Abstract
Obesity is considered a mild inflammatory state, and the secretion of inflammation-related cytokines rises as adipose tissue expands. Inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interlukin 6 (IL-6) and monocyte-chemoattractant protein 1 (MCP-1), are modulated by adipose tissue and known to play an important role in insulin resistance which is the common characteristics of obesity related disorders. In this study we analyzed the effects of Sasa borealis leaves extract on inflammatory cytokines and insulin resistance in diet induced obese C57/BL6J mice. The obese state was induced by a high fat diet for 20 weeks and then the mice were divided into two groups; obese control group (OBC, n = 7) and experimental group (OB-SBE, n = 7). The OBC group was fed a high fat diet and the OB-SBE group was fed a high fat diet containing 5% Sasa borealis leaves extract (SBE) for 12 weeks. We also used mice fed a standard diet as a normal control (NC, n = 7). The body weight and adipose tissue weight in the OB group were significantly higher than those in the NC group. The effects of the high fat diet were reduced by SBE treatments, and the body weight and adipose tissue deposition in the OB-SBE group were significantly decreased compared to the OBC group. The OBC group showed higher serum glucose and insulin levels which resulted in a significant increase of incremental area under the curve (IAUC) and HOMA-IR than the NC group. Also, serum leptin, TNF-α, and IL-6 levels were significantly higher in the OBC group than in the NC group. In contrast, the OB-SBE group showed a reversal in the metabolic defects, including a decrease in glucose, insulin, IAUC, HOMA-IR, TNF-α, IL-6 and leptin levels. These results suggest that BSE can suppress increased weight gain and/or fat deposition induced by a high fat diet and theses effects are accompanied by modulation of the inflammatory cytokines, TNF-α and IL-6 secretion resulting in improved insulin resistance.
Keywords: Obesity, insulin resistance, inflammation, cytokine, Sasa borealis
Introduction
Obesity and associated comorbidities are worldwide health problems. It is widely accepted that obesity results from disequilibrium between energy intake and expenditure, however nowadays adipose tissue is recognized as a major endocrine and secretory organ, releasing a wide range of protein factors and signals termed adipokines. Obesity is characterized by a state of mild inflammation, and the expression and release of inflammation-related adipokines generally rise as adipose tissue expands [1,2]. Inflammatory markers such as tumor necrosis factor-α (TNF-α), interlukin 6 (IL-6), and monocyte-chemoattractant protein 1 (MCP-1) are increased in obese individuals compared with lean subjects, although not to the same extent observed in classic inflammatory conditions. The presence of systemic inflammation has been linked to the increased risk of development of insulin resistance, type II diabetes, cardiovascular disease and other metabolic disorders associated with obesity [3-5].
There has been a substantial effort toward understanding the mechanisms underlying obesity induced metabolic disorders and numerous studies have been conducted looking for natural active products, especially medicinal plant sources, for therapeutic intervention to prevent and/or cure obesity and related disorders with few side effects. However, mechanistic studies related to cytokine modulation by medicinal plant sources are rare. Bamboo has been used for medicinal purposes for centuries in Korea and other Asian countries and its efficacy has been recorded in the materia medica, DongEuiBoGam in Korea [6-8]. Recent scientific research has shown health benefits of bamboo leaves [8-16], culm [17], shoot [18], shavings [19] and oils [20,21]. It has been shown that the medicinal effects of Sasa borealis, a species of bamboo, are predominantly anti-diabetic through enhancing insulin secretion [11], hypoglycemic and hypolipidemic effects [10,14-16], anti-obesity [10] and/or anti-oxidant effects [13]. It has recently been used as plain extraction or blend tea, hamburger patties, and complementary and alternative agent to demonstrate beneficial health effects [12,14]. In spite of the usefulness in the treatment for obesity and related metabolic disorders, the mechanism of these effects, focused on cytokine regulation, is rare and still remains to be solved. Our previous study revealed that the intake of Sasa borealis leaf extracts had a beneficial effect on adiponectin and resistin levels and related molecules such as C-reactive protein and homocysteine levels which are involved in cardiovascular disease [10].
