Abstract
Background
This study examined the prognostic factors that affect the surgical outcome of laminoplasty in cervical spondylotic myelopathy patients by comparative analysis.
Methods
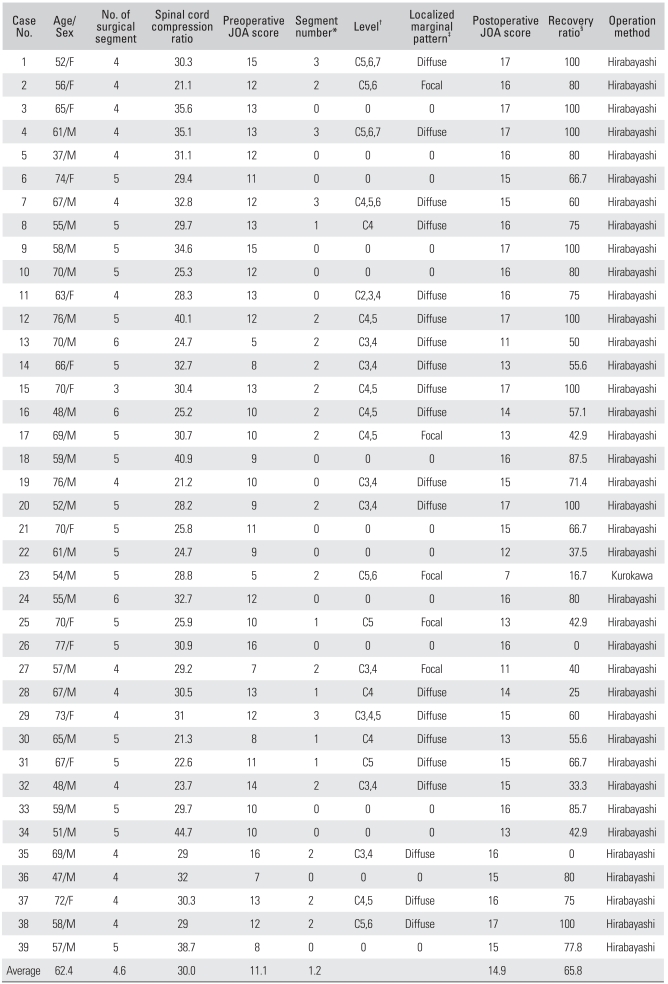
Thirty nine patients, 26 males and 13 females, who were treated with laminoplasty for cervical myelopathy from September 2004 to March 2008 and followed up for 12 months or longer, were enrolled in this study. The mean age of the subjects was 62.4 years (range, 37 to 77 years). The patients' age, number of surgical segments, spinal cord compression ratio, segment number, level, localized marginal pattern of high signal intensity within the spinal cord in the T2 image, preoperative Japanese Orthopaedic Association Scoring System (JOA) score with the recovery ratio were compared respectively. The JOA score was used for an objective assessment of the patients' preoperative and postoperative clinical status. The recovery ratios of surgery were graded using the Hirabayashi equation. Statistical analysis was carried out using Pearson correlation analysis.
Results
The patients' JOA score increased from a preoperative score of 11.1 (range, 5 to 16) to a postoperative score of 14.9 (range, 7 to 17). The average recovery ratio was 65.8% (range, 0 to 100%). The number of segments with high signal changes in the T2 image, a localized marginal pattern with high signal change, signal intensity changes in the upper cervical spinal cord were inversely associated with the recovery ratio, whereas the spinal cord compression ratio showed a significant positive correlation. However, the currently known prognostic factors, such as number of surgical segment, age, and preoperative JOA score, showed no statistically significant correlation.
Conclusions
The number of segments, localized marginal pattern, rostral location of signal intensity changes with a high signal change in the T2 image and a low spinal cord compression ratio in cervical spondylotic myelopathy patients treated by laminoplasty can indicate a poor prognosis.
