Abstract
The effects of grape seeds extract and grape peels extract prepared from grape pomace on the activity of antioxidant enzymes, degree of lipid peroxidation in serum and liver tissue were investigated in rabbits fed on high cholesterol diet. New Zealand white rabbits were divided as follows ; 1) NOR (normal group); 2) CHOL (cholesterol group); 3) GSH (cholesterol + grape seed extract group); 4) GPE (cholesterol + grape peel extract); 5) GSP (cholesterol + grape seed powder); 6) GPP (cholesterol + grape peel powder); 7) GE (cholesterol + grape seed and peel extract); 8) GP (cholesterol + grape seed and peel powder). Eight groups of rabbits were studied for 8 weeks. At the end of the experimental period, rabbits were sacrificed and the liver tissue were removed. Then, GSH, GPx, GST, CAT and MDA in the liver were measured. In liver tissues, total glutathione contents (GSH), glutathione peroxidase (GPx) and catalase (CAT) activity, which was significantly higher by grape seed extract supplementation. The level of malondialdehyde (MDA) was lower in the serum of rabbits fed grape seed extract or grape peel powder plus cholesterol than in the serum of rabbits fed cholesterol alone. It is therefore likely that grape seed extract prepared from grape pomace functioned as antioxidants in vivo, negating the effects of the oxidative stress induced by 1% cholesterol diet. The grape seed extract was found effective in converting the oxidized glutathione into reduced glutathione, and in removing H2O2 that is created by oxidative stress. The grape peel powder was found to have small influence on reduced glutathione content, CAT and GPX activity, but it increased GST activity in liver tissues, resulting in promoting the combination of lipid peroxide and glutathione (GSH), and further, lowering the formation of lipid peroxide in the serum. Therefore, grape pomace (grape seed extract and grape peel powder) supplementation is considered to activate the antioxidant enzyme system and prevent damage with hypercholesterolemia.
Keywords: Glutathione, glutathione peroxidase, grape pomace, malondialdehyde, rabbit
Introduction
All organisms are exposed to reactive oxygen species (ROS) or reactive oxygen metabolites (ROM), such as hydrogen peroxide (H2O2), superoxide anions (·O2-) or hydroxyl radicals (·OH), as a byproduct of oxidative metabolism or through exposure to radical-generating compounds [1]. Free radicals and ROS are closely associated with various degenerative diseases including atherosclerosis, ischemic heart disease, and aging, etc. [2,3]. Oxygen free radicals have been implicated in the development of atherosclerosis during hypercholesterolemia [4,5]. Hypercholesterolemic atherosclerosis was associated with the increase in aortic tissue and blood malondialdehyde (MDA) content, a lipid peroxidation product, and antioxidant enzyme and a decrease in antioxidant reserve [6,7]. These results suggested that levels of oxygen free radicals are increased in hypercholesterolemia. The increase in oxygen free radicals could be due to their overproduction and/or decreased antioxidant reserve and antioxidant enzyme activity. There is also an increasing amount of evidence that supports the "oxidation hypothesis" for atherosclerosis [8]. Carew et al. [9] and Hanna et al. [10] reported that probucol treatment lowers the rate of aortic atherosclerosis by limiting the oxidative modification of LDL and exerting other antioxidant properties.
Flavonoids, which are another antioxidant class, are a large group of polyphenolic antioxidants present in a variety of foods from vegetable sources, such as onions, apples, citrus, grapes, tea, and wine. Most naturally occurring flavonoids express various antioxidant effects, e.g., the scavenging of free radicals, chelation of transition metals, and inhibition of radical producing enzymes [11,12]. A flavonoid-rich diet has also been related to a reduced incidence of coronary heart disease (CHD) [13].