In this study, we tested whether the intake of extracts of Sasa borealis leaves modulates the production of the inflammatory cytokines TNF-α, IL-6 and MCP-1, which are involved in insulin resistance, on diet induced obesity.
Materials and Methods
Sasa borealis leaves extract (SBE)
Sasa borealis leaves collected from the area of Chuwall mountain, at Damyang province in Korea were steamed for 30min, dried and ground to a fine powder, and then extracted with 70% ethanol for 20 hours at 95℃. The resulting extracts were filtered, concentrated under vacuum at 60℃, and stored at 4℃ until used. The extraction yield of SBE was 10.4% (g/100 g, w/w).
Animals and diets
Twenty one male C57BL/6J mice, aged 4 wk, were purchased from Charles River Laboratories (Tokyo, Japan). The animals were maintained on a standard chow diet (Jeil-jedang, Suwon, Korea) for 1 week, and then divided into two groups, normal diet (NC, n = 7) and high fat diet (OBC, n = 14) group. To make diet induced obese mice, OBC group was fed a high fat diet, with 60% of calories coming from fat (D12451, Research Diets, Ins., New Brunswick, NJ, USA) for 20 weeks. The NC group was fed a standard fat diet, with 10% of calories coming from fat (D12450B, Research Diets, Inc., New Brunswick, NJ, USA). The induction of obesity was confirmed by checking the body weight (Fig. 1). The obese mice were randomly divided into two groups such that the average weight in each group was comparable, OBC group (n = 7) and high fat diet containing 5% Sasa borealis leaves extract (OB-SBE, n = 7) group. During the 12 week experimental periods, NC and OBC groups were fed the standard fat and high fat diet, respectively, and the OB-SBE group was fed high fat diet containing 5% SBE. The optimal level of BSE in the diet was determined in previous studies [9], which showed that the anti-obesity effect of Sasa borealis leaves extract was highest in 5% ethanol extracts (70%). The food consumption and body weight were measured daily and weekly, respectively. The mice were maintained in accordance with Guidelines for Care and Use of Laboratory Animals of Chonnam National University. The animals were housed in a polycarbonate cage in a temperature, humidity-controlled (23 ± 1℃, 53 ± 2%) room and maintained on a light cycle (12 h/12 h light/dark) with free access to food and water. At the end of the experiment, the mice were deprived of food for 12 hours and then anesthetized. The experimental protocols were approved by the Animal Ethics Committee of Chonnam National University.
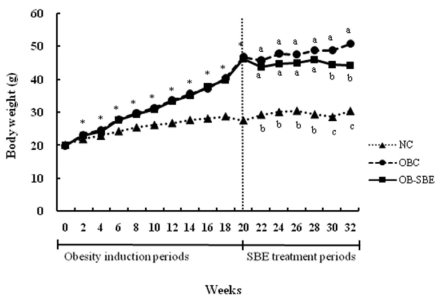
Fig. 1.
Changes of body weight during the experimental periods. Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract. Mean values with * or different letters are significantly different by repeated-measures ANOVA at P < 0.05.
Collection of blood and tissue samples
Blood was collected after 12 hours of fasting by orbital cardiac puncture and put on ice water for 1 hour. Serum was separated by centrifugation at 1,100 × g for 15 min. at 4℃ (Micro 17R, Vision Scientific Co., Seoul, Korea), and stored at -20℃ until analyzed. After blood collection, organs such as liver, kidney and pancreas were surgically removed, and the adipose tissue was collected from epididymal and retroperitoneal fat. The samples were rinsed with phosphate-buffered saline solution, wiped with a paper towel, weighed, immediately frozen in liquid nitrogen and stored at -80℃ until analyzed.
