Abstract
Interleukin-6 (IL-6) is necessary for cachexia in ApcMin/+ mice, but the mechanisms inducing this myofiber wasting have not been established. The purpose of this study was to examine gastrocnemius muscle wasting in the ApcMin/+ mouse and to determine IL-6 regulated mechanisms contributing to muscle loss. Gastrocnemius type IIB mean fiber cross-sectional area (CSA) from ApcMin/+ mice decreased 32% between 13- and 22-wks of age. ApcMin/+ mice lacking IL-6 did not have type IIB fiber atrophy, while over-expression of circulating IL-6 exacerbated the loss of type IIB fiber CSA in ApcMin/+ mice. Muscle Atrogin-I mRNA expression was induced at least 9-fold at 18- and 22-wks of age compared to 13-wk-old mice. Atrogin-I gene expression was also induced by over-expression of circulating IL-6. These data suggest that high circulating IL-6 levels induce type IIB fiber CSA loss in ApcMin/+ mice, and circulating IL-6 is sufficient to regulate Atrogin-I gene expression in cachectic mice.
Keywords: SKELETAL MUSCLE, CYTOKINE, INTERLEUKIN, INFLAMMATION, INTESTINE, COLON
INTRODUCTION
Patients suffering from cancer cachexia exhibit skeletal muscle wasting and the loss of adipose tissue. Gastrointestinal cancer patients are extremely susceptible to cachexia and can lose up to 30% of their original body weight [1, 2]. The muscle wasting condition is also associated with many other symptoms, such as chronic inflammation, fatigue, anemia, and metabolic disturbances [3-6]. Although many cachectic factors have been examined for the induction of muscle wasting, the inflammatory cytokine interleukin-6 (IL-6) is an important mediator of these processes. Elevating IL-6 levels in animals reduces body mass [7, 8], muscle mass [9, 10], and stimulates protein degradation [9, 11]. Conversely, inhibiting IL-6 action can attenuate muscle wasting [12-14]. The ApcMin/+ mouse is a genetic model of colorectal cancer and cachexia [15, 16]. High circulating IL-6 levels promote tumorgenesis, while ablation of IL-6 lessens the tumor burden in ApcMin/+ mice [17]. The changes in polyp burden induced with IL-6 are also directly correlated with skeletal muscle mass [17]. However, the muscle signaling pathways activated or repressed during IL-6-induced skeletal muscle wasting have not been defined in this model.
It has previously been shown that different muscle fiber types do not undergo wasting at the same rate. Fast-twitch muscles, such as the gastrocnemius muscle, are more susceptible to muscle wasting during cancer than are slow-twitch muscles, like the soleus [18-20]. The mouse gastrocnemius muscle consists of myofibers that differ in their oxidative and metabolic capacities. The mouse gastrocnemius muscle is fast-twitch with approximately 80% of the fibers classified as type IIB [21]. Fast-twitch type IIA fibers contain more mitochondria and demonstrate higher rates of oxidative metabolism compared to type IIB fibers, which have relatively few mitochondria and are predominately glycolytic. Peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) is a transcriptional coactivator that regulates mitochondrial biogenesis, glucose/fatty acid metabolism, and fiber type conversion to a more oxidative phenotype in skeletal muscle [22, 23]. In fact, an oxidative phenotype is actually a protective mechanism during cachexia [18, 19]. Mice that over-express PGC-1α during fasting and denervation-induced atrophy have an attenuation of skeletal muscle mass loss [18]. We have shown that the ApcMin/+ mouse gastrocnemius muscle undergoes significant wasting, while the soleus demonstrates myofiber regeneration with minimal myofiber area loss [16]. However, since the gastrocnemius muscle has a mixed phenotype, it is unknown whether type IIA and type IIB fibers atrophy at a similar rate and if IL-6 plays a role in this process.
The majority of intracellular skeletal muscle proteins are degraded by the ubiquitin-proteasome system and this is the main pathway contributing to skeletal muscle loss during cancer cachexia [24-28]. In the ATP-dependent ubiquitin-proteasome pathway, small ubiquitin proteins are attached to lysine residues by E3 ubiquitin ligases on proteins targeted for degradation by the 26S proteasome [25]. Recently, two muscle-specific E3 ubiquitin ligases, Atrogin-I/MAFBx and MuRF-I, have been identified as important mediators of skeletal muscle loss [29, 30]. Atrogin-I and MuRF-I gene expression are increased in several cell culture and animal models of cachexia [31-33]. Likewise, depletion of the MuRF proteins or Atrogin-1 in cells or animals result in hypertrophy and also confer resistance to atrophy [34, 35]. Certain cytokines, such as TNF-α and IL-6 levels can also increase markers of the ubiquitin-proteasome pathway, such as E3 ubiquitin ligase gene expression and proteasome activity, both in vivo [9, 11] and in vitro [24, 36]. While IL-6 appears to be an important mediator of cachexia, it is currently unknown if IL-6 is can induce Atrogin-I or MuRF-I during cancer cachexia.
Elevated circulating IL-6 levels during cachexia in the ApcMin/+ mouse are necessary for muscle mass loss, but the cellular events that lead to muscle mass loss have not been defined. The overall purpose of this study was to determine if the ApcMin/+ mouse gastrocnemius muscle undergoes wasting in a fiber-type specific manner and if IL-6 regulates this process. An additional purpose was to determine if activation of the ATP-dependent ubiquitin-proteasome pathway contributes to this atrophy. We hypothesized that type IIB fibers would undergo more myofiber atrophy than type IIA fibers, IL-6 would exacerbate type IIB myofiber loss, and this would coincide with activation of MuRF-1 and Atrogin-1 gene expression.
METHODS
Animals
Mice were originally purchased from Jackson Laboratories (Bar Harbor, ME) and breeding was maintained at the University of South Carolina's animal resource facility as previously described [16]. ApcMin/+ mice were group housed and were randomly assigned to be sacrificed at 13-wks-old (pre-cachexia; n=13), 18-wks-old (early-cachexia; n=11), and 22-wks-old (late-cachexia; n=7). To examine the loss of IL-6 a separate group of ApcMin/+ / IL-6-/- mice (n=8), ApcMin/+ mice (n=7), and C57BL/6 mice (n=5) were sacrificed at 26 weeks of age. To increase circulating IL-6 levels, ApcMin/+ mice (Control; n=8 and + IL-6; n=10) and C57BL/6 mice (Control; n=10 and + IL-6; n=10) were used for IL-6 over-expression experiments (See procedure below). The room was maintained on a 12:12 light:dark cycle with the light period starting at 0700. Mice were provided standard rodent chow (Harlan Teklad Rodent Diet, #8604, Madison, WI) and water ad libitum. Body weights and food intake were measured weekly. All animal experimentation was approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Grip strength and RotoRod performance
Combined hindlimb and forelimb rodent grip strength was measured with the Grip Tester (Columbus Instruments, Columbus, OH). Mice were placed with all 4 limbs on a metal grid mounted at a 45° angle connected to a force transducer. Mice were pulled by the tail until they let go of the grid. Each mouse went through a series of 2 sets of 5 repetitions of force measurements, with a 2-3 minute rest period between each set. The highest score from each set of 5 repetitions was averaged together for a maximum force measurement for each mouse.
