Abstract
In this study, we compared corn gluten hydrolyzates, BCAAs, and leucine for their effects on body weight reduction in high fat-induced obese rats in order to determine the major active components in the corn gluten hydrolyzates. After obesity was induced for 13 weeks with high fat diet, the overweight-induced SD rats (n = 64) were stratified according to body weight, randomly blocked into eight treatments, and raised for 8 weeks. Four groups were changed to a normal diet and the other groups remained on the high fat diet. Each of the groups within both diets was fed either casein, corn gluten hydrolyzates, leucine, or branched chain amino acids, respectively. Daily food intake, body weight gain, and food efficiency ratio were significantly lower in the corn gluten hydrolyzate groups compared to the other groups, regardless of the high fat diet or normal fat diet. The rats fed the corn gluten hydrolyzates diet had the lowest perirenal fat pad weights whereas muscle weight was significantly increased in the corn gluten hydrolyzates groups. Plasma triglyceride, hepatic total lipid, and total cholesterol contents were significantly reduced in the corn gluten hydrolyzates groups. Other lipid profile measurements were not significantly changed. Plasma triglyceride and hepatic total lipid were also significantly reduced in the BCAA and leucine groups. Leptin levels were significantly lower and adiponectin was significantly higher in the corn gluten hydrolyzates groups. Fasting blood glucose, insulin, C-peptide, and HOMA-IR levels were also significantly reduced in the corn gluten hydrozylates groups, regardless of fat level.
Keywords: Corn gluten hydrolyzates, branched chain amino acids, leucine, body weight, insulin resistance
Introduction
Obesity is defined as "abnormal or excessive fat accumulation in the body due to an imbalance between energy intake and consumption." This obesity can lead to chronic disease complications such as cardiac disorders, hypertension, hyperlipidemia, and diabetes, which threaten the health of humankind. The cause of obesity is very complicated results from interactions among a variety of factors, however, two reasons are suggested as the main cause: over-nutrition and insufficient energy consumption. Diet control, in particular, is emphasized to prevent and treat obesity [1,2]. In Korea, the obesity occurrence rate (above 19 years old) was increased 5.7% over 10 years, from 26.0% in 1998 to 31.7% in 2007 [3].
In the early stage of obesity research, the focus was merely on cutting calorie intake to prevent obesity, but this method may cause loss of not only body-fat but also body-protein. As a result, research is now focused on the effects of specific nutrients and substances. As plant proteins usually have inferior functional qualities compared to animal proteins, particular attention has been given to the enzymatic hydrolysis of plant proteins. Among plant sources, soybeans are widely used to obtain protein hydrolyzates, but several studies on the preparation of corn gluten hydrolyzates have also been reported [4,5,6,7,8,9,10,11].
Corn is a major cereal crop throughout the world. Corn gluten is used mainly for animal feed because it lacks functional properties essential for food. However, based on its biochemical structure such as high hydrophobicity, as well as its low price and high abundance, corn gluten is becoming an interesting source for food and non-food applications [4]. Corn has a high amount of branched chain amino acids (BCAAs), especially leucine, which plays an important role in body metabolism [12, 13]. Leucine is well known to stimulate muscle protein synthesis, on the other hand, it has been recently demonstrated to decrease food intake and body weight by stimulating hypothalamic mammalian target of rapamycin (mTOR) signaling [14]. Thus, the potential role of corn gluten and its hydrolyzates containing high amount of BCAAs especially leucine in body weight control makes this an interesting area for further investigation.
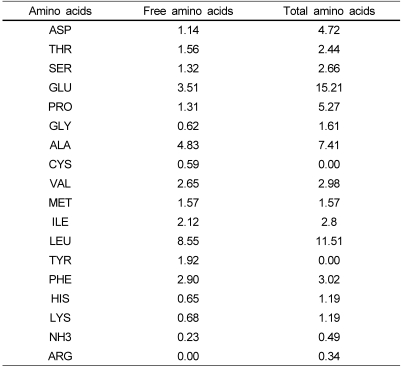
In our previous research we studied the effects of various vegetable proteins and protein hydrolyzates on weight loss in rats with obesity induced by a high-fat diet [11]. That study revealed that corn gluten hydrolyzate, having high free amino acids, reduced weight gain; however, it showed negative nitrogen balance. We cautiously speculated that the effects of the corn gluten hydrolyzate may be explained by its high amounts of BCAA and leucine (Table 1). Thus in this study, we compared corn gluten hydrolyzate, BCAAs, and leucine for their effects on body weight reduction in high fat-induced obese rats in order to determine the major active components of the corn gluten hydrolyzates.