Wine-making affords grape pomace as a by-product in an estimated amount of 13% by weight of the grapes. This waste, consisting of peels, seed and stems, should be treated as a special solid residue, due to its high levels of residual phenolic compounds [14]. Thus, grape seed and peel are increasingly being used to obtain functional food ingredients such as natural antioxidants and dietary supplements [15]. Simonetti et al. [16] reported that the ingestion of grape seed extract increase the levels of α-tocopherol in red blood cell membranes and suggested that grape seed extract exert their antioxidant protection by sparing liposoluble vitamin E in vivo. Koga et al. [17] reported that the intake of proanthocyanidins increases the resistance of plasma against oxidative stress and may contribute to physiological functions of plant food including wine through their in vivo antioxidant activities. Yamakoshi et al. [18] suggested that proanthocyanidins, the major polyphenol in red wine, might trap reactive oxygen species in plasma and interstitial fluid of the arterial wall, thereby inhibit the oxidation of LDL and show an antiatherosclerotic activity. The grape peel is rich in anthocyanins, which are glycosidic-linked flavonoids responsible for the red, violet, purple, and blue colors of many plants [19]. As with other plant polyphenols, many anthocyanins have marked antioxidant activity in vitro [20]. Some anthocyanidins are bioavailable, as they have been detected in human plasma, however, little is known about their potential antioxidant properties in vivo [21,22]. Thus, the present study was undertaken to compare the action and effects of grape seed extract and peel extract supplements on various antioxidative changes in high-cholesterol-fed rabbits.
Materials and Methods
Chemicals
Red grapes (Vitis labruscana L.) were purchased at commercial maturity at a local store. Grape seed powder was defatted with hexane. After shaking the mixture for 6 hour at room temperature, the liquid was separated from the solid by vacuum filtration. The solid residue was evenly distributed over a tray and kept under the hood in the dark to evaporate the hexane. Grape peel was ground with the help of a grinder to obtain a powder for extraction and a feed additive. Grape seed and peel extract were extracted from powder of grape pomace. Briefly, grape seed extracts were extracted with methanol at 70℃ for 6 hour and grape peel extracts were extracted with ethanol at 25℃ for 6 hour. To obtain each powder, the aqueous layers were lyophilized by freeze-dry.
Animals and diets
Forty-eight male New Zealand white rabbits (Samtako Co., LD, Korea) with a mean weight 2.30 ± 0.13 kg were divided into eight groups. They were individually housed in steel cages in an air-conditioned room (23 ± 1℃, 55 ± 5%, humidity). The diets were prepared freshly every 8 weeks and stored at 4℃ until use to prevent oxidation and loss of antioxidants. Fresh feed was provided as powder once a day. Throughout the experimental period, they were given restricted amounts (250 g/ head per day) of each diet, and access to water ad libitum. A normal group (NOR) of six rabbits were fed the commercial rabbit chow diet (Purina Co., Korea). The six rabbits in the cholesterol group (CHOL) were fed the standard diet containing 1% cholesterol (w/w). The six rabbits in the grape seed extract group (GSE) were fed the standard diet containing 1% cholesterol plus 0.2% grape seed extract, the six rabbits in the grape peel extract group (GPE) were fed the standard diet containing 1% cholesterol plus 0.2% grape peel extract, the six rabbits in the grape seed powder group (GSP) were fed the standard diet containing 1% cholesterol plus 10% grape seed powder, the six rabbits in the grape peel powder group (GPP) were fed the standard diet containing 1% cholesterol plus 10% grape peel powder, the six rabbits in the grape extract group (GE) were fed the standard diet containing 1% cholesterol plus 0.1% grape seed extract with 0.1% grape peel extract, and the six rabbits in the grape powder group (GP) were fed the standard diet containing 1% cholesterol plus 5% grape seed powder with 5% grape peel powder. Body weight was determined every week.
Blood samples
At the end of the experimental period, the rabbits were anesthetized with katamine-HCl following a 12 h fast. Serum was obtained by centrifuging the blood at 3,000 rpm for 20 min at 4℃. The livers were then removed, rinsed with 0.1 mM PBS (pH 7.4), and weighed. The serum and livers were stored at -70℃ until analyzed. Breeding of experimental animals was carried out by observing the regulations on use and management of laboratory animals by the Korea Testing & Research Institute (announcement of amendment by Institutional Animal Care and Use Committee, 2006).
Liver antioxidant enzyme activities
The enzyme sources were isolated using the following procedure. One gram of liver tissue was homogenized with 9 mL of 0.1 mM PBS buffer, and the homogenates were then centrifuged at 10,000 rpm for 60 min to obtain the cytosol supernatant. Protein level was determined in diluted aliquots of the homogenates/cytosol by the method of Lowry et al. [23] using bovine serum albumin as standard. The concentration of GSH was determined by the method of Simons and Johnson [24] based on the development of a yellowed color when DTNB (5,5'-dithiobis-(2-nitrobenzoic acid)) is added to compounds containing sulfhydryl groups. The activity of GST was determined as described by Habig et al. [25] with modification. The reaction was started by the addition of 0.1 mL of 10 mM GSH to the reaction mixture containing 0.5 mL of GST buffer, 0.1 mL of 10 mM CDNB, 0.1 mL of cytosol, and 0.2 mL distilled water. The increase in absorbance at 340 nm was red using CDNB as substrate. In the assay of glutathione peroxidase, the enzyme catalyzed the reduction of organic peroxide through formation of oxidized glutathione (GSSG). GSSG was reduced to glutathione by glutathione reductase (GR) which oxidized NADPH to NADP+ in the catalytic cycle. The change in absorbance at 340 nm due to the oxidation of NADPH was the basis for quantitating the activity of GPx by the method of Paglia and Valentine [26]. Catalase activity was measured by the method of Aebi [27] using hydrogen peroxide (H2O2) as substrate. The disappearance of H2O2 was followed at 240 nm. Enzyme activity was expressed as U/mg protein at 25℃.