Neuromuscular performance was assessed with the RotoRod (Columbus Instruments, Columbus, OH). The protocol consisted of a ramping protocol from 0 to 30 rpm over a period of 45 seconds. The protocol continued at 30 rpm from 45 s to 120 s. Each mouse performed the protocol twice, with each trial separated by a 2-3 minute rest period. The longest time of the 2 tests was recorded for each mouse. ApcMin/+ mice were traced for grip strength and RotoRod performance on a weekly basis from 18 to 22-weeks of age. Muscles in mice electroporated with IL-6 mice were tested prior to their first treatment and then every 2 weeks.
IL-6 over-expression
In vivo intramuscular electroporation of an IL-6 plasmid was used to increase circulating IL-6 levels in mice as previously described [17, 37]. The quadriceps muscle was used as a vessel to produce IL-6 and secrete it into circulation, and was not used for any analyses in the study. The gastrocnemius muscle used in the study was not subjected to electroporation. Briefly, mice were injected with 50 μg of the IL-6 plasmid driven by the CMV promoter, or empty control vector, into the quadriceps muscle. Briefly, mice were anesthetized with a 2% mixture of isoflurane and oxygen (1 L/min). The leg was shaved and a small incision was made over the quadriceps muscle. Fat was dissected away from the muscle and the plasmids were injected in a 50 μl volume of PBS. A series of eight 50 ms, 100 V pulses was used to promote uptake of the plasmid into myofibers, and then the incision was closed with a wound clip. Both Control and +IL-6 groups of all strains of mice received the appropriate plasmid starting at 16 weeks of age. Preliminary data demonstrated that circulating IL-6 levels can substantially decline 2 and 3 weeks following electroporation, and others have reported similar findings related to over-expression with this procedure [37]. To maintain circulating IL-6 levels, the entire plasmid injection and electroporation procedure was repeated at 2-wk intervals, alternating between the right and left quadriceps muscles. Mice were sacrificed after 4 weeks (ApcMin/+) or 10 weeks (C57BL/6) of IL-6 over-expression.
Plasma IL-6
Plasma IL-6 levels were measured with a mouse specific ELISA (Biosource, Carlsbad, CA) by taking blood samples under brief isoflurane anesthesia from the retroorbital eye sinus 7 days following each electroporation and at sacrifice to ensure validity of the procedure. The IL-6 level for each animal was averaged for all time points assessed over the experimental period. Plasma IL-6 levels were also determined in mice that were not electroporated at sacrifice.
Tissue collection
Mice were given a subcutaneous injection of ketamine/xylazine/acepromazine cocktail (1.4 ml/kg BW). Gastrocnemius muscles, soleus muscles, epididymal fat pad, spleen, and tibias were excised. The tissues were rinsed in PBS, snap frozen in liquid nitrogen, weighed, and stored at -80 °C until further analysis. Tibia length was measured as an indicator of animal body size. The small intestines were removed as described previously (32). Briefly, the intestines were dissected, cleared of mesentery adipose tissue, and fixed in 10% buffered formalin for 24 hours. Plasma was collected via the inferior vena cava with heparinized needles, stored on ice, centrifuged at 1,000 g for 10 min at 4 °C, and the plasma was stored at -80 °C until further analysis.
Polyp counts
To verify the tumor burden in the mice polyp counts were performed as previously described [38]. Briefly, formalin-fixed intestinal sections from all animals were rinsed in deionized water, stained in 0.1% methylene blue, and counted by a single investigator who was blinded to the treatments. Polyps were counted under a dissecting microscope, using tweezers to pick through the intestinal villi and identify polyps. Polyps were also categorized as large (>2 mm), medium (1-2 mm), or small (<1 mm) in the small and large intestine.
Gastrocnemius morphology
Sectioning of muscle and staining was performed the same as described previously [16]. Briefly, transverse sections (10 μm) were cut from the mid-belly of the medial gastrocnemius on a cryostat at -20°C. Myosin ATPase staining was performed on the sections to delineate the type IIA and type IIB fibers to measure fiber cross-sectional area. Digital images were taken from each section with a Nikon spot camera and fibers were traced with imaging software (Scion Image, Frederick, MD). Approximately 200 fibers per mouse were traced at a 200X magnification in a blinded fashion.
RNA isolation, cDNA synthesis, and real time PCR
RNA isolation, cDNA synthesis, and real-time PCR was performed as previously described [17], using reagents from Applied Biosystems (Foster City, CA). Quantitative real-time PCR analysis was carried out in 25 μl reactions consisting of 2x SYBR green PCR buffer (AmpliTaq Gold DNA Polymerase, Buffer, dNTP mix, AmpErase UNG, MgCl2), 0.1 μl cDNA, RNase-free water, and 60 nM of each primer. The sequence for the primers were as follows: MuRF-I Forward, 5’ – ATG AAC TTC ACG GTG GGT TT –3’; MuRF-I Reverse, 5’ – GGG ATT GCC ACA GAG GAT TA – 3’, Atrogin-I Forward, 5’ – TTC AGC AGC CTG AAC TAC GA – 3’; Atrogin-I Reverse, 5’ – GGC AGT CGA GAA GTC CAG TC – 3’, Cyclophilin Forward: 5’ – TGT GCC AGG GTG GTG ACT T – 3’; Cyclophilin Reverse, 5’ – TCA AAT TTC TCT CCG TAG ATG GAC TT – 3’. Samples were analyzed on an ABI 7300 Sequence Detection System. Reactions were incubated for 2 min at 50°C and 10 min at 95°C, followed by 40 cycles consisting of a 15-s denaturing step at 95°C and 1-min annealing/extending step at 60°C. Data were analyzed by ABI software using the cycle threshold (CT), which is the cycle number at which the fluorescence emission is midway between detection and saturation of the reaction. The 2-ΔΔ CT method [39] was used to determine changes in gene expression between treatment groups with the cyclophilin CT as the correction factor [17]. IL-6 mRNA was also measured as previously described [17].
Western blotting
Western blot analysis was performed as previously described [16]. Briefly, frozen gastrocnemius muscle was homogenized in Mueller buffer and protein concentration determined by the Bradford method [40]. Crude muscle homogenate (30-60 μg) was fractionated on 8%-12% SDS-polyacrylamide gels. Gels were transferred to PVDF membranes overnight. Membranes were Ponceau stained to verify equal loading of each gel. Membranes were blocked overnight in 5% milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T). Primary antibodies for phosphorylated STAT-3 (TYR 705) and for Atrogin-I were purchased from Cell Signaling (Danvers, MA) and ECM Biosciences (Versailles, KY), respectively. Antibodies were diluted 1:1000 to 1:2000 in 5% BSA in TBS-T followed by overnight incubation with membranes at 4 °C. Anti-rabbit IgG horseradish-peroxidase conjugated secondary antibodies (GE Healthcare Life Sciences, Piscataway, NJ) were incubated with the membranes at 1:2000 to 1:5000 dilutions for 2 hours in 5% milk in TBS-T. Enhanced chemiluminescence (ECL) (GE Healthcare Life Sciences, Piscataway, NJ) was used to visualize the antibody-antigen interactions. Film was digitally scanned and blots were quantified by densitometry using scientific imaging software (Scion Image, Frederick, MD). The Ponceau stained membranes were also digitally scanned and the 45 kDa actin bands were quantified by densitometry and used as a protein loading correction factor for each lane.