Analysis of glucose levels and glucose tolerance test (OGTT)
Glucose tolerance test was carried out at the end of the experiment during the fasting state. A 75% glucose solution (3.0 g/kg body weight) was orally applied to each mouse using an intubation tube. Blood from the tail (20 ul) was taken every 30 min from 0 min to 120 min after the glucose solution load. Blood glucose levels were measured by glucose oxidase method using a portable blood glucose monitor (model Super Glucocard II, KDK, Japan). The positive incremental area under the curve (IAUC) was calculated using the Tapezoidal rule [22].
Analysis of serum insulin and leptin levels and homeostasis model assessment of insulin resistance (HOMA-IR).
Serum insulin and leptin levels were analyzed by Enzyme-Linked Immunosorbent Assay (ELISA). The analysis was conducted utilizing a mouse insulin assay kit (Mercodia AB, Uppsala, Sweden) and a mouse leptin assay kit (BioVendor Laboratories Std., Modrice, Czech Republic). The sample was thawed, appropriately diluted with assay diluents, and assayed. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as described by Hosker et al. [23], HOMA-IR = [fasting insulin (µU/mL) × fasting glucose (mmol/L)] / 22.5.
Analysis of serum inflammatory cytokines levels
Serum inflammatory cytokines levels were analyzed by ELISA. The analysis was conducted utilizing a mouse TNF-α assay kit (BioSource International, Inc., California, USA), a mouse IL-6 assay kit (BioSource International, Inc., California, USA), or a mouse MCP-1 assay kit (BioSource International, Inc., California, USA). The sample was thawed, appropriately diluted with assay diluents, and assayed.
Statistical analysis
All values were expressed as group means ± SE. The statistical analyses were performed on an SPSS version 12.0 (SPSS Inc, Chicago, IL, USA). One way analysis of variance (ANOVA) followed by Tukey's test was used to examine the differences between groups. All time dependent data were analyzed by repeated-measures ANOVA. Statistical significance was considered at P < 0.05.
Results
Body weight and weight gain
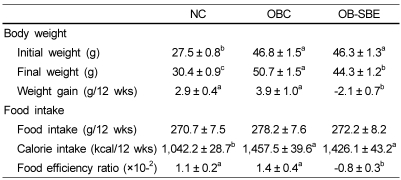
As shown in Table 1, at the beginning of the experiment, the weight of NC group was significantly lower than those of OBC and OB-SBE groups whereas there was no significant difference in weight between the OBC and OB-SBE group. At the end of the experiment, SBE treatment led a significant weight reduction resulting that the weight of OB-SBE group was significantly lower than that of the OBC group.
Table 1.
The effects of SBE on body weight and food intake
Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract.
Values are mean ± SE of 7 mice. Mean values with different letters are significantly different between the three groups by ANOVA with Tukey's test at P < 0.05.
Food efficiency ratio = weight gain (g/12 wk) / food intake (g/12 wk)
Food intake and feed efficiency
The caloric intake of the animals is shown in Table 2. There was no significant difference in feed consumption among the NC and OBC groups, either with or without SBE, however the caloric intake was significantly lower in the NC group than in both the OBC and OB-SBE groups. The feed efficiency ratio was significantly lower in the OB-SBE group than in the NC and OBC groups. Treatment with SBE significantly decreased the feed efficiency ratio and resulted in a negative feed efficiency ratio.
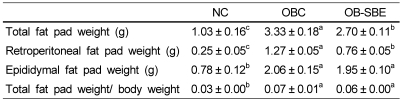
Table 2.
The effects of SBE on adipose tissue weight
Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract.
Values are mean ± SE of 7 mice. Mean values different letters are significantly different between the three groups by ANOVA with Tukey's test at P < 0.05.
Body fat pad weight and organ weight
Body fat pad weight is shown in Table 2. The epididymal and retroperitoneal fat pad weights were significantly lower in the NC group than in the OBC group. BSE treatment led to lower retroperitoneal fat deposition resulting that the retroperitoneal fat pad weight was significantly lower in OB-SBE than OBC group. However, there was no significant difference in epididymal fat pad deposition between the OBC and OB-SBE group. The body fat pad weight to body weight ratio was significantly lower in NC group than in OBC and OB-SBE groups. SBE treatment showed no significant effect on the body fat pad weight to body weight ratio in OB-SBE group.