Serum and liver lipid peroxidation (TBARS assay)
Endogenous lipid peroxidation in serum was assessed by measuring malondialdehyde (MDA) level according to the method of Buege and Aust [28]. Briefly, 100 µl of serum was well mixed with 2.5% trichloroacetic acid (TCA) and 1% thiobarbituric acid (TBA). After boiling at 100℃ for 15 min, the samples were cooled at room temperature, centrifuged at 3,000 rpm for 10 min at 4℃, and the supernatant absorbance was read at 532 nm.
The levels of liver lipid peroxide were determined using the method of Ohkawa et al. [29] with a slight modification. Briefly, 0.1 mL of liver homogenate (1 g liver/9 mL of 0.1 mM PBS) was well mixed with 0.2 mL of 8.1% sodium dodecyl sulfate, 0.7 mL of distilled water, 0.8% TBA and 20% acetic acid. The reaction mixture was heated at 100℃ for 60 min. After cooling the mixture, 4 mL of n-butanol : pyridine (14:1, v/v) was added and centrifuged at 3,000 rpm for 10 min. Absorbance of the resulting colored layer was measured at 532 nm using 1,1,3,3-tetramethoxypropane (Sigma, USA) as the standard.
Serum and liver α-tocopherol concentration
The serum and liver vitamin E concentrations were simultaneously determined using the HPLC method of Choi [30]. 0.5 mL of serum/liver homogenate was added to ethanol and extracted by hexane. The collected hexane layer was then evaporated, dissolved in 1 mL hexane, and separated by HPLC. The chromatography was equipped with a Supelcosil™ LC-NH2 (25 cm × 4.6 mm, 5 µm) column and detected at UV 290 nm.
Statistical analysis
Results are presented as mean value ± standard deviation. Analysis of variance was performed using ANOVA procedures. Significant differences between means were determined by Duncan's multiple range test at a level of α = 0.05.
Results
Effect on weight gain and organ weights
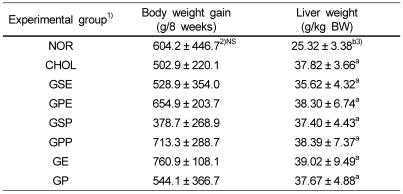
There were no significant differences in the weight gain among the groups, but liver and kidney weights were significantly increased in cholesterol supplement groups compared to the normal diet group (Table 1).
Table 1.
Body weight gain and liver weight of rabbits fed experimental diets
1)NOR : normal diet, CHOL : 1% cholesterol diet, GSE : 1% cholesterol + 0.2% grape seed extract diet, GPE : 1% cholesterol + 0.2% grape peel extract diet, GSP : 1% cholesterol + 10% grape seed powder diet, GPP : 1% cholesterol + 10% grape peel powder diet, GE : 1% cholesterol + 0.1% grape seed extract + 0.1% grape peel extract diet, GP : 1% cholesterol + 5% grape seed powder + 5% grape peel powder diet
2)Mean ± SD, n = 6
3)Values within a column with different superscripts are significantly different at α= 0.05 by Duncan's multiple range test (NS: not significant).
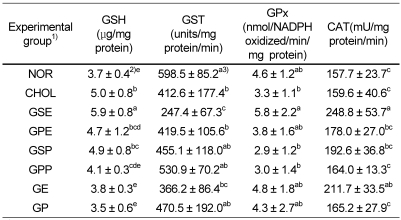
Liver glutathione contents and antioxidant enzyme activities
The glutathione contents and antioxidant enzyme activities of grape seed and peel extract or powder feeding groups are shown in Table 2. Glutathione content of NOR (normal group) was significantly lower than that of CHOL (cholesterol group) (P < 0.05). Also, GE (grape seed extract group) and GP (grape seed and peel powder group) were observed as significantly lower than CHOL (P < 0.05). But glutathione content of GSE was not significantly different compared with CHOL.