Table 1.
Amino acid composition of corn gluten hydrolyzates (g/100 g)
Materials and Methods
Corn gluten peptide, leucine, and branched chain amino acids
The corn gluten peptide used in this study was provided by Sempio Food Company (Seoul, Korea) and was the same as that used in our previous study [11], which was manufactured using alcase, protamex, and flavourzyme (Novo Nordisck's Enzyme Business, Denmark). Food grade leucine and branched chain amino acids (BCAAs; valine, isoleucine, leucine) were kindly provided by Vixxol Korea (Ajinomoto, Japan). The total amino acid composition of the corn gluten peptides is shown in Table 1.
Animals
Eight-week-old male Sprague-Dawley rats (CD (SD)IGS, Outbred, Charles River Origin; Jung-Ang Lab. Animal, Inc., Seoul, Korea) were placed in individual stainless steel wire-mesh cages in an air-conditioned room (22-24℃) with a 12 h light/dark cycle and 45 ± 5% humidity in compliance with the Guide for the Care and Use of Laboratory Animals [15]. Feed (chow or experimental diets) and water (boiled and filtered) were provided ad libitum at all times. All animals were used in the experiments after 10 days of acclimatization and were sacrificed after the experiments.
Experimental design and diets
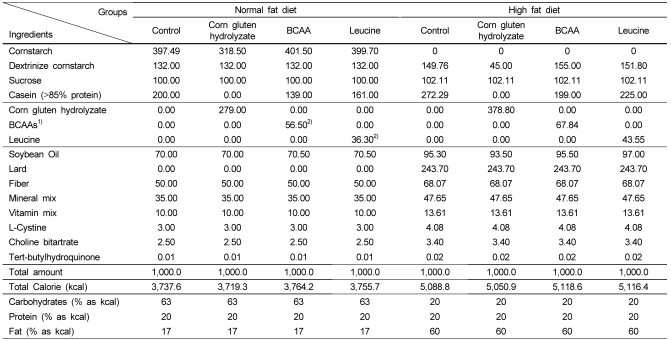
The SD rats (n = 72) were fed a modified American Institute of Nutrition (AIN)-93M diet [16] with high fat (60% of energy as fat) for 13 weeks. The lipid sources in the high fat diet were lard and soybean oil. Each amount of protein, fiber, minerals, and vitamins per total calories in the high fat diet was equalized to that of the AIN-93M diet (Table 2) [17]. Besides the high fat group, in order to confirm overweight, 8 SD rats were fed a normal fat diet as a control group. After 13 weeks, 8 SD rats were randomly selected and sacrificed with the control diet group in order to confirm that overweight was induced using body weight and fat mass. Body weight, body weight gain, and fat mass were significantly higher in the high fat diet group than in control diet group (Table 3). We confirmed that overweight was induced based on these results. The overweight-induced SD rats (n = 64) were then stratified according to body weight, randomly blocked into eight treatments, and raised for 8 weeks. Four groups were changed to a normal fat diet and the other groups remained on the high fat diet. Each of the groups within both diets was fed either control protein, corn gluten peptide, leucine, or branched chain amino acids, respectively. The corn gluten peptide group was fed corn gluten peptide as the only protein source, and the amounts of leucine and BCAAs in the corn gluten peptide were calculated and then adjusted with casein for the same protein concentration in all experimental diets. The composition and proportion of BCAAs (valine : isoleucine : leucine = 1.25 : 1 : 4.04) also corresponded to that of the amino acid composition of the corn gluten hydrolyzate. The compositions of the experimental diets are shown in Table 2. All other materials were purchased from Dyets Inc. (Bethlehem, PA, USA). Body weight and food intake were measured every week. After the 8-week period, the rats were sacrificed by exsanguination from the heart under light ether anesthesia. The plasma was separated by centrifugation and the livers were removed, weighed, and cut into small pieces. All the samples were then stored at -80℃ until further analysis. Feces were collected using metabolic cages for the final three days of the experimental period. Perirenal fat pads, epididymal fat pads, interscapular brown adipose tissue, and soleus muscle tissue were removed and weighed after sacrificing the animals.