Statistical analysis
A repeated measures ANOVA was used to assess changes in grip strength and RotoRod performance. Chi-square analysis was used to determine shifts in gastrocnemius type IIA and type IIB fiber frequency distribution. A Pearson correlation was used to draw correlations between circulating IL-6, Atrogin-I mRNA, and gastrocnemius muscle mass. One-way ANOVAs or independent t-tests were used to determine significance for all other variables. Post-hoc analyses were performed with Student-Newman-Keuls methods. If the assumption of normality or equal variance failed, an ANOVA on ranks was used. Significance was set at P<0.05.
RESULTS
Time course of cachexia-general animal characteristics
Three cohorts of different aged ApcMin/+ mice were examined to characterize the overall wasting and inflammatory phenotype. There were no significant changes in body weight between 13-wk-old mice (pre-cachexia), 18-wk-old (early-cachexia), and 22-wk-old (late-cachexia) ApcMin/+ mice (Table 1; P=0.911). The lack of body weight change may reflect the increased inflammatory state in older animals. Spleen weight increased 2-3-fold between 13 to18 and 22-wks of age, demonstrating increased inflammation and splenomegaly associated with the diseased condition. Tibia length increased after 13 weeks of age as a function of maturation, regardless of adipose and skeletal muscle mass loss (Table 1; P=0.001). There was significant loss of lean and fat tissue over the time course. Epididymal fat pad weight was 78% lower in 22-wk-old (P=0.034) than in 13-wk-old mice (Table 1). We have determined that the loss of fat mass in other primary fat pads, such as the mesentery and retroperitoneal fat pads, also occurs in addition to epididymal fat pad mass in ApcMin/+ mice (data not shown).
Table 1.
Body weight, tibia length, and spleen weight in 13, 18, and 22-wk-old ApcMin/+ mice. Large polyps were greater or equal to 2 mm in diameter. Values are means ± SE.
| Age (weeks) | 13 | 18 | 22 |
|---|---|---|---|
| Body weight (g) | 23.4 ± 0.6 | 23.1 ± 1.1 | 22.6 ± 1.1 |
| Tibia length (mm) | 15.8 ± 0.1 | 16.6 ± 0.1* | 16.7 ± 0.1* |
| Epididymal fat pad (mg) | 214 ± 24 | 171 ± 56 | 47 ± 45* |
| Spleen weight (mg) | 167 ± 13 | 346 ± 37* | 484 ± 71* |
| Polyp number (#) | 105 ± 14 | 119 ± 17 | 102 ± 10 |
| Large polyp no. (#) | 7 ± 2 | 40 ± 8* | 56 ± 7* |
| Plasma IL-6 (pg/ml) | 3.8 ± 0.8 | 17.5 ± 5.6* | 12.5 ± 3.1* |
Signifies different from 13-wk-old.
Time course of cachexia-tumor burden
ApcMin/+ mice have an intestinal polyp burden that advances with age and is thought to plateau at approximately 13-15 weeks of age [41]. A greater intestinal polyp burden is associated with greater skeletal muscle mass loss in the ApcMin/+ mouse [17]. The polyp burden was determined in the current study in 13-wk-old, 18-wk-old, and 22-wk-old ApcMin/+ mice. The total number of polyps was not different between the 3 age groups (Table 1; P=0.695). However, older mice did exhibit more large polyps (>2 mm in diameter). Twenty-two-wk-old and 18-wk-old mice had 6-8 times as many large polyps compared to 13-wk-old mice (P<0.001). There were no differences in the number of small polyps (<1 mm in diameter) between the different age groups (data not shown). The increase in polyp size was associated with an approximately 3-4-fold increases in circulating IL-6 in 18 and 22-wk-old mice (Table 1; P=0.008).
Time course of cachexia-skeletal muscle mass loss
Skeletal muscle tissue weights were examined in ApcMin/+ mice during wasting. Soleus muscle mass did not change during the time course of cachexia between 13-wk-old (8 ± 1 mg), 18-wk-old (7 ± 0 mg), or 22-wk-old mice (8 ± 1 mg; P=0.609). The gastrocnemius muscle, predominately a fast oxidative and fast glycolytic muscle, was also examined for changes in skeletal muscle mass over time (Fig. 1a). Gastrocnemius muscle mass was 26% less in 22-wk-old ApcMin/+ mice (P=0.043) than in 13-wk-old mice.
Fig. 1.
Time course of gastrocnemius muscle wasting in ApcMin/+ mice. Gastrocnemius muscle mass (a), mean gastrocnemius fiber CSA of type IIA and type IIB fibers (b), frequency distribution for type IIB gastrocnemius fibers (c), and frequency distribution of type IIA fibers (d). Muscles were harvested from 13-wk-old (n=13), 18-wk-old (n=11), and 22-wk-old (n=7) ApcMin/+ mice. Values are means ± SE. *Signifies different from 13-wk-old mice.
Grip strength and RotoRod performance
To determine if the decrements in muscle mass over time were associated with changes in muscle performance, the combined forelimb and hindlimb grip strength and the RotoRod test were used. Coinciding with the decrease in gastrocnemius muscle size, combined forelimb and hindlimb rodent grip strength decreased 21% over time (Table 2; P=0.001). RotoRod performance did not change over the time course of cachexia (P=0.166). These data demonstrate that decreases in overall muscle strength are not due to impairment in neuromuscular coordination, but rather to decreases in muscle mass.
Table 2.
Combined forelimb and hindlimb grip strength and RotoRod performance in ApcMin/+ mice. Values are means ± SE.
| Age (weeks) | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|
| Grip strength (N) | 1.40 ± 0.06 | 1.31 ± 0.05 | 1.34 ± 0.07 | 1.25 ± 0.07 | 1.10 ± 0.05* |
| RotoRod (s) | 31.08 ± 2.60 | 32.13 ± 1.89 | 37.15 ± 1.82 | 36.55 ± 3.15 | 31.03 ± 2.32 |
Signifies different from 18 weeks.