Table 2.
Glutathione (GSH) contents, GST, GPx and CAT activity in liver tissue of rabbits fed experimental diets
1)NOR : normal diet, CHOL : 1% cholesterol diet, GSE : 1% cholesterol + 0.2% grape seed extract diet, GPE : 1% cholesterol + 0.2% grape peel extract diet, GSP : 1% cholesterol + 10% grape seed powder diet, GPP : 1% cholesterol + 10% grape peel powder diet, GE : 1% cholesterol + 0.1% grape seed extract + 0.1% grape peel extract diet, GP : 1% cholesterol + 5% grape seed powder + 5% grape peel powder diet
2)Mean ± SD, n = 6
3)Values within a column with different superscripts are significantly different at α= 0.05 by Duncan's multiple range test.
The GST activity was higher in the grape peel powder group (GPP) than in the high cholesterol diet group (CHOL). The GPx and CAT activities were significantly higher in the grape seed extract group (GSE) than those in the high cholesterol diet group (CHOL). The supplementation of grape seed extract caused a 52% and 56% increase, respectively, in the GPx and CAT activities compared to the high cholesterol diet group (CHOL).
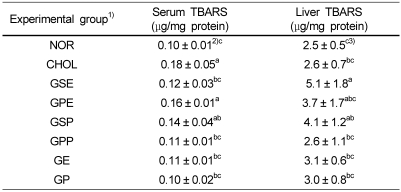
Serum and liver lipid peroxidation (TBARS assay)
The serum and lipid peroxide concentrations were determined by measuring the TBARS concentration (Table 3). The serum TBARS concentration was significantly lower in grape seed extract group (GSE), grape peel powder group (GPP), mixed group of grape seed and peel extract (GE) and mixed group of grape seed and peel powder (GP). However, the hepatic TBARS concentration was lower in high cholesterol diet group (CHOL) rather than in grape seed and peel supplement groups. The grape seed and peel supplementation had no effect on reducing TBARS concentrations.
Table 3.
The contents of TBARS in serum, liver and kidney of rabbits fed experimental diets
1)NOR : normal diet, CHOL : 1% cholesterol diet, GSE : 1% cholesterol + 0.2% grape seed extract diet, GPE : 1% cholesterol + 0.2% grape peel extract diet, GSP : 1% cholesterol + 10% grape seed powder diet, GPP : 1% cholesterol + 10% grape peel powder diet, GE : 1% cholesterol + 0.1% grape seed extract + 0.1% grape peel extract diet, GP : 1% cholesterol + 5% grape seed powder + 5% grape peel powder diet
2)Mean ± SD, n = 6
3)Values within a column with different superscripts are significantly different at α= 0.05 by Duncan's multiple range test (NS: not significant).
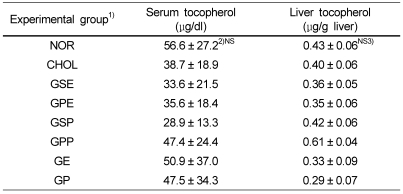
Serum and liver α-tocopherol concentration
There was no difference in the vitamin E concentration of serum and liver between the groups. However, the grape peel powder supplement increased the concentrations of serum vitamin E by 52%, compared to the high cholesterol diet group (CHOL) (Table 4). Also, the mixed grape seed and peel extracts supplement increased the concentrations of hepatic vitamin E by 31%, compared to the high cholesterol diet group (CHOL).
Table 4.
α-Tocopherol levels in serum and liver tissue of rabbits fed the experimental diets
1)NOR : normal diet, CHOL : 1% cholesterol diet, GSE : 1% cholesterol + 0.2% grape seed extract diet, GPE : 1% cholesterol + 0.2% grape peel extract diet, GSP : 1% cholesterol + 10% grape seed powder diet, GPP : 1% cholesterol + 10% grape peel powder diet, GE : 1% cholesterol + 0.1% grape seed extract + 0.1% grape peel extract diet, GP : 1% cholesterol + 5% grape seed powder + 5% grape peel powder diet
2)Mean ± SD, n = 6
3)Values within a column with different superscripts are significantly different at α= 0.05 by Duncan's multiple range test (NS: not significant).