Table 2.
The composition of the experimental diets (g/kg diet)
1)The composition and proportion of BCAAs (valine : isoleucine : leucine = 1.25 : 1 : 4.04) corresponded to the amino acid composition of the corn gluten hydrolyzates.
2)The amounts of leucine and BCAAs were the same as the amounts in the corn gluten peptide.
Table 3.
Body weight and Fat pad weights in rats fed diets with different levels of dietary fat during and after an overweight induction period
1)CON: 17% calories from fat, HF: 60% calories from fat
2)Mean ± standard error (S.E)
*Significantly different at the level of α=0.05 by using Student's t-test.
Biochemical analysis
The total lipid concentrations in plasma, liver and feces were measured using the Frings & Dunn's method [18] and Bligh & Dyer's method [19], respectively. Plasma concentrations of triglycerides, total cholesterol, and high density lipoprotein (HDL) cholesterol, as well as hepatic and fecal concentrations of triglyceride and total cholesterol were measured using a commercial kit (Asan Pharmaceutical, Seoul, Korea). Serum glucose was measured using an enzymatic method with a test kit from Kodak Ektachem (Rochester, NY, USA). Insulin was determined by ELIZA with a kit from Mercodia (Uppsala, Sweden). Insulin sensitivity was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) [Fasting plasma insulin (µU/ml) x fasting blood glucose (mmol/l)/22.5]. Leptin and adiponection in the plasma were analyzed using two-site enzyme immunoassay kit (Linco Research, USA; B-bridge, Japan, respectively).
Statistical analysis
All statistical analyses were performed using the SAS program package version 9.1. Differences between the control and treatment groups were analyzed by Student's t-test. As a way to compare several groups, one-way analysis of variance (ANOVA) with Duncan's multiple range tests were used at P < 0.05. Data were also analyzed using two-way ANOVA to test for main effects. All results are expressed as the mean ± standard error (SE).
Results
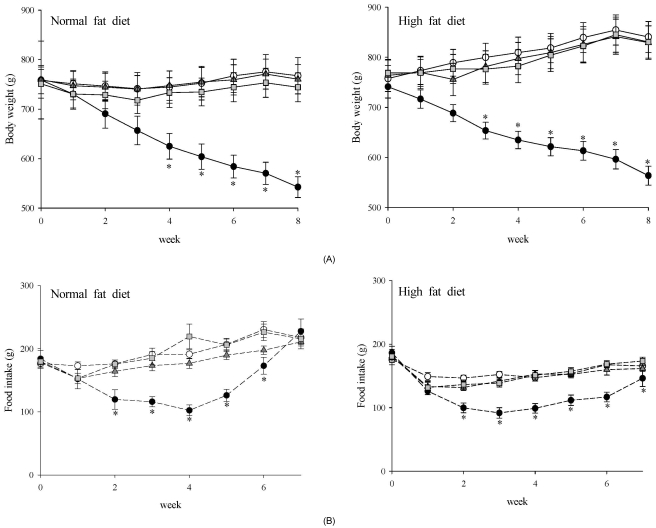
Body weight changes and food intakes are shown in Fig. 1. Body weight in the group of corn gluten hydrolyzates was reduced regardless of a high fat or normal fat diet and significant effects began to appear at 2 weeks (Fig. 1A). Food intake was significantly influenced by corn gluten hydrolyzates (P < 0.05). Groups fed the corn gluten hydrolyzates had significantly lower calorie intakes than other groups (data not shown). Because of scarifying and collecting metabolic samples, we did not measure daily food intake at 8 weeks. With body weight decrease, food intake was also decreased in the group of corn gluten hydrolyzates, however, from 4 weeks, and the level of food intake was increased and reached the same level as other groups (Fig. 1B). Food intake was not significantly influenced by dietary fat levels (data not shown), and rats fed high fat diet showed increasing tendency slightly. BCAAs and leucine did not affect the body weight reduction and daily food intake in this study.
Fig. 1.
