Abstract
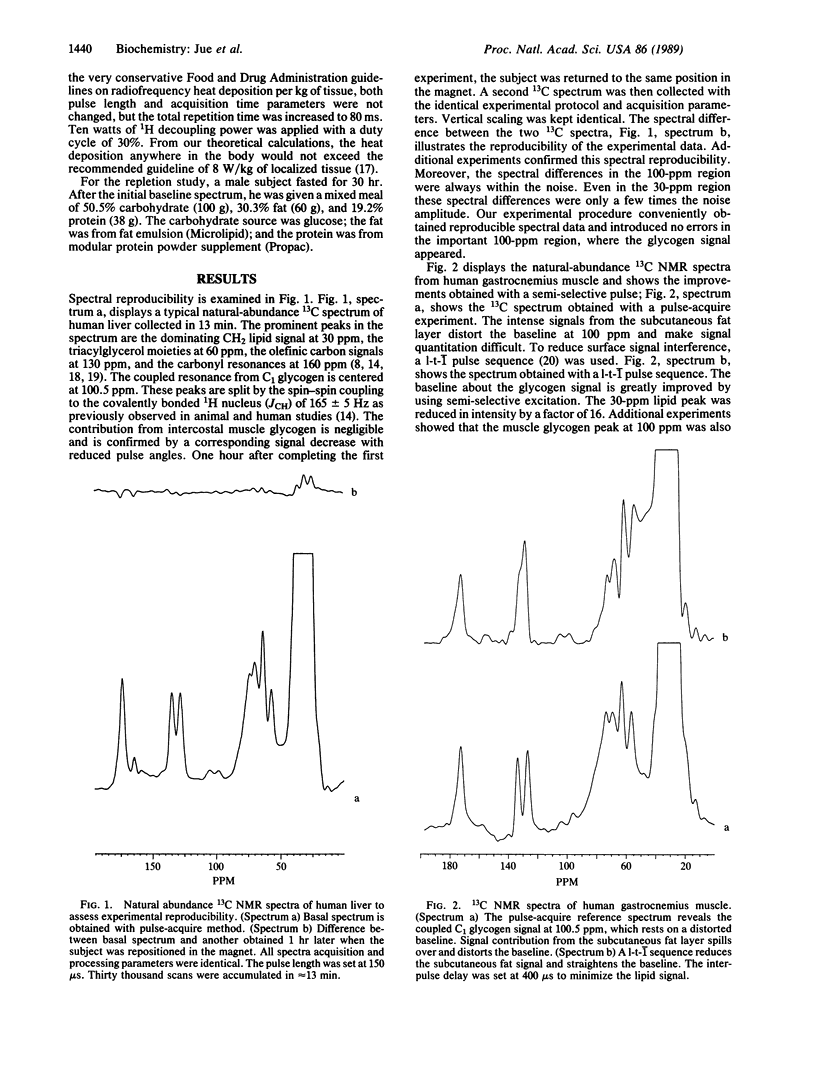
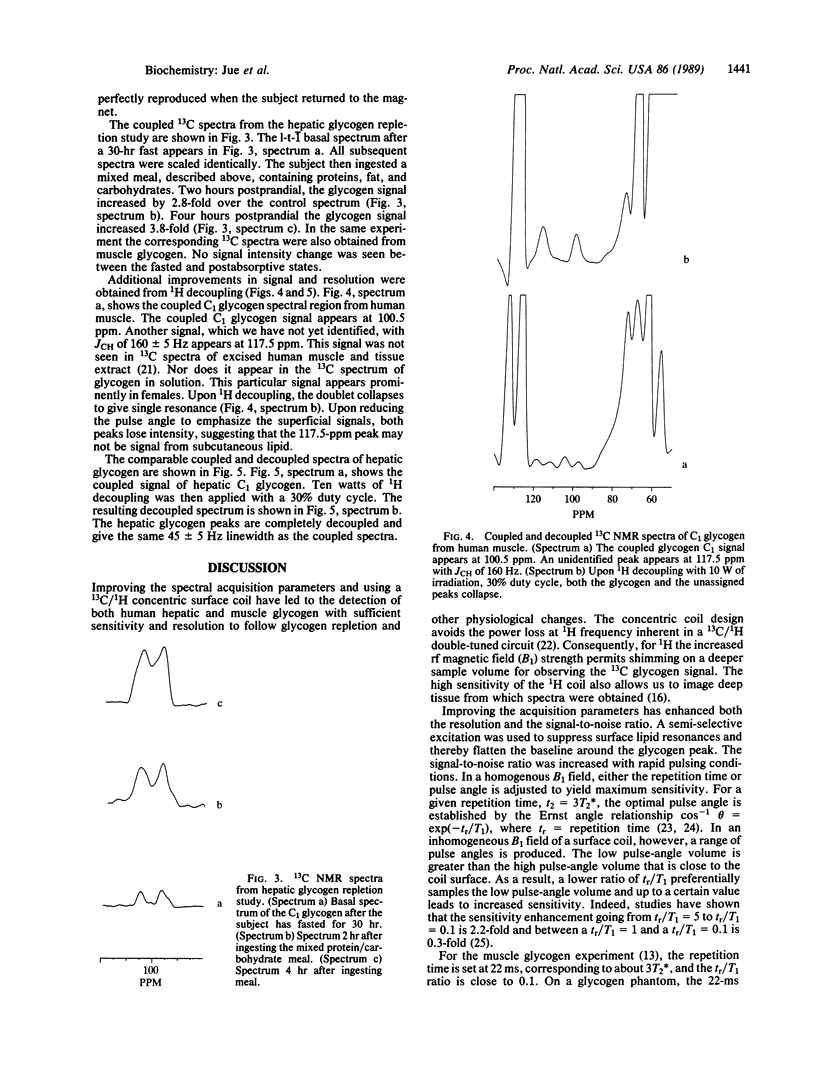
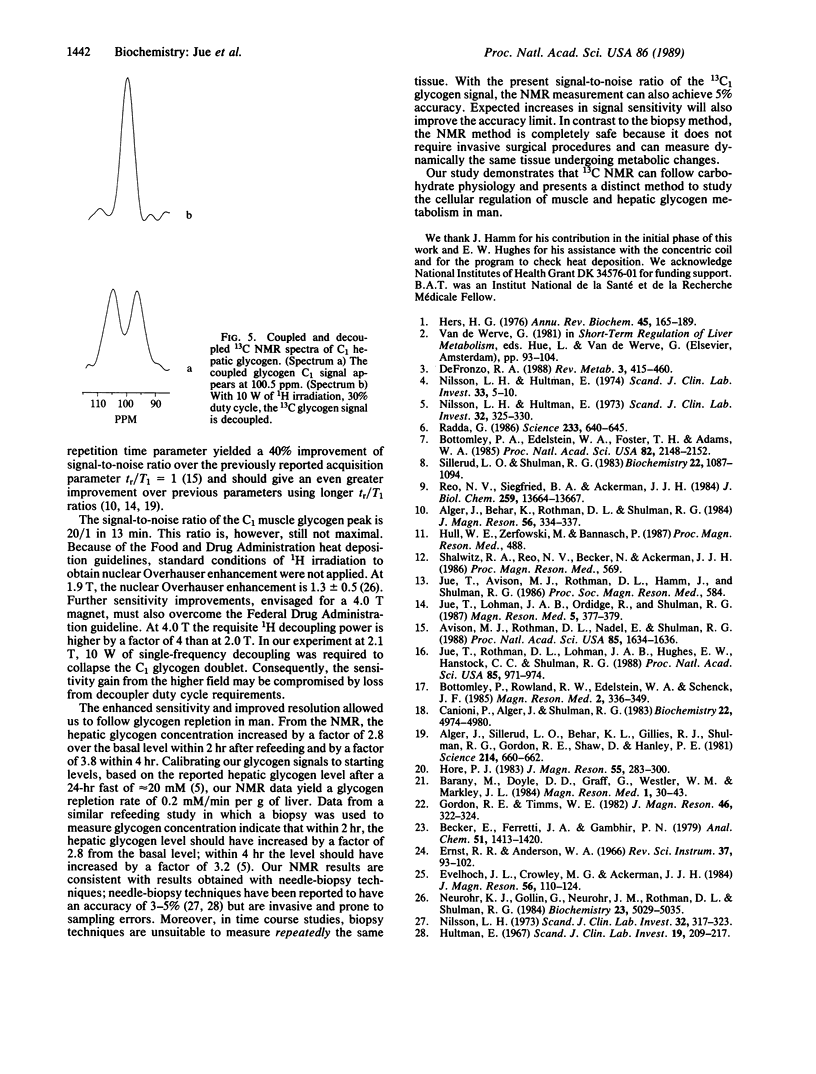
Optimizing the surface-coil design and spectral-acquisition parameters has lead to the observation of the 13C NMR natural abundance glycogen signal in man at 2.1 T. Both the human muscle and hepatic glycogen signals can be detected definitively with a time resolution of approximately equal to 13 min. A 1H/13C concentric surface coil was used. The 1H outer coil was 11 cm in diameter; the 13C inner coil was 8 cm in diameter. The coils were tuned to 89.3 MHz and 22.4 MHz, respectively. The 1H coil was used for optimizing field homogeneity (shimming) the magnet and for single-frequency decoupling of the C1 glycogen signal. Total power deposition from both the transmitter pulse and the continuous wave decoupling did not exceed the Food and Drug Administration guideline of 8 W/kg of tissue. Experiments were done for which healthy subjects returned to the magnets at different times for 13C NMR measurement. The spectral difference between experiments was within the noise in the C1 glycogen region. Because of the spectral reproducibility and the signal sensitivity, hepatic glycogen repletion can be followed. Four hours postprandial, hepatic glycogen increases by 3.8 times from the basal fasted state. The hepatic glycogen data correspond directly to previous biopsy results and support the use of 13C NMR as a noninvasive probe of human metabolism.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger J. R., Sillerud L. O., Behar K. L., Gillies R. J., Shulman R. G., Gordon R. E., Shae D., Hanley P. E. In vivo carbon-13 nuclear magnetic resonance studies of mammals. Science. 1981 Nov 6;214(4521):660–662. doi: 10.1126/science.7292005. [DOI] [PubMed] [Google Scholar]