Keywords: Cervical spondylosis, Myelopathy, Laminoplasty, Prognostic factor
Cervical spondylotic myelopathy is a condition in which narrowing and dysfunction of the spinal cord occur as a result of degenerative changes to the cervical spine. The condition is caused by hard disc herniation or ligamentum flavum thickening in most cases, and by ossification of the ligamentum longitudinale posterius, soft disc herniation, congenital spinal stenosis, or spinal cord tumor in some cases. It often develops progressively without apparent predisposing factors and progresses slowly. However, in some cases, it suddenly occurs as a result of trauma and advances rapidly. Long standing spinal cord compression can eventually result in irreversible, histological, and physiological changes, such as neurofibrosis, demyelination, and a loss of neurons.1)
In most cases of cervical spondylotic myelopathy, the symptoms rarely improve with conservative treatment and progress steadily. Therefore, early surgical treatment before the occurrence of irreversible changes is strongly recommended. While satisfactory surgical treatment outcomes have been reported by many authors, there is still some controversy regarding the prognostic factors. The most common surgical options include anterior fusion, laminectomy and laminoplasty. Surgical treatment can relieve the neurological symptoms and spinal cord compression, but the postoperative outcomes are affected by a range of factors.2,3)
In this study, the various prognostic factors that could affect the outcomes of laminoplasty were compared and analyzed retrospectively.
METHODS
Thirty-nine cervical spondylotic myelopathy patients (39 cases), who were treated with laminoplasty between September 2004 and March 2008 and followed up for ≥ 12 months, were enrolled in this study. The inclusion criteria were the clear identification of a lesion on MRI and the presence of symptoms. Patients with an indistinct lesion on MRI or were asymptomatic were excluded. The mean follow-up period was 19.1 months (range, 12 to 36 months) with an average age of 62.4 years (range, 37 to 77 years) at the time of surgery. There were 26 males and 13 females. The mean illness duration was 12.8 months (range, 1 to 60 months). Expansive open door laminoplasty using the method reported by Hirabayashi4) was performed in 38 patients and laminoplasty using the method reported by Kurokawa was performed in 1 (Table 1).
Table 1.
Data of Cervical Myelopathy Patients Treated by Laminoplasty
JOA: Japanese Orthopaedic Association Scoring System.
*The number of segments with high signal change in the T2 images. †The level of segments with high signal change in the T2 images. ‡Localized marginal pattern of segments with high signal changes in the T2 images. §Recovery ratio of surgery was graded using the Hirabayashi equation (preoperative score - postoperative score/17 - preoperative score × 100).
The patients' age, number of surgical segments, number, level, and localized marginal pattern of segments with high signal intensity on the T2-weighted images, spinal cord compression ratio, and preoperative Japanese Orthopaedic Association Scoring System (JOA) score were measured and compared with the recovery rate to identify the prognostic factors affecting the surgical outcome (Table 1). The preoperative and postoperative clinical condition was assessed using JOA score. The recovery rate was graded using the Hirabayashi equation.
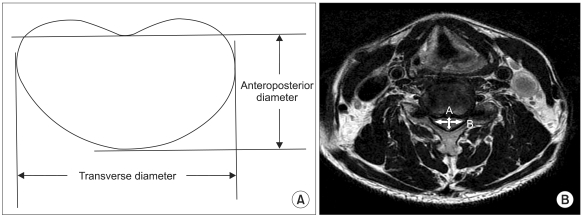
Preoperative MRI scans were performed in all patients. T2 images showing high signal intensity within the compressed spinal cord were used to assess the number of segments with high signal intensity, marginal pattern of segments with high signal intensity (localized or diffuse), and the level of segments with high signal intensity. The anteroposterior compression ratio of the spinal cord was calculated using the measurements obtained in the middle of the level of maximum compression (sagittal diameter/transverse diameter × 100%) (Fig. 1). The number of segments with high signal intensity on MRI was defined as the number of segments where the high signal intensity starts and ends on the T2-weighted images. For example, if high signal intensity extends from C3 to C5, the number recorded was 3. For an assessment of the marginal pattern of segments with high signal intensity, the difference from the adjacent tissues on the T2-weighted images was examined. The grades assigned according to pattern (localized pattern = 2, diffuse pattern = 1) were tested for their association with the recovery rate.
Fig. 1.
(A) Compression ratio of the spinal cord = anteroposterior diameter/transverse diameter × 100. (B) Method for drawing "A" (anteroposterior diameter) and "B" (transverse diameter) in the compression ratio of this patient. "A" and "B" are the widest anteroposterior and transverse diameter of the spinal cord, respectively.
Pearson's correlation analysis was performed to determine the correlations between these parameters and the recovery rate. Statistical significance was set at p ≤ 0.05.