Myofiber area
The medial gastrocnemius muscle was sectioned and myosin ATPase stained to determine fiber cross-sectional area in type IIA and type IIB fibers in 13-wk-old, 18-wk-old, and 22-wk-old ApcMin/+ mice. The mouse gastrocnemius muscle contains less than 1% of type I fibers [21]. Type I fiber area was not quantified due to the low number of these fibers on each section. Type IIB fiber cross-sectional area decreased 32% between 13 and 22-wks of age (Fig. 1b; P=0.027). There was also a shift in the frequency distribution of type IIB fiber size (Fig. 1c). The percentage of small type IIB fibers (<750 μm2) increased from 1% to 20% and the percentage of large type IIB fibers (>3000 μm2) decreased from 3% in to 0% in 13 and 22-wk-old mice (P<0.001). There was a trend for mean type IIA fiber cross-sectional area to decrease between 13 and 22-wks of age (P=0.062). While mean type IIA fiber CSA did not change, there was a shift in the type IIA fiber frequency distribution (Fig. 1d). The percentage of small type IIA fibers (<400 μm2) increased from 3% to 15% and the percentage of large type IIA fibers (>1200 μm2) decreased from 14% to 0% in 13 and 22-wk-old mice (P<0.001). These data demonstrate that both type IIA and IIB fibers undergo significant atrophy, but there is a preferential wasting of type IIB fibers.
IL-6, Atrogin-I, and MuRF-I gene expression
Since circulating IL-6 increases with age in the ApcMin/+ mouse and it is associated with wasting, we measured IL-6 gene expression in the gastrocnemius muscle. IL-6 mRNA did not change with age in the gastrocnemius muscle of ApcMin/+ mice (Fig. 2a; P=0.440), similar to our previous findings [17]. However, phosphorylated STAT-3 was higher in the gastrocnemius muscle of 22-wk-old mice compared to 13-wk-old mice (Fig. 2b). This indicates that IL-6 signaling was occurring in the gastrocnemius muscle, but it was not being driven by muscle-produced IL-6. To examine the ubiquitin-proteasome protein degradation pathway, real-time PCR was used to measure Atrogin-I and MuRF-I mRNA expression in the gastrocnemius muscle. MuRF-I mRNA expression did not change between the different age groups (Fig. 2c; P=0.871). Atrogin-I mRNA was induced early in the progression of cachexia in ApcMin/+ mice, increasing 10-fold in 18-wk-old mice, when compared to 13-wk-old mice (Fig. 2d). Atrogin-I mRNA remained increased 9-fold in 22-wk-old mice (P<0.001). Atrogin-I protein levels were also induced approximately 2-fold at 18 and 22 wks of age compared to 13-wk-old ApcMin/+ mice (Fig 2e; P<0.029). There was also a significant inverse correlation (r=-0.675; P=0.002) between Atrogin-I mRNA levels and gastrocnemius muscle mass (Fig 2f). These data show that Atrogin-I activation is associated with skeletal muscle wasting in the ApcMin/+ mouse.
Fig. 2.
Gene and protein expression related to protein degradation in cachectic ApcMin/+ mouse gastrocnemius muscle. IL-6 mRNA (a) and STAT-3 phosphorylation (b) were indicators of IL-6 signaling. MuRF-I mRNA (c) and Atrogin-I mRNA (d) were measured in 13-, 18-, and 22-wk-old ApcMin/+ mice. Atrogin-I protein levels were also elevated during cachexia (e). There was a significant correlation (r=-0.675; P=0.002) between Atrogin-I mRNA and gastrocnemius muscle mass (f). Values are means ± SE. *Signifies different from 13-wk-old mice.
Gastrocnemius fiber CSA during IL-6 depletion
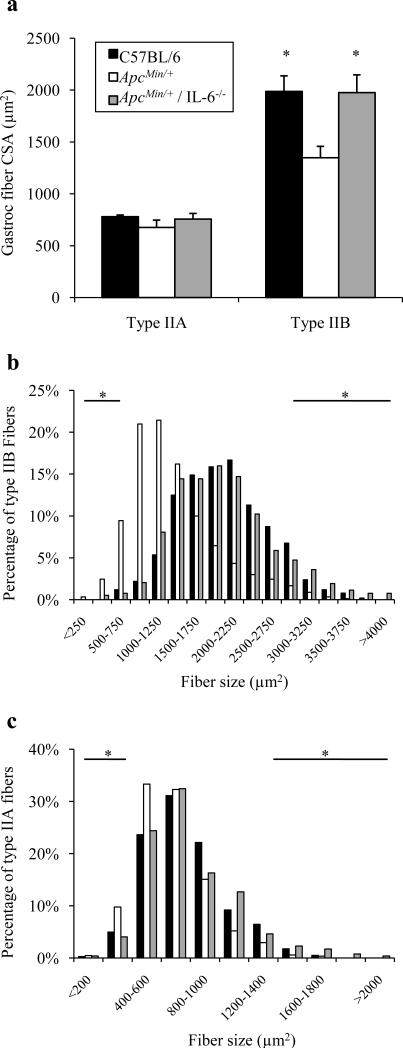
Although still having a significant intestinal and colon tumor burden, ApcMin/+ / IL-6-/- mice do not exhibit cachectic symptoms by 6 months of age [17]. For this portion of the study, a separate group of ApcMin/+ and ApcMin/+ / IL-6-/- mice were compared at 6 months of age, when a large percentage of ApcMin/+ mice are cachectic. The medial gastrocnemius muscle was sectioned and myosin ATPase stained to determine type IIB myofiber size in ApcMin/+ mice lacking IL-6. At 6 months of age, type IIB mean fiber cross-sectional area was 32% lower in the ApcMin/+ mouse, when compared to age-matched C57BL/6 mice (Fig. 3a; P=0.016). ApcMin/+ / IL-6-/- mice had type IIB mean fiber cross-sectional areas similar to wild-type values (P=0.961). There was also a shift in the frequency distribution of type IIB fibers when ApcMin/+ mice were compared to both C57BL/6 and ApcMin/+ / IL-6-/- mice. In ApcMin/+ mice, the percentage of small type IIB fibers was much less compared to age-matched C57BL/6 mice (1% vs. 12%), as expected. ApcMin/+ / IL-6-/- mice had a much greater percentage of large type IIB fibers compared to ApcMin/+ mice (8% vs. 1%; Fig. 3b; P<0.001), similar to non-tumor-bearing C57BL/6 mice.
Fig. 3.
Gastrocnemius fiber CSA in 26-week-old ApcMin/+ mice lacking IL-6. Mean gastrocnemius type IIA and type IIB fiber CSA (a) in 26-week old C57BL/6, ApcMin/+, and ApcMin/+ / IL-6-/- mice. Type IIB (b) and type IIA (c) fiber frequency distributions in C57BL/6, ApcMin/+, and ApcMin/+ / IL-6-/- mice. Values are means ± SE. *Signifies both C57BL/6 and ApcMin/+ / IL-6-/- different from ApcMin/+ mice.
Type IIA gastrocnemius mean fiber cross-sectional area was not different between 6-mo-old C57BL/6, ApcMin/+, or ApcMin/+ / IL-6-/- mice (Fig. 3a; P=0.327). While the overall mean of type IIA fibers was not different, there were shifts in fiber frequency of type IIA fibers (Fig. 3c). The percentage of small type IIA fibers in C57BL/6, ApcMin/+, and ApcMin/+ / IL-6-/- mice was 5%, 10%, and 4%, respectively (P<0.001). The percentage of large type IIA fibers in C57BL/6, ApcMin/+, and ApcMin/+ / IL-6-/- mice was 9%, 4%, and 10%, respectively (P<0.001). These data indicate that in ApcMin/+ mice lacking IL-6, there is no loss of type IIB and IIA myofiber cross-sectional area.
