Abstract
The mechanisms underlying the association between air pollution and cardiovascular morbidity and mortality are unknown. This study aimed to determine whether controlled exposure to elemental carbon ultrafine particles (UFP) affects electrocardiogram (ECG) parameters describing heart rate variability; repolarization duration, morphology, and variability; and changes in the ST segment. Two separate controlled studies (12 subjects each) were performed using a crossover design, in which each subject was exposed to filtered air and carbon UFP for 2 hours. The first protocol involved 2 exposures to air and 10 µg/m3 (~ 2 × 106 particles/cm3, count median diameter ~25 nm, geometric standard deviation ~1.6), at rest. The second protocol included 3 exposures to air, 10, and 25 µg/m3 UFP (~ 7 × 106 particles/cm3), with repeated exercise. Each subject underwent a continuous digital 12-lead ECG Holter recording to analyze the above ECG parameters. Repeated measures analysis of variance (ANOVA) was used to compare tested parameters between exposures. The observed responses to UFP exposure were small and generally not significant, although there were trends indicating an increase in parasympathetic tone, which is most likely also responsible for trends toward ST elevation, blunted QTc shortening, and increased variability of T-wave complexity after exposure to UFP. Recovery from exercise showed a blunted response of the parasympathetic system after exposure to UFP in comparison to air exposure. In conclusion, transient exposure to 10–25 µg/m3 ultrafine carbon particles does not cause marked changes in ECG-derived parameters in young healthy subjects. However, trends are observed indicating that some subjects might be susceptible to air pollution, with a response involving autonomic modulation of the heart and repolarization of the ventricular myocardium.
INTRODUCTION
Air pollution is associated with increased morbidity and mortality, with an estimated 500,000 deaths each year related to air pollution worldwide (Committee on Research Priorities for Airborne Particulate Matter, 2004; Rom & Samet, 2006; US Environmental Protection Agency, 2005). Increased levels of air pollution are associated with death not only from lung cancer but also from cardiopulmonary disease (Brook, 2007; Pope III et al., 2004). Daily variations of PM10 (particles measuring 10 µm or less) and carbon monoxide were linked to daily hospital admissions for cardiovascular disease in the elderly (Schwartz, 1999). Increases in levels of airborne particulate matter (Peters et al., 2001), and exposure to traffic (Peters et al., 2004), are both associated with hospitalization for myocardial infarction. Air pollution is also associated with increased risk for cardiac arrhythmias. In patients with implanted cardiac defibrillators, device interrogation revealed relationships between ventricular arrhythmias and various indices of air pollution (Dockery et al., 2005; Peters et al., 2000).
Despite these epidemiological observations, the mechanisms underlying the association between increased air pollution and increased risk of cardiovascular morbidity and mortality have not been fully defined, and further research is required (Committee on Research Priorities for Airborne Particulate Matter, 2004; Utell et al., 2002). Many studies reported to date have shown associations between air pollution levels and changes in heart rate variability, propensity to ischemia, and arrhythmias in elderly subjects or in patients with apparent cardiovascular disease (reviewed in Bhatnagar, 2004; Brook, 2007; Godleski, 2006; Maitre et al., 2006). They indicate that electrical activity of the heart or its regulation could be affected by air pollution. There are limited data regarding the influence of air pollution on ECG parameters in young healthy subjects, and findings from clinical exposure studies have not shown consistent effects on ECG parameters (Devlin et al., 2003; Gong Jr. et al., 2008; Samet et al., 2007). The aim of this study was to determine the effects of controlled exposure to laboratory-generated ultrafine elemental carbon particles, as surrogates of ambient air ultrafine particles, on a series of prespecified ECG parameters describing heart rate variability; repolarization duration, morphology, and variability; and changes in the ST segment (Zareba et al., 2001). Other findings from these exposure studies have been reported elsewhere, including ultrafine particle deposition (Daigle et al., 2003) and effects on pulmonary function, blood parameters, and vascular function (Frampton et al., 2006; Pietropaoli et al., 2004a; Pietropaoli et al., 2004b).
METHODS
Study Population
Written informed consent was obtained from all subjects, and the studies were approved by the University of Rochester Research Subjects Review Board. Twenty-four healthy nonsmoking subjects aged 18–40 years (equal numbers by gender) participated, and were paid a stipend. Subjects were not studied within 6 weeks of a respiratory infection, and were required to have normal spirometry and a normal 12-lead electrocardiogram.
Two separate randomized, double-blinded, controlled studies were performed using a crossover design, in which each subject was exposed to filtered air and elemental carbon ultrafine particles (UFP) for 2 hours. Exposures were separated by at least 2 weeks, orders of exposure were randomized, and the randomization was blocked by order of presentation and gender. The first study involved 12 subjects (mean age 30 ± 9 years, six females) exposed at rest to 10 µg/m3 (~2 × 106 particles/cm3) UFP and filtered air; the second study involved 12 subjects (mean age 27 ± 6 years, six females) with three exposures for each subject, 10 µg/m3 UFP, 25 µg/m3 (~7 × 106 particles/cm3) UFP, and filtered air. In the second study, for safety reasons, the order of exposure was randomized in a restricted fashion, so that each subject received the 10-µg/m3 exposure before the 25-µg/m3. To simulate outdoor activities, subjects exercised on a bicycle ergometer for 15 minutes of each half hour of a 2-hour exposure at an intensity adjusted to increase the minute ventilation to approximately 20 L/min/m2 body surface area. All exposures took place in the morning hours to minimize the effect of circadian variation of the studied parameters.
Exposure to Ultrafine Carbon Particles
The details of the exposure system have been described elsewhere (Daigle et al., 2003). Briefly, elemental carbon ultrafine particles (count median diameter ~25 nm, geometric standard deviation (GSD) ~1.6) were generated in an argon atmosphere using an electric spark discharge between two graphite electrodes, and then deionized and diluted with filtered air to the desired concentration. Particle number, mass, and size distribution were monitored on both the inspiratory and expiratory sides of the subject. Electronic integration of a pneumotachograph signal provided tidal volume, respiratory frequency, and minute ventilation measurements. Air for the control exposures, and for dilution of the particles, was passed through charcoal and high efficiency particle filters, and was essentially free of contaminating gases and particles (0–10 particles/cm3).
ECG Monitoring
Each subject underwent a continuous digital 12-lead ECG Holter recording (Mortara Instruments, Milwaukee, MN), which was started in the morning prior to exposure and ended the following morning. During this continuous ECG recording, 5-minute supine resting ECG recording sessions were performed prior to exposure, immediately after exposure, and 3.5 hours and 21 hours after exposure to evaluate ECG parameters in controlled conditions unaffected by physical activity or body position. Each 5-minute segment was preceded by a 3–5-minute resting period. In addition, 5-minute ECG segments were identified for detailed analysis during the final 15 minutes of exposure, and during the night (2 a.m.). In the protocol involving exercise, the 5-minute monitoring segment of ECG data was accrued during the last exercise session. The 5-minute analysis segments preceded other subject procedures at each time point, in order to avoid effects of those procedures on the ECG recording.
