endocannabinoid content in OBX rats. Anandamide and 2-AG content in the limbic forebrain is suppressed by dopaminergic agonism and increased by dopaminergic antagonism [19]. These observations also suggest that endocannabinoid release may be regulated by dopamine receptor activity. Brain regions not innervated by the olfactory bulb (e.g. cerebellum) showed no change in endocannabinoid content following OBX. Importantly, other regions known to be deafferented by OBX also showed no changes in endocannabinoid content relative to sham-operated rats. These latter observations reinforce a critical role for endocannabinoids in the ventral striatum in response to a novel open field. In our studies, endocannabinoid content was measured from brains extracted immediately after the 30 min open field exposure. The OBX-induced decrease in endocannabinoid content may, therefore, be due to loss of synaptic activity-induced mobilization of 2-AG and anandamide and/or induction of hyperdopaminergic tone in the striatum. Conditional mutant mice lacking cortical glutamatergic CB1 receptors display increased novelty-induced behavioral inhibition (decreased interaction with and approach to novel object) [52]. By contrast, those lacking cortical GABAergic CB1 receptors display increased interaction and approach to a novel object [52]. In sham rats, 2-AG may suppress the behavioral response during the novelty phase by activating CB1 receptors on GABAergic terminals of medium spiny neurons of the ventral striatum. Future studies should determine whether OBX preferentially alters endocannabinoid signaling at glutamatergic or GABAergic neurons in the striatum.
The present results suggest that endocannabinoid signaling in the ventral striatum may suppress novelty-induced hyperlocomotion in intact rats. Consistent with this interpretation, 2-AG levels are negatively correlated with locomotor activity in sham rats, suggesting that individual variability in normal endocannabinoid signaling predicts the locomotor response to a novel open field. In contrast to intact rats, endocannabinoid signaling in the ventral striatum of OBX rats is altered, as reflected by decreased endocannabinoid accumulation following open field exposure. It is, therefore, reasonable to propose that in the dysregulated state induced by OBX, the normal relationship between endocannabinoid signaling and locomotor activity no longer holds. This interpretation is supported by the finding of a modest positive correlation between 2-AG levels and locomotor activity in OBX rats. Although 2-AG levels are lower overall in the endocannabinoid-compromised OBX rats compared to controls, the positive correlation may reflect a compensatory response to the novelty stress or perhaps the hyperlocomotor behavior itself. Modest or delayed mobilization of endocannabinoids may thus occur in some OBX rats, possibly due to recruitment of cortical inputs beyond the olfactory bulb/ventral striatal circuit. However, such compensation does not achieve the levels required to suppress locomotor activity, thereby accounting for a lack of negative correlation between 2-AG and locomotion in OBX rats. Thus, in OBX rats, blockade of cannabinoid receptors with multiple CB1 antagonists increases locomotor activity only during the habituation phase of the open field locomotor response. By contrast, blockade of cannabinoid receptors with multiple CB1 antagonists had no effect on the locomotor response to the open field during the habituation phase in intact rats.
A closer examination of the different temporal patterns of associations between 2-AG levels and activity in sham and OBX rats provides further insight into the function of endocannabinoids in the ventral striatum. Specifically, in sham-operated, but not OBX animals, 2-AG content was negatively correlated with distance traveled during the novelty phase (0–3 min). In OBX, but not sham-operated animals, 2-AG content in the ventral striatum was positively correlated with distance traveled during the habituation phase (3–30 min). This correlational evidence indicates that the relationship between activity levels and 2-AG is altered by olfactory bulbectomy. It remains to be determined whether release of 2-AG is delayed or reduced in OBX rats relative to sham-operated rats in response to novelty. Future studies employing microdialysis should enable direct measurements of changes in 2-AG accumulation in ventral striatum of awake behaving animals during open field exposure.
