Abstract
Background and Purpose
The authors previously reported on walking recovery in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury (SCI) after 76 sessions of locomotor training (LT). Although clinical measures did not predict his recovery, reciprocal patterned leg movements developed, affording recovery of independent walking with a reverse rolling walker. The long-term functional limitations and secondary complications often associated with pediatric-onset SCI necessitate continued follow-up of children with SCI. Therefore, the purpose of this case report is to describe this child's walking function and musculoskeletal growth and development during the 2 years since his participation in an LT program and subsequent walking recovery.
Case Description
Following LT, the child attended elementary school as a full-time ambulator. He was evaluated 1 month (baseline), 1 year, and 2 years after LT. Examination of walking function included measures of walking independence, gait speed and spatiotemporal parameters, gait kinematics, and daily step activity. Growth and development were assessed by tracking his height, weight, incidence of musculoskeletal complications, and gross motor task performance.
Outcomes
Over the 2 years, the child continued to ambulate independently with a reverse rolling walker, increasing his fastest gait speed. Spatiotemporal and kinematic features of his walking improved, and daily step activity increased. Height and weight remained on their preinjury trajectory and within age-appropriate norms. The child experienced only minor musculoskeletal complications. Additionally, he gained the ability to use reciprocal patterned leg movements during locomotor tasks such as assisted stair climbing and independent tricycle pedaling.
Conclusions
Two years after recovery of walking, this child with incomplete SCI had maintained and improved his walking function and experienced age-appropriate growth and development.
Pediatric spinal cord injury (SCI) represents 3% to 5% of all SCI cases, resulting in nearly 2 cases per 100,000 children in the United States.1 Children with SCI experience motor and sensory impairments,2 resulting in long-term functional limitations endured across their life spans.3 Furthermore, as these children grow and develop, they experience additional complications such as bone and joint deformities resulting from reduced weight bearing after injury.4 Although increasing functional mobility is a primary goal of rehabilitation, it traditionally has been accomplished using compensatory movement strategies, as well as wheelchairs, assistive devices, and braces to compensate for residual motor deficits.2,5 In contrast, rehabilitation to promote walking recovery (ie, relative to preinjury walking patterns) relies on the intrinsic neurobiology of walking control and experience-dependent neuroplasticity.6,7
Locomotor training (LT) is a rehabilitation intervention that aims to restore walking after incomplete SCI.7 The principles of LT are based on basic and translational investigations of spinal pattern generators and walking recovery.8 Afferent sensory information critical for producing a basic stepping pattern is optimized in a permissive environment that uses a treadmill and partial body-weight support to enable repetitive walking practice.9 The benefits of this intervention have been reported in adults with SCI10 and described recently in 2 reports of children with severe, incomplete SCI.11,12 Both reports described significant walking recovery in children less than 6 years old with severe, incomplete, cervical SCI. Prosser11 reported the feasibility of carrying out an LT program as part of inpatient rehabilitation and initiated LT with a 5-year-old 1 month after injury. This child with acute SCI made rapid gains in walking function and a concomitant improvement in leg strength (force-generating capacity). The second case report, published by our research group,12 described LT with a child with chronic injury (16 months after injury) who had no prognosis for walking recovery. This child recovered a reciprocal stepping pattern that enabled him to attend elementary school as a full-time ambulator, but he achieved no parallel improvements in clinical measures of strength or volitional, fractionated movement.
Although the children described in these reports made significant gains, both were injured at a young age, which is associated with a greater incidence and severity of secondary complications13 such as scoliosis, hip dysplasia, and lower-extremity bone deformities. In particular, scoliosis and hip dislocation occur in nearly all children who are injured before adolescence.14 Secondary complications are a critical issue in the rehabilitation of children with SCI, especially as medical complications and reduced functional independence are associated with increased depression, lower levels of community participation,15 and difficulty transitioning into adulthood.3 The secondary complications commonly experienced by children with SCI,2 combined with the long-term consequences of pediatric SCI,3 necessitate ongoing assessment of these children.16
This case report is a follow-up to our previous report of a child who recovered reciprocal stepping and independent ambulation 16 months after incomplete cervical SCI.12 The purposes of this case report are to describe this child's walking function and to report on his growth and development during the 2 years since his participation in an LT program and recovery of full-time ambulatory status. Because this child reached a critical threshold of walking independence with a reverse rolling walker, he was able to regularly practice walking and thus “self-train” under varying conditions.17 Therefore, we hypothesized that over this 2-year period, he would maintain his ability to ambulate independently with an assistive device and improve his walking function, as evidenced by increased gait speed, improved spatiotemporal features of his gait pattern, improved gait kinematics, and an increased number of daily steps taken in his home and community. We also hypothesized that his growth and development would be characterized by: (1) age-appropriate increases in height and weight and only minor musculoskeletal complications secondary to his incomplete SCI and (2) ongoing gross motor development, as evidenced by improved scores on standardized assessments of gross motor function and clinical assessments of other locomotor tasks.
