Abstract
The B1 subunit of Escherichia coli ribonucleotide reductase (EC 1.17.4.1) has been overexpressed using the pT7-5/pGP1-2 system developed by Tabor and Richardson [Tabor, S. & Richardson, C. (1985) Proc. Natl. Acad. Sci. USA 82, 1074-1078]. This method has allowed the preparation of two mutant B1 subunits in which two of the four thiols postulated to be within the active site of the enzyme, Cys-225 and Cys-759, have been changed to serines. Incubation of the [Ser225]B1 mutant with the B2 subunit, [U-14C]CDP, and the allosteric effector ATP results in production of cytosine, destruction of the tyrosyl radical in B2, radiolabeling of the protein, and cleavage of the B1 subunit into two pieces of 26 and 61.5 kDa. This process is independent of the identity of reductant. The [Ser759]B1 mutant reduces CDP in the presence of thioredoxin/thioredoxin reductase at 7.7% the rate of wild-type B1. When dithiothreitol is utilized as reductant, however, the rate of CDP reduction with [Ser759]B1 is identical to that observed with wild type.
Full text
PDF
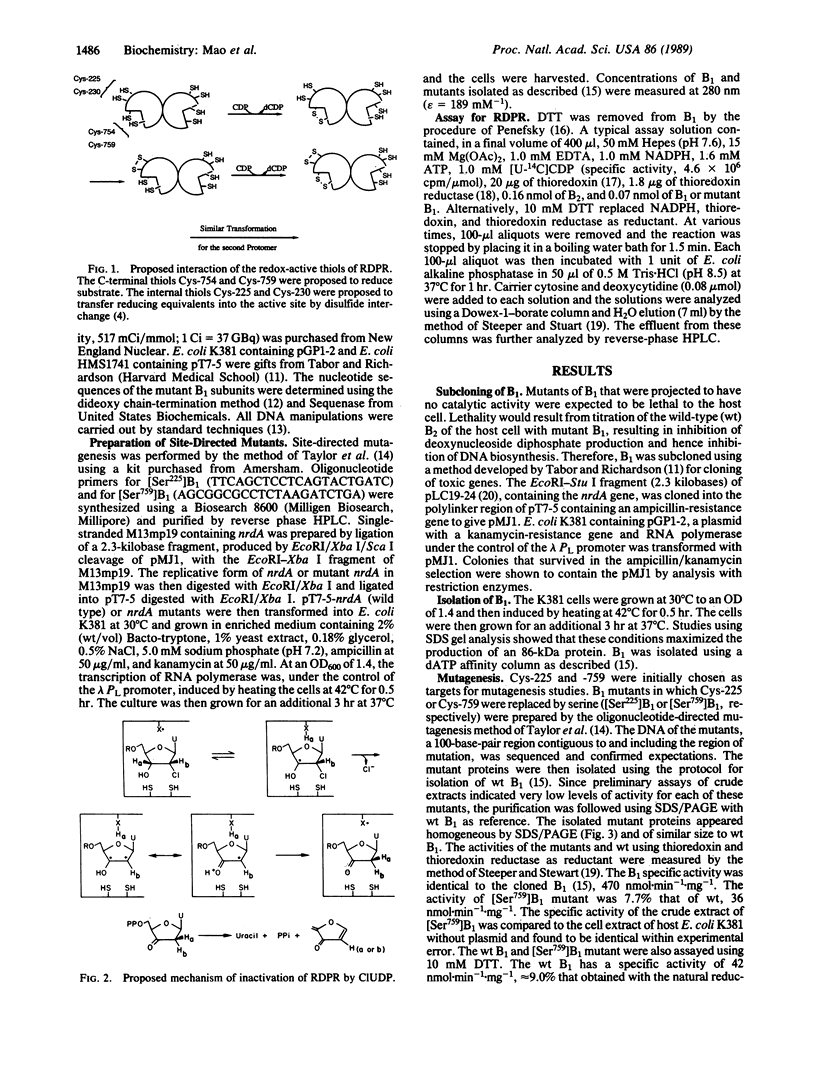
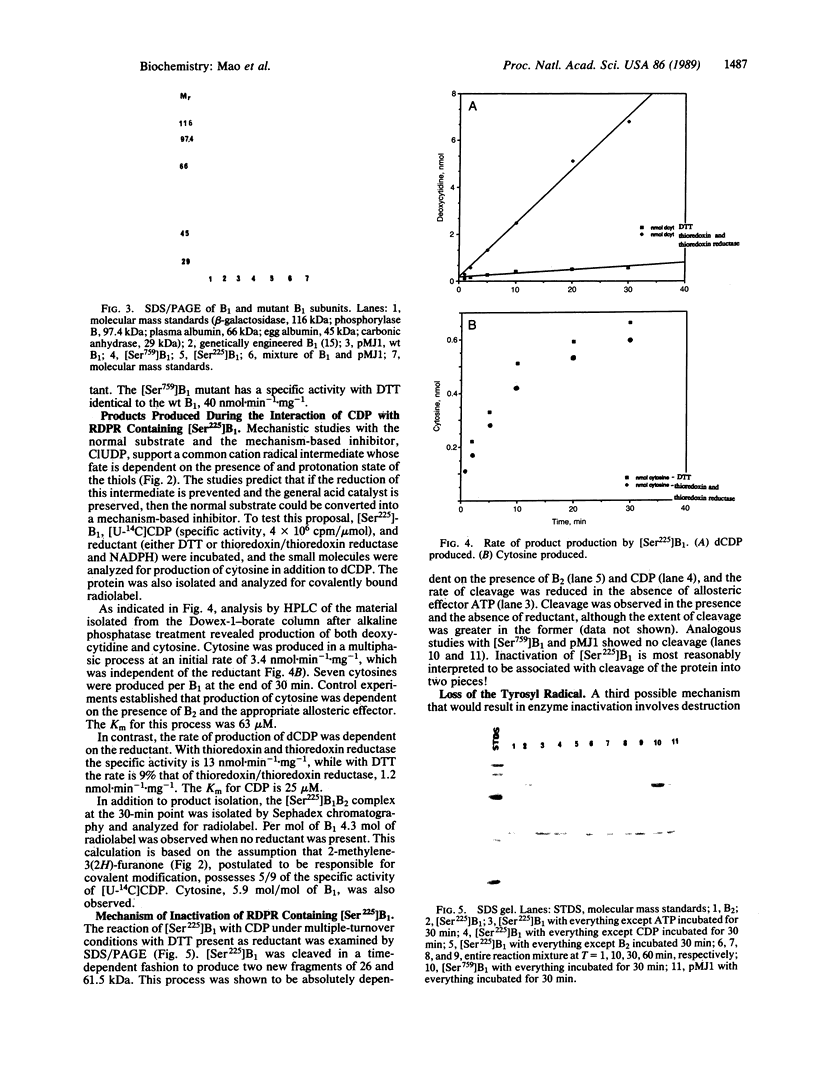

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley G. W., Stubbe J. Current ideas on the chemical mechanism of ribonucleotide reductases. Pharmacol Ther. 1985;30(3):301–329. doi: 10.1016/0163-7258(85)90054-3. [DOI] [PubMed] [Google Scholar]
- Ator M. A., Stubbe J. Mechanism of inactivation of Escherichia coli ribonucleotide reductase by 2'-chloro-2'-deoxyuridine 5'-diphosphate: evidence for generation of a 2'-deoxy-3'-ketonucleotide via a net 1,2 hydrogen shift. Biochemistry. 1985 Dec 3;24(25):7214–7221. doi: 10.1021/bi00346a029. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Sjöberg B. M., Hahne S. Ribonucleoside diphosphate reductase from Escherichia coli. An immunological assay and a novel purification from an overproducing strain lysogenic for phage lambdadnrd. J Biol Chem. 1977 Sep 10;252(17):6132–6138. [PubMed] [Google Scholar]
- Harris G., Ator M., Stubbe J. Mechanism of inactivation of Escherichia coli and Lactobacillus leichmannii ribonucleotide reductases by 2'-chloro-2'-deoxynucleotides: evidence for generation of 2-methylene-3(2H)-furanone. Biochemistry. 1984 Oct 23;23(22):5214–5225. doi: 10.1021/bi00317a020. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sjöberg B. M. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. EMBO J. 1986 Aug;5(8):2037–2040. doi: 10.1002/j.1460-2075.1986.tb04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. N., Ashley G. W., Stubbe J. Location of the redox-active thiols of ribonucleotide reductase: sequence similarity between the Escherichia coli and Lactobacillus leichmannii enzymes. Biochemistry. 1987 Nov 3;26(22):6905–6909. doi: 10.1021/bi00396a006. [DOI] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P., THELANDER L. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES.V. PURIFICATION AND PROPERTIES OF THIOREDOXIN REDUCTASE FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3445–3452. [PubMed] [Google Scholar]
- Marcus F., Moberly L., Latshaw S. P. Comparative amino acid sequence of fructose-1,6-bisphosphatases: identification of a region unique to the light-regulated chloroplast enzyme. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5379–5383. doi: 10.1073/pnas.85.15.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Lundqvist T., Hahne S., Sjöberg B. M. Structure-function studies of the large subunit of ribonucleotide reductase from Escherichia coli. Biochem Soc Trans. 1988 Apr;16(2):91–94. doi: 10.1042/bst0160091. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Salowe S. P., Ator M. A., Stubbe J. Products of the inactivation of ribonucleoside diphosphate reductase from Escherichia coli with 2'-azido-2'-deoxyuridine 5'-diphosphate. Biochemistry. 1987 Jun 16;26(12):3408–3416. doi: 10.1021/bi00386a024. [DOI] [PubMed] [Google Scholar]
- Salowe S. P., Stubbe J. Cloning, overproduction, and purification of the B2 subunit of ribonucleoside-diphosphate reductase. J Bacteriol. 1986 Feb;165(2):363–366. doi: 10.1128/jb.165.2.363-366.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeper J. R., Steuart C. D. A rapid assay for CDP reductase activity in mammalian cell extracts. Anal Biochem. 1970 Mar;34:123–130. doi: 10.1016/0003-2697(70)90092-8. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L. Reaction mechanism of ribonucleoside diphosphate reductase from Escherichia coli. Oxidation-reduction-active disulfides in the B1 subunit. J Biol Chem. 1974 Aug 10;249(15):4858–4862. [PubMed] [Google Scholar]
- Tseng M. J., Hilfinger J. M., Walsh A., Greenberg G. R. Total sequence, flanking regions, and transcripts of bacteriophage T4 nrdA gene, coding for alpha chain of ribonucleoside diphosphate reductase. J Biol Chem. 1988 Nov 5;263(31):16242–16251. [PubMed] [Google Scholar]