Serum glucose, insulin and leptin levels
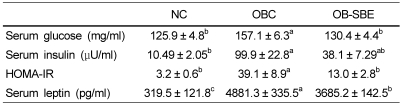
The effects of high fat diet and SBE treatment on serum glucose, insulin and leptin levels are shown in Table 3. Fasting serum glucose, insulin and leptin levels were significantly higher in the OBC group than in the NC group. SBE treatment significantly lowered all levels in the OB-SBE group compared to OBC group. The predominant effects of high fat diet were on serum leptin levels, which were significantly increased almost tenfold.
Table 3.
The effects of SBE on serum glucose, insulin and leptin levels and HOMA-IR
Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract.
Values are mean ± SE of 7 mice. Mean values with different letters are significantly different between the three groups by ANOVA with Tukey's test at P < 0.05.
Oral glucose tolerance test (OGTT) and homeostasis model assessment of insulin resistance (HOMA-IR)
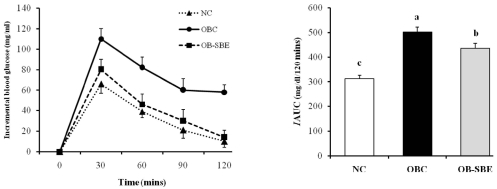
The area under the curve (AUC) calculated from the oral glucose tolerance test is shown in Fig 2. The incremental AUC was significantly high in the OBC group compared to the NC group. BSE treatment significantly decreased the incremental AUC in the OB-SBE group compared to the OBC group. The effects of high fat diet and SBE treatment on HOMA-IR corresponded with insulin levels, which were significantly higher in the OBC group than in the NC group, and BSE treatment significantly decreased HOMA-IR in the OB-SBE group compared to the OBC group.
Fig. 2.
The effects of SBE on incremental blood glucose levels (left) and incremental area under the curve (right) after oral glucose tolerance test. Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract. Mean values with different letters are significantly different between the three groups by ANOVA with Tukey's test at P < 0.05.
Serum inflammatory cytokines levels
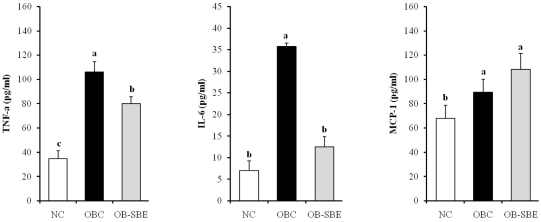
Serum inflammatory cytokines levels are shown in Fig 3. The TNF-α and IL-6 levels were significantly higher in the OBC group than in the NC group whereas there was no difference in MCP-1 levels between the OBC and NC group. SBE treatment significantly decreased TNF-α and IL-6 levels and there was no significant change in MCP-1 levels in the OB-SBE group.
Fig. 3.
The effects of SBE on serum inflammatory cytokine levels. Group abbreviation: NC; mice fed normal diet, OBC; mice fed the high fat diet, OB-SBE; mice fed the diet containing 5% Sasa borealis leaves extract. Mean values with different letters are significantly different between the three groups by ANOVA with Tukey's test at P < 0.05.