ECG Analyses
Analysis of the 24-hour Holter ECG recordings was performed using the H-Scribe Mortara System (Mortara Instruments, Milwaukee, MN). After automatic beat annotation, verified by a technician, the ECG analysis was performed using a research version of Mortara’s program called MISHA, yielding quantitative measures of several ECG parameters, including beat-to-beat RR intervals, lead-specific and beat-to-beat ST segment levels, T-wave amplitude, and T-wave complexity. The QT interval was measured as the longest interval for each beat among all leads. Next, an 8-beat-segment average was computed for the QT interval, adjusted for heart rate with Bazett’s formula (Bazett, 1920). Subsequently, means of 8-beat averages from the 5-minute analysis segments were obtained. The measurements of QTc interval duration were also performed manually in lead II. For T-wave amplitude, the original ECG leads I, II, and V1–V6 were used, and the median value from these eight original ECG leads was taken for each beat and averaged over 5 minutes. T- wave complexity, describing morphology of the T-wave, was measured in each beat by principal component analysis (PCA) based on the above eight original leads, and averaged over the 5-minute period (Priori et al., 1997). Variability of T-wave complexity was measured as a standard deviation over the 5-minute period. ST segment analysis was focused on leads II, V2, and V5, and the median ST segment level over the 5-minute period was used.
The time-domain heart rate variability (HRV) parameters: SDNN (standard deviation of normal-to-normal sinus beat intervals) and rMSSD (root mean square of successive differences in NN intervals) were calculated for each 5-minute segment of interest, and for a 16-hour period starting 3.5 hours after exposure. The following frequency-domain HRV parameters were computed for each 5-minute segment using a fast Fourier technique: high frequency power (HF: 0.15–0.40 Hz), low frequency power (LF: 0.04–0.15 Hz), both expressed in normalized units, and the LF/HF ratio (Malik & Camm, 1995).
Arrhythmias were quantified first automatically, using the standard Mortara H-Scribe scanning system. Subsequently, annotation of the beats was performed by a trained technician under the direction of a cardiologist. The number of supraventricular and ventricular arrhythmias was based on the annotated ECG recordings.
Statistical Analysis
The first protocol utilized a standard, two-period crossover design in which each subject received both 10 µg/m3 UFP and air. The second protocol utilized a three-period crossover design in which each subject received both low (10 µg/m3) and high (25 µg/m3) concentrations of particles, and air. There were then three possible exposure sequences, depending on where in the sequence the air exposure was placed. In both protocols, equal numbers of subjects were randomly assigned to each sequence, and the randomization was balanced by gender. The wash-out period of at least 2 weeks between exposures was felt to be of sufficient duration to prevent any carry-over effects from one exposure to the next.
For the ECG parameters, differences were calculated by subtracting the pre-exposure values from those at each subsequent time point. These differences were then compared between the air and UFP exposures. As suggested by Jones and Kenward (2003), the primary analysis was based on “mixed models,” with adjustment for baseline measurements. The ANOVA included tests for an effect of time as well as interactions with other effects in the model. Order of presentation was a between-subjects factor, while exposure, period, and time were within-subject factors. The analysis included tests for period and carry-over effects, although the latter were expected to be non-existent (Jones & Kenward, 2003). Model checking included an examination of residuals as a check on the required assumptions of normally distributed errors with constant variance. If these assumptions were not satisfied, data transformations were considered. Paired t-tests were also performed comparing UFP vs. filtered air exposure for each time point. A level of 5% was required for statistical significance. Because multiple comparisons were involved and many endpoints were related, the congruence and plausibility of the results were considered in interpreting significance, and marginally statistically significant differences that were isolated, implausible, or inconsistent with other findings were not considered significant. Data are shown as means ± SE, unless otherwise indicated.
RESULTS
Exposure to 10 µg/m3 UFP at Rest
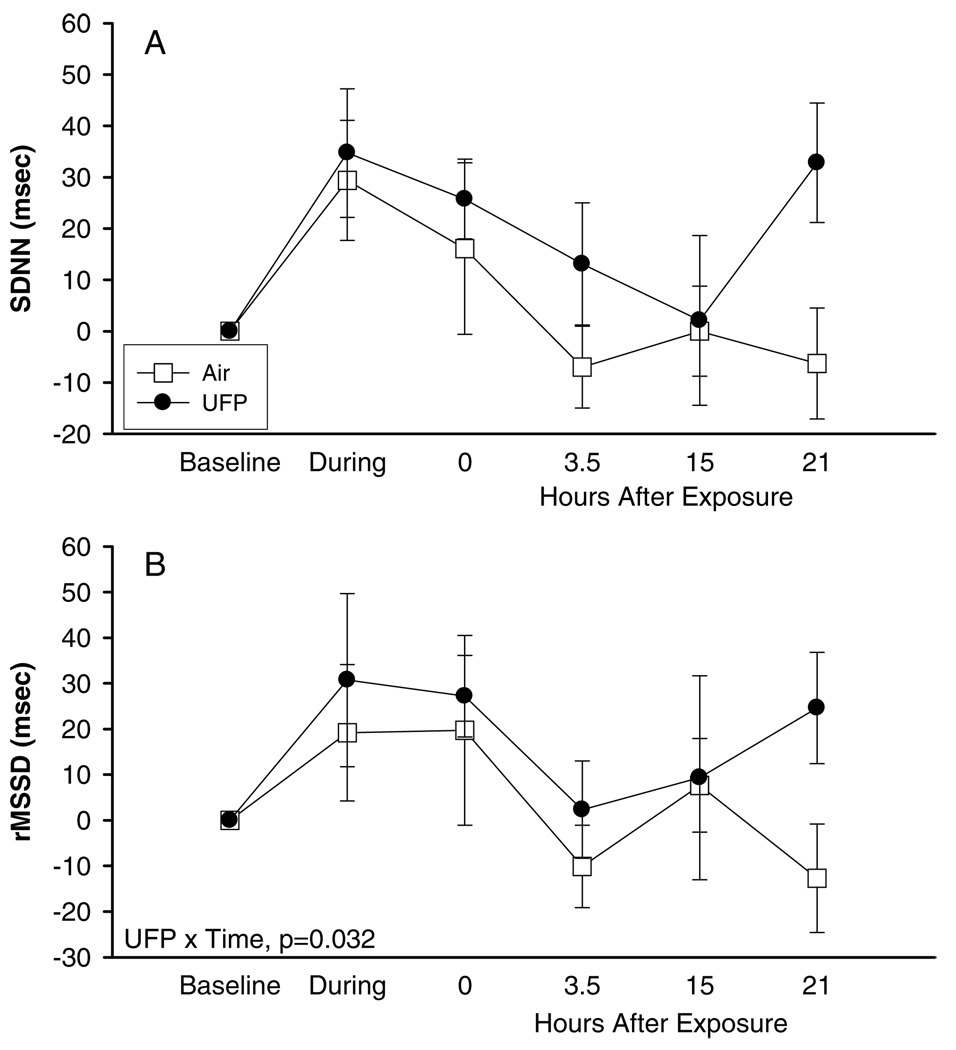
As shown in Tables 1 and 2, few significant changes in ECG parameters were observed with exposures to 10 µg/m3 UFP at rest. In particular, there were no significant changes in heart rate or heart rate variability parameters analyzed in the frequency domain. SDNN and rMSSD were somewhat more increased during, immediately after, and 3 hours after UFP compared with air exposure (Figure 1); the differences were significant only for rMSSD (p = 0.032).
TABLE 1.