Increased circulating IL-6 affects gastrocnemius myofiber area
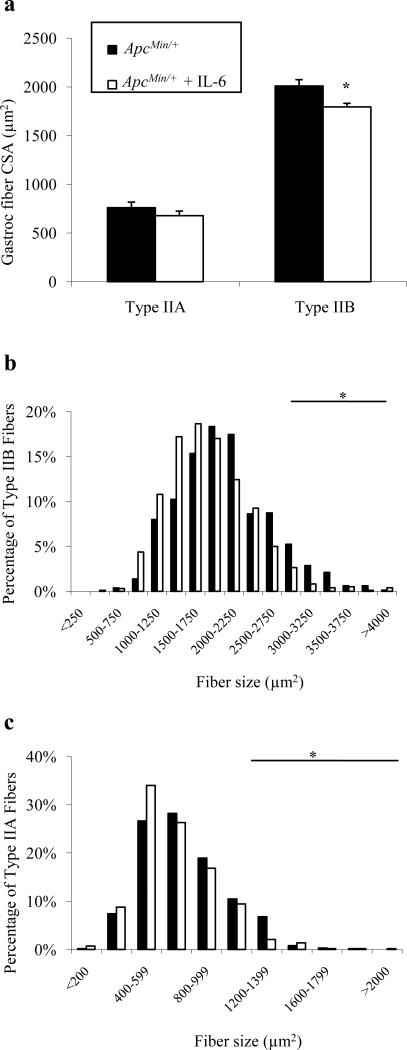
We have previously shown that there is a negative correlation between circulating IL-6 levels and gastrocnemius muscle mass in ApcMin/+ mice, and more severely cachectic animals have higher plasma IL-6 levels [17]. Since a dose-dependent response of IL-6 appears to mediate muscle wasting, an in vivo plasmid-based IL-6 over-expression system was used to increase circulating IL-6 levels in ApcMin/+ mice to try to accelerate wasting. We have previously published that this methodology increases circulating IL-6 levels, and is sufficient to accelerate wasting in ApcMin/+ mice and induce wasting in ApcMin/+/ IL-6-/- mice [17]. IL-6 levels in these mice have been previously published and were significantly increased with the IL-6 over-expression plasmid 18-fold (95-328 pg/ml) over ApcMin/+ levels (0-24 pg/ml) [17]. Although not used in any analyses, the quadriceps muscle was 28% lower in ApcMin/+ mice (149 ± 19 vs. 108 ± 8 mg; P=0.048). The gastrocnemius muscle was sectioned and myosin ATPase stained to determine fiber-type. In ApcMin/+ mice subjected to IL-6 over-expression there was an 11% reduction in type IIB mean fiber CSA, but no effect on type IIA mean fiber area (Fig. 4a). Circulating IL-6 over-expression also decreased the incidence of large diameter type IIB fibers (Fig. 4b; 6% vs. 2%; P<0.001), but did not increase the incidence of small fibers (P=0.324). Increased circulating IL-6 also decreased the number of large type IIA fibers (Fig. 4c; 8% vs. 4%; P=0.003), but had no effect on the number of small type IIA fibers (P=0.274). Grip strength was also measured over the electroporation period. Combined forelimb and hindlimb grip strength decreased 9-14% during IL-6 treatment (Table 3; P=0.009). These data indicate that increased circulating IL-6 can accelerate atrophy of large type IIB and IIA myofibers and this is associated with decreased muscle grip strength.
Fig. 4.
Over-expression of circulating IL-6 in ApcMin/+ mice. Mean gastrocnemius type IIA and type IIB fiber CSA (a) and fiber frequency distributions of type IIB (b) and type IIA fibers (c) following 4 weeks of increased IL-6. Values are means ± SE. *Signifies different from ApcMin/+ Control.
Table 3.
Changes in combined forelimb and hindlimb grip strength over time following increased circulating IL-6 in tumor-bearing and non-tumor-bearing mice.
| Grip Strength (N) | ||
|---|---|---|
| Time (wks) | ApcMin/+ + IL-6 (n=7) | C57BL/6 + IL-6 (n=5) |
| 0 | 1.48 ± 0.02 | 1.44 ± 0.03 |
| 2 | 1.27 ± 0.08* | 1.46 ± 0.05 |
| 4 | 1.34 ± 0.05* | 1.47 ± 0.05 |
| 6 | NA | 1.50 ± 0.03 |
| 8 | NA | 1.44 ± 0.04 |
| 10 | NA | 1.51 ± 0.02 |
Time refers to weeks during electroporation. Data were analyzed with a repeated measures one-way ANOVA within each strain.
Signifies different from 0 wks.
Circulating IL-6 does not affect muscle size or function in non-tumor-bearing mice
We have previously shown that our IL-6 electroporation procedure does not induce muscle wasting in wild-type mice, as measured by a lack of a change in body mass, epididymal fat pad mass, and gastrocnemius muscle mass with up to 10 weeks of increased circulating IL-6 [17]. Similarly, the gastrocnemius muscle mass to tibia length ratio was not altered with 10 weeks of IL-6 administration in C57BL/6 mice (8.2 ± 0.3 vs. 8.4 ± 0.1 mg/mm; P=0.542). Other skeletal muscles, such as the soleus (9 ± 0 vs. 8 ± 0 mg; P=0.401) and plantaris muscles (19 ± 1 vs. 19 ± 1; P=0.808), also did not differ between mice receiving the empty vector and the IL-6 vector. While the quadriceps not used for analysis, it was 16% lower in C57BL/6 mice (215 ± 7 vs. 180 ± 7; P=0.019) with muscle-specific IL-6 expression compared to control mice. Plasma IL-6 levels from these mice have been previously published and were increased 85-fold (44-244 pg/ml) over C57BL/6 mice receiving the empty vector (0-8 pg/ml). Functional testing was also performed in C57BL/6 receiving IL-6. Combined forelimb and hindlimb grip strength did not change over the course of 10 weeks of IL-6 over-expression (Table 3; P=0.404). These data show that IL-6 is not sufficient to alter skeletal muscle mass or function in non-tumor-bearing mice.
