Abstract
Our previous study demonstrated that methanolic extract of Chrysanthemum zawadskii Herbich var. latilobum Kitamura (Compositae) has the potential to induce detoxifying enzymes such as NAD(P)H:(quinone acceptor) oxidoreductase 1 (EC 1.6.99.2) (NQO1, QR) and glutathione S-transferase (GST). In this study we further fractionated methanolic extract of Chrysanthemum zawadskii and investigated the detoxifying enzyme-inducing potential of each fraction. The fraction (CZ-6) shown the highest QR-inducing activity was found to contain (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro [4,5] decane and increased QR enzyme activity in a dose-dependent manner. Furthermore, CZ-6 fraction caused a dose-dependent enhancement of luciferase activity in HepG2-C8 cells generated by stably transfecting antioxidant response element-luciferase gene construct, suggesting that it induces antioxidant/detoxifying enzymes through antioxidant response element (ARE)-mediated transcriptional activation of the relevant genes. Although CZ-6 fraction failed to induce hepatic QR in mice over the control, it restored QR activity suppressed by CCl4 treatment to the control level. Hepatic injury induced by CCl4 was also slightly protected by pretreatment with CZ-6. In conclusion, although CZ-6 fractionated from methanolic extract of Chrysanthemum zawadskii did not cause a significant QR induction in mice organs such as liver, kidney, and stomach, it showed protective effect from liver damage caused by CCl4.
Keywords: Quinone reductase; detoxifying enzymes; Chrysanthemum zawadskii; (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]decane; carbon tetrachloride-induced liver injury
Introduction
Chrysanthemum zawadskii var. latilobum (Compositae), known as "Gu-Jeol-Cho" in Korea, has been used in traditional medicine in Korea for the treatment of pneumonia, bronchitis, cough, common cold, pharyngitis, bladder-related disorders, gastroenteric disorders and hypertension [1]. Our previous study showed that the methanolic extract of Chrysanthemum zawadskii strongly induced NAD(P)H:(quinone acceptor) oxidoreductase 1 (EC 1.6.99.2) (NQO1, QR) activity [2,3]. NQO1 has been known to be anticarcinogenic marker enzyme because it contains antioxidant response element (ARE), a cis-element bound by a transcriptional activator Nrf2, like other detoxifying phase 2 enzymes. Therefore, the induction of QR not only protects against quinone-mediated cytotoxicity, but also acts as a potential mechanism in the prevention of chemical carcinogenesis [4]. Antioxidant response element (ARE) is present in the promoter region of genes encoding for phase 2 detoxification/antioxidant enzymes such as heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase (EC 1.6.99.2) (NQO1, QR), and glutathione S-transferase. In unstressed states, Nrf2 is present in the cytoplasm in association with Kelch-like ECH-associated protein 1 (Keap1). Disturbance of the interaction between Nrf2 and Keap1 including covalent or oxidative modification of cysteine thiols in Keap1 by electrophiles or oxidative stress results in Nrf2 release and its translocation into the nucleus. Binding of Nrf2 to the ARE sequence in genes encoding phase 2/antioxidant enzymes causes transcriptional activation of the relevant genes, promoting removal of ROS or toxic chemicals. Many natural compounds such as curcumin, caffeic acid phenethyl ester (CAPE) and sulphoraphane are known to act as electrophiles in Nrf2/ARE activation [5-9].
In this study we further fractionated the methanolic extract of Chrysanthemum zawadskii into 13 different fractions by silica gel chromatography. One of those fractions (hereafter referred to as CZ-6) showed the highest QR-inducing activity and was found to contain (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]decane. We also investigated the protective effect of CZ-6 on CCl4-induced hepatotoxicity in mouse.
Materials and Methods
Materials
All cell culture reagents and fetal bovine serum were obtained from Gibco BRL (Gaitherburg, MD, USA) and Hyclone (Logan, UT, USA), respectively. Hepa1c1c7 and BPRc1 cells were from American Type Culture Collection (Rockville, MD, USA). All other chemicals were of reagent grade. Chrysanthemum zawadskii var. latilobum were purchased from DeaGuang in Chuncheon in 2008. A voucher (No. 325) was deposited at the Hallym University RIC center in Chuncheon, Republic of Korea.