The changes of body weight (A) and food intake (B) during 8 weeks treatment of corn gluten hydrolyzates, BCAAs, and leucine. Because of scarifying and collecting metabolic samples, food intake was measured for 7 weeks. Values are expressed as means ± SE shown by vertical bars. Statistical significance was calculated by one-way ANOVA. Comparison were done among different groups (*P < 0.05) (○ casein as control, • corn gluten hydrolyzates, ▴ BCAAs, ▪ Leucine).
The weights of the perirenal fat pads, epididymal fat pads, brown adipose tissue, and muscle tissue are shown in Table 4. The rats fed the corn gluten hydrolyzate diet had the lowest weight for perirenal fat pads, whereas muscle weight was significantly increased in the corn gluten hydrolyzate groups. On the contrary, the BCAA and leucine groups showed no significant changes in body weight or body fat reduction.
Table 4.
Fat pad weights and muscle weights in rats fed diets with corn gluten hydrolyzates, BCAAs, and leucine
1)Mean ± SE
2)Values are not significantly different among the groups by Duncan's multiple range test.
3)Values with different levels within a cell are significantly different at P < 0.05 by Duncan's multiple range test.
4)Statistical significance of experimental factors was calculated using two-way ANOVA.
A: Effect of dietary fat level was significant at α=0.05.
B: Effect of protein source was significant at α=0.05.
AB: Interaction of dietary fat and protein source was significant at α=0.05.
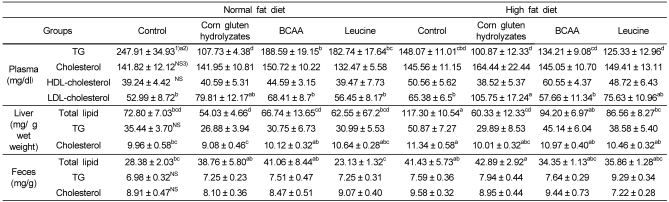
Lipid concentrations in the plasma, liver, and feces are shown in Table 5. Plasma triglyceride and hepatic total lipid were significantly reduced in the corn gluten hydrolyzate groups. Other lipid profile measurements were not changed significantly. Plasma triglyceride and hepatic total lipid were also significantly reduced in the BCAA and leucine groups.
Table 5.
Lipid concentrations in plasma, liver, and feces of rats fed diets with corn gluten hydrolyzates, BCAAs, and leucine
1)Mean ± SE
2)Values with different levels within a cell are significantly different at P < 0.0001 by Duncan's multiple range test.
3)Values are not significantly different among the groups by Duncan's multiple range test.
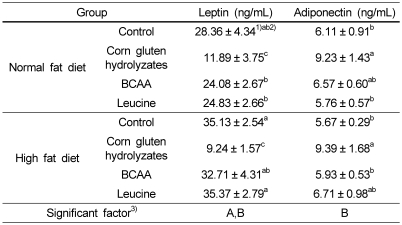
We also examined levels of adipokines such as leptin and adiponectin, which are hormones secreted by adipose tissue. Leptin levels were high in the high fat diet group, however, adiponection showed no effect depending on the fat level. Levels of both of these hormones were significantly affected by protein source, in which leptin levels were significantly lower and aiponectin levels significantly higher in the corn gluten hydrolyzate groups (Table 6).
Table 6.
Adipokine concentrations in plasma of rats fed diets with corn gluten hydrolyzates, BCAAs, and leucine
1)Mean ± SE
2)Values with different levels within a cell are significantly different at P < 0.001 by Duncan's multiple range test.
3)Statistical significance of experimental factors was calculated using two-way ANOVA.
A: Effect of dietary fat level was significant at α=0.05.
B: Effect of protein source was significant at α=0.05.
AB: Interaction of dietary fat and protein source was significant at α=0.05.
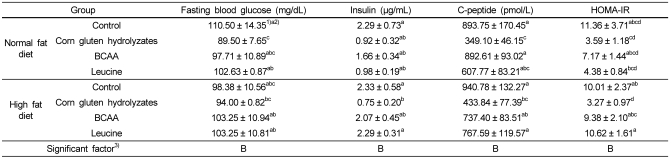
Fasting blood glucose, insulin, C-peptide, and HOMA-IR levels were also significantly reduced in the corn gluten hydrolyzates groups, regardless of fat level (Table 7). Fasting blood glucose did not show significant differences between dietary fat levels but was significantly reduced depending on the protein source, and the rats fed the corn gluten hydrolyzate had significantly lower fasting blood glucose levels. Plasma insulin level showed a similar pattern to fasting blood glucose and HOMA-IR, an index of insulin resistance, and was significantly improved in the corn gluten hydrolyzate groups. C-peptide, which is a precursor of insulin, was also significantly reduced depending on the protein source and regardless of dietary fat level. C-peptide was significantly lower in the corn gluten hydrolyzate and leucine groups, and the corn gluten hydrolyzate groups had the lowest levels.