Case Description and Rehabilitation History
A 3.5-year-old boy sustained an accidental, self-inflicted gunshot wound, resulting in a cervical (C6–7) SCI. Prior to the accident, the child's motor development was normal, based on his ability to walk, run, jump, bike, and swim. Sixteen months postinjury, he was nonambulatory; his injury was classified using the American Spinal Injury Association (ASIA) Impairment Scale (AIS) as motor-incomplete AIS C, C8 bilaterally, with a Lower Extremity Motor Score (LEMS)18,19 of 4/50. He enrolled in an ongoing study of walking recovery after incomplete SCI and participated in 76 sessions of LT using both the treadmill and over-ground environments.12 During the course of LT, patterned, voluntary stepping emerged that enabled him to achieve over-ground walking with an assistive device. Following completion of LT, he returned to his home community where he attended kindergarten, walking independently with a reverse rolling walker. The complete details of the child's medical history, his progression throughout and immediately following LT, and LT methods were described by Behrman et al.12
Throughout the 2 years following LT, the child attended outpatient physical therapy and occupational therapy sessions 2 to 3 times per week. Therapy did not include LT, but instead targeted skills necessary to function at home and school, such as standing balance, tall kneeling, transfers from sitting to standing and into and out of a vehicle, and upper-body strength. Interventions that focused on walking were directed at using less restrictive assistive devices, such as forearm crutches. Otherwise, there was little focus on locomotor skills or training of reciprocal lower-extremity tasks. Over the 2 years, the child also received standard medical care from his pediatrician and medical specialists following him for his SCI.
Examination
Tests and measures were conducted by a licensed physical therapist at 1 month (baseline), 1 year, and 2 years after LT. Examinations at 1 year and 2 years included about 10 sessions of daily LT to reassess the child's stepping and make recommendations to his parents. The child's mother provided informed consent for participation and use of medical records in compliance with the US Health Information Portability and Accountability Act (HIPAA). Medical records were obtained in order to track the child's musculoskeletal development and to review the amount and type of outpatient therapy received.
Tests and Measures
Neurologic Status
The AIS is a standard assessment of neurologic function for individuals with SCI. Its validity has been established for adults with SCI,18,19 but is questionable when used with young children.20 An alternative assessment for children with SCI has not been established. Therefore, annual assessments included the AIS to classify this child's spinal cord lesion and level of sensory and motor impairment.18,19
Walking Function
Walking independence.
Walking independence was categorized using the Walking Index for Spinal Cord Injury–Version II (WISCI-II).21,22 This 21-item scale (0–20) categorizes walking function based on level of physical assistance required and use of braces and assistive devices required to walk 10 m on a level surface.23
Gait speed and spatiotemporal parameters.
Gait speed and spatiotemporal parameters of the child's walking pattern were examined using computerized, pressure-sensitive mats that record footfalls (GaitMat II* and GAITRite†).23,24 The child walked across the 3.6-m walkway using a reverse rolling walker for a minimum of 2 trials at his fastest comfortable walking speed. Gait speed, cadence, step length, and stride length were calculated using the associated software.
Observational gait analysis.
Walking trials were video recorded from a lateral view and reviewed separately by 2 licensed physical therapists. Qualitative analyses of the child's gait pattern and use of compensatory strategies were performed independently by each therapist and then reviewed for comparison.25
Daily step activity in the home and community.
The number of daily steps taken by the child in his home and community were monitored using the Step Activity Monitor.‡ The Step Activity Monitor is a small accelerometer worn around the ankle that can accurately and reliably count steps in adults with SCI26 and children who are healthy.27 During each assessment, the child wore the device during waking hours for a minimum of 2 days. The total number of steps and activity bouts (number of times the child switched from inactivity to activity) was averaged for the 2 days with the greatest number of steps.28 The total number of steps practiced during LT also was counted from a video recording of session 73 of LT for comparison with the number of steps practiced outside of training. This 1-hour session was representative of the amount of stepping practice the child performed daily in the LT environment.
Growth and Development
Musculoskeletal growth and development.
The child's medical records were reviewed to track his height, weight, and incidence of musculoskeletal impairment over time. Height and weight were compared with national averages for children of the same age.29 Radiographic reports and medical assessments were reviewed for incidence of spine and bone deformity.
Gross motor skill development.