Heart rate variability, exposure to 10 µg/m3 at rest, difference from baselinea
| ECG parameter/ exposure |
During exposure |
Hours after exposure |
ANOVA | |||
|---|---|---|---|---|---|---|
| 0 | 3.5 | 15 | 21 | |||
| RR (msec) | ||||||
| Air | 20 ± 42 | 169 ± 34 | –4 ± 25 | 80 ± 48 | –34 ± 39 | NS |
| UFP | 16 ± 40 | 140 ± 31 | –37 ± 45 | 71 ± 28 | 0.6 ± 21 | |
| SDNN (msec) | ||||||
| Air | 29 ± 12 | 16 ± 17 | –7 ± 8 | 0 ± 9 | –6 ± 11 | NS |
| UFP | 35 ± 13 | 26 ± 8 | 13 ± 12 | 2 ± 17 | 33 ± 12 | |
| rMSSD (msec) | ||||||
| Air | 19 ± 15 | 20 ± 21 | –10 ± 9 | 8 ± 10 | –13 ± 11 | UFP × time: p = 0.032 |
| UFP | 31 ± 19 | 27 ± 9 | 2 ± 11 | 9 ± 22 | 25 ± 12 | |
| Low frequency (Hz) | ||||||
| Air | 1411 ± 707 | 1213 ± 1435 | –401 ± 511 | 366 ± 529 | –228 ± 791 | NS |
| UFP | 1692 ± 449 | 693 ± 336 | 508 ± 321 | 458 ± 635 | 833 ± 482 | |
| High frequency (Hz) | ||||||
| Air | 2022 ± 1706 | 672 ± 1665 | –917 ± 680 | 686 ± 537 | –1188 ± 925 | NS |
| UFP | 2566 ± 1459 | 1315 ± 696 | 44 ± 779 | 1022 ± 1655 | 2407 ± 1261 | |
| Low frequency/high frequency | ||||||
| Air | 0.68 ± 0.45 | –0.46 ± 0.29 | 0.15 ± 0.25 | 0.12 ± 0.59 | 0.42 ± 0.42 | NS |
| UFP | 0.30 ± 0.23 | –0.28 ± 0.24 | 0.31 ± 0.58 | 0.10 ± 0.31 | –0.03 ± 0.17 | |
Data are means ± SE.
TABLE 2.
Cardiac repolarization parameters, exposure to 10 µg/m3 at rest, difference from baseline
| ECG parameter/ exposure |
During exposure |
Hours after exposure |
ANOVA | |||
|---|---|---|---|---|---|---|
| 0 | 3.5 | 15 | 21 | |||
| QTc (msec) | ||||||
| Air | −8 ± 5 | −11 ± 5 | 1 ± 3 | −7 ± 12 | 3 ± 4 | NS |
| UFP | −3 ± 4 | −4 ± 4 | −0.4 ± 7 | −6 ± 11 | 0.8 ± 5 | |
| T- wave amplitude (µV) | ||||||
| Air | 26 ± 19 | 43 ± 21 | −44 ± 24 | −44 ± 21 | 2 ± 18 | NS |
| UFP | 31 ± 17 | 23 ± 16 | −50 ± 18 | −37 ± 16 | 24 ± 12 | |
| PCA | ||||||
| Air | −3 ± 1 | −0.7 ± 0.9 | −0.06 ± 1.9 | 1.5 ± 1 | −1 ± 1 | NS |
| UFP | −3 ± 0.7 | −0.4 ± 0.7 | 0.9 ± 0.5 | 0.5 ± 1 | −0.8 ± 0.9 | |
| PCA variability | ||||||
| Air | 0.21 ± 0.14 | 0.02 ± 0.16 | 0.07 ± 0.13 | 0.15 ± 0.10 | 0.04 ± 0.08 | NS |
| UFP | 0.37 ± 0.13 | 0.13 ± 0.08 | 0.61 ± 0.42 | 0.08 ± 0.11 | 0.37 ± 0.10 | |
| ST in lead II (µV) | ||||||
| Air | 8 ± 5 | −1 ± 3 | −12 ± 4 | 3 ± 4 | −1 ± 5 | NS |
| UFP | 10 ± 5 | 2 ± 3 | −12 ± 2 | 10 ± 4 | 6 ± 4 | |
| ST in V2 (µV) | ||||||
| Air | −4 ± 2 | −3 ± 3 | −6 ± 4 | −10 ± 5 | −9 ± 4 | NS |
| UFP | −2 ± 2 | 0 ± 2 | −3 ± 2 | −10 ± 4 | −7 ± 4 | |
| ST in V5 (µV) | ||||||
| Air | −2 ± 4 | −4 ± 3 | −16 ± 6 | −7 ± 6 | −12 ± 7 | NS |
| UFP | 7 ± 2 | 4 ± 2 | −7 ± 3 | 4 ± 3 | 0.3 ± 3 | |
FIG. 1.
Heart rate variability, exposure to 10 µg/m3 at rest, difference from baseline, means ± SE: (A) SDNN, (B) rMSSD.
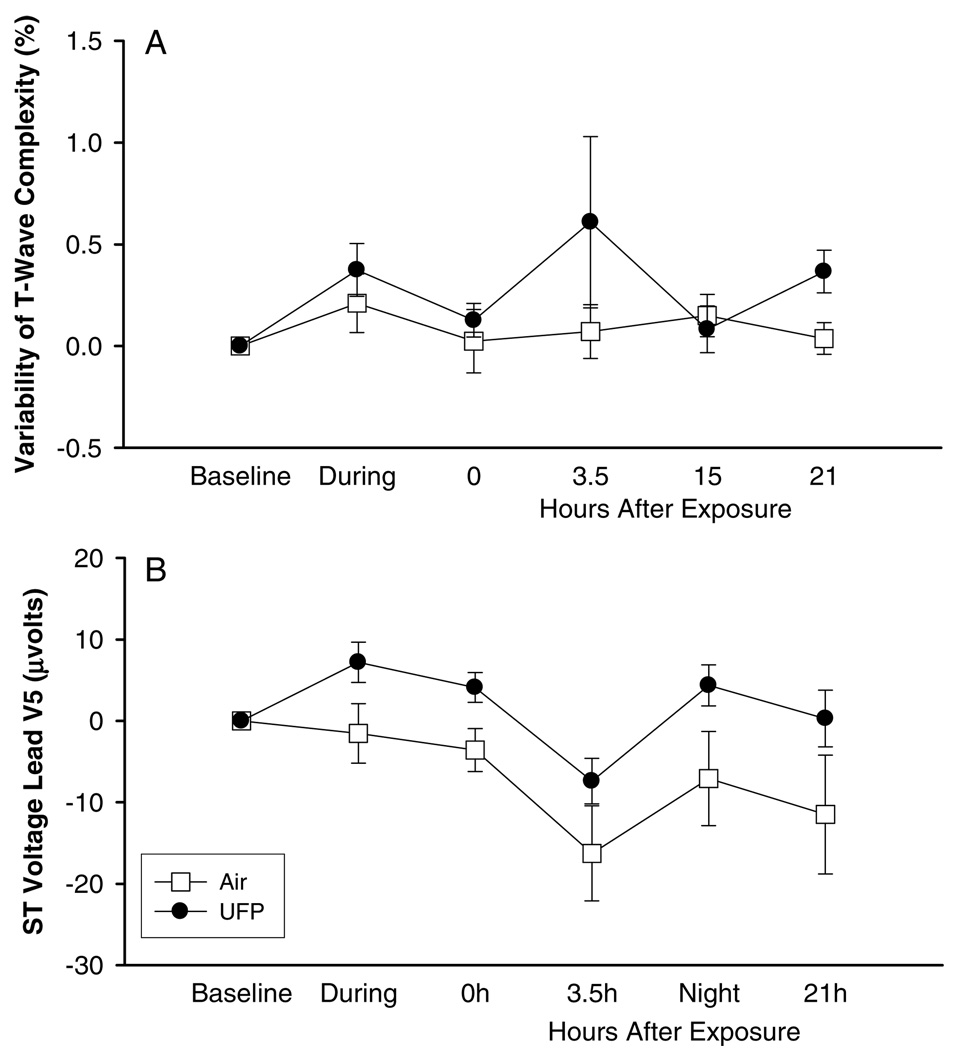
Repolarization duration, measured by the QTc corrected using Bazett’s formula, showed less shortening during and immediately after exposure with UFP than with pure air, again with no significant differences in response (Table 2). No changes were found in T-wave amplitude or T-wave complexity. However, the variability of repolarization, measured by beat-to-beat variability of T-wave complexity, showed some trends toward increased values during and after exposure with UFP when compared to values recorded during pure air exposure (Figure 2A). The ST segment, measured in lead V5, also showed a slight elevation after UFP but not air exposure (Figure 2B). The difference was not statistically significant, and was not observed in lead II or V2.