Table 7.
Fasting blood glucose, insulin, C-peptide, and HOMA-IR in rats fed diet with corn gluten hydrolyzates, BCAAs, and leucine
1)Mean ± SE
2)Values with different levels within a cell are significantly different at P < 0.05 by Duncan's multiple range test.
3)Statistical significance of experimental factors was calculated using two-way ANOVA.
A: Effect of dietary fat level was significant at α=0.05.
B: Effect of protein source was significant at α=0.05.
AB: Interaction of dietary fat and protein source was significant at α=0.05.
Discussion
Several studies have examined the effects of plant proteins and their hydrolyzates on weight reduction in experimental animals [1,20-23]. Some soybean protein isolates showed weight reduction effects [1,20] but other plant protein hydrolyzates did not [21-23]. Our previous report indicated that body weight was decreased by corn gluten hydrolyzate; however, corn gluten and other hydrolyzates did not decrease body weight [11]. In that report, we proposed that the different results of various studies examining the effects of plant proteins and their hydrolyzates on body weight might be explained by several possible differences, such as the ratio of free amino acids and peptides in the experimental diets, the amounts of BCAAs and indispensible amino acids in the experimental diets, the fecal lipid excretions or the activities of hepatic enzymes related to lipid metabolism in the experimental animals. In the previous report, we showed that down-regulation rather than up-regulation of hepatic lipogenic enzyme activities seemed to contribute to decreased body and adipose tissue weights, but we could not determine the exact mechanism [11]. In addition, we did not determine the working mechanism of each dietary factor, or how much each dietary factor had contributed to weight reduction. Thus, in this study, we studied the effects of corn gluten hydrolyzate, having high BCAA and leucine contents, compared to BCAA and leucine using high fat-induced obese rats, respectively.
In this study, we confirmed that corn gluten hydrolyzate was significantly effective for body weight reduction and improvements of lipid metabolism. However, we did not reveal whether the effectiveness of the corn gluten hyrolyzate was attributed to BCAAs or leucine. Although corn gluten hydrolyzate has high amounts of BCAAs and leucine compared to other amino acids as free amino acid, it still seems to have many small peptides inferred by its free amino acid and total amino acid contents (Table 1). Therefore, in order to find the active compounds for body weight reduction in corn gluten hydrolyzate, further studies on the purification and isolation of active peptides are required. With decreased body weight, daily food intake was also decreased (Fig. 1B). At the first half period of this research, daily food intake and body weight were reduced with a slight curve, however, from 4 weeks, and the daily food intake started to increase while body weight has continued to decrease. Cota et al. [14] reported that high leucine intakes affected to decrease food intake and body weight by stimulating the hypothalamic mammalian target of rapamycin (mTOR) signaling and there are relatively specific interactions among mTOR, leptin and insulin resistance. Therefore, we studied the effect of corn gluten hydrolyzate on insulin sensitivity and adipokine levels.
Although leucine is known to be an enhancer of insulin sensitivity, it seems that prolonged, very high intakes of leucine may lead to insulin resistance and ultimately lead to a blunting of the stimulation of muscle protein synthesis [24]. However, in our other previous study, we showed that leucine supplementation for 5 weeks improved insulin resistance [25]. In this study, we also confirmed that the corn gluten hydrolyzate, which has a high amount of leucine, improved insulin resistance, and the leucine diet also showed a slight tendency for improving insulin resistance. However, the amount of leucine may not have been high enough to raise insulin resistance.