In our study, relationships between activity and endocannabinoid levels were specific to 2-AG. Anandamide content did not correlate with activity levels in either sham-operated or OBX rats at these behaviorally relevant time points. Recent studies by our group also demonstrate that OBX animals are relatively insensitive to acute and chronic effects of URB597, an inhibitor of the anandamide-hydrolyzing enzyme FAAH [24]. Thus, FAAH inhibition may be unable to reinstate anandamide levels in endocannabinoid-compromised OBX rats to levels observed in sham-operated rats. Further work is necessary to determine whether inhibition of 2-AG deactivation in the ventral striatum is able to suppress hyperlocomotion induced by OBX. Examination of the impact of OBX on the levels and activity of enzymes that degrade anandamide and 2-AG (i.e. FAAH and monoacylglycerol lipase, respectively) would aid in further characterizing the dysregulation in the endocannabinoid signaling observed here. Finally, CB2 receptors may be functionally relevant to drug abuse and depression [53]. Given 2-AG’s high affinity for CB2 [3], a possible contribution of CB2 receptors to the locomotor response to novelty could be explored. Future studies are required to determine whether alterations in expression of CB2 receptors are also observed following OBX. Our findings, nonetheless, demonstrate that endocannabinoid signaling, and 2-AG signaling in particular, is dysregulated by OBX.
4.4. CB1 Blockade Differentially Affects Responsivity to Novelty in Sham-operated and OBX Rats
Differences between OBX and sham-operated rats, and between CB1 antagonist treatments, were revealed during the novelty phase of the open field locomotor response. As expected, OBX produced hyperactivity during the novelty phase of the open field response. The novelty phase in our study likely corresponds to the same initial interval (i.e. the first 10 min block following exposure to a novel open field) during which striatal glutamate levels are transiently elevated in OBX relative to sham-operated rats [54]. Furthermore, the novelty phase corresponds to the same time interval in which a negative correlation between locomotor activity and ventral striatal endocannabinoid content was observed in sham but not OBX animals. Thus, it is plausible that direct infusion of exogenous endocannabinoids into the ventral striatum would attenuate novelty-induced hyperactivity in OBX to sham-operated levels.
In sham rats, CB1 blockade selectively altered locomotor activity during the novelty phase without altering locomotor activity during the habituation phase. During the novelty phase, AM251 (1 mg kg−1 i.p.) increased, whereas rimonabant (1 mg kg−1 i.p.) decreased, distance traveled in sham-operated but not OBX rats. Rimonabant unexpectedly decreased distance traveled in sham rats during the novelty phase of the open field session without altering velocity of travel. Comparable doses of rimonabant are reported to block hypolocomotor effects of CB1 agonists without altering motor behavior when administered alone [32,55,56]. However, these studies did not report effects of antagonists specifically during the initial period of exposure to the open field (i.e. the novelty phase in our study), which make comparisons to the present results difficult. It is possible that rimonabant paradoxically inhibits exploratory behavior in the open field of sham-operated rats by producing an anxiogenic effect via blockade of endocannabinoid tone. Support for this hypothesis is derived from the observation that mice lacking CB1 receptors are prone to anxiety- and depression-like behaviors [47,57–59]. Thus, it is noteworthy that only low doses of rimonabant and AM251, which do not typically produce anxiogenic effects [31,60] or alter open arm exploratory behavior in the elevated plus maze [31,60], were employed in our study. Differences between effects of AM251 and rimonabant may also reflect differences in cannabinoid receptor binding properties or off-target effects (for review, see [61]). For example, rimonabant and AM251 may act as agonists at a putative novel cannabinoid receptor, the G protein-coupled orphan receptor GPR55, with rimonabant being the more potent of the two [62]. This receptor is mainly localized to the striatum [63] and thus represents a possible off-target site capable of mediating unexpected effects of rimonabant on locomotor activity. Consistent with this hypothesis, opposing effects of AM251 and rimonabant on locomotor activity have been reported previously; AM251 attenuated, whereas rimonabant facilitated, amphetamine sensitization in mice [64,65]. Finally, it is particularly noteworthy that rimonabant partially antagonizes, whereas AM251 fully antagonizes, the inhibition of release and uptake of striatal glutamate by cannabinoid agonists [18]. Mechanisms underlying differences observed in effects of rimonabant and AM251 on locomotor activity during the novelty phase warrant further investigation.