RESULTS
From the 2.4th postoperative day on average, the patients could walk with a Philadelphia cervical orthosis. A postoperative wound infection was encountered in 2 patients. In one patient, the condition improved when the infected area was incised, irrigated and closed accordingly. In the other, the infection was treated with antibiotics. No improvement in the JOA score was observed in these two patients (the 26th and 35th patients in Table 1), who had a high JOA score of 16. In the remaining patients, the JOA score improved and symptomatic improvement to varying degrees was observed.
The mean JOA score increased from 11.05 (range, 5 to 16) preoperatively to 14.90 (range, 7 to 17) postoperatively. The mean recovery rate was 65.8% (range, 0 to 100%). The mean age at the time of surgery was 62.4 years (range, 47 to 77 years) and there was no significant association with the recovery rate (p = 0.399). The mean number of surgical segments was 4.6 (range, 3 to 5) and there was no remarkable correlation with the recovery rate (p = 0.366). The mean preoperative JOA score (11.1) had a correlation coefficient of 0.95 with the recovery rate, which was not significant (p = 0.566). However, the number of segments with high signal intensity on the T2-weighted images, 1.2 (range, 0 to 3), as well as the localized marginal pattern of the segments with high signal intensity on the T2-weighted images showed a significant inverse correlation with the recovery rate (r = -0.31, p = 0.00; r = -2.42, p = 0.00, respectively). The negative correlation became more significant with increasing height of the upper segment with high signal intensity (rostral) (r = 2.42, p = 0.03). The mean spinal cord compression ratio was 29.9% (range, 21.1 to 44.7%) and there was a positive correlation (r = 2.54, p = 0.00).
DISCUSSION
Old age and a low preoperative JOA score have been associated with unsatisfactory surgical outcome.2,5) However, there were no correlations between the two factors and the final results (p = 0.399, p = 0.566). Of the patients, 9 were over 70-years-old and their preoperative and postoperative JOA scores were similar to the mean JOA score of the total study population (preoperative JOA score, 11.1; postoperative JOA score, 14.9). Many studies reported that the outcome deteriorated with the patient's age, but there was no correlation between them in the present study. According to Lee et al.,6) the surgical results for the treatment of cervical spondylotic myelopathy were more associated with the age at the onset of symptoms and the level of spinal nerve paralysis than the patient's age. Meanwhile, Kim et al.7) suggested that the illness duration that was dependent on the patient's memory could not be an objective indicator. In addition, they reported that the analysis results might also differ according to which of the present symptoms was used as a parameter. In this study, the correlation between the illness duration and recovery rate could have been investigated if most patients had remembered their age at the onset of the disease.
In many studies, the JOA score was described as an important prognostic factor that can be used for objective evaluation of the level of symptoms.8,9) Hamburger et al.10) reported that a good preoperative clinical condition led to satisfactory surgical outcomes and highlighted the importance of the preoperative JOA score, particularly if it was ≥ 10. However, the present study found no significant relationship between the preoperative JOA score and recovery rate (p = 0.566). The recovery rate was 0 in the 2 patients with a relatively high preoperative JOA score of 16. This was attributed to surgery being performed without major symptoms. It is believed that a correlation between the preoperative JOA score and the recovery rate could have been observed if the patient selection process had been more discriminating.
Regarding the correlation between the anteroposterior spinal cord diameter, inner diameter, and myelopathy, severe spinal stenosis is associated with severe symptoms and the stage of myelopathy.5,11) The Pavlov ratio12) and anteroposterior compression ratio along with the transverse area at the maximum compression level on MRI have been used to evaluate such correlation.5) In this study, the anteroposterior compression ratio was measured and found to be associated with the outcome (p = 0.00).