In this study, we measured different adipokine levels such as leptin and adiponectin depending on dietary fat level and protein source. Leptin levels were high by feeding the high fat diet; however, adiponectin showed no effects depending on fat level. Levels of both of these hormones were significantly affected by protein source, and leptin levels were significantly lower and aiponectin was significantly higher in the corn gluten hydrolyzate groups (Table 6). Leptin is a hormone secreted by adipose tissue in direct proportion to the amount of body fat. The circulating leptin levels serve as a gauge of energy stores, thereby directing the regulation of energy homeostasis, neuroendocrine function, and metabolism [26]. In the most diet induced obesity animal models, leptin resistance is occurred. Obese rodents have elevated leptin levels but blunted leptin sensitivity. It is presumably reversible if the high fat diet is withdrawn [27]. In our study, although high fat induced obese rats had high leptin levels in their plasma, the food intake was not decreased but increased slightly, however, when they were fed normal fat diet, and the daily food intake seemed to be maintained. In the group of corn gluten hydrolyzates, the daily food intake was decreased until 4 weeks regardless of dietary fat level, and then it became the normal level at 7 weeks (Fig. 1B). The leptin level in plasma at 8 weeks was the lowest in the group of corn gluten hydrolyzates (Table 6). These results suggest that leptin resistance induced by high fat diet might be diminished by feeding corn gluten hydrolyzates. Inferred from daily food intake pattern, corn gluten hydrolyzates showed the effectiveness in improving leptin resistance until 4 weeks, after then, the signaling and metabolic pathway seemed to be normal. Moreover, it also affected to improve insulin sensitivity. However, we did not measure weekly leptin levels and other signaling pathway, and more researches are needed. Adiponectin is an adipose tissue-secreted endogenous insulin sensitizer, which plays a key role as a mediator of peroxisome proliferator-activated receptor gamma action. Adiponectin alters glucose metabolism and insulin sensitivity, exhibits antiinflammatory and antiatherogenic properties, and has been linked to several malignancies. Circulating concentrations of adiponectin are determined primarily by genetic factors, nutrition, exercise, and abdominal adiposity. Adiponectin concentrations are lower in subjects with obesity, metabolic syndrome, and cardiovascular disease [28]. It is also reported to stimulate fatty acid oxidation through the phosphorylation of AMPK and acetyl CoA carboxylase [29]. Our previous study showed that hepatic enzymes related to lipid metabolism contribute to the role of corn gluten hydrolyzates in the body weight reduction [11]. In this study, the corn gluten hydrolyzate also elevated adiponectin levels compared to control and other amino acid groups (Table 6). These results indicate that this peptide mixture might signal energy homeostasis as well as insulin sensitivity. However, the exact mechanism on signaling pathway must be studied further.
In conclusion, the corn gluten hydrolyzate diet, which had relatively high amounts of free amino acids and BCAAs, especially leucine, had weight reduction effects by lowering adipose tissue weights and levels of adipokines such as leptin and adiponectin. Moreover, it improved insulin resistance by lowering fasting blood glucose levels, plasma insulin levels, C-peptide, and HOMA-IR in the experimental animals, but major compounds such as BCAAs and leucine did not show these effects. Therefore, further studies to identify the active compounds such as small peptides and to determine the exact mechanisms for body weight reduction and improvements of insulin resistance are needed.
Footnotes
This project was supported by the Korean Institute of Planning and Evaluation for Technology of Food, Agriculture, Forestry and Fisheries (Project No. 609003-03-1-SB320) and the Ministry of Education, Science and Technology (Brain Korea 21, Project No. 2006-0519-4-7).
References
- 1.Aoyama T, Fukui K, Nakamori T, Hashimoto Y, Yamamoto T, Takamatsu K, Sugano M. Effect of soy and milk whey protein isolates and their hydrolysates on weight reduction in genetically obese mice. Biosci Biotechnol Biochem. 2000;64:2594–2600. doi: 10.1271/bbb.64.2594. [DOI] [PubMed] [Google Scholar]
- 2.Frank BH. Protein, body weight, and cardiovascular health. Am J Clin Nutr. 2005;82:242S–247S. doi: 10.1093/ajcn/82.1.242S. [DOI] [PubMed] [Google Scholar]
- 3.2007 Korea national health and nutrition examination surveys. Ministry for Health, Welfare and Family Affairs & Korea Center for Disease Control and Prevention [Internet] [cited 2009 March 06]. Available from: http://knhanes.cdc.go.kr.