Gross motor skill development was assessed in 2 ways. First, the Gross Motor Function Measure (66-item version) (GMFM-66)30 was administered to quantify changes in motor skills across 5 dimensions: lying and rolling; sitting; crawling and kneeling; standing; and walking, running, and jumping. Although the psychometric properties of this measure have not been reported for use in children with SCI, this measure is a standardized assessment of children with cerebral palsy and is valid and reliable for this population,31 as well as children with traumatic brain injury.32
Second, clinical examination of the child's ability to perform reciprocal lower-extremity tasks also was conducted. When the child initially completed LT, a gross assessment of his ability to negotiate steps was performed. Based on his unexpected ability to negotiate 3 to 4 steps, performance of reciprocal leg movements during stair climbing was reassessed 1 month after LT (baseline). Examinations at 1 year and 2 years after LT expanded to assess reciprocal leg movements in a supine position and while pedaling an adapted tricycle, crawling, stair climbing, and swimming.
Outcomes
Over the 2-year period, the child's AIS scores remained relatively stable, with no notable changes in segmental sensory or motor impairment. Lower-extremity motor control remained characterized by mass synergistic movements and little to no isolated joint control. Despite this persistent lack of isolated lower-extremity joint control, follow-up evaluations at 1 and 2 years after LT supported our hypotheses. The child's recovered walking pattern was maintained and improved and his height and weight advanced normally compared with age-matched norms for children who are healthy, with only minor musculoskeletal complications. He continued to develop gross motor skills and demonstrated reciprocal patterned leg movements in the context of other locomotor tasks.
Neurologic Status
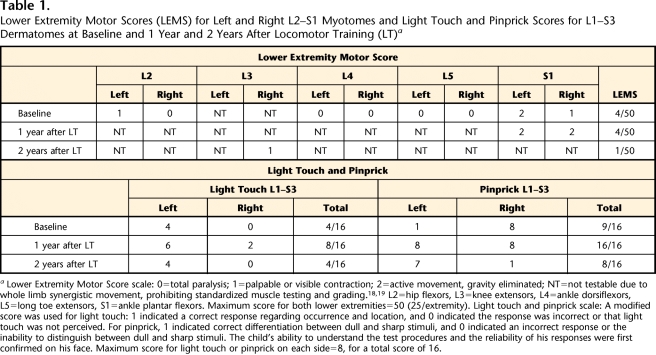
The child's SCI neurological level remained unchanged from baseline (AIS C, C8).12 Lack of isolated lower-extremity movements (noted as “not testable”18,19 in Tab. 1) persisted 1 and 2 years later (Tab. 1). The AIS Motor Examination uses standardized manual muscle testing and grading of key muscles to assess segmental myotome function during voluntary, single-joint movements, such as knee extension. When standardized muscle testing cannot be performed, the muscle is graded as not testable.18,19 Therefore, during instances when the child was unable to perform isolated lower-extremity joint motions and could perform only multijoint, synergistic movements (eg, hip flexion accompanied by knee flexion and ankle dorsiflexion), the myotome was scored as not testable. Variations in sensory scores for light touch and pinprick were observed (Tab. 1) and attributed to the use of this measure in a young child.20
Table 1.
Lower Extremity Motor Scores (LEMS) for Left and Right L2–S1 Myotomes and Light Touch and Pinprick Scores for L1–S3 Dermatomes at Baseline and 1 Year and 2 Years After Locomotor Training (LT)a
Lower Extremity Motor Score scale: 0=total paralysis; 1=palpable or visible contraction; 2=active movement, gravity eliminated; NT=not testable due to whole limb synergistic movement, prohibiting standardized muscle testing and grading.18,19 L2=hip flexors, L3=knee extensors, L4=ankle dorsiflexors, L5=long toe extensors, S1=ankle plantar flexors. Maximum score for both lower extremities=50 (25/extremity). Light touch and pinprick scale: A modified score was used for light touch: 1 indicated a correct response regarding occurrence and location, and 0 indicated the response was incorrect or that light touch was not perceived. For pinprick, 1 indicated correct differentiation between dull and sharp stimuli, and 0 indicated an incorrect response or the inability to distinguish between dull and sharp stimuli. The child's ability to understand the test procedures and the reliability of his responses were first confirmed on his face. Maximum score for light touch or pinprick on each side=8, for a total score of 16.
Walking Function
Walking independence.