Discussion
The aim of this study was to evaluate the antioxidant enzymatic activity of extracts and powders prepared from grape pomace in hypercholesterolemic-rabbits. In this study, we demonstrated that although grape pomace did not increase antioxidant enzyme activities, grape peel powder intake was a favorable effect on the increase of GST activity, which resulted in high tocopherol content. The extract and powder of grape seed and peel supplement were not significantly different among rabbit groups, however, showed the highest level in GE and the lowest level in GSP. The liver weight increased of CHOL, GSE, GPE, GSP, GPP, GE and GP would result by cholesterol intake. The glutathione content within the liver tissue was significantly higher in grape seed extracts group (GSE) than other groups, but significantly lower than the control group (CHOL) in normal diet groups (NOR) and mixed group of grape seed and peel powder (GP). GST activation was significantly low in grape seed extracts group (GSE) which differed from the results of glutathione content, and showed a high activation in normal diet group (NOR) and mixed group of grape seed and peel powder (GP). The activation of GPx and CAT showed similar results with glutathione content, having a significantly high value in grape seed extracts group (GSE).
GSH content functions as the substrate of GST and GPx, which are enzymes that show defensive reaction of cells due to external oxidative damage. As a substance that removes lipid peroxide within the cells and other foreign bodies and as a storage and carrier of amino acid, it performs various cell functions [31]. It decreases by chronically taking ethanol and is known to increase cytosolic GST activation of the liver cell [32]. GST is an enzyme which catalyzes the reaction of glutathione and thioether formation by including reduced GSH to lipotropic and electrophilic substances within the body. It participates in detoxification and excretion of lipotropic and electrophilic substances among mutagen substances, carcinogenic substances, toxic and pharmacologically active materials, metabolites of these substances, and endogenous toxins [33]. It also is related to the biosynthesis of prostaglandin and leukotriene, and combines with bilirubin, heme, steroid hormone, bile acid and others to induce transmigration within cells, transportation, and excretion [34]. The activation of enzyme in blood increases in acute and fulminant hepatitis, hepatocirrhosis, and hepatoma when poisoned by carbon tetrachloride. It is known to be reduced in liver tissues that are intoxicated by carbon tetrachloride and hepatoma tissues [35].
In case of rabbits, the GSH content within liver tissues was significantly highest in grape seed extracts group (GSE), and GST activation was shown to be high compared to the control group (CHOL) in grape peel power group (GPP). The GSH content results were similar to Cahide and Tannaz's results, which was increased in white mice with hypercholesterolemia when administrating vitamin E for a long period [36]. The GSH content was significantly increased in hypercholesterolemic rabbits that ate bamboo coated rice [37]. GPx is a selenium dependent enzyme that catalyzes the formation of GSSG and water from hydrogen peroxide and GSH, and GSSG, alcohol, and water from other peroxides and GSH within the body. It prevents oxidative damage of tissues and counteracts oxidative stress [38]. For rabbits, the GPx activation was increased in grape seed extracts group (GSE) like the GSH content. This is consistent with the result reported by Tebib et al. [39] that supplying grape seed extracts increases GPx activation within the liver tissue. The GSH content within liver tissue was high in grape seed extracts group (GSE) along with GPx and CAT activation. However, it is considered that GST activation was low because grape seed extracts participated in increasing activation of enzymes which eliminate hydroxyl radical that formed from grape seed extracts and hydrogen peroxide, before GST activation was increased.