FIG. 2.
Cardiac repolarization, exposure to 10 µg/m3 at rest, difference from baseline, means ± SE: (A) variability of T-wave complexity, (B) ST voltage lead V.
Exposure to 10 and 25 µg/m3 with Exercise
Very similar analyses were performed for the ECG recordings obtained during the second protocol, involving randomized exposures to air and to UFP at 10 and 25 µg/m3. The results are shown in Tables 3 and 4 for HRV parameters and repolarization parameters, respectively.
TABLE 3.
Heart rate variability, exposures to 10 and 25 µg/m3 with exercise, difference from baseline
| ECG parameter/ exposure |
During exposure |
Hours after exposure |
ANOVA | |||
|---|---|---|---|---|---|---|
| 0 | 3.5 | 15 | 21 | |||
| RR (msec) | ||||||
| Air | −300 ± 40 | −110 ± 35 | −117 ± 37 | 63 ± 53 | −22.8 ± 30 | UFP × gender: |
| 10 µg/m3 | −275 ± 26 | −84 ± 26 | −91 ± 28 | 77 ± 35 | 27 ± 19 | p = 0.019 |
| 25 µg/m3 | −333 ± 50 | −70 ± 44 | −101 ± 30 | 37 ± 54 | −72 ± 29 | |
| SDNN (msec) | ||||||
| Air | −12 ± 12 | 3 ± 6 | −7 ± 12 | −9 ± 13 | 7 ± 7 | UFP: |
| 10 µg/m3 | 15 ± 10 | 5 ± 4 | 7 ± 8 | 3 ± 5 | 12 ± 7 | p = 0.013 |
| 25 µg/m3 | −17 ± 15 | 4 ± 17 | −18 ± 13 | −11 ± 15 | −6 ± 7 | |
| rMSSD (msec) | ||||||
| Air | −33 ± 13 | 11 ± 15 | −20 ± 17 | −5 ± 18 | 5 ± 7 | NS |
| 10 µg/m3 | −8 ± 7 | −3 ± 4 | −2 ± 10 | 12 ± 7 | 14 ± 10 | |
| 25 µg/m3 | −33 ± 19 | −3 ± 23 | −30 ± 18 | −9 ± 20 | −12 ± 6 | |
| Low frequency (Hz) | ||||||
| Air | −622 ± 249 | −583 ± 438 | −354 ± 499 | −272 ± 531 | −41 ± 303 | UFP × gender: |
| 10 µg/m3 | 380 ± 388 | 480 ± 329 | 534 ± 416 | 345 ± 287 | 389 ± 219 | p = 0.002 |
| 25 µg/m3 | — | 147 ± 1657 | — | −961 ± 1153 | −436 ± 695 | |
| High frequency (Hz) | ||||||
| Air | — | — | — | — | 518 ± 506 | UFP × gender: |
| 10 µg/m3 | −383 ± 151 | 175 ± 197 | −32 ± 221 | 470 ± 323 | 1418 ± 1056 | p = 0.011 |
| 25 µg/m3 | — | 100 ± 2071 | — | — | −111 ± 252 | |
| Low frequency/High frequency | ||||||
| Air | 3 ± 1 | −0.4 ± 0.6 | 0.1 ± 0.9 | −0.5 ± 0.9 | −1.3 ± 0.9 | UFP: |
| 10 µg/m3 | 3 ± 0.6 | 0.5 ± 0.3 | 1 ± 0.4 | 0 ± 0.3 | −0.3 ± 0.2 | p <0.0001 |
| 25 µg/m3 | 2 ± 1 | −0.5 ± 1 | −0.1 ± 1 | −0.9 ± 0.8 | −0.2 ± 0.4 | |
TABLE 4.
Cardiac repolarization parameters, exposures to 10 and 25 µg/m3 with exercise, difference from baseline
| ECG parameter/ exposure |
During exposure |
Hours after exposure |
ANOVA | |||
|---|---|---|---|---|---|---|
| 0 | 3.5 | 15 | 21 | |||
| QTc (msec) | ||||||
| Air | 37 ± 10 | 19 ± 9 | 21 ± 7 | 10 ± 5 | 23 ± 9 | UFP: |
| 10 µg/m3 | −53 ± 5 | 6 ± 5 | −17 ± 8 | −3 ± 10 | 1 ± 5 | p <0.001 |
| 25 µg/m3 | 27 ± 11 | 0.1 ± 4 | 5 ± 3 | −5 ± 9 | 7 ± 4 | |
| T- wave amplitude (µV) | ||||||
| Air | −75 ± 42 | 74 ± 26 | −41 ± 36 | −42 ± 22 | 6 ± 22 | UFP: |
| 10 µg/m3 | −41 ± 38 | 106 ± 24 | 3 ± 25 | −13 ± 22 | 38 ± 18 | p = 0.026 |