Increased circulating IL-6 increases Atrogin-I gene expression
To determine if IL-6 regulates the ubiquitin-proteasome pathway, Atrogin-I and MuRF-I gene expression was measured in ApcMin/+ mice gastrocnemius muscle following IL-6 over-expression. Atrogin-I mRNA was increased nearly 3-fold (P=0.048) and MuRF-I mRNA expression was unchanged (P=0.384) in muscles from ApcMin/+ mice following increased circulating IL-6 (Fig. 5a). In fact, there was a positive correlation (r=0.55; P=0.018) between circulating IL-6 and Atrogin-I mRNA in these mice (Fig. 5b). Atrogin-I protein levels were also 2-fold greater in ApcMin/+ mice with high circulating levels of IL-6 (P<0.001; Fig. 5c). To determine if IL-6 can directly induce the ubiquitin-proteasome pathway independent of an underlying tumor burden, Atrogin-I and MuRF-I mRNA was measured in wild-type mice following circulating IL-6 over-expression. Despite a lack of atrophy or change in grip strength, elevated circulating IL-6 increased Atrogin-I mRNA 60% (Fig. 5d), but did not change MuRF-I mRNA (P=0.287) expression. There was a trend (P=0.11; Fig 5e) for Atrogin-I protein levels to change with IL-6 treatment, but there was considerable variability in the response. These data show that Atrogin-I protein levels appear to be induced by IL-6 only when concurrent wasting is present.
Fig. 5.
Atrogin-I and MuRF-I mRNA and protein expression in gastrocnemius muscle from mice over-expressing circulating IL-6. Atrogin-I and MuRF-I gene expression during increased circulating IL-6 in cachectic ApcMin/+ mice (a). There was a positive correlation between Atrogin-I mRNA levels and plasma IL-6 in ApcMin/+ mice (b). Atrogin-I protein levels during IL-6 over-expression in ApcMin/+ mice (c). Atrogin-I and MuRF-I gene expression during increased circulating IL-6 in non-cachectic, wild-type mice (d). Atrogin-I protein levels during IL-6 over-expression in C57BL/6 mice (e). Values are means ± SE. *Signifies different from Control.
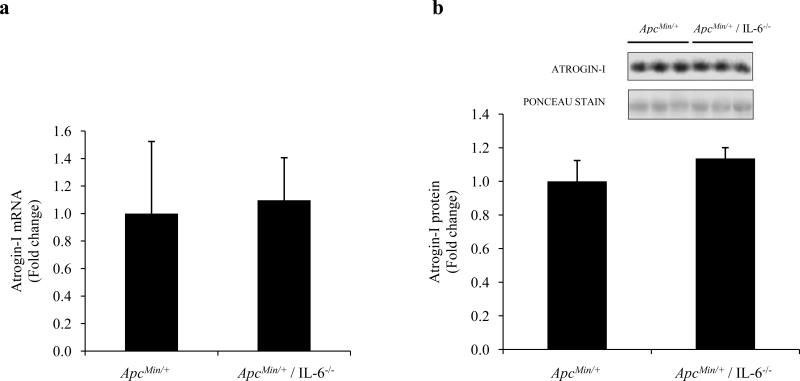
Atrogin-I levels are not repressed in ApcMin/+/ IL-6-/- mice
Since ApcMin/+ / IL-6-/- mice do not undergo atrophy by 6 months of age, we decided to determine if lower Atrogin-I levels were responsible for the prevention of cachexia in these mice. To our surprise, Atrogin-I mRNA levels were similar between ApcMin/+ and ApcMin/+ / IL-6-/- mice (Fig. 6a; P=0.380). Protein levels were also determined. Atrogin-I protein levels were similar between ApcMin/+ and ApcMin/+ / IL-6-/- mice (Fig. 6b; P=0.365). These data demonstrate that the mechanism of muscle mass retention in ApcMin/+ / IL-6-/- mice is not mediated via the down-regulation of Atrogin-I.
Fig. 6.
Atrogin-I mRNA and protein expression in 6-mo-old ApcMin/+ and ApcMin/+ / IL-6-/- mice. Atrogin-I mRNA (a) and Atrogin-I protein (b) in 6-mo-old cachectic (ApcMin/+) and non-cachectic (ApcMin/+ / IL-6-/-) mice. Values are means ± SE.
DISCUSSION
Using the ApcMin/+ mouse to study cachexia is advantageous because of the relatively long duration of muscle wasting, chronic low levels of inflammation, and lack of anorexia. This study presents several novel findings related to myofiber atrophy, IL-6 regulation, and the induction of Atrogin-I gene expression during cancer cachexia. Although other cachectic models have demonstrated that fast-twitch, glycolytic muscles are more susceptible to wasting than oxidative, slow-twitch muscles [19, 20, 42], the current study extends previous work by demonstrating the wasting of type IIB fibers in the ApcMin/+ mouse is a major contributor to overall muscle mass loss in this cachectic animal. The type IIB fast-glycolytic fibers from cachectic ApcMin/+ mice demonstrated a more consistent and larger degree of atrophy when compared to oxidative type IIA fibers of the gastrocnemius muscle. Additionally, the ApcMin/+ mouse soleus muscle, which is a highly oxidative muscle with a much higher percentage of slow-type fibers, did not demonstrate atrophy over this same time period. The soleus muscle may not have shown atrophy during this time period because it typically does not have type IIB fibers. An important finding of the current study was that circulating IL-6 was a mediator of this muscle wasting process. ApcMin/+ mice lacking IL-6 did not undergo type IIB myofiber atrophy and increasing circulating IL-6 levels exacerbated the atrophy of type IIB fibers.
IL-6 has been implicated as an important stimulus for initiating muscle mass loss during cachexia [7-11]. We have recently shown that IL-6 is necessary and sufficient for the development of muscle wasting in ApcMin/+ mice. This wasting is closely related to polyp formation and growth, since mice with a larger tumor burden exhibit more cachectic symptoms [17]. Although there are many possible sources of elevated circulating IL-6 in ApcMin/+ mice, the tumors and related systemic immune response are a likely source. In the current study, when circulating IL-6 levels were manipulated there was a direct impact on mean type IIB myofiber size, but there was a much weaker effect on type IIA fibers size. Additionally, ApcMin/+ mice lacking IL-6, while still having a significant tumor burden, had type IIB myofiber cross-sectional areas similar to wild-type, non-tumor bearing mice. While other cachexia studies have shown that IL-6 induces muscle wasting [9, 12, 13, 43, 44], this is the first in vivo study to demonstrate that IL-6 has a stronger and more consistent effect on type IIB muscle fiber wasting. It is possible that primary myofiber metabolism related to an elevated glycolytic capacity and diminished oxidative capacity create a more susceptible environment for IL-6 induced muscle wasting. This may coincide with the overall metabolic state of the animal, which is being affected by the intestinal/colon tumor burden. We know from the loss of adipose stores in the cachectic animal that fat oxidation should be increased in muscle. IL-6 is also a tumor growth factor [45-47] and we have previously shown that IL-6 increases the tumor burden in ApcMin/+ mice [17]. Others have shown that colon tumors are very glycolytic and this is also true of ApcMin/+ mouse intestinal polyps [48, 49]. It is possible that type IIB fibers are more susceptible to atrophy because of IL-6-induced tumor growth and subsequent high glucose utilization by the tumors. The redirection of glucose towards the intestinal polyps may render type IIB fibers susceptible to atrophy because of the lack of an energy source and others have reported that oxidative fibers are protected from atrophy during cachexia [18, 19]. It remains a significant question to delineate the properties of IIB fibers that render them more susceptible to wasting stimuli with cancer.