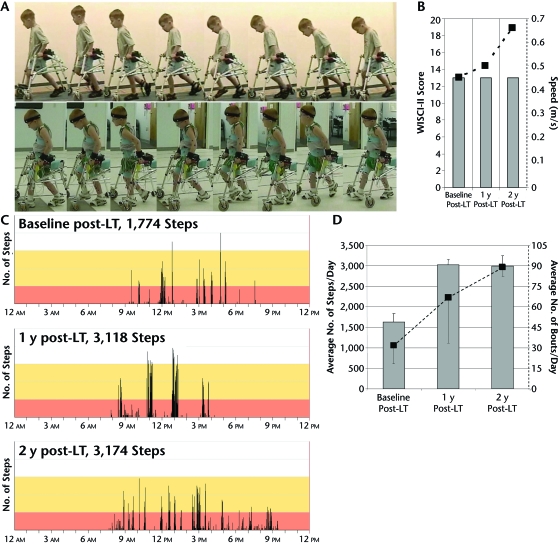
One and 2 years after LT, the child's walking independence remained unchanged (see video). He still required the use of a reverse rolling walker to independently ambulate; therefore, his WISCI-II score remained 13/20 (Fig. 1).
Figure 1.
Walking function. Walking independence was achieved with the use of a reverse rolling walker at baseline (A, top panel). Walking independence remained relatively unchanged at 1 year after locomotor training (LT) (A, bottom panel) and 2 years after LT (Walking Index for Spinal Cord Injury–Version II [WISCI-II] scores; B). Fastest gait speed (dashed line) continued to increase over time, despite no changes in the WISCI-II score (B). The number of steps taken across the 24-hour period increased substantially from baseline to 1 year after LT and was sustained at 2 years after LT (C). This trend also was evident in the number of steps averaged from 2 days (D, black bar graphs) and the corresponding average number of total stepping bouts (D, dashed line). Error bars denote standard deviation.
Gait speed and spatiotemporal parameters.
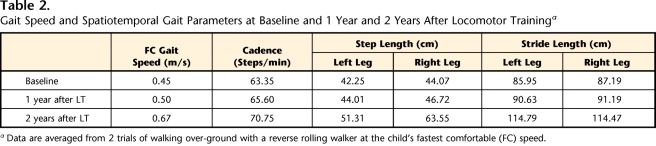
The child's fastest gait speed increased from 0.45 m/s at baseline to 0.67 m/s 2 years after LT (Tab. 2, Fig. 1). Concurrent with his advancing height and leg length, step and stride length increased.33 Although step length became more asymmetrical over the 2 years, stride length remained symmetrical and cadence increased (Tab. 2).
Table 2.
Gait Speed and Spatiotemporal Gait Parameters at Baseline and 1 Year and 2 Years After Locomotor Traininga
Data are averaged from 2 trials of walking over-ground with a reverse rolling walker at the child's fastest comfortable (FC) speed.
Observational gait analysis.
Qualitative analysis of the child's gait pattern during over-ground walking using a reverse rolling walker at baseline (Fig. 1 and video) revealed his ability to walk with upright trunk posture, with minimal weight bearing through his arms while stepping reciprocally. Gait deviations were evident throughout the gait cycle as he used his arms and trunk to shift his weight and exaggerate his stepping to clear his toes during swing. Most notable was the use of his upper extremities to reciprocally push into his walker, moving his shoulder and upper trunk anteriorly to exaggerate his weight shift to the contralateral side and facilitating ipsilateral swing. These compensatory movements were more evident on his left side. During swing, his foot occasionally crossed midline, catching behind the contralateral lower leg. This movement also was more pronounced for the left leg. The child's stepping pattern is shown in Figure 1 and is reviewed in the video.
Two years after LT, the child's gait pattern was improved (Fig. 1 and video). He was able to generate reciprocal stepping with a noticeable absence of shoulder and trunk compensations that were used at baseline. This improvement was particularly evident on his left side, where compensations previously dominated. Although he was able to step reciprocally and perform basic gait adaptations for stopping and turning, he was unable to walk backward, side step, or maintain upright balance without upper-extremity support.
Daily step activity in the home and community.
Over the 2 years, the average number of daily steps this child took at home and in the community increased from about 1,600 steps at baseline to more than 3,000 steps at 1 year and 2 years after LT (Fig. 1). In comparison, the number of steps practiced during a 1-hour LT session on the treadmill with manual assistance and body-weight support totaled 2,732 steps. The average number of stepping bouts steadily increased from 31 at baseline to 88 per day at 2 years after LT (Fig. 1).
Growth and Development
Musculoskeletal growth and development.