Cell culture
Hepa1c1c7 and its mutant (BPRc1) cells were plated at density of 3×105 and 5×105 cells per 100 mm plate (Nunc, Rochester, NY) in 10 mL of α-MEM supplemented with 10% FBS, respectively. The HepG2-C8 cell line established in Dr. Kong's lab at Rutgers, The State University of New Jersey, by transfecting human hepatoma HepG2 cells with pARE-TI-luciferase construct was used for reporter assay [9]. HepG2-C8 cells were maintained in modified DMEM supplemented with 10% FBS and 0.5 mg/mL neomycin. Cells were normally starved overnight in 0.5% FBS-containing medium before treatment. The cells were normally incubated for 3~4 days in a humidified incubator in 5% CO2 at 37℃. Cells were cultured for 16 h, starved 12 h, exposed to various concentrations of the sample for another 16 h, followed by biochemical assays.
Isolation of QR inducer from Chrysanthemum zawadskii var. latilobum
Leaves (1.5 kg) of C. zawadskii were air-dried followed by grinding in a Willey-Mill plant grinder. Ground plant material was soaked in n-hexane (8.5 L) for 24 h. The solvent was decanted from the plant residue and evaporated in vacuo to yield 40.8 g of crude extract. A portion of the n-hexane root extract (5.2 g) was adsorbed to silica gel and applied to a silica gel chromatography column (40-63 µm, 60 × 300 mm, 60 Å). Elution of the column was performed using increasing polarity mixtures of n-hexane/EtOAc in a series of three linear gradient steps. Step 1 consisted of 100/0 to 90/10 using 2 L with step 2 consisting of 90/10 to 75/25 using 1 L. Step 3 consisted of 75/25 to 0/100 using 1 L and the column was washed with 2 L of EtOAc. Column eluate was collected in 30 mL test tubes and, based on TLC similarities, recombined into 13 fractions [1, 1-60, 80 mg; 2, 61-75, 85 mg; 3, 76-89, 1.3 g; 4, 90-105, 620 mg; 5, 106-145, 45 mg; 6, 1-60, 55 mg; 7, 61-75, 86 mg; 8, 76-89, 1.2 g; 9, 90-105, 520 mg; 10, 106-145, 52 mg; 11, 1-60, 65 mg; 12, 61-75, 96 mg; 13, 76-89, 1.1 g]. Further purification of the bioactive fractions 8 and 13 was accomplished using repeated VLC procedures on silica gel to yield compound 1 (57 mg) and compound 2 (85 mg; Fig. 1), respectively, with a purity of 98.8% as determined by HPLC. The compound 2 was most active in inducing quinone reductase and its chemical name is (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]decane.
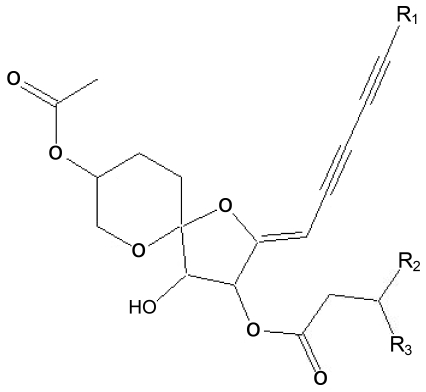
Fig. 1.
Structure of (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]decane isolated from Chrysanthemum zawadskii
Molecular formula : C21H26O7 (390.1679)
Appearance : Yellowish oil
[α]D : +62°(c, 5.66; CHCl3)
IR max(KBr) : 3450(OH), 2229, 2139(C=C), 1733(ester C=O), 1650(C=C) cm-1
UV max(MeOH): 216, 224, 250, 263, 279, 291 nm
EI-MS m/z (% rel. int) : 390(M+, 25), 372(M+-H2O, 12), 306(M+-C5H8O2), 288(M+-C5H10O2, 100), 246(74), 228(32) 13C-NMR(CDCl3) : 64.9(C-1), 66.3(C-2), 23.2(C-3), 25.2(C-4), 103.9(C-5), 80.0(C-6), 76.2(C-7), 164.5(C-8), 84.6(C-9), 68.8 (C-10), 78.8(C-11), 65.0(C-12), 80.7(C-13), 5.0(C-14), 173.1 (C-1'), 43.0(C-2'), 25.8(C-3'), 22.8, 22.9(C-4', 5'), 171.0(C-1"), 21.6(C-2")
1H-NMR(CDCl3) : 3.90, 3.98(H-1), 4.87(H-2), 1.92, 2.07(H-3), 1.62, 2.21(H-4), 3.91(H-6), 6.01(H-7), 5.13(H-9), 1.95(C-14), 2.32, 2.40(H-2'), 2.19(H-3'), 0.99, 1.0(H-4', 5'), 2.17(H-2")
Biochemical assays
QR activity was measured by a spectrophotometric assay in which the rate of reduction of 2,6-dichlorophenolindophenol was monitored at 600 nm [10]. tert-Butylhydroquinone (TBHQ, 20 µM or 3.3 µg/mL), a known QR inducer, was used as a positive control in all biochemical assays. The specific activity of enzymes was normalized to the protein concentration, which was determined in triplicate using the Lowry assay [11].