OBX and sham-operated rats exhibited similar levels of locomotor activity during the habituation phase of the open field locomotor response, but differed markedly in their responses to CB1 antagonists. Blockade of endocannabinoid actions with CB1 antagonists produced a hyperactive locomotor state in OBX rats specifically during the habituation phase. In OBX rats, rimonabant and AM251 may compete more effectively for CB1 receptors in the diminished endocannabinoid environment of the ventral striatum to alter locomotor activity. CB1 antagonists may block behavioral effects associated with delayed endocannabinoid mobilization in OBX rats, thereby producing a hyperlocomotor state that is manifest as a failure of acute habituation of the locomotor response to the open field. Thus, when both endocannabinoid mobilization and signaling is impaired or blocked, a failure of stress habituation may be observed (for review see [66]). Further studies are required to determine whether the pattern of changes in locomotor behavior produced by systemic administration of antagonists is mimicked by local injection of antagonists in the ventral striatum. The ability of the FAAH inhibitor URB597 to exert CB1-mediated anxiolytic effects requires saliently aversive experimental conditions, including shifting illumination intensity and a novel experimental environment; constant light illumination and habituation to the experimental environment diminishes this effect [67]. Thus, sensitivity induced by OBX, combined with exposure to the novel open field, may induce an aversive state that delays CB1-sensitive endocannabinoid release until after the novelty phase.
4.5. Olfactory Bulbectomy as a Model of Loss of Endocannabinoid Function
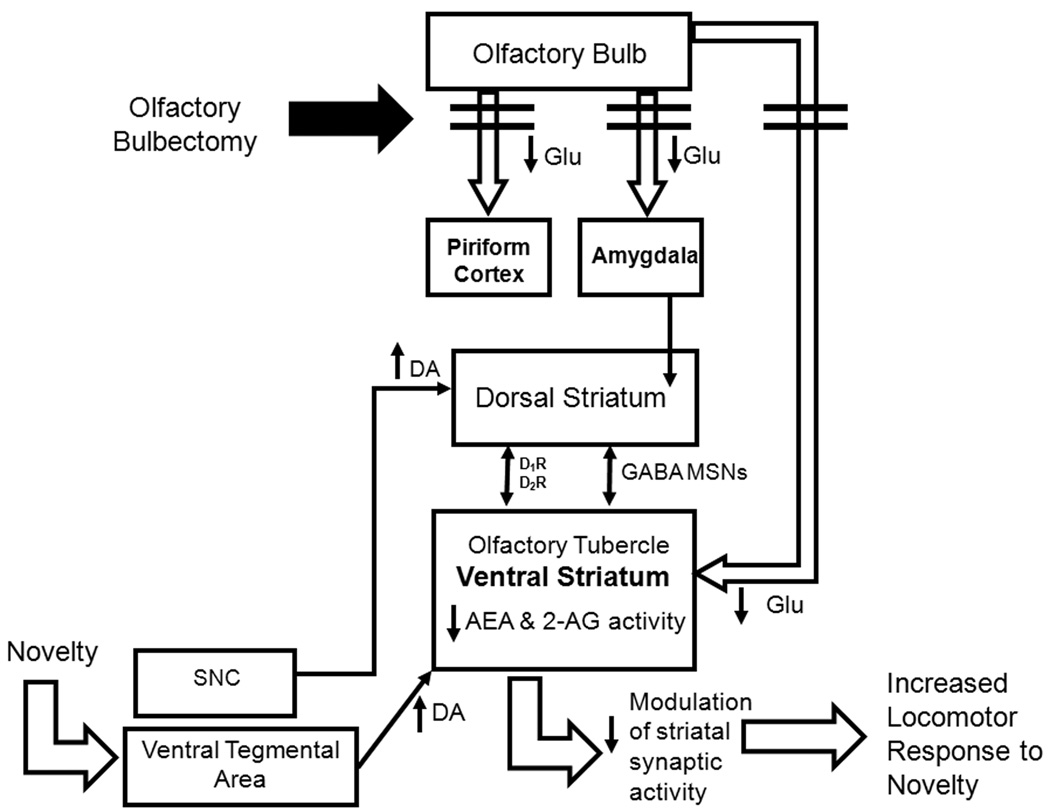
In a recent study, OBX increased cannabinoid receptor density and signaling in the prefrontal cortex and central-medial amygdala [48]. These effects were attenuated by chronic administration of the selective serotonin reuptake inhibitor fluoxetine which also normalized hyperactivity in the open field relative to sham-operated rats [48]. In our study, decreases in endocannabinoid content were detected in OBX relative to sham animals under conditions in which upregulations of cannabinoid receptors were not observed. Differences in the open field locomotor response of subjects evaluated in binding studies, the level of section and the postsurgical delay evaluated may contribute to differences between the studies [48]. In this latter study, acute administration of the cannabinoid agonist Δ9-tetrahydrocannabinol attenuated hyperlocomotor activity in the open field in OBX rats to sham levels [48]. These latter observations are consistent with the increase in locomotor activity induced by the CB1 antagonist AM251 in sham animals in our study. These observations, taken together, support the hypothesis