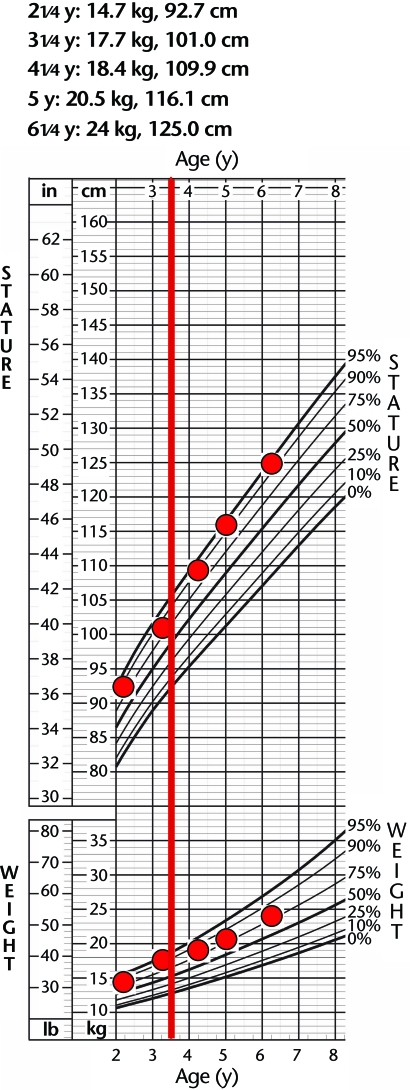
Prior to injury, the child ranked in the 90th to 95th percentiles in both weight and height. Following injury, his height remained in the 90th to 95th percentiles, and his weight was in the 80th percentile (Fig. 2). Medical records of the child's radiology reports indicated minimal changes in his spine curvature and hip joint alignment. Over the 2-year period, he was not diagnosed with scoliosis, but mild coxa valga was noted at both hip joints; radiology reports indicated that all findings were stable. One year after LT, the child returned for his annual evaluation wearing bilateral ankle-foot orthoses. The child's mother explained that the orthoses were prescribed by the child's physician because of concern for the child's “flat feet.” She reported that he wore the orthoses during waking hours to improve foot and ankle alignment, but that he had difficulty walking initially while wearing the orthoses and had to adapt his stepping pattern. All assessments in this report were conducted without the use of orthoses.
Figure 2.
Growth percentiles maintained after spinal cord injury. Measurements for height and weight were plotted against the national standards from the Centers for Disease Control and Prevention29 for children of the same age and sex. The red line at 3.5 years denotes the time at which injury occurred.
Gross motor skill development.
The results from the GMFM-66 suggest this child's total gross motor function remained stable during the 2 years following LT. The total percentage score, reflecting performance across the 5 dimensions, was 65% across all time points (Tab. 3). However, within each dimension of gross motor function, there were variations in the child's performance. The child's fatigue level and lower-extremity muscle tone (resistance to passive stretch) were noted to affect performance on specific test items. For instance, compared with baseline, the child's score in the standing dimension at 1 year after LT decreased 10%, likely due to increased lower-extremity extensor tone that prohibited him from flexing one leg while standing. Although the child continued to require upper-extremity support for balance (eg, a reverse rolling walker) during upright tasks, it was notable that at 1 year after LT, his score increased 11% in the walk, run, jump dimension. Higher scores in this dimension were achieved on tasks requiring greater gait adaptability, such as stopping and turning and kicking a ball.
Table 3.
Gross Motor Function Measure (GMFM-66) Scores at Baseline and 1 Year and 2 Years After Locomotor Training (LT)a
Composite scores were calculated for each dimension of the GMFM-66. Dimension percentage scores were determined by dividing the dimension score by the total possible in each dimension. Dimension percentage scores were summed and divided by the number of dimensions (5) to calculate the total percentage score.
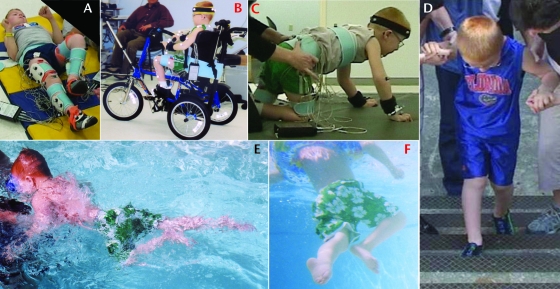
Clinical examination of reciprocal lower-extremity tasks conducted at baseline demonstrated the child's ability to negotiate 3 to 4 steps with maximal assistance for balance and perform reciprocal 4-point crawling (examined on the GMFM-66). At 1 year and 2 years after LT, he was able to generate reciprocal leg movements in a supine position and in the context of tricycle pedaling, negotiation of stairs, crawling, and swimming (Fig. 3 and video). Alternating flexion and extension synergistic leg movements were used to perform these tasks. For instance, in a supine position, leg flexion movements incorporated hip flexion with lateral (external) rotation, knee flexion, and ankle dorsiflexion. During extension, the hip and knee extended as the ankle moved into plantar flexion (Fig. 3A and video).