| 25 µg/m3 | −66 ± 40 | 86 ± 34 | −9 ± 21 | −18 ± 26 | 28 ± 18 | |
| PCA | ||||||
| Air | −1 ± 2 | −2 ± 2 | 1 ± 2 | −1 ± 0.8 | −1 ± 1 | NS |
| 10 µg/m3 | −2 ± 1 | −3 ± 1 | −0.1 ± 0.8 | 1 ± 2 | −0.3 ± 0.8 | |
| 25 µg/m3 | 0 ± 2 | −2 ± 0.6 | 0.3 ± 1 | −0.4 ± 0.9 | −1 ± 1 | |
| PCA variability | ||||||
| Air | 3 ± 1 | −0.01 ± 0.48 | 0.7 ± 0.6 | −0.2 ± 0.4 | −0.1 ± 0.5 | NS |
| 10 µg/m3 | 4 ± 1 | −0.6 ± 0.4 | −0.4 ± 0.5 | 0.2 ± 0.9 | −0.03 ± 0.72 | |
| 25 µg/m3 | 4 ± 0.7 | 0.1 ± 0.4 | 0.5 ± 0.7 | −0.1 ± 0.3 | 0.4 ± 0.5 | |
| ST in lead II (µV) | ||||||
| Air | −20 ± 8 | −0.3 ± 4 | 0.7 ± 6 | 0.8 ± 3 | 0 ± 5 | NS |
| 10 µg/m3 | −18 ± 12 | 0.7 ± 5 | 2 ± 4 | −1 ± 3 | −3 ± 5 | |
| 25 µg/m3 | −17 ± 7 | 2 ± 5 | 1 ± 4 | −0.7 ± 2 | 0.5 ± 2 | |
| ST in V2 (µV) | ||||||
| Air | −19 ± 7 | −7 ± 4 | −5 ± 4 | −12 ± 5 | −11 ± 5 | NS |
| 10 µg/m3 | −19 ± 5 | −4 ± 4 | −2 ± 2 | −10 ± 5 | −4 ± 5 | |
| 25 µg/m3 | −25 ± 6 | −6 ± 4 | −3 ± 3 | −8 ± 5 | −6 ± 3 | |
| ST in V5 (µV) | ||||||
| Air | −10 ± 5 | −3 ± 3 | −2 ± 4 | 7 ± 5 | −0.6 ± 4 | NS |
| 10 µg/m3 | −14 ± 7 | −3 ± 4 | −2 ± 3 | 11 ± 4 | 2 ± 4 | |
| 25 µg/m3 | −16 ± 5 | −4 ± 4 | −5 ± 3 | 7 ± 4 | 2 ± 2 | |
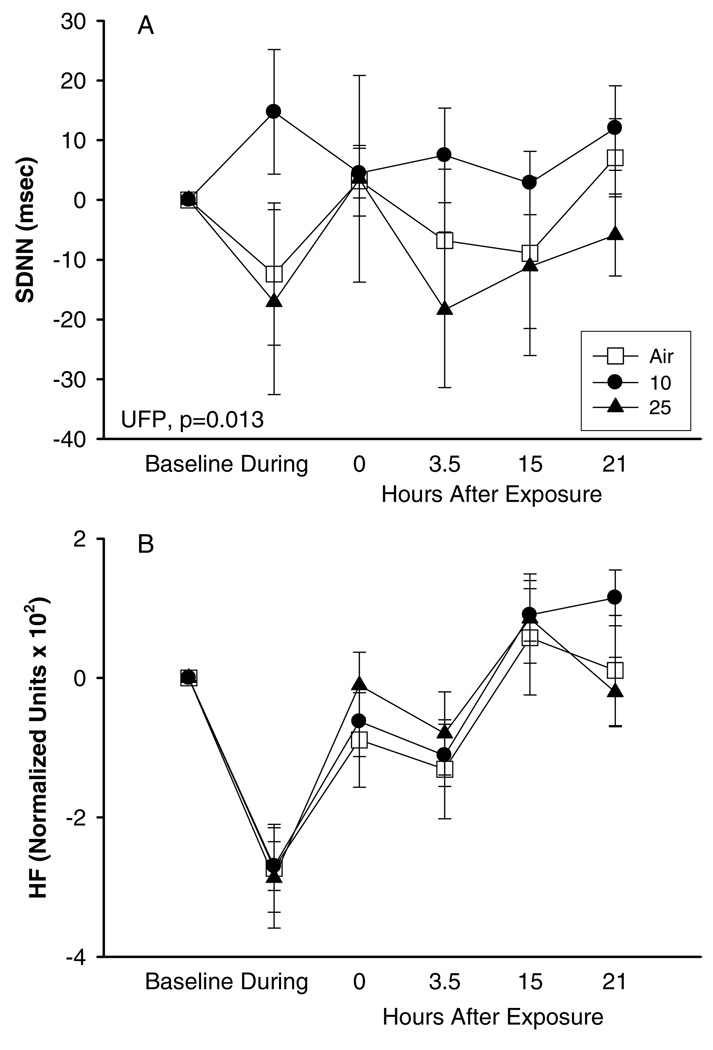
Exercise had a profound effect on all ECG parameters recorded during exposure with pure air or UFP. Similar to the observations for the protocol with exposure at rest, SDNN (Figure 3A) showed higher values with exposure to 10 µg/m3UFP than with exposure to air. Frequency-domain HRV parameters showed a similar pattern. However, this effect was not observed with exposure to UFP at 25 µg/m3 (Figure 3A). When analyzing HRV parameters using normalized units, we observed that the exercise-associated response of the parasympathetic system (measured by normalized units of HF components) seen with air exposure was blunted 0 hours after exposure to UFP (Figure 3B), although again the difference was not statistically significant.
FIG. 3.
Heart rate variability, exposures to 10 and 25 µg/m3 with exercise, difference from baseline, means ± SE: (A) SDNN, (B) HF.
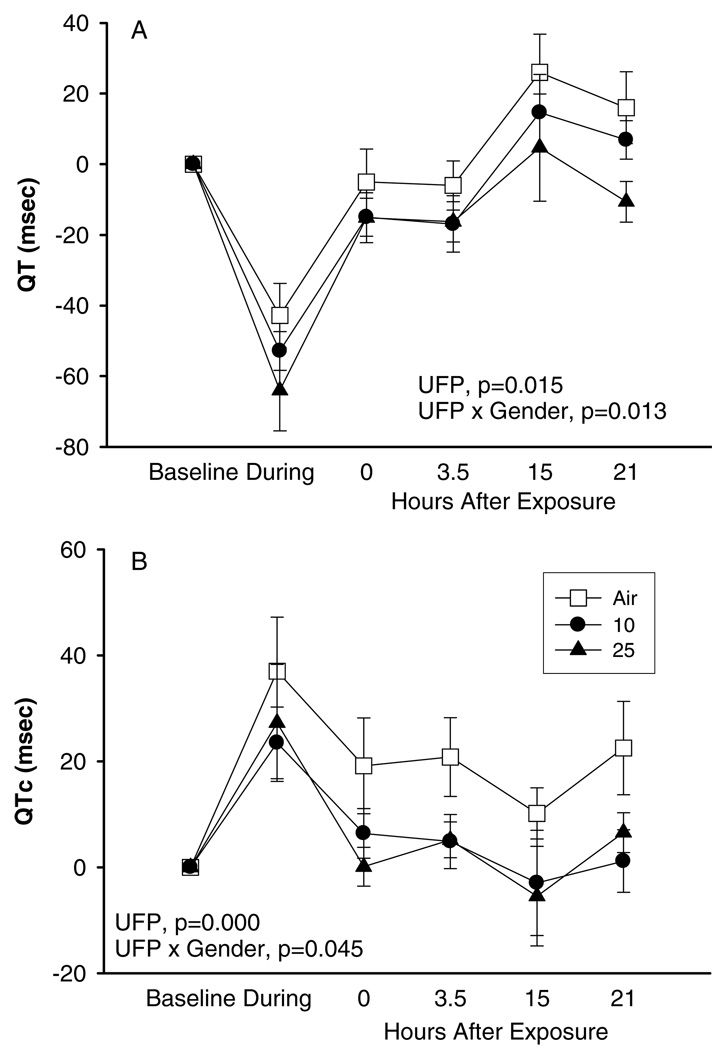
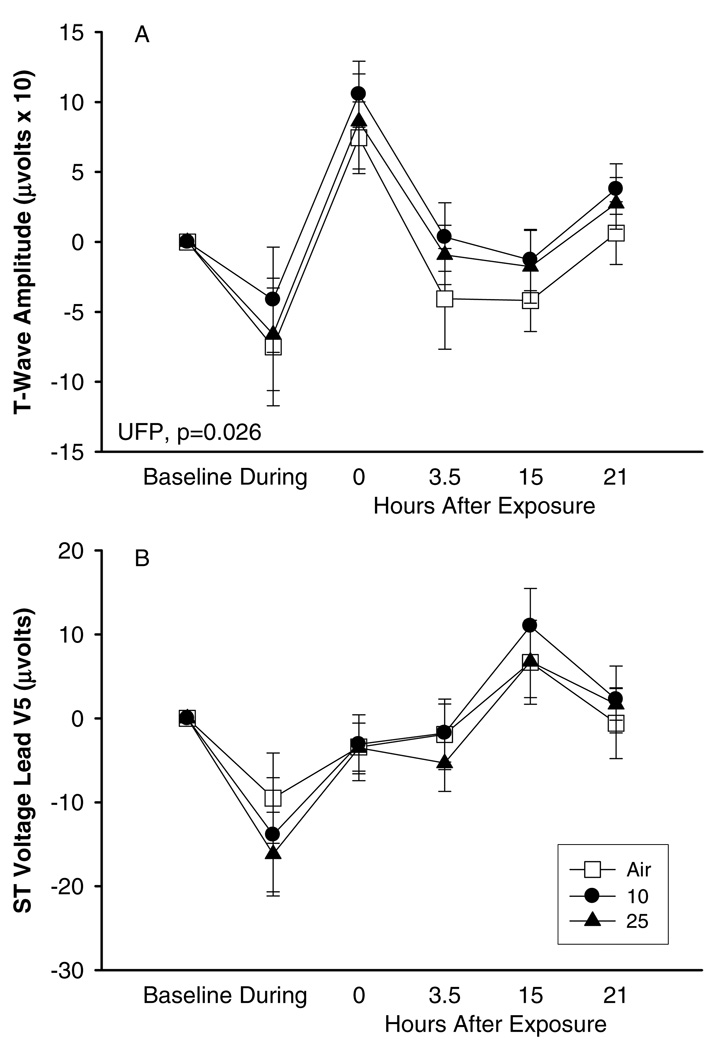
The analysis of QT interval duration and T-wave amplitude also showed a blunted response after UFP exposure in comparison to pure air exposure. Figure 4 shows that QT and QTc were shortened during exercise more substantially during UFP particle exposure than during pure air exposure, and that the QT and QTc intervals remained shortened for several hours after UFP exposure but not after pure air exposure (Figure 4). Simultaneously, T-wave amplitude was also higher after exercise with UFP than after exercise with pure air (Figure 5). Exercise during UFP exposure induced a somewhat more pronounced effect on ST segment level in lead V5 (minimal ST depression) than during exposure to pure air.