Discussion
The present study clearly shows that dietary fat has an effect on the development of obesity in mice. Our results support previous studies which showed that a high fat diet induced higher body fat deposition and increased body weight. Although feed consumption was not significantly different, the increased caloric density of high fat diet led to significantly higher weight gain compared with the normal diet which resulted in higher feed efficiency. Thus, mice fed high fat diet in the OBC group showed more rapid weight gain and greater fat pad deposition than mice fed normal diet in the NC group. However, the higher weight gain and feed efficiency exhibited by the OBC group were reduced by SBE treatment. Although feed and caloric intakes were not significantly different, the body weight of the OBC group was increased whereas that of the OB-SBE group was decreased and resulted in negative feed efficiency ratio. The SBE treatment significantly decreased retroperitoneal fat deposition whereas epididymal fat deposition was not changed. Changes in body fat deposition and organ weights were reflected to body weight changes (data were not shown). These results suggest that SBE can suppress the increase in weight gain or fat deposition, especially in retroperitoneal fat induced by high fat diet. Adipose tissue has been long considered as an active tissue, primarily responsible for storing lipids in times of food plenty and releasing them when energy is restricted. Recent studies have demonstrated that adipose tissue plays an important role in energy regulation as well as whole body homeostasis via endocrine, paracrine and autocrine signals [24,25]. Leptin is a well-known obesity index produced by adipose tissue. In this study body fat accumulation was increased by high fat diet and correlated with body weight, fat content, and a marker of obesity, serum leptin levels increased accompanying a high fat diet. Thus, at the end of the experimental period, serum leptin levels were significantly higher in the OBC group compared to the NC group, whereas serum leptin levels were significantly decreased by BSE treatment in the OB-BSE group. However, leptin levels were still higher in the OB-SBE group compared to the NC group. These changes parallel with changes in adipose tissue depositions, especially in the retroperitoneal fat. In this study, we showed that SBE has a potential for weight reduction, especially in the retroperitoneal fat deposition, in mice fed a high fat diet, which is in agreement with other studies [9,10].
Several studies, including in vitro [11], animal studies [9,10] and human studies [12,14], have reported that BSE has beneficial effects in controlling serum glucose levels. However, in those studies only one or two indices were checked among the serum glucose, insulin levels, IAUC from OGTT or HOMA-IR. In this study we analyzed all of the integral indices to confirm the effects of SBE on glucose regulation by measuring serum glucose and insulin levels as well as calculating IAUC and HOMA-IR as an index of insulin resistance. Insulin resistance is defined as a decreased response of peripheral tissues to insulin action resulting in a hyperglycemic state and has been recognized as the integral feature of metabolic syndrome, which includes glucose intolerance, insulin resistance, obesity, hypertriglyceridemia, low HDL-cholesterol and hypertension. Growing evidence has pointed to a correlative and causative relationship between inflammation and insulin resistance. Xu et al. [26] reported that an inflammatory response is a general phenomenon of the obese state regardless of genetic or diet induced obesity (DIO) and the phenomenon was white adipose tissue (WAT) specific and was not observed in any other tissues including liver, lung, spleen and muscle. Also, Xu et al. [26] revealed that numerous inflammation and macrophage-specific genes are dramatically up-regulated in the white adipose tissue in both obese models. The up-regulation is progressively increased in WAT in DIO and precedes a dramatic increase in the circulating insulin level. Xu et al. [26] proposed a hypothesis that obesity-related insulin resistance is, at least in part, a chronic inflammatory disease initiated in adipose tissue. In the present study, the OBC group gained more adipose tissue and showed higher serum glucose and insulin levels compared to the NC group and resulted in a significantly increased insulin resistance as defined by HOMA-IR. The results from our study were in agreement with the above hypothesis. Also, our results showed that BSE treatment improves all of the indices and suggests that BSE has the potential to improve insulin resistance induced by a high fat diet.