Assay of reporter gene activity
HepG2-C8 cells (kindly provided from Dr. Kong at Rutgers University) were plated in 6-well plates at a density of 5×105 cells/well. After 16 h incubation, cells were cultured in fresh modified DMEM with high glucose containing 0.5% FBS for 12 h before sample treatment. After cells were cultured for another 16 h in the presence of various concentrations of the sample, cells were collected and the luciferase activity was determined according to the protocol provided by the manufacturer (Promega Corp., Madison, WI) [9]. Briefly, after sample treatment, cells were washed twice with ice-cold PBS and harvested in reporter lysis buffer. The homogenates were centrifuged at 12,000× g for 2 min at 4℃. A 20 µL supernatant was assayed for luciferase activity using TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Luciferase activity was normalized against protein concentration.
Animal experiment
ICR mice (Samtako, Osan, Gyeonggi, Korea) weighing 20-30 g at the initiation of the experiment were used. Mice were given free access to a standard diet (Chow, Purina, Korea) and distilled water. Chronic liver injury was induced by injection of carbon tetrachloride (CCl4) (Wako Pure Chemical, Osaka, Japan) according to a standard method described by Proctor and Chatamra [12]. Briefly, CCl4 was injected subcutaneously twice at 7th and 9th days at a dose of 0.6 ml/kg of body weight while CZ-6 fraction (5 mg/kg bw) was orally injected for 3 times at 7th, 9th, 11th days, followed by sacrifice at 13th day. Right after anesthetizing mice by CO2 asphyxiation, organs were collected from 5-8 mice per group and the rest of mice were subjected to perfusion for histological examination.
Statistical analysis
Statistical significance of data was tested by analysis of variance, followed by Duncan's multiple range test, using SPSS software (SPSS Inc., Chicago, IL). P < 0.05 was considered to be statistically significant.
Results
Effect of CZ-6 on QR activity in murine hepatoma cells
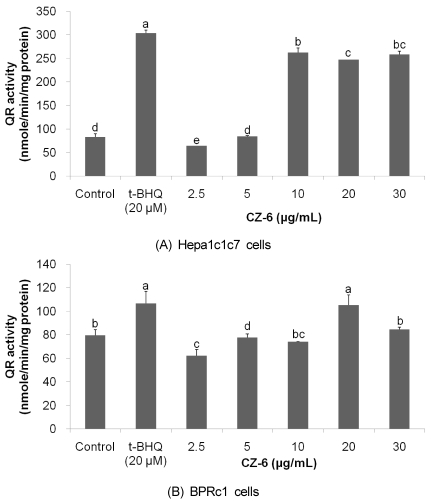
Since QR is a biomarker enzyme for phase 2 detoxifying/antioxidant enzymes, we determined whether CZ-6 fraction induces QR activity in Hepa1c1c7 and its mutant BPRc1 cells lacking arylhydrocarbon receptor nuclear translocator (ARNT), which are typical murine hepatoma cell lines highly responsive to phase 2 enzyme inducers and thereby widely used for screening phase 2 enzyme inducers [9,10,12,13,14]. As shown in Fig. 1, cytosolic QR enzyme activity in Hepa1c1c7 cell line was increased in a dose-dependent manner in the range of 2.5 to 10 µg/mL of CZ-6 fraction (Fig. 2A) while the enzyme activity in BPRc1 cells was induced at a single dose of 20 µM CZ-6 fraction alone but not the other doses used (Fig. 2B).
Fig. 2.
Induction of quinone reductase by CZ-6 fraction in Hepa1c1c7 (A) and BPRc1 cells. After culturing 48 h in alpha-MEM containing 10% FBS, cells were exposed to various doses of CZ-6 fraction for another 24 h and subjected to QR assay.