that endocannabinoids, rather than being released tonically to regulate locomotor activity, are released on demand in a stimulation-contingent fashion, possibly in response to dopamine release triggered by exposure to a novel environment. A theoretical model is proposed in Figure 5 to account for our findings and illustrate our hypothesis that OBX disrupts glutamatergic innervation of the ventral striatum, which in turn decreases endocannabinoid mobilization and modulation of synaptic activity in this brain region. The consequent behavioral phenotype is one of locomotor hyperactivity in response to novelty (see Figure 5).
Figure 5.
Hypothetical model showing impact of olfactory bulbectomy on endocannabinoid levels and locomotor behavior. Olfactory bulbectomy disrupts glutamatergic projections to limbic areas including the piriform cortex, amygdala and olfactory tubercle of the ventral striatum. We hypothesize that exposure to a novel environment increases dopaminergic input into the striatum. In sham animals, the endocannabinoid system is intact and endocannabinoids are mobilized in the ventral striatum to act at CB1 receptors localized to glutamatergic terminals (derived from the hippocampus and cingulate cortex) and GABAergic terminals (localized to striatal medium spiny projection neurons) to modulate behavioral responsivity in response to this increase in dopaminergic transmission. However, this system is dysfunctional (i.e. low endocannabinoid content in the ventral striatum) in OBX rats due to deafferentation of glutamatergic inputs to the ventral striatum. The endocannabinoid system is unable to efficiently modulate dopaminergic responsivity in OBX animals, resulting in increased locomotor response to the novel environment.
Our data extend the results of electrophysiological and pharmacological studies which document a role for endocannabinoids in modulating basal ganglia responsivity [17,18,50]. Our results suggest that the hyperlocomotor response elicited by exposure of OBX rats to an open field specifically involves endocannabinoid dysfunction in brain regions implicated in regulating responsivity to stimuli such as novelty that enhance dopaminergic transmission [68]. The endocannabinoid signaling system thus represents a possible pharmacological target for the treatment of disorders involving dopaminergic dysfunction such as depression and schizophrenia.
Acknowledgments
Supported by DA021644, DA022478, DA022702 (to A.G.H. and D.P.) and an ARCS Foundation Scholarship and University of Georgia Dissertation Completion Assistantship (to S.A.E.).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide
- ANCOVA
analysis of covariance
- ANOVA
analysis of variance
- AP
anterior-posterior to bregma
- CB1
type 1 cannabinoid receptor
- DV
dorsal-ventral to bregma
- FAAH
fatty-acid amide hydrolase
- GABA
gamma-aminobutyric acid
- i.p.