To explore the relationship of inflammatory cytokines in obesity and insulin resistance, we analyzed TNF-α, IL-6 and MCP-1 levels. The inflammatory cytokine TNF-α has been demonstrated to mediate insulin resistance as a result of obesity in many rodent obesity models [26,27]. TNF-α is the first adipocytokine suggested to be a molecular link between obesity and inflammation since its levels are increased in the adipose tissue in the obese state and its increase impairs insulin sensitivity [27,28]. Recently, the MCP-1 was also shown to impair adipocyte insulin sensitivity [3,29,30]. It has been estimated that WAT contributes about 30% of circulating IL-6, thus, directly correlated with adiposity and insulin resistance. One of the best candidates for a connection between adiposity and insulin resistance is adiponectin which is the adipokine that circulates at the highest levels. In the previous study, we confirmed that high fat diet induced obese mice have lower adiponectin levels compared with lean mice fed a standard diet [10]. In the present study, the levels of TNF-α and IL-6 were significantly higher in the OBC group than in the NC group, and SBE treatment in the OB-SBE group significantly reduced TNF-α and IL-6 levels. However, there were no significant changes in the MCP-1 level between NC, OBC and OB-SBE groups. MCP-1 has already been shown to be clearly associated to the obese state in human and rodents [31]. The role of TNF-α and IL-6 in weight loss related to insulin resistance is well established; for instance, studies in obese human, diet therapy and drug treatment have both been shown to improve insulin sensitivity and correlate with a decreased adipose tissue production of TNF-α and IL-6 [1-3,26-28]. However, the studies in weight loss subsequent to MCP-1 production are still need to be established [29,30]. German et al. [32] reported that although it is known that MCP-1 levels in circulation are correlated with body weight, weight loss led no notable decrease in those levels in contrast to TNF-α and IL-6. It is of particular note that the degrees of reduction in circulating insulin and cytokine levels were of magnitude similar to those seen in our study. Further work, for explaining these changes, would be required to determine the significance of these results. The changes in cytokines levels measured in our study paralleled those of body weight and adipose tissue weight, and also reflected changes in HOMA-IR. Although we didn't track directly the sequence of adipose tissue deposition, glucose/insulin resistance and inflammatory cytokine modulation, the results which showed changes in serum inflammatory cytokine levels, insulin and leptin levels, adipose tissue weight and HOMA-IR by high fat diet are in agreement with previous reports [26,27,31] which explained the subsequence and relation between cytokines and insulin resistance in obesity induction. Xu et al. [26] tracked the expression level of inflammatory cytokine genes in WAT of DIO mice to determine whether gene regulation occurs prior to the development of systemic insulin resistance, which is characterized by hyperinsulinemia. Xu et al. [26] reported an increase in inflammatory gene expression as early as 3 weeks on high fat diet and a more dramatic up-regulation occurred around 16 weeks which was correlated with a marked increase in fasting insulin levels. Also, Xu et al. [26] concluded that the adipose inflammatory response was increased with an increase of adiposity, prior to the increase of fasting insulin level, but intensified at the onset of hyperinsulinemia. Wang et al. [31] examined the integrated effect of TNF-α on inflammatory cytokine expression in adipocytes and reported that the most substantial responses occurred with TNG-α, IL-6 and MCP-1 itself, expression of each which was strongly upregulated by the cytokine. In this study, we confirmed that SBE has the potential to reduce adipose tissue deposition and insulin secretion, and improve insulin resistance through modulation of secretion of the inflammatory cytokines TNF-α and IL-6. However, Ko et al. [11] reported that crude extract of Sasa borealis leaves contains a potential insulin sensitizer with functions similar to that of PPAR-γ agonist and possibly improves glucose utilization by enhancing insulin-stimulated glucose uptake and inhibiting carbohydrate digestion without affecting insulin secretion in vitro. The present study, conducted in vivo and mice were fed high fat diet with SBE for 12 weeks, showed significantly decreased fasting glucose and insulin levels and resulted in favorable changes in HOMA-IR and oral glucose tolerance. Therefore, to clarify these conflicting effects, additional work will be required to explore which effects of SBE, secretion, sensitizer or both contribute to improve glucose homeostasis/insulin resistance in obesity.