ARE activation by CZ-6 fraction
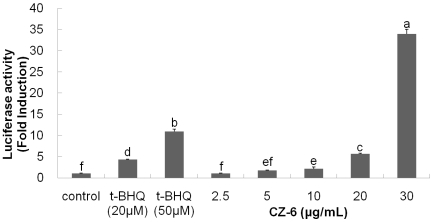
To examine whether induction of phase 2 enzymes is mediated by antioxidant responsive element (ARE) in the promoter region of genes encoding the enzymes, HepG2-C8 cells harboring pARE-luciferase gene construct were exposed to various concentrations of the sample. As shown in Fig. 3, the reporter assay showed that CZ-6 fraction increased luciferase gene expression proportionately with the increasing concentrations of the compound. The luciferase activity was increased by 5 and 35-folds in HepG2-C8 cells exposed to CZ-6 at the concentrations of 20 and 30 µg/mL, respectively.
Fig. 3.
Dose-dependent increase of reporter luciferase activity in HepG2-C8 cells by CZ-6 fraction. HepG2-C8 cells stably transfected with pARE-TI-luciferase construct were exposed to various doses of CZ-6 and assayed for luciferase activity using luminometer.
Effect of CZ-6 on body and organ weights of mice exposed to CCl4
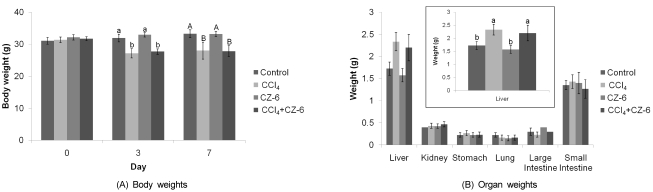
As CZ-6 fraction caused a significant induction of QR activity, a biomarker for anticarcinogenic and antioxidative potential, we examined whether it also had QR-inducing activity as well as protective effect on CCl4-induced hepatotoxicity in mice. The body weight of mice was significantly decreased at 3rd and 7th day after CCl4 injection regardless of CZ-6 application. However, liver weight was increased by CCl4 treatment while co-treatment with CZ-6 did not repress liver enlargement induced by CCl4 (Fig. 4).
Fig. 4.
>Effect of treatment of carbon tetrachloride and CZ-6 fraction on body (A) and organ (B) weights of mice. Mice weighing 20-30 g were insulted with CCl4 twice (7th and 9th days) in a week with and without CZ-6, and weighed for body and organs on 11th day after initiating the study.
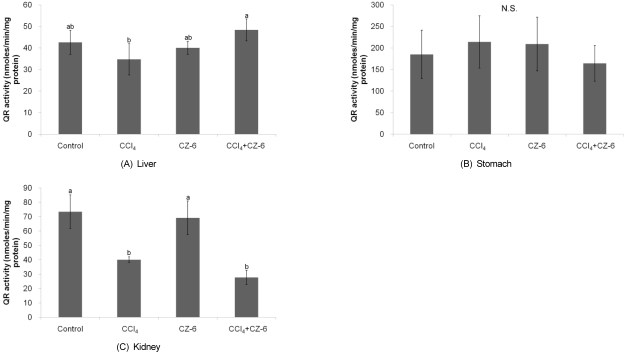
Effect of CZ-6 fraction on QR activity in organs from mice exposed to CCl4
Treatment with CCl4 slightly decreased QR activity in mouse kidney, without significant effect on QR activity in other organs (Fig. 5). The suppression of liver QR induced by CCl4 injection was relieved by pretreatment with CZ-6 fraction. However, CZ-6 fraction failed to restore kidney QR reduction caused by CCl4. The QR activities in stomach, small intestine, and large intestine were not influenced by treatment with either CCl4 or CZ-6 fraction.
Fig. 5.
Effect of CCl4 and CZ-6 fraction on QR activities in mouse organs. Mice weighing 20-30 g were insulted with CCl4 twice (7th and 9th days) in a week with and without CZ-6, and assayed for QR activities of tissues on 11th day after initiating the study.