- 4.Lu XX, Chen XH, Tang JZ. Studies on the functional property of enzymatic modified corn protein. Food Science. 2000;21:13–15. [Google Scholar]
- 5.Miyoshi S, Ishikawa H, Kaneko T, Fukui F, Tanaka H, Maruyama S. Structures and activity of angiotensin-converting enzyme inhibitors in an α-zein hydrolysate. Agric Biol Chem. 1990;55:1313–1318. [PubMed] [Google Scholar]
- 6.Suh HJ, Whang JH, Suh DB, Bae SH, Noh DO. Preparation of angiotensin I converting enzyme inhibitory from corn gluten. Process Biochem. 2003;39:1239–1244. [Google Scholar]
- 7.Yamaguchi M, Nishikiori F, Ito M, Furukawa Y. Effect of corn peptide on alcohol metabolism and plasma free amino acid concentrations in healthy men. Eur J Clin Nutr. 1996;50:682–688. [PubMed] [Google Scholar]
- 8.Yamaguchi M, Takada M, Nozaki O, Ito M, Furukawa Y. Preparation of corn peptide from corn gluten meal and its administration effect on alcohol metabolism in stroke-prone spontaneously hypertensive rats. J Nutr Sci Vitaminol. 1996;42:219–231. doi: 10.3177/jnsv.42.219. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Tao G, Liu P, Liu J. Peptide with angiotensin I - converting enzyme inhibitory activity from hydrolyzed corn gluten meal. J Agric Food Chem. 2007;55:7891–7895. doi: 10.1021/jf0705670. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XQ, Li LT, Liu XL, Wang XJ, Lin J, Li D. Production of hydrolysate with antioxidative activity by enzymatic hydrolysis of extruded corn gluten. Appl Microbiol Biotechnol. 2006;73:763–770. doi: 10.1007/s00253-006-0537-9. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Park J, Hong S, Kim MK. Effect of corn gluten and its hydrolysate consumptions on weight reduction in rats fed a high-fat diet. Nutr Res Pract. 2009;3:200–207. doi: 10.4162/nrp.2009.3.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133:261S–267S. doi: 10.1093/jn/133.1.261S. [DOI] [PubMed] [Google Scholar]
- 13.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 14.Cota D, Proulx K, Blake Smith KA, Kozma SC, Thomas G. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 15.Nutrition Research Council. Guide for the care and use of laboratory animals. 4th Edition. Washington DC: National Academy Press; 1997. [Google Scholar]
- 16.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets Nutrition Ad Hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 17.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces on obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 18.Frings CS, Dunn RT. A colorimetric method for determination of total serum lipid based on the sulfuric-phosphovanillin reaction. Am J Clin Nutr. 1970;53:89–90. doi: 10.1093/ajcp/53.1.89. [DOI] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipids extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK) Nutrition. 2000;16:349–354. doi: 10.1016/s0899-9007(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez MA, Mitsuya T, Hatta H, Koketsu M, Kobayashi R, Juneja LR, Kim M. Comparision of egg-yolk protein hydrolystate and soybean protein hydrolysate in terms of nitrogen utilization. Br J Nutr. 1998;80:477–484. [PubMed] [Google Scholar]
- 22.Hwang EH, Kang BG, Kim BR, Lee HJ. Protein quality evaluation and effect of plasma lipid contents of acid hydrolysates of cocoon in rats fed by high cholesterol, high triglyceride and high sucrose diet. Journal of the Korean Society of Food Science and Nutrition. 2001;30:1004–1009. [Google Scholar]
- 23.Lee HM, Chang UJ. Effect of corn peptide on the lipid metabolism in rats. The Korean Journal of Dietary Culture. 2001;16:416–422. [Google Scholar]
- 24.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005;135:1553S–1556S. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 25.Park HJ, Lee EJ, Kim J, Kim JY, Kwon O, Kim MK. Effect of leucine intake on body weight reduction in rats fed high fat diet. The Korean Journal of Nutrition. 2009;42:714–722. [Google Scholar]
- 26.Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. doi: 10.1059/0003-4819-152-2-201001190-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpace PJ, Zhang Y. Leptin resistance: a predisposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyck DJ. Adipokines as regulators of muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab. 2009;34:396–402. doi: 10.1139/H09-037. [DOI] [PubMed] [Google Scholar]