The lipid peroxide content within serum showed a decrease due to the supply of grape seed extracts (GSE) and grape power mixture (GP), but peroxide formation could not be suppressed in liver tissues. Lipid peroxidation within the body acts with proteins, RNA, and DNA which are the components of cells, and causes biochemical change and dysfunction of mitochondria that has antioxidant enzymes. This accelerates degenerative diseases such as arteriosclerosis [40]. Among this, MDA is a main byproduct of lipid peroxidation, and is being reported as a LDL oxidation inducer since an antibody for MDA-modified LDL was discovered in the lipid peroxidation of LDL that occurred in vivo [41]. Jeon et al. [42] reported that TBARS contents showed a higher value in rabbits that took high cholesterol meals than those that did not. The lipid peroxidation was sufficiently suppressed within the liver tissue when the antioxidants naringin and probucol were added to the high cholesterol meal by 0.5 g/kg diet concentration. Choe and Kim [43] examined lipid peroxidation in the liver and kidney of hypercholesterolemic rabbits, and reported that wheat controlled the peroxidation of rabbit tissue. Tamer et al. [44] also reported that for atherosclerosis patients, GSH in erythrocytes and total antioxidant functions within plasma was decreased while MDA blood content was increased within plasma. Yamakoshi et al. [18] reported that TBARS content within serum was significantly decreased at 4 weeks after breeding from administrating grape seed extracts. Also, when grape seed extracts full of proanthocyanidin are administered, it will inhibit MDA formation within liver tissues in cataract ICR/f rat, and significantly reduce ChE-OOH (cholesteryl ester hydroperoxides) formation in plasma oxidation that is induced by Cu [45]. Ahn et al. [46] reported the favorable effects of tannin substances in persimmon and grape seeds which well scavenge DPPH radical, and are effective in reducing TBARS and phosphatidlycholine hydroperoxide formation within the body and liver. In this study, the grape seed extracts reduced the TBARS amount in the serum of the rabbit. Not like in serum, the grape seed extract was not effective in controlling TBARS formation within the liver tissue of the rabbit. This is considered to be caused by the monomeric flavanol from the extracts which were given to the rabbit, as mentioned in Tebib et al. [39], so the MDA formation was not significantly decreased within the liver tissue.
Osada et al. [47] reported that apple polyphenol fed rat slightly increased serum tocopherol contents compared with control group. Also, (+)catechin increased the level of α-tocopherol in rats by a mechanism independent of tocopherol-ω-hydroxylase, which is a key enzyme in tocopherol catabolism [48]. Therefore, the metabolites of procyanidins, rather than internal α-tocopherol, may play critical free radical-scavenging roles.
In conclusion, grape seed extracts were effective in increasing CAT and GPx activation in the liver tissue, but not so effective in controlling the formation of lipid peroxides within the liver tissue. Thus it is considered that the activation of GST which mainly participates in drug detoxification influences lipid peroxidation control in the first stage, compared to other antioxidant enzyme activation. Also, this is the result of not presenting sufficient connection of grape seed extracts to the metabolism of antioxidant enzyme activation, and is considered to be deeply related to the polymerization of proanthocyanidin which exists within the grape seed extract.
Through this study, lipid peroxidation inhibition activity of grape pomace was not identified, but the activation for antioxidant enzyme can play an important role. In the future, MDA (malondialdehyde) formation level of various organ included heart, spleen, kidneys, arteries as well as liver, which is analyzed to be clearly identified on metabolic pathway.
Fig. 1.
The change of rabbit's body weight for experimental period. (-○- NOR : Normal diet, -△- CHOL : 1% cholesterol diet, -◆- GSE : 1% cholesterol + 0.2% grape seed extract diet , -□- GPE : 1% cholesterol + 0.2% grape peel extract diet, -×- GSP : 1% cholesterol + 10% grape seed powder diet, -●- GPP : 1% cholesterol + 10% grape peel powder diet, -◇- GE : 1% cholesterol + 0.1% grape seed extract + 0.1% grape peel extract diet,, -■- GP : 1% cholesterol + 5% grape seed powder + 5% grape peel powder diet)
Footnotes
This study was supported by ARPC, Republic of Korea.
References