Several studies have suggested various biological and pharmacological properties of bamboo leaves extract which may be due to the presence of flavones glycosides, phenolic acids, coumarone lactones, anthraquinones, and amino acids, and has been utilized as therapeutic agents [33]. Park [13] reported that Sasa borealis leaves extract have high polyphenol (85.5 mg/g) and flavonoid (30.9 mg/g) contents compared to other bamboo species or medicinal plants and the major phenolic compounds were protocatechuic acid, p-hydroxy benzoic acid, caffeic acid, syringic acid, p-cumaric acid, leuteolin-6-glucoside and tricine-7-glucoside. Yoon et al. [34] reported that Sasa borealis have friedelin and glutiol, a class of triterpenoids and isoorientin, tricine 7-O-β-D-glucopyranoside, a class of flavonoid, and luteolin 6-C-α-L-arabinopyranoside. Koo et al. [11] suggested that triterpenoids present in Sasa borealis leaves extract have a potential to inhibit fat deposition. They also proposed that flavonoidal glucoside containing Sasa borealis leaves extract might have a potential insulin sensitizer. Triterpenoids, which are made up of 30 carbons, are considered as a condensation compound of six isoprenes [35]. Many kinds of triterpenoid extracted from several important Chinese herbal drugs such as Panax ginseng and bamboo shavings have been studied increasingly and their physiological activities and medicinal values have been focused [19]. Jung et al. [36] reported that triterpenoids extracted from the root of Rosa rugosa have an anti-inflammatory potential. Taken together, these results suggest that the effects of SBE in our study might be due to the active components, tritepenoids and flavonoids present in BSE. However, the exact component and their mechanism of SBE in vivo remain to be studied in the future.
In summary, we showed that SBE has beneficial effects on weight reduction, retroperitoneal fat deposition and insulin secretion and resistance which are accompanied by reduction in the secretion of the inflammatory cytokines, TNF-α and IL-6 except MCP-1in high fat diet fed obese mice.
References
- 1.Laclaustra M, Corella D, Ordovas J. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis. 2007;17:125–139. doi: 10.1016/j.numecd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Trayhurn P. Endocrine and signaling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 3.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Wang CC, Galstone ML, Drazin B. Molecular mechanism of insulin resistance that impact cardiovascular biology. Diabetes. 2004;53:2735–2740. doi: 10.2337/diabetes.53.11.2735. [DOI] [PubMed] [Google Scholar]
- 5.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hae J. DongEuBoGam. Seoul: Namsangdang; 1990. pp. 140–142. [Google Scholar]
- 7.Lu B, Wu X, Tie X, Zhang Y, Zhang Y. Toxicology and safty of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol. 2005;43:783–792. doi: 10.1016/j.fct.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Wu X, Shi J, Dong Y, Zhang Y. Toxicology and safty of anti-oxidant of bamboo leaves. Part 2: Developmental toxicity test in rat with antioxidant of bamboo leaves. Food Chem Toxicol. 2006;44:1739–1743. doi: 10.1016/j.fct.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Jung EY. Effect of the Sasa Borealis leaves extract on metabolic syndrome in C57/BL6j mice fed a high fat diet [master's thesis] Gwanju: Chonnam National University; 2006. [Google Scholar]
- 10.Kim EY, Jung EY, Lim HS, Heo YR. The effects of the Sasa Borealis leaves extract on plsma adiponectin, resistin, C-reactive protein and homocyteine levels in high fat diet-induced obese C57/BL6J mice. The Korean Journal of Nutrition. 2007;40:303–311. [Google Scholar]
- 11.Ko BS, Jun DW, Jang JS, Kim JH, Park S. Effect of Sasa borealis and white lotus root and leaves on insulin action and secretion In Vitro. Korean Journal of Food Science and Technology. 2006;38:114–120. [Google Scholar]
- 12.Oh HK. Development of the hamburger patties containing Sasa Borealis bamboo leaves, Laminaria japonica or rice [doctor's thesis] Gwangju: Chonnam National University; 2008. [Google Scholar]
- 13.Park YO. Antioxidant activities of extracts of Sasa Borealis leaves [doctor's thesis] Gwangju: Chonnam National University; 2008. [Google Scholar]