FIG. 4.
Cardiac QT (A) and QTc intervals (B), exposures to 10 and 25 µg/m3 with exercise, difference from baseline, means ± SE.
FIG. 5.
Cardiac repolarization, exposures to 10 and 25 µg/m3 with exercise, difference from baseline, means ± SE: (A) T-wave amplitude, (B) ST voltage lead V5.
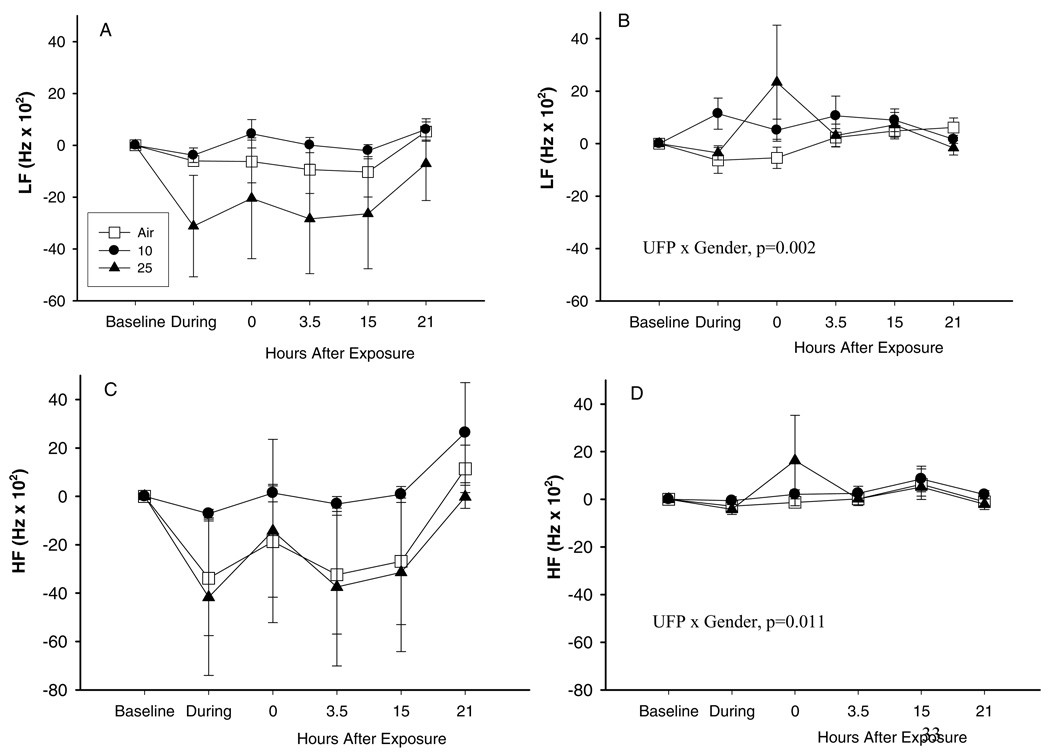
The ANOVA suggested a few significant differences in response based on gender (see Tables 2 and 3). However, the examination of gender-specific results did not indicate an overall pattern of increased susceptibility to ECG effects based on gender. For example, changes in HF and LF both showed significant gender interactions (Figure 6). However, there was no concentration-related effect, and there was no significant gender interaction for changes in LF/HF.
FIG. 6.
Low frequency (LF, panels A, B) and high frequency (HF, panels C, D) heart rate variability in females (A, C) and males (B, D) exposed to 10 and 25 µg/m3 with exercise. Data are differences from baseline, means ± SE.
No UFP-induced cardiac arrhythmias were seen in any exposure protocol.
DISCUSSION
This is the first study of controlled human exposures to elemental carbon UFP, using a comprehensive set of ECG parameters that describe autonomic regulation of the heart, myocardial substrate, and vulnerability. ECG monitoring was chosen to elucidate cardiac effects of UFP based on the concept that electrical signals of the heart might be affected by air pollution through either indirect or direct mechanistic pathways (Utell et al., 2002; Zareba et al., 2001). As expected, young healthy subjects did not show dramatic changes in the studied ECG parameters, but some interesting trends were observed.
Exposure to 10 µg/m3 UFP at rest was associated with some, mostly non-significant, changes in ECG parameters. These changes indicate an increase in parasympathetic tone, which is most likely also responsible for the trend toward ST elevation and blunted QTc shortening. Increased variability of T-wave complexity after exposure to UFP could also be attributed to an enhanced parasympathetic response. The heavy breathing associated with exercise physiologically increases parasympathetic modulation of the heart, and this response seems to be exaggerated by UFP exposure.
Interestingly, similar findings have been reported in other clinical studies of relatively young, healthy subjects. Gong Jr. et al. (2003) studied healthy subjects and subjects with mild asthma exposed to concentrated ambient fine particles (at a concentration of 174 µg/m3). They observed an air pollution-related increase in parasympathetic measures of autonomic regulation of the heart. This group found similar effects following exposures of healthy and asthmatic subjects to concentrated ambient ultrafine particles (Gong Jr. et al., 2008). Riediker et al. (2004) studied highway patrol officers during work shifts, and found that exposure to PM2.5 in their vehicles was associated with increased HRV parameters the next morning, indicating increased vagal tone. In contrast, elderly subjects showed reduced HRV in response to exposure to concentrated ambient fine particles (Devlin et al., 2003), indicating a loss of vagal control.
In the second protocol, the impact of exercise was seen on most ECG variables. As with exposures at rest, the parasympa-thetic measures of HRV increased during exposure at the lower UFP level (10 µg/m3) but not the higher UFP level (25 µg/m3). This finding may indicate that low-level exposure to UFP triggers some increase in parasympathetic tone, while higher concentrations might lead to a more balanced effect on both the sympathetic and parasympathetic systems. Recovery from exercise showed a blunted response of the parasympathetic system (measured by normalized units of HF components) after exposure to UFP in comparison to air exposure. This diminished vagal response was not observed 3.5 hours later.