Figure 3.
Reciprocal lower-extremity tasks. One and 2 years following locomotor training, the child was able to perform rhythmic, reciprocal lower-extremity movements in the context of other locomotor tasks. Lower-extremity activities in addition to walking included reciprocal lower-extremity flexion and extension in a supine position (A), tricycle pedaling (B), crawling (C), stair climbing (D), and swimming (E, F).
Discussion
Reciprocal patterned leg movement enabling independent ambulation with a rolling walker was maintained 2 years after LT in a 6.5-year-old child with severe, incomplete cervical SCI. Although clinical assessments did not predict this child would recover walking,34 16 months after severe SCI, a stepping pattern developed that afforded independent community ambulation with a reverse rolling walker. Two years after this initial recovery, he continued to ambulate full-time, and his fastest walking speed and the number of steps taken daily progressively increased.
Because repetition and practice are critical factors in skill acquisition and motor learning, achievement of independent community ambulation was likely a critical threshold that enabled ongoing stepping practice in varying environments to reinforce his stepping pattern.17 Furthermore, during the time since his recovery, he took nearly 3,000 steps per day, which exceeds the more than 2,500 steps he practiced during a 1-hour session of LT. Although 3,000 daily steps is considerably less than the 8,000 daily steps taken by uninjured children,35 this level of daily walking practice and upright activity is a notable contrast to children who use a wheelchair full-time. Moreover, it is likely that upright physical activity, including load-bearing steps, contributed to his healthy physical development. Nearly 4 years after injury, he had not been diagnosed with scoliosis, a pervasive condition occurring in 97% of children injured before age 10 years.14
Although the child continued to require a reverse rolling walker, this device was specifically selected and used in a manner to minimize compensatory gait strategies and promote ongoing stepping recovery. Compared with a forward walker, the reverse rolling walker places the support base behind the body, which minimizes load bearing on the arms and encourages an upright trunk posture and appropriate limb kinematics. This task-specific sensorimotor experience enhances reciprocal leg muscle activation to generate a rhythmic stepping pattern for walking and was emphasized during his initial LT program on the treadmill as well as over-ground walking practice.12
As the child matured and became more socially active over the 2 years, his desire to keep up with his peers motivated the development of alternative strategies for using his walker. The child adapted his gait pattern and compensated with his arms to “skate” or “ride” on his rolling walker to gain increased speed. As the child becomes more engaged with his peers, especially as he approaches adolescence, the social demands of keeping up with his peers may further influence his stepping pattern and ultimately his physical development. These complex interactions will continue to be studied as this child enters adolescence and young adulthood.
An unexpected outcome of this 2-year follow-up was the child's ability to produce reciprocal patterned leg movements in the context of other tasks such as tricycle pedaling, crawling, stair climbing, and swimming. When he initially enrolled in the LT program 16 months after injury, he could only initiate mass extension leg movements and could not reciprocally flex and extend his legs. Reciprocal leg movements developed through the course of LT (after 20 sessions) and were dependent on task-specific cues such as upright posture and hip joint kinematics.12 Thus, the ability to perform reciprocating leg movements during other tasks suggests that he was no longer dependent on the afferent information associated with walking. Interestingly, recent investigations suggest that there may be similar control mechanisms for varied rhythmic, reciprocal lower extremity tasks.36 Evidence of potential neural mechanisms contributing to this child's ongoing recovery, however, is not provided in this report. Furthermore, outcomes in this case report have been limited to clinical assessments without detailed biomechanical analyses of the child's locomotor pattern over time. These methods are currently in development as we follow this child's progression into adolescence and adulthood.
It is critical to further investigate the efficacy of LT in promoting walking recovery in children with incomplete SCI, and conducting comprehensive, quantitative assessments over time provides a longitudinal view of the maintenance and progression of recovery. Rehabilitation interventions that target recovery of function and activation of the neuromuscular system after SCI may be beneficial in reducing the incidence and severity of secondary complications. However, long-term investigations of the complex interactions among injury, recovery, and musculoskeletal development are necessary to better understand the current and potential trajectory of this population.
Conclusions
After being severely injured at 3.5 years, at 16 months postinjury, this child was predicted (by a LEMS of 4/50) to remain nonambulatory and require full-time use of a wheelchair. His initial recovery following 76 sessions of LT suggests that voluntary, isolated limb movements are not a prerequisite for walking recovery.12,37 This follow-up report describes the child's ongoing recovery 2 years following LT and suggests that children with SCI have the potential to sustain the skills recovered following LT. These outcomes are particularly important in a pediatric case given the detrimental impact of long-term, full-time wheelchair use and lack of load bearing on growth and development.