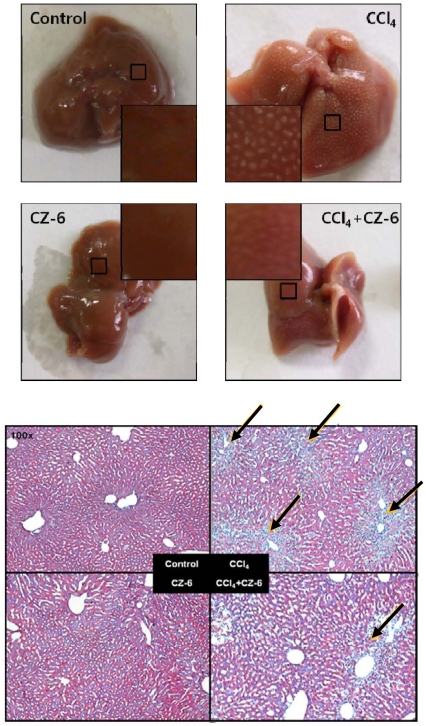
Effect of CZ-6 fraction on CCl4-induced liver damage
In order to investigate whether CZ-6 fraction has preventive effect from CCl4-induced hepatic injury, mice were injected with CCl4 in combination with CZ-6 fraction and subjected to histological examination of liver. As shown in Fig. 6, CCl4 treatment caused severe necrosis in hepatic tissues. However, the cotreatment with CZ-6 fraction did have weak yet obvious protective effect from hepatic damage induced by CCl4.
Fig. 6.
Liver histology in mice after CCl4 and CZ-6 treatments. The method for the administration of CZ-6 and induction of liver injury are described in 'Materials and Methods' section. The liver sections obtained from the mice were subjected to hematoxylin-eosin staining. Arrows indicate necrotic areas.
Discussion
In the previous study we found that the methanolic extract of Chrysanthemum zawadskii induced QR, an anticarcinogenic biomarker enzyme [2,3]. Further chromatographic fractionation to search for active component(s) resulted in active fraction (CZ-6) which contains (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]de cane as QR inducer, belonging to acetylene family. Although the fraction showed relatively weak activity in QR induction compared to other known phytochemicals [8,13-16], its QR-inducing potential of the fraction at the 20 µg/mL was compatible with 10 µM tert-butylhydroquinone (tBHQ), a known QR inducer, in BPRc1 cells. Furthermore, its relatively low QR-inducing potential in BPRc1 cells with defective arylhydrocarbon receptor translocator (arnt) indicates that the fraction consists of a mixture of bifunctional inducer and monofunctional inducer. A monofunctional inducer does not depend on a competent Ah receptor (AHR) or arylhydrocarbon hydroxylase (AHH) activity and appears to involve an electrophilic chemical signal. In contrast, bifunctional inducers require competent AHRs to induce both AHH and QR, although the latter process appears to be regulated by more than one mechanism [7].
The induction of phase 2 detoxifying and antioxidant enzymes by most monofunctional inducers has been reported to be mediated by interaction between NF-E2-related factor-2 (Nrf2) and antioxidant response element (ARE) in the promoter region of phase 2 detoxifying and antioxidant enzyme genes. Considering that CZ-6 stimulated luciferase activity in the reporter assay representing ARE-binding activity of Nrf2, the fraction appears to liberate Nrf2 from Nrf2-keap1 complex, and promote nuclear migration and ARE-binding of Nrf2 to activate transcription of antioxidant enzyme genes [17,18].
It is generally believed that CCl4 toxicity results from the bioactivation of the CCl4 molecule to the trichloromethyl free radical by cytochrome P450 2E1 of the endoplasmic reticulum [19]. Once the trichloromethyl radical is formed, it reacts with molecular oxygen to form the highly toxic trichloromethyl peroxy radical [20-22]. The free radicals then attack on polyunsaturated fatty acids of membrane lipids to propagate a chain reaction, leading to lipid peroxidation. These chains of events result in the breakdown of membrane structure, disrupting cell energy processes and protein synthesis [23-25].
As shown in Fig. 5, CZ-6 failed to induce QR over the control in liver, kidney and stomach of the mouse model although the fraction caused significant induction of phase 2 detoxifying enzymes in cultured cells. The limited QR induction by CZ-6 fraction in the whole animal model may be due to the rapid metabolism and/or poor transport of active component(s) into cells. It is also possible for the active components to induce some CYP450s including 2E1, resulting in reinforcement of CCl4 toxicity and negating the phase 2 detoxifying-inducing activity. However, the recovery of hepatic QR activity suppressed by CCl4 treatment to the control level may explain the partial protection of liver from CCl4-induced necrosis as shown in Fig. 6.
Meanwhile, (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro[4,5]decan e, a compound isolated from CZ-6 fraction, induced QR in a dose-dependent manner in hepa1c1c7 and BPRc1 cells (data not shown).
Although CZ-6 fraction failed to show significant QR induction in mouse organs, it somehow inhibited hepatic necrosis caused by CCl4 as shown in Fig. 6. While its protection from CCl4-induced liver damage in mouse may be associated with QR activity, its detailed protective mechanism remains to be elucidated.