- 1.Yu BP. Cellular defences against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 3.Steinbrecher UP, Zhang H, Lougheed M. Role of oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Prasad K, Kalra J. Oxygen free radicals and hypercholesterolemic atherosclerosis: effect of vitamin E. Am Heart J. 1993;125:958–973. doi: 10.1016/0002-8703(93)90102-f. [DOI] [PubMed] [Google Scholar]
- 5.Henning B, Chow CK. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med. 1988;4:99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 6.Prasad K, Kalra J. Experimental atherosclerosis and oxygen free radicals. Angiology. 1989;40:835–840. doi: 10.1177/000331978904000911. [DOI] [PubMed] [Google Scholar]
- 7.Prasad K, Kalra J, Lee P. Oxygen free radicals as a mechanism of hypercholesterolemic atherosclerosis: effects of probucol. Int J Angiol. 1994;3:100–112. [Google Scholar]
- 8.Jeon SM, Bok SH, Jang MK, Kim YH, Nam KT, Jeong TS, Park YB, Choi MS. Comparison of antioxidant effects of naringin and probucol in cholesterol-fed rabbits. Clin Chim Acta. 2002;317:181–190. doi: 10.1016/s0009-8981(01)00778-1. [DOI] [PubMed] [Google Scholar]
- 9.Carew T, Schwenke D, Steinburg D. Antiatherogenic effect of probucol unrelated to its hypercholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hypercholesterolemic rabbits. Proc Natl Acad Sci U S A. 1987;84:7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanna AN, Feller DR, Witiak DT, Newman HA. Inhibition of low density lipoprotein oxidation by thyronines and probucol. Biochem Pharmacol. 1993;45:753–762. doi: 10.1016/0006-2952(93)90151-l. [DOI] [PubMed] [Google Scholar]
- 11.Lanningham-Foster L, Chen C, Chance DS, Loo G. Grape extract inhibits lipid peroxidation of human low density lipoprotein. Biol Pharm Bull. 1995;18:1347–1351. doi: 10.1248/bpb.18.1347. [DOI] [PubMed] [Google Scholar]
- 12.Hollman PC, van Trijp JM, Mengelers MJ, de Vries JH, Katan MB. Bioavailbility of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997;114:139–140. doi: 10.1016/s0304-3835(97)04644-2. [DOI] [PubMed] [Google Scholar]
- 13.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Eldarly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 14.Amico V, Napoli EM, Renda A, Ruberto G, Spatafora C, Tringali C. Constituents of grape pomace from the Sicilian cultivar "Nerello Mascalese". Food Chem. 2004;88:599–607. [Google Scholar]
- 15.Goñi I, Martín N, Saura-Calixto F. In vitro digestibility and intestinal fermentation of grape seed and peel. Food Chem. 2005;90:281–286. [Google Scholar]
- 16.Simonetti P, Ciappellano S, Gardana C, Bramati L, Pietta P. Procyanidins from Vitis vinifera seed: In vivo effects on oxidative stress. J Agric Food Chem. 2002;50:6217–6221. doi: 10.1021/jf011412+. [DOI] [PubMed] [Google Scholar]
- 17.Koga T, Moro K, Nakamori K, Yamakoshi J, Hosoyama H, Kataoka S, Ariga T. Increase of antioxidative potential of rat plasma by oral administration of proanthocyanidin-rich extract from grape seed. J Agric Food Chem. 1999;47:1892–1897. doi: 10.1021/jf9810517. [DOI] [PubMed] [Google Scholar]
- 18.Yamakoshi J, Kataoka S, Koga T, Ariga T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 1999;142:139–149. doi: 10.1016/s0021-9150(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Cao GH, Prior RL. Oxygen radical absorbing capacity of anthocyanidns. J Agric Food Chem. 1997;45:304–309. [Google Scholar]
- 20.Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T. Inhibition of lipid peroxidation and the activie oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris. Biochem Pharmacol. 1996;52:1033–1039. doi: 10.1016/0006-2952(96)00421-2. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa T, Nakagawa K, Kudo M, Kayo M, Someyo K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3-5-diglucoside, into rats and humans. J Agric Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 22.Cao G, Prior RL. Anthocyanins are detected in human plasma after oral administration of an elderberry extract. Clin Chem. 1999;45:554–576. [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Rendall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Simons SS, Johnson DF. Reaction of o-phtal-alaldehyde and thiols with primary amines: Fluoroscence properties of 1-alkyl (and aryl) thio-2-alkylisoindoles. Anal Biochem. 1978;90:705–725. doi: 10.1016/0003-2697(78)90163-x. [DOI] [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MP, Jakoby WB. Glutathione S-transferase. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxides. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 27.Aebi A. Catalase, methods of enzymatic analysis II. New York: Academic press; 1974. pp. 674–684. [Google Scholar]