Supplementary Material
Footnotes
Dr Fox, Dr Howland, and Dr Behrman provided concept/idea/project design. Dr Fox, Dr Tester, Dr Howland, and Dr Behrman provided writing. All authors provided data collection and analysis. Dr Fox and Dr Behrman provided project management. Dr Behrman provided fund procurement. Dr Phadke, Dr Nair, and Dr Senesac provided consultation (including review of manuscript before submission). The authors extend their sincere appreciation to the University of Florida undergraduate student volunteers, Doctor of Physical Therapy students, and Rehabilitation Science Doctoral (PhD) students who assisted in this study and to the child and his family for their ongoing support of rehabilitation and recovery research.
The University of Florida Health Science Center Institutional Review Board approved this study.
This work, in part, was presented at the Howard H. Steel Conference: Pediatric Spinal Cord Injuries and Dysfunction; December 3–5, 2009; Lake Buena Vista, Florida; the Combined Sections Meeting of the American Physical Therapy Association; February 14–18, 2007; Boston, Massachusetts; the III STEP Conference, sponsored by the Neurology and Pediatrics sections of the American Physical Therapy Association; July 19, 2005, Salt Lake City, Utah; and the Pediatric Spinal Cord Injury Conference, sponsored by Tingley Children's Hospital and the Department of Pediatrics, College of Medicine, University of New Mexico; July 10, 2005; Albuquerque, New Mexico.
This work was supported by the Craig H. Neilsen Foundation, the University of Florida Brooks Center for Rehabilitation Studies, the Florida Department of Health Brain and Spinal Cord Injury Program, NICHD-NCMRR grant K01 HD 0134801, and NIH (OD) NCRR grant TL1RR029889.
EQ Inc, 3469 Limekiln Pike, Chalfont, PA 18914.
CIR Systems Inc, 60 Garlor Dr, Havertown, PA 19083.
Cyma Corp, 6405 218th St SW, Suite 100, Mountlake Terrace, WA 98043.
References
- 1.Vitale MG, Goss JM, Matsumoto H, Roye DP., Jr Epidemiology of pediatric spinal cord injury in the United States: years 1997 and 2000. J Pediatr Orthop 2006;26:745–749 [DOI] [PubMed] [Google Scholar]
- 2.Vogel LC, Hickey KJ, Klaas SJ, Anderson CJ. Unique issues in pediatric spinal cord injury. Orthop Nurs 2004;23:300–308; quiz 309–310 [DOI] [PubMed] [Google Scholar]
- 3.Anderson CJ, Vogel LC, Betz RR, Willis KM. Overview of adult outcomes in pediatric-onset spinal cord injuries: implications for transition to adulthood. J Spinal Cord Med 2004;27(suppl 1):S98–S106 [DOI] [PubMed] [Google Scholar]
- 4.Bergstrom EM, Short DJ, Frankel HL, et al. The effect of childhood spinal cord injury on skeletal development: a retrospective study. Spinal Cord 1999;37:838–846 [DOI] [PubMed] [Google Scholar]
- 5.Vogel LC, Mendoza MM, Schottler JC, et al. Ambulation in children and youth with spinal cord injuries. J Spinal Cord Med 2007;30(suppl 1):S158–S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am 2007;18:183–202 [DOI] [PubMed] [Google Scholar]
- 7.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: an emerging paradigm shift in rehabilitation and walking recovery. Phys Ther 2006;86:1406–1425 [DOI] [PubMed] [Google Scholar]
- 8.Edgerton VR, Tillakaratne NJ, Bigbee AJ, et al. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 2004;27:145–167 [DOI] [PubMed] [Google Scholar]
- 9.Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair 2003;17:3–11 [DOI] [PubMed] [Google Scholar]
- 10.Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 2000;80:688–700 [PubMed] [Google Scholar]
- 11.Prosser LA. Locomotor training within an inpatient rehabilitation program after pediatric incomplete spinal cord injury. Phys Ther 2007;87:1224–1232 [DOI] [PubMed] [Google Scholar]
- 12.Behrman AL, Nair PM, Bowden MG, et al. Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury. Phys Ther 2008;88:580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel LC, Krajci KA, Anderson CJ. Adults with pediatric-onset spinal cord injury, part 1: prevalence of medical complications. J Spinal Cord Med 2002;25:106–116 [DOI] [PubMed] [Google Scholar]
- 14.Betz RR, Mulcahey MJ. Spinal cord injury rehabilitation. In: Weinstein SL. ed. The Pediatric Spine: Principles and Practice 2nd ed.New York, NY: Raven Inc; 2001:601–618 [Google Scholar]