- Avison M. J., Rothman D. L., Nadel E., Shulman R. G. Detection of human muscle glycogen by natural abundance 13C NMR. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1634–1636. doi: 10.1073/pnas.85.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Edelstein W. A., Foster T. H., Adams W. A. In vivo solvent-suppressed localized hydrogen nuclear magnetic resonance spectroscopy: a window to metabolism? Proc Natl Acad Sci U S A. 1985 Apr;82(7):2148–2152. doi: 10.1073/pnas.82.7.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley P. A., Redington R. W., Edelstein W. A., Schenck J. F. Estimating radiofrequency power deposition in body NMR imaging. Magn Reson Med. 1985 Aug;2(4):336–349. doi: 10.1002/mrm.1910020404. [DOI] [PubMed] [Google Scholar]
- Bárány M., Doyle D. D., Graff G., Westler W. M., Markley J. L. Natural abundance 13C NMR spectra of human muscle, normal and diseased. Magn Reson Med. 1984 Mar;1(1):30–43. doi: 10.1002/mrm.1910010106. [DOI] [PubMed] [Google Scholar]
- Canioni P., Alger J. R., Shulman R. G. Natural abundance Carbon-13 nuclear magnetic resonance spectroscopy of liver and adipose tissue of the living rat. Biochemistry. 1983 Oct 11;22(21):4974–4980. doi: 10.1021/bi00290a015. [DOI] [PubMed] [Google Scholar]
- Hultman E. Muscle glycogen in man determined in needle biopsy specimens: method and normal values. Scand J Clin Lab Invest. 1967;19(3):209–217. doi: 10.3109/00365516709090628. [DOI] [PubMed] [Google Scholar]