intraperitoneal or intraperitoneally
- LC/MS
liquid chromatography-mass spectrometry
- ML
medial-lateral to bregma
- OBX
olfactory bulbectomy/bulbectomized
- Fisher’s PLSD
Protected Least-Significant Difference
Footnotes
Disclosure/Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakur GA, Tichkule R, Bajaj S, Makriyannis A. Latest advances in cannabinoid receptor agonists. Expert Opin Ther Pat. 2009;19:1647–1673. doi: 10.1517/13543770903436505. [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 4.Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 5.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 8.Piomelli D. The endocannabinoid system: A drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- 9.Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 12.Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 13.Mora F, Segovia G, Del Arco A. Glutamate-dopamine-GABA interactions in the aging basal ganglia. Brain Res Rev. 2008;58:340–353. doi: 10.1016/j.brainresrev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 15.Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohmann AG, Herkenham M. Localization of cannabinoid CB(1) receptor mRNA in neuronal subpopulations of rat striatum: A double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlagh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: A combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- 20.Gilad GM, Reis DJ. Collateral sprouting in central mesolimbic dopamine neurons: Biochemical and immunocytochemical evidence of changes in the activity and distrubution of tyrosine hydroxylase in terminal fields and in cell bodies of A10 neurons. Brain Res. 1979;160:17–26. doi: 10.1016/0006-8993(79)90597-3. [DOI] [PubMed] [Google Scholar]
- 21.Lingham RB, Gottesfeld Z. Deafferentation elicits increased dopamine-sensitive adenylate cyclase and receptor binding in the olfactory tubercle. J Neurosci. 1986;6:2208–2214. doi: 10.1523/JNEUROSCI.06-08-02208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers RA, Sheehan T, Taylor JR. Locomotor sensitization to cocaine in rats with olfactory bulbectomy. Synapse. 2004;52:167–175. doi: 10.1002/syn.20017. [DOI] [PubMed] [Google Scholar]
- 23.Masini CV, Holmes PV, Freeman KG, Maki AC, Edwards GL. Dopamine overflow is increased in olfactory bulbectomized rats: An in vivo microdialysis study. Physiol Behav. 2004;81:111–119. doi: 10.1016/j.physbeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Eisenstein SA, Holmes PV, Hohmann AG. Endocannabinoid modulation of amphetamine sensitization is disrupted in a rodent model of lesion-induced dopamine dysregulation. Synapse. 2009;63:941–950. doi: 10.1002/syn.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 26.Primeaux SD, Wilson MA, Wilson SP, Guth AN, Lelutiu NB, Holmes PV. Herpes virus-mediated preproenkephalin gene transfer in the ventral striatum mimics behavioral changes produced by olfactory bulbectomy in rats. Brain Res. 2003;988:43–55. doi: 10.1016/s0006-8993(03)03337-7. [DOI] [PubMed] [Google Scholar]
- 27.Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Klein D, Brown TS. Exploratory behavior and spontaneous alternation in blind and anosmic rats. J Comp Physiol Psychol. 1969;68:107–110. doi: 10.1037/h0027657. [DOI] [PubMed] [Google Scholar]
- 29.van Riezen H, Leonard BE. Effects of psychotropic drugs on the behavior and neurochemistry of olfactory bulbectomized rats. Pharmacol Ther. 1990;47:21–34. doi: 10.1016/0163-7258(90)90043-2. [DOI] [PubMed] [Google Scholar]
- 30.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 31.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: Further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 32.Jarbe TU, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Delta 9-THC and the CB1 receptor antagonist SR-141716 in rats: Open-field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. 1 v. San Diego: Academic Press; 1998. (unpaged). [Google Scholar]
- 34.Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2755–2767. doi: 10.1016/j.jchromb.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- 36.Hohmann AG, Herkenham M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci Lett. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- 37.Saigusa T, Tuinstra T, Koshikawa N, Cools AR. High and low responders to novelty: Effects of a catecholamine synthesis inhibitor on novelty-induced changes in behaviour and release of accumbal dopamine. Neuroscience. 1999;88:1153–1163. doi: 10.1016/s0306-4522(98)00275-9. [DOI] [PubMed] [Google Scholar]
- 38.Verheij MM, de Mulder EL, De Leonibus E, van Loo KM, Cools AR. Rats that differentially respond to cocaine differ in their dopaminergic storage capacity of the nucleus accumbens. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes PV. Olfactory bulbectomy increases prepro-enkephalin mRNA levels in the ventral striatum in rats. Neuropeptides. 1999;33:206–211. doi: 10.1054/npep.1999.0031. [DOI] [PubMed] [Google Scholar]