Epidemiological and panel studies have shown effects of air pollution on HRV. Gold et al. (2000) found a reduction in parasympathetic (vagal) tone in elderly subjects associated with exposure to outdoor air particles. Adar et al. (2007) also found evidence for reduced parasympathetic tone in elderly subjects associated with 24-hour exposures to PM2.5, black carbon, and UFP (particle number). Pope 3rd et al. (1999) measured HRV parameters and levels of PM10 and found that elevations of PM10 were associated with increased heart rate and decreased HRV. Chuang et al. (2007) reported reduced HRV in association with ambient sulfate and ozone levels in healthy subjects. Baccarelli et al. (2008) found that heart rate variability was reduced in association with exposures to PM2.5 in elderly men, with the effects most pronounced in men with specific susceptibility genotypes and reduced dietary intake of vitamins B6 and B12 and methio-nine. Exposure to coarse particles (PM10–2.5) also appears to affect HRV in elderly people (Lipsett et al., 2006). In contrast, exposure to diesel exhaust containing 200 µg/m3 of PM for 2 hours at rest did not induce significant changes in autonomic control of the heart (Peretz et al., 2008).
Animal studies also indicate that concentrated ambient particles induce HRV changes in autonomic regulation of the heart, including a decrease in parasympathetic modulation (Wellenius et al., 2002). On the other hand, rats exposed to on-road aerosols showed increased high-frequency power and decreased vago-sympathetic balance (Elder et al., 2007). Our observations indicate that some effect of UFP on parameters of HRV, reflecting control of the heart by the autonomic nervous system, is present in healthy subjects, although the exact mechanism for these changes is not yet understood.
The repolarization changes in response to UFP exposure with exercise could have a complex mechanism, which remains to be elucidated. Blunted response of vagal modulation on the sinus node does not fully explain the observed blunted response of QTc duration after UFP exposure. It is known that heart rate (sinus node function under the influence of the autonomic nervous system) provides only a partial explanation for changes in QT duration (Merri et al., 1993). Possibly, UFP have an additional effect on repolarization either through a direct effect of the autonomic nervous system on ventricular myocardium (apart from that on the sinus node) or by directly affecting ion channel function in ventricular myocardium through a yet unknown mechanism (Utell et al., 2002).
The reduction in QT duration with concomitant increase in T- wave amplitude after UFP exposure provides evidence that repolarization is affected by air pollution. These preliminary findings require confirmation in further studies in groups likely to demonstrate more pronounced effects (for example, elderly and coronary disease patients).
Lengthening of the QTc interval predisposes to an increased potential for arrhythmias. However, shortening of repolarization is known to be caused by hypoxia and ischemia, and to be arrhythmogenic (Safi et al., 2001). Calcium, potassium, and chloride channels may contribute significantly to shortening of the action potential duration. For example, the action potential shortening by chloride current activation may perpetuate re-entry by shortening the refractory period. The other possible explanation for observed QT shortening may be the result of cardiac myocyte functional responses to subtle changes in systemic vascular tone. These changes, in turn, may be related to increased endothelin production and/or reduced NO release by endothelium in response to particles. Alternatively, UFP may gain access to pulmonary capillary blood, where they could be transported to the heart and cause direct effects on membrane ion channel function.
Slight ST segment changes in studied young subjects should not be considered as measures of ischemic burden. Rather, the ST segment reflects the plateau phase of repolarization of the myocardium, with several ion channels operating that might be vulnerable to air pollution. Brugada syndrome is an example of an arrhythmogenic disorder manifested by ST segment elevation caused by abnormal kinetics of ion channels involved in the repolarization process (Antzelevitch, 2001).
Evidence for air pollution effects on cardiac repolarization comes from a panel study in Erfurt, Germany (Henneberger et al., 2005). Fifty-six male patients with coronary artery disease showed significant increases in QT duration in response to exposure to organic carbon; significant decreases in T-wave amplitude with exposure to ultrafine, accumulation mode, and PM2.5 particles; and a significant increase in T-wave complexity in association with PM2.5 particles for the 24 hours before the ECG recordings.
Limitations of the studies we report here include the use of laboratory-generated elemental carbon UFP, which do not contain metal, organic compounds, and other chemical species present in ambient UFP. Our findings could therefore underestimate the cardiovascular effects of ambient ultrafine particles. A limited number of subjects were included in each tested protocol (six males and six females in each). However, carefully designed protocols with multiple randomized exposures to pure air and UFP at different concentrations, with individuals serving as their own controls, are strengths of the studies. We used sophisticated measures of electrical activity of the heart including novel digital Holter technology and novel parameters quantifying T-wave morphology and repolarization variability, in addition to HRV and ST segment Holter parameters. These sensitive parameters are increasingly used in studies aiming to detect subtle changes in myocardial electrical activity. For the majority of the analyses, we standardized recording conditions (5 minutes supine) to diminish the influence of confounding factors (change in body position, activity, meals, stress) known to affect studied ECG parameters. We also analyzed whole 24-hour recordings for HRV parameters, which yielded results similar to those based on multiple 5-minute segments.
The mass concentrations of carbon UFP used in this study, 10 and 25 µg/m3, are representative of ambient mass concentrations in US cities, are below the current US 24-hour National Ambient Air Quality Standard for PM2.5 of 35 µg/m3, and are about 10-fold lower than mass concentrations used in human clinical studies of concentrated fine particles. The particle numbers are higher than those generally found in ambient air, although particle number concentrations in traffic on major highways may reach or exceed these numbers. Thus, the particle concentrations used in these studies are relevant to real-world UFP exposures.
In summary, transient exposure to ultrafine carbon particles in concentrations of 10–25 µg/m3 does not cause marked changes in ECG-derived parameters in young healthy subjects. However, trends are observed indicating that some subjects might be susceptible to such exposures, with responses involving autonomic modulation of the heart and repolarization of the ventricular myocardium. It is highly likely that individuals with compromised health status (or possibly genetic predisposition) might show significant changes in studied ECG parameters, reflecting mechanistic pathways for the cardiovascular effects of air pollution. Our findings in these studies of laboratory-generated ultrafine carbon particles, together with those using concentrated ambient particle exposures (Devlin et al., 2003; Gong Jr. et al., 2003; Gong Jr. et al., 2008), suggest that, in young healthy subjects, ambient levels of ultrafine and fine particles do not have substantial effects on the electrical activity of the heart and its central regulation.
Acknowledgments
This study was supported by grants R01ES13394, P30ES01247, and RR00044 from the National Institutes of Health, assistance agreement RD 832415 from the US Environmental Protection Agency, and contract 98-19 from the Health Effects Institute.
Research described in this article was conducted under contract to the Health Effects Institute (HEI), an organization jointly funded by the US Environmental Protection Agency (EPA) (assistance agreement X-812059) and automotive manufacturers. The contents of this article do not necessarily reflect the views of the HEI, nor do they necessarily reflect the policies of the US EPA or of automotive manufacturers.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Wojciech Zareba, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA.
Jean Philippe Couderc, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA.
Günter Oberdörster, Department of Environmental Medicine, University of Rochester Medical Center, Rochester, NY, USA.
David Chalupa, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA.
Christopher Cox, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA.
Li-Shan Huang, Department of Biostatistics and Computational Biology, University of Rochester Medical Center, Rochester, NY, USA.
Annette Peters, Helmholtz Zentrum München – German Research Center for Environmental Health, Neuherberg, Germany.