The ubiquitin-proteasome pathway is activated during cachexia [12, 50, 51] and is responsible for the degradation of most skeletal muscle proteins [24]. There is a biphasic response, with proteasome activation during early muscle loss, but suppressed during severe muscle mass loss [27, 28, 52]. Atrogin-I and MuRF-I are E3 ubiquitin ligases that are normally activated during atrophy [26]. In the current study, Atrogin-I mRNA, but not MuRF-I mRNA, was elevated from 18-22 weeks of age. Additionally, Atrogin-I protein levels were also higher in 18 and 22-wk-old mice compared to 13-wk-old mice. Increased circulating IL-6 levels were associated with increased gastrocnemius muscle Atrogin-I expression, while STAT-3 phosphorylation was only elevated in muscle from mice at 22-wks of age. Although we have previously shown an association between STAT-3 phosphorylation and muscle mass loss [17], further work is required to determine if IL-6-induced activation of STAT-3 can directly activate Atrogin-I expression in wasting muscle. Atrogin-I and MuRF-I expression in this model is also interesting since expression of both of these ligases is typically increased together during atrophy [26, 28, 32]. However, recent evidence has shown that MuRF-1 is responsible for the degradation of slow MHC [34]. Furthermore, there has been a disconnect between Atrogin-I and MuRF-I during disuse atrophy [53] and resistance exercise in humans [54]. These data support the hypothesis that these two ubiquitin ligases are activated by different stimuli, including cancer. Since type IIB myofibers underwent significantly more atrophy, these data also suggest that Atrogin-I may specifically target the degradation of fast MHC or other proteins found in type IIB fibers. However, further work is needed to assess the specific substrates of Atrogin-I and how they relate to fiber type.
Since IL-6 is necessary and sufficient for muscle wasting in ApcMin/+ mice and Atrogin-I is induced in cachectic muscle, we next determined if IL-6 activates Atrogin-I. Atrogin-I and MuRF-I mRNA were also measured in gastrocnemius muscles from wild-type and ApcMin/+ mice following IL-6 over-expression. Atrogin-I mRNA and protein were induced in ApcMin/+ mice with high circulating IL-6 levels and this was associated with type IIB myofiber loss. Atrogin-I mRNA, but not protein, was also induced in wild-type mice, but these mice do not undergo atrophy following IL-6 over-expression [17]. These data suggest that circulating IL-6 can induce skeletal muscle Atrogin-I gene expression, but activation of this pathway alone is not sufficient to induce muscle wasting. This is in agreement with work showing the Atrogin-I over-expression in skeletal muscle is not sufficient to induce atrophy [33]. Furthermore, mice lacking IL-6 preserved gastrocnemius muscle fiber area, but still had Atrogin-I protein levels similar to cachectic ApcMin/+ mice. Atrogin-I activation without subsequent atrophy may point to an additional secondary function for Atrogin-I. Jagoe and colleagues [55] have shown that 1-2 days of fasting leads to 8-10 fold increases in Atrogin-I mRNA, but not MuRF-I mRNA, in mouse gastrocnemius muscle. This is associated with a decrease in gene expression associated with glycolysis and glycogen breakdown. Similarly, both IL-6 and Atrogin-I are stimulated in human skeletal muscle immediately after a bout of endurance exercise [54]. One may hypothesize that Atrogin-I may serve as a sensor of ATP supply and a director of fuel source, directing the muscle to switch to fatty acid oxidation at a time of limited glucose. This may be an important feed-forward mechanism of providing glucose to the tumor, sacrificing skeletal muscle in the process. However, when this high metabolic demand is not present, such as the absence of a tumor, the skeletal muscle is spared. Further work is required to understand the IL-6 specific induction Atrogin-I in cachectic muscle, and whether this is a valid target for anti-wasting therapies.
These data demonstrate type IIB fibers in the ApcMin/+ mouse are highly susceptible to wasting and this may be partially mediated through IL-6 induction of Atrogin-I gene expression. The induction of Atrogin-I mRNA increased concurrently with the loss of gastrocnemius muscle mass and IIB fiber cross-sectional area loss. IL-6 over-expression accelerated type IIB myofiber atrophy in ApcMin/+ mice, and ApcMin/+ mice lacking IL-6 had no reduction in type IIB myofiber cross-sectional area. Atrogin-I gene expression was also regulated by IL-6. Increased circulating IL-6 caused an induction of Atrogin-I protein in ApcMin/+ mice, but not in wild-type mice. Muscle MuRF-I mRNA expression was not induced by cachexia or circulating IL-6 over-expression. In conclusion, IL-6-induced type IIB myofiber atrophy appears to be partially mediated through induction of muscle Atrogin-I expression, and a yet unidentified stimulus related to the intestinal/colon tumor burden in the ApcMin/+ mouse.
ACKNOWLEDGEMENTS
The authors would like to thank Tia Davis, Joseph McClung, and April Wilson for technical assistance. The research described in this report was supported by the National Institutes of Health (NIH) Grant P20 RR-017698 from the National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Price SA, Tisdale MJ. Mechanism of inhibition of a tumor lipid-mobilizing factor by eicosapentaenoic acid. Cancer Res. 1998;58:4827–4831. [PubMed] [Google Scholar]
- 2.Giordano A, Calvani M, Petillo O, Carteni M, Melone MR, Peluso G. Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem. 2003;90:170–186. doi: 10.1002/jcb.10601. [DOI] [PubMed] [Google Scholar]
- 3.al-Majid S, McCarthy DO. Resistance exercise training attenuates wasting of the extensor digitorum longus muscle in mice bearing the colon-26 adenocarcinoma. Biol Res Nurs. 2001;2:155–166. doi: 10.1177/109980040100200301. [DOI] [PubMed] [Google Scholar]
- 4.Ardies CM. Exercise, cachexia, and cancer therapy: a molecular rationale. Nutr Cancer. 2002;42:143–157. doi: 10.1207/S15327914NC422_1. [DOI] [PubMed] [Google Scholar]
- 5.Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589–597. [PubMed] [Google Scholar]
- 6.Argiles JM, Busquets S, Lopez-Soriano FJ. Cytokines in the pathogenesis of cancer cachexia. Curr Opin Clin Nutr Metab Care. 2003;6:401–406. doi: 10.1097/01.mco.0000078983.18774.cc. [DOI] [PubMed] [Google Scholar]
- 7.Espat NJ, Auffenberg T, Rosenberg JJ, Rogy M, Martin D, Fang CH, Hasselgren PO, Copeland EM, Moldawer LL. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am J Physiol. 1996;271:R185–190. doi: 10.1152/ajpregu.1996.271.1.R185. [DOI] [PubMed] [Google Scholar]