MRI is the optimal modality that allows an accurate assessment of the level of spinal cord stenosis and the spinal cord condition in patients with cervical spondylotic myelopathy. High signal intensity on the T2-weighted MRI images and low signal intensity on the T1-weighted MRI images indicate spinal cord lesions. There are some reports showing that the signal changes on the T2-weighted images indicate reversible edema or irreversible changes to the spinal cord.13,14) However, the authors are divided on the relationship between high signal intensity on the T2-weighted MRI images and the postoperative outcome. For one, the reversibility of a condition cannot be examined using the MRI signal changes alone, and intervertebral disc herniation and a short illness duration are associated with reversible changes.2) According to Matsuda et al.15) and Okada et al.,16) the preoperative T2-weighted images are related to the prognosis because patients with no signal changes on the postoperative T2-weighted MRI images showed clinical improvement. On the other hand, Morio et al.17) and Yone et al.18) disputed the correlation between the signal changes on the preoperative T2-weighted images and the clinical outcome. Morio et al.19) reported that the signal changes in both the T2-weighted and T1-weighted images indicated an irreversible spinal cord injury and were associated with a poor prognosis. Chen et al.20) examined the signal changes on T2-weighted images only because objective detection is difficult using the T1-weighted images. In the current study, the number of segments with high signal intensity on the T2-weighted images (r = -0.31) and localized marginal pattern of the segments with high signal intensity (r = -2.42) were inversely associated with the recovery rate whereas the anteroposterior spinal cord compression ratio (Fig. 1) had a significant positive correlation (r = 2.54, p = 0.00). Chen et al.20) reported that the pattern of high signal intensity on the T2-weighted MR images were related to the prognosis. They classified the different signal intensity patterns into 3 types: type 0 was defined as no appearance of high signal intensity on the T2-weighted MR images; type 1 as the appearance of a > 50% faint and fuzzy border of high signal intensity; and type 2 as the appearance of > 50% of intense and well-defined border of high signal intensity. Their conclusion was that cervical spondylotic patients with a type 2 high signal intensity pattern had a poor prognosis. In the present study, high signal intensity images were also subdivided into diffuse and localized types to test the efficacy of the border pattern as a prognostic factor; a localized border pattern was associated with a poor outcome.
According to Flanders et al.,21) poor recovery from high cervical injury was associated with the presence of spinal cord hemorrhage and long edema on MRI. In this study, a negative correlation was also found between the recovery rate and high location of cervical spondylotic myelopathy (or the location of the segments with high signal intensity on the T2-weighted MRI images). Therefore, a study involving cases with edema is needed.
One of the limitations of this study was that there was no objective standard for classifying the patterns of high signal intensity, which were subdivided into two, diffuse and localized types. It is believed that it will be important to provide an objective classification standard in order to show the correlation between localized high signal intensity and the recovery rate in future studies. Another limitation is that the study focused only on the correlations between the findings on the MRI images and the recovery rate. In other studies, the cases subdivided according to their symptoms were also tested for possible correlations with the recovery rate. Although it is difficult to objectify and grade symptoms that are highly subjective, as reported in many other studies, it will be important to examine that relationship in a future study. The other limitation is that only the spinal cord compression ratio (anteroposterior diameter/transverse diameter × 100) was used, not the area of the spinal cord. Considering that a reduction is observed in the anteroposterior and transverse diameter in severe cases, the absolute correlation coefficient would be lower in such cases. Therefore, it is believed that studies involving severe cases should deal with the correlation between the recovery rate and the area of the spinal cord, not the spinal cord compression ratio.
In conclusion, there are a range of prognostic factors of laminoplasty in patients with cervical spondylotic myelopathy. Based on these results, it is believed that unsuccessful treatment outcomes can be associated with a high number of segments with high signal intensity on the T2-weighted images, a localized marginal pattern of high signal intensity, rostral location of high signal intensity and low spinal cord compression ratio.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79(4):550–561. doi: 10.3171/jns.1993.79.4.0550. [DOI] [PubMed] [Google Scholar]