- 14.Yun EK. Effects of Sasa Borealis leaves extract on glucose tolerance of major contributing foods to carbohydrate intake [master's thesis] Gwanju: Chonnam National University; 2009. [Google Scholar]
- 15.Choi YJ, Choi JS, Shin SY, Bae JY, Kang SW, Kang IJ, Kang YH. Blocked of chronic high glucose-induced endotherial apoptosis by Sasa borealis bamboo extract. Exp Biol Med. 2008;233:580–591. doi: 10.3181/0707-RM-205. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JY, Han JS. Inhibitory effects of Sasa borealis leaves extracts on carbohydrate digestive enzyme and postprandial hyperglycemia. Journal of the Korean Society of Food Science and Nutrition. 2007;36:989–994. [Google Scholar]
- 17.Lee MJ, Park WH, Song YS, Lee YW, Song YO, Moon GS. Effect of bamboo culm extract on oxidative stress and genetic expression: Bamboo culm extract ameliorates cell adhesion molecule expression and NFκB activity through the suppression of the oxidative stress. Clin Nutr. 2008;27:755–763. doi: 10.1016/j.clnu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Park EJ, Jhon DY. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. LWT-Food Science and Technology. 2010;43:655–659. [Google Scholar]
- 19.Zang Y, Yao X, Bao B, Zhang Y. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam) Phytother Res. 2006;20:872–878. doi: 10.1002/ptr.1965. [DOI] [PubMed] [Google Scholar]
- 20.Choi DB, Cho KA, Na MS, Choi HS, Kim YO, Lim DH, Cho SJ, Cho H. Effect of bamboo oil on antioxidative activity and nitrite scavenging activity. Journal of Industrial and Engineering Chemistry. 2008;14:765–770. [Google Scholar]
- 21.Cho H, Cho KA, Jia S, Cho SJ, Choi DB. Influence of bamboo oil supplementation on blood lipid concentration in serum. Journal of Industrial and Engineering Chemistry. 2009;15:281–284. [Google Scholar]
- 22.Pham B, Cranney A, Boers M, Verhoeven AC, Wells G, Tugwell P. Validity of area-under-the-curve analysis to summarize effect in rheumatoid arthritis clinical trials. J Rheumatol. 1999;26:712–716. [PubMed] [Google Scholar]
- 23.Hosker JP, Matthews DR, Rudenski AS, Burnett MA, Darling P, Bown EG, Turner RC. Continuous infusion of glucose with model assessment: measurement of insulin resistance and beta-cell function in men. Diabetologia. 1985;28:401–411. doi: 10.1007/BF00280882. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Moustaid-Mossa N. Secretory, endocrine and autocrine/paracrine function of adipocyte. J Nutr. 2000;130:3110S–3115S. doi: 10.1093/jn/130.12.3110S. [DOI] [PubMed] [Google Scholar]
- 25.Urs S, Heo YR, Kim S, Kim JH, Jones BH, Moustaid-Moussa N. Genomics, proteomics and nutrition: Applications to obesity research. Nutritional Sciences. 2002;5:129–133. [Google Scholar]
- 26.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Sharigill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 28.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;46:1526–1531. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 29.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocyte, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 30.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-α. Am J Physiol Endocrinol Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- 32.German AJ, Hervera M, Hunter L, Holden SL, Morris PJ, Biourge V, Trayhurn P. Improvement in insulin resistance and reduction in plsma inflammatory adipokines after weight loss in obese dogs. Domest Animal Endocrinol. 2009;37:214–226. doi: 10.1016/j.domaniend.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Panee J. Bamboo extract in the prevention of diabetes and breast cancer. In: Watson RR, editor. Complementary and Alternative Therapies and the Aging Population: An Evidence-Based Approach. San Diego: ELSEVIER; 2008. pp. 159–177. [Google Scholar]
- 34.Yoon KD, Kim CY, Huh H. The flavone glycosides of Sasa Borealis. Korean Journal of Pharmacognosy. 2000;31:224–227. [Google Scholar]
- 35.Mahato SB, Nandy AK, Roy G. Triterpenoids. Phytochemistry. 1992;31:2199–2249. doi: 10.1016/0031-9422(92)83257-y. [DOI] [PubMed] [Google Scholar]
- 36.Jung HJ, Nam JH, Choi J, Lee KT, Park HJ. 19α-hydroxyursane-type triterpenoids: antinociceptive anti-inflammatory principles of the roots of Rosa rugosa. Biol Pharm Bull. 2005;28:101–104. doi: 10.1248/bpb.28.101. [DOI] [PubMed] [Google Scholar]