- 8.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 9.Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun. 1995;207:168–174. doi: 10.1006/bbrc.1995.1168. [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567–1570. doi: 10.3748/wjg.v9.i7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- 12.Fujita J, Tsujinaka T, Yano M, Ebisui C, Saito H, Katsume A, Akamatsu K, Ohsugi Y, Shiozaki H, Monden M. Anti-interleukin-6 receptor antibody prevents muscle atrophy in colon-26 adenocarcinoma-bearing mice with modulation of lysosomal and ATP-ubiquitin-dependent proteolytic pathways. Int J Cancer. 1996;68:637–643. doi: 10.1002/(SICI)1097-0215(19961127)68:5<637::AID-IJC14>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Tsujinaka T, Fujita J, Ebisui C, Yano M, Kominami E, Suzuki K, Tanaka K, Katsume A, Ohsugi Y, Shiozaki H, Monden M. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J Clin Invest. 1996;97:244–249. doi: 10.1172/JCI118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111:592–595. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- 15.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 16.Mehl KA, Davis JM, Berger FG, Carson JA. Myofiber degeneration/regeneration is induced in the cachectic ApcMin/+ mouse. J Appl Physiol. 2005;99:2379–2387. doi: 10.1152/japplphysiol.00778.2005. [DOI] [PubMed] [Google Scholar]
- 17.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 18.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol. 2007;170:599–608. doi: 10.2353/ajpath.2007.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulland TJ. Histochemical and morphometric evaluation of skeletal muscle of cachectic sheep. Vet Pathol. 1981;18:279–298. doi: 10.1177/030098588101800301. [DOI] [PubMed] [Google Scholar]
- 21.Allen DL, Harrison BC, Sartorius C, Byrnes WC, Leinwand LA. Mutation of the IIB myosin heavy chain gene results in muscle fiber loss and compensatory hypertrophy. Am J Physiol Cell Physiol. 2001;280:C637–645. doi: 10.1152/ajpcell.2001.280.3.C637. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Advances in physiology education. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 24.Kwak KS, Zhou X, Solomon V, Baracos VE, Davis J, Bannon AW, Boyle WJ, Lacey DL, Han HQ. Regulation of protein catabolism by muscle-specific and cytokine-inducible ubiquitin ligase E3alpha-II during cancer cachexia. Cancer Res. 2004;64:8193–8198. doi: 10.1158/0008-5472.CAN-04-2102. [DOI] [PubMed] [Google Scholar]
- 25.Tisdale MJ. The ‘cancer cachectic factor’. Support Care Cancer. 2003;11:73–78. doi: 10.1007/s00520-002-0408-6. [DOI] [PubMed] [Google Scholar]
- 26.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. Faseb J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 27.Khal J, Wyke SM, Russell ST, Hine AV, Tisdale MJ. Expression of the ubiquitinproteasome pathway and muscle loss in experimental cancer cachexia. Br J Cancer. 2005;93:774–780. doi: 10.1038/sj.bjc.6602780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. Faseb J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 29.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 31.Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano FJ, Doglietto GB, Argiles JM, Baccino FM, Rossi Fanelli F. Igf-1 Is Down-Regulated in Experimental Cancer Cachexia. Am J Physiol Regul Integr Comp Physiol. 2006;291:R674–683. doi: 10.1152/ajpregu.00104.2006. [DOI] [PubMed] [Google Scholar]
- 32.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–1597. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- 33.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. 2007;117:2486–2495. doi: 10.1172/JCI32827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanai JI, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme VP, Lecker SH. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- 37.Fattori E, Cappelletti M, Zampaglione I, Mennuni C, Calvaruso F, Arcuri M, Rizzuto G, Costa P, Perretta G, Ciliberto G, La Monica N. Gene electro-transfer of an improved erythropoietin plasmid in mice and non-human primates. The journal of gene medicine. 2005;7:228–236. doi: 10.1002/jgm.652. [DOI] [PubMed] [Google Scholar]
- 38.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.Tucker JM, Davis C, Kitchens ME, Bunni MA, Priest DG, Spencer HT, Berger FG. Response to 5-fluorouracil chemotherapy is modified by dietary folic acid deficiency in Apc(Min/+) mice. Cancer Lett. 2002;187:153–162. doi: 10.1016/s0304-3835(02)00402-0. [DOI] [PubMed] [Google Scholar]
- 42.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieskovska J, Guo D, Derman E. Growth impairment in IL-6-overexpressing transgenic mice is associated with induction of SOCS3 mRNA. Growth Horm IGF Res. 2003;13:26–35. doi: 10.1016/s1096-6374(02)00135-1. [DOI] [PubMed] [Google Scholar]
- 44.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brozek W, Bises G, Girsch T, Cross HS, Kaiser HE, Peterlik M. Differentiation-dependent expression and mitogenic action of interleukin-6 in human colon carcinoma cells: relevance for tumour progression. Eur J Cancer. 2005;41:2347–2354. doi: 10.1016/j.ejca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27:1507–1515. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 47.Steiner H, Godoy-Tundidor S, Rogatsch H, Berger AP, Fuchs D, Comuzzi B, Bartsch G, Hobisch A, Culig Z. Accelerated in vivo growth of prostate tumors that up-regulate interleukin-6 is associated with reduced retinoblastoma protein expression and activation of the mitogen-activated protein kinase pathway. Am J Pathol. 2003;162:655–663. doi: 10.1016/S0002-9440(10)63859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herzfeld A, Legg MA, Greengard O. Human colon tumors: enzymic and histological characteristics. Cancer. 1978;42:1280–1283. doi: 10.1002/1097-0142(197809)42:3<1280::aid-cncr2820420337>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 49.Leclerc D, Deng L, Trasler J, Rozen R. ApcMin/+ mouse model of colon cancer: gene expression profiling in tumors. J Cell Biochem. 2004;93:1242–1254. doi: 10.1002/jcb.20236. [DOI] [PubMed] [Google Scholar]
- 50.Williams A, Wang JJ, Wang L, Sun X, Fischer JE, Hasselgren PO. Sepsis in mice stimulates muscle proteolysis in the absence of IL-6. Am J Physiol. 1998;275:R1983–1991. doi: 10.1152/ajpregu.1998.275.6.R1983. [DOI] [PubMed] [Google Scholar]
- 51.Costelli P, Bossola M, Muscaritoli M, Grieco G, Bonelli G, Bellantone R, Doglietto GB, Baccino FM, Rossi Fanelli F. Anticytokine treatment prevents the increase in the activity of ATP-ubiquitin- and Ca(2+)-dependent proteolytic systems in the muscle of tumour-bearing rats. Cytokine. 2002;19:1–5. doi: 10.1006/cyto.2002.1036. [DOI] [PubMed] [Google Scholar]
- 52.Galban VD, Evangelista EA, Migliorini RH, do Carmo Kettelhut I. Role of ubiquitin-proteasome-dependent proteolytic process in degradation of muscle protein from diabetic rabbits. Mol Cell Biochem. 2001;225:35–41. doi: 10.1023/a:1012260605910. [DOI] [PubMed] [Google Scholar]
- 53.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 55.Jagoe RT, Lecker SH, Gomes M, Goldberg AL. Patterns of gene expression in atrophying skeletal muscles: response to food deprivation. Faseb J. 2002;16:1697–1712. doi: 10.1096/fj.02-0312com. [DOI] [PubMed] [Google Scholar]