- 2.Kohno K, Kumon Y, Oka Y, Matsui S, Ohue S, Sakaki S. Evaluation of prognostic factors following expansive laminoplasty for cervical spinal stenotic myelopathy. Surg Neurol. 1997;48(3):237–245. doi: 10.1016/s0090-3019(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 3.Koyanagi T, Hirabayashi K, Satomi K, Toyama Y, Fujimura Y. Predictability of operative results of cervical compression myelopathy based on preoperative computed tomographic myelography. Spine (Phila Pa 1976) 1993;18(14):1958–1963. doi: 10.1097/00007632-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi K, Watanabe K, Wakano K, Suzuki N, Satomi K, Ishii Y. Expansive open-door laminoplasty for cervical spinal stenotic myelopathy. Spine (Phila Pa 1976) 1983;8(7):693–699. doi: 10.1097/00007632-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K, Yonenobu K, Ebara S, Yamashita K, Ono K. The prognosis of surgery for cervical compression myelopathy: an analysis of the factors involved. J Bone Joint Surg Br. 1989;71(3):393–398. doi: 10.1302/0301-620X.71B3.2722928. [DOI] [PubMed] [Google Scholar]
- 6.Lee KB, Park IH, Song KW, Lee EJ, Lee JS. Surgical treatment of cervical spondylotic myelopathy. J Korean Orthop Assoc. 1997;32(5):1181–1188. [Google Scholar]
- 7.Kim YT, Lee CS, Lee SW, Choi DS, Lee SW. Prognostic factors affecting the results of the surgery for cervical spondylotic myelopathy. J Korean Soc Spine Surg. 2005;12(4):255–261. [Google Scholar]
- 8.Handa Y, Kubota T, Ishii H, Sato K, Tsuchida A, Arai Y. Evaluation of prognostic factors and clinical outcome in elderly patients in whom expansive laminoplasty is performed for cervical myelopathy due to multisegmental spondylotic canal stenosis. A retrospective comparison with younger patients. J Neurosurg. 2002;96(2 Suppl):173–179. doi: 10.3171/spi.2002.96.2.0173. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka J, Seki N, Tokimura F, Doi K, Inoue S. Operative results of canal-expansive laminoplasty for cervical spondylotic myelopathy in elderly patients. Spine (Phila Pa 1976) 1999;24(22):2308–2312. doi: 10.1097/00007632-199911150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hamburger C, Buttner A, Uhl E. The cross-sectional area of the cervical spinal canal in patients with cervical spondylotic myelopathy: correlation of preoperative and postoperative area with clinical symptoms. Spine (Phila Pa 1976) 1997;22(17):1990–1994. doi: 10.1097/00007632-199709010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura Y, Nishi Y, Nakamura M. Dorsal shift and expansion of the spinal cord after expansive open-door laminoplasty. J Spinal Disord. 1997;10(4):282–287. [PubMed] [Google Scholar]
- 12.Ogino H, Tada K, Okada K, et al. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976) 1983;8(1):1–15. doi: 10.1097/00007632-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Fujimura Y. Magnetic resonance imaging of the spinal cord in cervical ossification of the posterior longitudinal ligament: can it predict surgical outcome? Spine (Phila Pa 1976) 1998;23(1):38–40. doi: 10.1097/00007632-199801010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ramanauskas WL, Wilner HI, Metes JJ, Lazo A, Kelly JK. MR imaging of compressive myelomalacia. J Comput Assist Tomogr. 1989;13(3):399–404. doi: 10.1097/00004728-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Miyazaki K, Tada K, et al. Increased MR signal intensity due to cervical myelopathy: analysis of 29 surgical cases. J Neurosurg. 1991;74(6):887–892. doi: 10.3171/jns.1991.74.6.0887. [DOI] [PubMed] [Google Scholar]
- 16.Okada Y, Ikata T, Yamada H, Sakamoto R, Katoh S. Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine (Phila Pa 1976) 1993;18(14):2024–2029. doi: 10.1097/00007632-199310001-00016. [DOI] [PubMed] [Google Scholar]
- 17.Morio Y, Yamamoto K, Kuranobu K, Murata M, Tuda K. Does increased signal intensity of the spinal cord on MR images due to cervical myelopathy predict prognosis? Arch Orthop Trauma Surg. 1994;113(5):254–259. doi: 10.1007/BF00443813. [DOI] [PubMed] [Google Scholar]
- 18.Yone K, Sakou T, Yanase M, Ijiri K. Preoperative and postoperative magnetic resonance image evaluations of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976) 1992;17(10 Suppl):S388–S392. doi: 10.1097/00007632-199210001-00008. [DOI] [PubMed] [Google Scholar]
- 19.Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y. Correlation between operative outcomes of cervical compression myelopathy and mri of the spinal cord. Spine (Phila Pa 1976) 2001;26(11):1238–1245. doi: 10.1097/00007632-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ. Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: prediction of prognosis with type of intensity. Radiology. 2001;221(3):789–794. doi: 10.1148/radiol.2213010365. [DOI] [PubMed] [Google Scholar]
- 21.Flanders AE, Spettell CM, Friedman DP, Marino RJ, Herbison GJ. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol. 1999;20(5):926–934. [PMC free article] [PubMed] [Google Scholar]