Mark J. Utell, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA
Mark W. Frampton, Department of Medicine, University of Rochester Medical Center, Rochester, NY, USA
REFERENCES
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18(1):95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- Antzelevitch C. Molecular biology and cellular mechanisms of Brugada and long QT syndromes in infants and young children. J. Electrocardiol. 2001;34 suppl:177–181. doi: 10.1054/jelc.2001.28865. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Cassano PA, Litonjua A, Park SK, Suh H, Sparrow D, Vokonas P, Schwartz J. Cardiac autonomic dysfunction: Effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117(14):1802–1809. doi: 10.1161/CIRCULATIONAHA.107.726067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazett HC. An analysis of time relations of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- Bhatnagar A. Cardiovascular pathophysiology of environmental pollutants. Am. J. Physiol. Heart. Circ. Physiol. 2004;286(2):H479–H485. doi: 10.1152/ajpheart.00817.2003. [DOI] [PubMed] [Google Scholar]
- Brook RD. Is air pollution a cause of cardiovascular disease? Updated review and controversies. Rev. Environ. Health. 2007;22(2):115–137. doi: 10.1515/reveh.2007.22.2.115. [DOI] [PubMed] [Google Scholar]
- Chuang K-J, Chan C-C, Su T-C, Lee C-T, Tang C-S. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med. 2007;176(4):370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Committee on Research Priorities for Airborne Particulate Matter. Research priorities for airborne particulate matter: IV. Continuing research progress. Washington, D.C: National Research Council; 2004. [Google Scholar]
- Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur. Respir. J. Suppl. 2003;40:76s, 80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Luttmann-Gibson H, Rich DQ, Link MS, Mittleman MA, Gold DR, Koutrakis P, Schwartz JD, Ver-rier RL. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ. Health. Perspect. 2005;113:670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Couderc JP, Gelein R, Eberly S, Cox C, Xia X, Zareba W, Hopke P, Watts W, Kittelson D, Frampton M, Utell M, Oberdorster G. Effects of on-road highway aerosol exposures on autonomic responses in aged, spontaneously hypertensive rats. Inhal. Toxicol. 2007;19(1):1–12. doi: 10.1080/08958370600985735. [DOI] [PubMed] [Google Scholar]
- Frampton MW, Stewart JC, Oberdörster G, Morrow PE, Chalupa D, Pietropaoli AP, Frasier LM, Speers DM, Cox C, Huang L-S, Utell MJ. Inhalation of carbon ultrafine particles alters blood leukocyte expression of adhesion molecules in humans. Environ. Health. Perspect. 2006;114:51–58. doi: 10.1289/ehp.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski JJ. Responses of the heart to ambient particle inhalation. Clin. Occup. Environ. Med. 2006;5(4):849–864. doi: 10.1016/j.coem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Linn WS, Sioutas C, Terrell SL, Clark KW, Anderson KR, Terrell LL. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in Los Angeles. Inhal. Toxicol. 2003;15:305–325. doi: 10.1080/08958370304455. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Linn WS, Clark KW, Anderson KR, Sioutas C, Alexis NE, Cascio WE, Devlin RB. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal. Toxicol. 2008;20(6):533–545. doi: 10.1080/08958370801911340. [DOI] [PubMed] [Google Scholar]
- Henneberger A, Zareba W, Ibald-Mulli A, Ruckerl R, Cyrys J, Couderc JP, Mykins B, Woelke G, Wichmann HE, Peters A. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ. Health Perspect. 2005;113(4):440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Kenward MG. Design and analysis of cross-over trials. 2nd ed. New York, NY: Chapman and Hall; 2003. [Google Scholar]
- Lipsett MJ, Tsai FC, Roger L, Woo M, Ostro BD. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ. Health Perspect. 2006;114(8):1215–1220. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre A, Bonneterre V, Huillard L, Sabatier P, de Gaudemaris R. Impact of urban atmospheric pollution on coronary disease. Eur. Heart J. 2006;27(19):2275–2284. doi: 10.1093/eurheartj/ehl162. [DOI] [PubMed] [Google Scholar]
- Malik M, Camm AJ. Heart rate variability. Armonk, NY: Futura Publishing Co; 1995. [Google Scholar]
- Merri M, Alberti M, Moss AJ. Dynamic analysis of ventricular repolarization duration from 24-hour Holter recordings. IEEE Trans. Biomed. Eng. 1993;40:1219–1225. doi: 10.1109/10.250577. [DOI] [PubMed] [Google Scholar]
- Peretz A, Kaufman JD, Trenga CA, Allen J, Carlsten C, Aulet MR, Adar SD, Sullivan JH. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ. Res. 2008;107:178–184. doi: 10.1016/j.envres.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, Baliff J, Oh JA, Allen G, Monahan K, Dockery DW. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, Löwel H. Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pietropaoli AP, Frampton MW, Hyde RW, Morrow PE, Oberdörster G, Cox C, Speers DM, Frasier LM, Chalupa DC, Huang L-S, Utell MJ. Pulmonary function, diffusing capacity and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal. Toxicol. 2004a;16 suppl. 1:59–72. doi: 10.1080/08958370490443079. [DOI] [PubMed] [Google Scholar]
- Pietropaoli AP, Frampton MW, Oberdörster G, Cox C, Huang L-S, Marder V, Utell MJ. Blood markers of co-agulation and inflammation in healthy human subjects exposed to carbon ultrafine particles. In: Heinrich U, editor. Effects of air contaminants on the respiratory tract—interpretations from molecular to meta analysis. Stuttgart, Germany: INIS Monographs, Fraunhofer IRB Verlag; 2004b. pp. 181–194. [Google Scholar]
- Pope CA, 3rd, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, Schwartz J, Villegas GM, Gold DR, Dockery DW. Heart rate variability associated with particulate air pollution. Am. Heart J. 1999;138(5 Pt 1):890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution. Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Priori SG, Mortara DW, Napolitano C, Diehl L, Paganini V, Cantu F, Cantu G, Schwartz PJ. Evaluation of the spatial aspects of T-wave complexity in the long-QT syndrome. Circulation. 1997;96:3006–3012. doi: 10.1161/01.cir.96.9.3006. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, Williams RW, Devlin RB. Particulate matter exposure in cars is associated with cardiovascular effects in healthy, young men. Am. J. Respir. Crit. Care Med. 2004;169:934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Rom WN, Samet JM. Small particles with big effects. Am. J. Respir. Crit. Care Med. 2006;173(4):365–366. doi: 10.1164/rccm.2601003. [DOI] [PubMed] [Google Scholar]
- Safi AM, Kwan T, Feit A, Gonzalez J, Stein RA. Use of intracoronary electrocardiography for detecting ST-T, QTc, and U wave changes during coronary balloon angioplasty. Heart Dis. 2001;3(2):73–76. doi: 10.1097/00132580-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Samet JM, Graff D, Bernsten J, Ghio AJ, Huang YCT, Devlin RB. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal. Toxicol. 2007;19 suppl. 1:29–32. doi: 10.1080/08958370701492706. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology. 1999;10(1):17–22. [PubMed] [Google Scholar]
- US Environmental Protection Agency. Air quality criteria for particulate matter. Washington, D.C: U.S. Environmental Protection Agency; 2005. [Google Scholar]
- Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. Cardiovascular effects associated with air pollution: Potential mechanisms and methods of testing. Inhal. Toxicol. 2002;14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Saldiva PH, Batalha JR, Krishna Murthy GG, Coull BA, Verrier RL, Godleski JJ. Electrocardio-graphic changes during exposure to residual oil fly ash (ROFA) particles in a rat model of myocardial infarction. Toxicol. Sci. 2002;66(2):327–335. doi: 10.1093/toxsci/66.2.327. [DOI] [PubMed] [Google Scholar]
- Zareba W, Nomura A, Couderc JP. Cardiovascular effects of air pollution: What to measure in ECG? Environ. Health Perspect. 2001;109 suppl. 4:533–538. doi: 10.1289/ehp.01109s4533. [DOI] [PMC free article] [PubMed] [Google Scholar]