- 40.Mar A, Spreekmeester E, Rochford J. Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology (Berl) 2000;150:52–60. doi: 10.1007/s002130000400. [DOI] [PubMed] [Google Scholar]
- 41.Mar A, Spreekmeester E, Rochford J. Fluoxetine-induced increases in open-field habituation in the olfactory bulbectomized rat depend on test aversiveness but not on anxiety. Pharmacol Biochem Behav. 2002;73:703–712. doi: 10.1016/s0091-3057(02)00881-x. [DOI] [PubMed] [Google Scholar]
- 42.Holmes PV. Rodent models of depression: Reexamining validity without anthropomorphic inference. Crit Rev Neurobiol. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- 43.Adamczyk P, Golda A, McCreary AC, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59:217–228. [PubMed] [Google Scholar]
- 44.Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Hill MN, Barr AM, Ho WS, Carrier EJ, Gorzalka BB, Hillard CJ. Electroconvulsive shock treatment differentially modulates cortical and subcortical endocannabinoid activity. J Neurochem. 2007;103:47–56. doi: 10.1111/j.1471-4159.2007.04688.x. [DOI] [PubMed] [Google Scholar]
- 46.Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maccarrone M, Valverde O, Barbaccia ML, Castane A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agro A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: Correlation with behaviour. Eur J Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Gaztelumendi A, Rojo ML, Pazos A, Diaz A. Altered CB receptor-signaling in prefrontal cortex from an animal model of depression is reversed by chronic fluoxetine. J Neurochem. 2009;108:1423–1433. doi: 10.1111/j.1471-4159.2009.05898.x. [DOI] [PubMed] [Google Scholar]
- 49.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- 50.Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41. doi: 10.1111/j.1460-9568.2008.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lafenetre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology. 2009;57:715–721. doi: 10.1016/j.neuropharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Liu QR, Chirwa SS, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci. 2008;1139:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho YJ, Chang YC, Liu TM, Tai MY, Wong CS, Tsai YF. Striatal glutamate release during novelty exposure-induced hyperactivity in olfactory bulbectomized rats. Neurosci Lett. 2000;287:117–120. doi: 10.1016/s0304-3940(00)01152-6. [DOI] [PubMed] [Google Scholar]
- 55.Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR, Rodriguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716a induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 56.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR141716a, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 57.Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 58.Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 59.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: Convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 60.Arevalo C, de Miguel R, Hernandez-Tristan R. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–131. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- 61.Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 62.Kapur A, Zhao P, Sharir H, Bai Y, Caron MG, Barak LS, Abood ME. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem. 2009;284:29817–29827. doi: 10.1074/jbc.M109.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, George SR, O'Dowd BF. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64:193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- 64.Thiemann G, Di Marzo V, Molleman A, Hasenohrl RU. The CB(1) cannabinoid receptor antagonist AM251 attenuates amphetamine-induced behavioural sensitization while causing monoamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 2008;89:384–391. doi: 10.1016/j.pbb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Thiemann G, van der Stelt M, Petrosino S, Molleman A, Di Marzo V, Hasenohrl RU. The role of the CB1 cannabinoid receptor and its endogenous ligands, anandamide and 2-arachidonoylglycerol, in amphetamine-induced behavioural sensitization. Behav Brain Res. 2008;187:289–296. doi: 10.1016/j.bbr.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress:Implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 67.Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sil'kis IG. The role of dopamine-dependent negative feedback in the hippocampus-basal ganglia-thalamus-hippocampus loop in the extinction of responses. Neurosci Behav Physiol. 2008;38:399–405. doi: 10.1007/s11055-008-0057-4. [DOI] [PubMed] [Google Scholar]
- 69.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. 1 v. Amsterdam; Boston: Elsevier Academic Press; 2005. (unpaged). [Google Scholar]