- 15.Anderson CJ, Kelly EH, Klaas SJ, et al. Anxiety and depression in children and adolescents with spinal cord injuries. Dev Med Child Neurol 2009;51:826–832 [DOI] [PubMed] [Google Scholar]
- 16.Edgerton VR. Invited commentary on “Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury.” Phys Ther 2008;88:590–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord 1998;36:744–749 [DOI] [PubMed] [Google Scholar]
- 18.Marino RJ, Barros T, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003;26(suppl 1):S50–S56 [DOI] [PubMed] [Google Scholar]
- 19.Maynard FM, Jr, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury: American Spinal Injury Association. Spinal Cord 1997;35:266–274 [DOI] [PubMed] [Google Scholar]
- 20.Mulcahey MJ, Gaughan J, Betz RR, Johansen KJ. The International Standards for Neurological Classification of Spinal Cord Injury: reliability of data when applied to children and youths. Spinal Cord 2007;45:452–459 [DOI] [PubMed] [Google Scholar]
- 21.Ditunno JF, Jr, Ditunno PL, Graziani V, et al. Walking Index for Spinal Cord Injury (WISCI): an international multicenter validity and reliability study. Spinal Cord 2000;38:234–243 [DOI] [PubMed] [Google Scholar]
- 22.Dittuno PL, Dittuno JF., Jr Walking Index for Spinal Cord Injury (WISCI II): scale revision. Spinal Cord 2001;39:654–656 [DOI] [PubMed] [Google Scholar]
- 23.Jackson AB, Carnel CT, Ditunno JF, Jr, et al. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med 2008;31:487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe DE, Dusing SC, Moore CG. Repeatability of temporospatial gait measures in children using the GAITRite electronic walkway. Arch Phys Med Rehabil 2005;86:2342–2346 [DOI] [PubMed] [Google Scholar]
- 25.Observational Gait Analysis Handbook Downey, CA: Professional Staff Association of Rancho Los Amigos Medical Center; 1989 [Google Scholar]
- 26.Bowden MG, Behrman AL. Step Activity Monitor: accuracy and test-retest reliability in persons with incomplete spinal cord injury. J Rehabil Res Dev 2007;44:355–362 [DOI] [PubMed] [Google Scholar]
- 27.McDonald CM, Widman L, Abresch RT, et al. Utility of a step activity monitor for the measurement of daily ambulatory activity in children. Arch Phys Med Rehabil 2005;86:793–801 [DOI] [PubMed] [Google Scholar]
- 28.Cavanaugh JT, Coleman KL, Gaines JM, et al. Using step activity monitoring to characterize ambulatory activity in community-dwelling older adults. J Am Geriatr Soc 2007;55:120–124 [DOI] [PubMed] [Google Scholar]
- 29.National Center for Health Statistics (developed with the National Center for Chronic Disease Prevention and Health Promotion). Available at: http://www.cdc.gov/growthcharts. Published 2000 Accessed May 3, 2009
- 30.Russell D, Rosenbaum P, Avery L, Lane M. The Gross Motor Function Measure (GMFM-66 & GMFM-88) User's Manual London, United Kingdom: Mac Keith Press; 2002 [Google Scholar]
- 31.Russell DJ, Avery LM, Rosenbaum PL, et al. Improved scaling of the Gross Motor Function Measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther 2000;80:873–885 [PubMed] [Google Scholar]
- 32.Linder-Lucht M, Othmer V, Walther M, et al. Validation of the Gross Motor Function Measure for use in children and adolescents with traumatic brain injuries. Pediatrics 2007;120:e880–e886 [DOI] [PubMed] [Google Scholar]
- 33.Sutherland D, Olshen R, Cooper L, Woo S. The development of mature gait. J. Bone Joint Surg Am 1980;62:336–353 [PubMed] [Google Scholar]
- 34.Burns AS, Ditunno JF. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine 2001;26(24 suppl):S137–S145 [DOI] [PubMed] [Google Scholar]
- 35.Song KM, Bjornson KF, Cappello T, Coleman K. Use of the StepWatch activity monitor for characterization of normal activity levels of children. J Pediatr Orthop 2006;26:245–249 [DOI] [PubMed] [Google Scholar]
- 36.Zehr EP. Neural control of rhythmic human movement: the common core hypothesis. Exerc Sport Sci Rev 2005;33:54–60 [PubMed] [Google Scholar]
- 37.Maegele M, Muller S, Wernig A, et al. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma 2002;19:1217–1229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.