Taken together, our current data demonstrated that CZ-6 fraction derived from Chrysanthemum zawadskii exerted some protective effect against CCl4-induced hepatotoxicity in mice although it showed only limited potential to induce QR, one of phase 2 detoxifying and antioxidant enzymes.
Footnotes
This study was supported by a grant from BioGreen 21 Program, Rural Development Administration, Republic of Korea.
References
- 1.Kwon HS, Ha TJ, Hwang SW, Jin YM, Nam SH, Park KH, Yang MS. Cytotoxic flavonoids from the whole plants of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. J Life Sci. 2006;16:746–749. [Google Scholar]
- 2.Kang HS, Park MJ, Jin KS, Kim YH, Jun M, Lim HJ, Jo WK, Kim JS, Jeong WS. Regulatory roles of Chrysanthemum zawadskii roots in nuclear factor E2-related factor 2/antioxidant response element pathway. Food Sci Biotechnol. 2008;17:367–372. [Google Scholar]
- 3.Kim JR, Kim JH, Lim HA, Jang CH, Kim JH, Kwon CS, Kim YK, Kim JS. Induction of quinone reductase, an anticarcinogenic marker enzyme, by extract from Chrysanthemum zawadskii var. latilobum K. Journal of Food Science and Nutrition. 2005;10:340–343. [Google Scholar]
- 4.Mehta RG, Pezzuto JM. Discovery of cancer preventive agents from natural products: from plants to prevention. Curr Oncol Rep. 2002;4:478–486. doi: 10.1007/s11912-002-0059-2. [DOI] [PubMed] [Google Scholar]
- 5.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prochaska HJ, De Long MJ, Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985;82:8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, Kong AN. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003;63:7520–7525. [PubMed] [Google Scholar]
- 10.Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P) H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1183–1190. [PubMed] [Google Scholar]
- 13.Yin H-Q, Lee BW, Kim YC, Sohn DH, Lee BH. Induction of the anticarcinogenic marker enzyme, quinone reductase, by Dalbergiae Lignum. Arch Pharm Res. 2004;27:919–922. doi: 10.1007/BF02975844. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Lee JS, Song KS, Kwon CS, Kim YK, Kim JS. Polyozellin isolated from Polyozellus multiplex induces phase 2 enzymes in mouse hepatoma cells and differentiation in human myeloid leukaemic cell lines. J Agric Food Chem. 2004;52:451–455. doi: 10.1021/jf034748n. [DOI] [PubMed] [Google Scholar]
- 15.Seo JY, Park J, Kim HJ, Lee IA, Lim JS, Lim SS, Choi SJ, Park JH, Kang HJ, Kim JS. Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes. J Med Food. 2009;12:1038–1045. doi: 10.1089/jmf.2009.0072. [DOI] [PubMed] [Google Scholar]
- 16.Seo JY, Lim SS, Kim JR, Lim JS, Ha YR, Lee IA, Kim EJ, Park JH, Kim JS. Nrf2-mediated induction of detoxifying enzymes by alantolactone present in Inula helenium. Phytother Res. 2008;22:1500–1505. doi: 10.1002/ptr.2521. [DOI] [PubMed] [Google Scholar]
- 17.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong F, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 20.Albano E, Tomasi A, Goria-Gatti L, Poli G, Vannini V, Dianzani MU. Free radical metabolism of alcohols by rat liver microsomes. Free Radic Res Commun. 1987;3:243–249. doi: 10.3109/10715768709069789. [DOI] [PubMed] [Google Scholar]
- 21.Connor HD, Lacagnin LB, Knecht KT, Thurman RG, Mason RP. Reaction of glutathione with a free radical metabolite of carbon tetrachloride. Mol Pharmacol. 1990;37:443–451. [PubMed] [Google Scholar]
- 22.McCay PB, Lai EK, Poyer JL, DuBose CM, Janzen EG. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism: Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984;259:2135–2143. [PubMed] [Google Scholar]
- 23.Packer JE, Slater TF, Willson RL. Reactions of the carbon tetrachloride-related peroxy free radical (CCl3O2.) with amino acids: Pulse radiolysis evidence. Life Sci. 1978;23:2617–2620. doi: 10.1016/0024-3205(78)90378-8. [DOI] [PubMed] [Google Scholar]
- 24.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967;19:145–208. [PubMed] [Google Scholar]