- Jue T., Lohman J. A., Ordidge R. J., Shulman R. G. Natural abundance 13C NMR spectrum of glycogen in humans. Magn Reson Med. 1987 Oct;5(4):377–379. doi: 10.1002/mrm.1910050410. [DOI] [PubMed] [Google Scholar]
- Jue T., Rothman D. L., Lohman J. A., Hughes E. W., Hanstock C. C., Shulman R. G. Surface coil localization of 31P NMR signals from orthotopic human kidney and liver. Proc Natl Acad Sci U S A. 1988 Feb;85(4):971–974. doi: 10.1073/pnas.85.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurohr K. J., Gollin G., Neurohr J. M., Rothman D. L., Shulman R. G. Carbon-13 nuclear magnetic resonance studies of myocardial glycogen metabolism in live guinea pigs. Biochemistry. 1984 Oct 9;23(21):5029–5035. doi: 10.1021/bi00316a031. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver glycogen in man--the effect of total starvation or a carbohydrate-poor diet followed by carbohydrate refeeding. Scand J Clin Lab Invest. 1973 Dec;32(4):325–330. doi: 10.3109/00365517309084355. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest. 1973 Dec;32(4):317–323. doi: 10.3109/00365517309084354. [DOI] [PubMed] [Google Scholar]
- Radda G. K. The use of NMR spectroscopy for the understanding of disease. Science. 1986 Aug 8;233(4764):640–645. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- Reo N. V., Siegfried B. A., Ackerman J. J. Direct observation of glycogenesis and glucagon-stimulated glycogenolysis in the rat liver in vivo by high-field carbon-13 surface coil NMR. J Biol Chem. 1984 Nov 25;259(22):13664–13667. [PubMed] [Google Scholar]
- Sillerud L. O., Shulman R. G. Structure and metabolism of mammalian liver glycogen monitored by carbon-13 nuclear magnetic resonance. Biochemistry. 1983 Mar 1;22(5):1087–1094. doi: 10.1021/bi00274a015. [DOI] [PubMed] [Google Scholar]