- 28.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–306. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 29.Yagi K, Matsuoka S, Ohkawa H, Ohishi N, Takeuchi YK, Sakai H. Lipoperoxide level of the retina of chick embryo exposed to high concentration of oxygen. Clin Chim Acta. 1977;80:355–360. doi: 10.1016/0009-8981(77)90044-4. [DOI] [PubMed] [Google Scholar]
- 30.Choi IS. Effects of some antioxidants added to sardine oil on tocopherols contents in plasma and liver of rats. The Korean Journal of Nutrition. 1990;23:44–51. [Google Scholar]
- 31.Lieber CS. Interaction of ethanol with drug, hepatotoxic agent, carcinogen and vitamins. Alcohol Alcohol. 1990;25:157–176. doi: 10.1093/oxfordjournals.alcalc.a044990. [DOI] [PubMed] [Google Scholar]
- 32.Lee JW. Effects of ethanol administration on glutathione and lipid peroxide level in rat liver and cerebellum. Journal of the Korean Society of Food Science and Nutrition. 1991;20:285–292. [Google Scholar]
- 33.Jokoby WB. The glutathione S-transferase A group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- 34.Marcus CJ, Habig WH, Jakoby WB. Glutathione S-transferase from human erythrocytes, non-identity with the enzymes from liver. Arch Biochem Biophys. 1978;188:267–273. doi: 10.1016/s0003-9861(78)80011-3. [DOI] [PubMed] [Google Scholar]
- 35.Mukhtar H, End JR. Serum glutathione S-transferase perinatal development, sex difference and effect of carbon terachloride administration on enzyme activity in the rat. Life Sci. 1977;21:1277–1285. doi: 10.1016/0024-3205(77)90008-x. [DOI] [PubMed] [Google Scholar]
- 36.Cahide G, Tannaz M. Changes of oxidative stress in various tissue by long term administration of vitamin E in hypercholesterolemic rats. Clin Chim Acta. 2003;328:155–161. doi: 10.1016/s0009-8981(02)00388-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee MJ, Kim EY, Moon GS. Effect of maengiong-juk extract coated rice supplementation on antioxidative system in rabbit fed high cholesterol diet. Journal of the Korean Society of Food Science and Nutrition. 2004;33:973–980. [Google Scholar]
- 38.Pierce S, Tappel AL. Glutathione peroxidase activites from rat liver. Biochem Biophys Acta. 1978;523:27–36. doi: 10.1016/0005-2744(78)90005-0. [DOI] [PubMed] [Google Scholar]
- 39.Tebib K, Rouanet JM, Besancon P. Antioxidant effects of dietary polymeric grape seed tannins in tissues of rats fed a high cholesterol-vitamin E-deficient diet. Food Chem. 1997;59:135–141. [Google Scholar]
- 40.Heinecke JW. Oxidized amino acids: culprits in human atherosclerosis and indicators of oxidative stress. Free Radic Biol Med. 2002;32:1090–1101. doi: 10.1016/s0891-5849(02)00792-x. [DOI] [PubMed] [Google Scholar]
- 41.Tornvall P, Wang G, Nilsson J, Hamsten A, Regnstrom J. Autoantibodies against modified low-density lipoproteins in coronary artery disease. Atherosclerosis. 2003;167:347–353. doi: 10.1016/s0021-9150(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 42.Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Jeong TS, Park YB, Rhee SJ, Choi MS. Antioxidant activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001;69:2855–2866. doi: 10.1016/s0024-3205(01)01363-7. [DOI] [PubMed] [Google Scholar]
- 43.Choe M, Kim HS. Effects of Korean wheat on LDL oxidation and atherosclerosis in cholesterol-fed rabbits. Journal of the Korean Society of Food Science and Nutrition. 2002;31:104–108. [Google Scholar]
- 44.Tamer L, Sucu N, Polat G, Ercan B, Barlas A, Yucebilgic G, Unlu A, Dikmengil M, Atik U. Decreased serum total antioxidant status and erythrocyte-reduced glutathione levels are associated with increased serum malondialdehyde in atherosclerotic patients. Arch Med Res. 2002;33:3257–3260. doi: 10.1016/s0188-4409(01)00381-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamakoshi J, Saito M, Kataoka S, Tokutake S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J Agric Food Chem. 2002;50:4983–4988. doi: 10.1021/jf0201632. [DOI] [PubMed] [Google Scholar]
- 46.Ahn HS, Jeon TI, Lee JY, Hwang SG, Lim YG, Park DK. Anitoxidative activity of persimmon and grape seed extract: in vitro and in vivo. Nutr Res. 2002;22:1265–1273. [Google Scholar]
- 47.Osada K, Suzuki T, Kawakami Y, Senda M, Kasai A, Sami M, Ohta Y, Kanda T, Ikeda M. Dose-dependent hypocholesterolemic actions of dietary apple polyphenol in rats fed cholesterol. Lipids. 2006;41:133–139. doi: 10.1007/s11745-006-5081-y. [DOI] [PubMed] [Google Scholar]
- 48.Frank J, Lundh T, Parker RS, Swanson JE, Vessby B, Kamal-Eldin A. Dietary (+)-catechin and BHT markedly increase α-tocopherol concentrations in rats by a tocopherol-ϖ-hydroxylase-independent mechanism. J Nutr. 2003;133:3195–3199. doi: 10.1093/jn/133.10.3195. [DOI] [PubMed] [Google Scholar]