Abstract
BRCA1 is an essential caretaker protein in the surveillance of DNA damage, is mutated in about 50% of all hereditary breast cancer cases and its expression is frequently decreased in sporadic breast cancer. β-catenin is a multifunctional protein that forms adhesion complex with E-cadherins, α-catenin and actin, and plays a central role in Wnt signaling through its nuclear translocation and activation of β-catenin-responsive genes. Although significant progress has been made in understanding the Wnt/β-catenin and BRCA1 signaling cascades, it is not known whether there is a link between β-catenin and BRCA1. We observed that the expression of the active nuclear form of β-catenin (also known as ABC, Ser37/Thr41-non phosphorylated β-catenin, dephosphorylated β-catenin) was lower or absent in the nucleus in most BRCA1 familial breast cancer tissues (17 cases), as compared to sporadic breast cancer (14 samples) and normal breast tissues. WT-BRCA1, but not mutated BRCA1, interacted with β-catenin and increased the levels of β-catenin protein expression in vitro. Furthermore, H2O2 induced the interaction of the nuclear form of β-catenin with BRCA1. The active form of β-catenin protein was down-regulated upon exposure to H2O2 in the nucleus of BRCA1-deficient HCC1937 breast cancer cells, whereas reconstitution of WT-BRCA1 in HCC1937 cells inhibited this down-regulation. This study provides evidence of a novel interaction between BRCA1 and β-catenin, and that loss of BRCA1 leads to impaired expression of the nuclear form of β-catenin, which may contribute to the pathogenesis of breast cancer.
Keywords: BRCA1, β-catenin, mutated BRCA1
Introduction
Humans heterozygous for BRCA1 mutations have a high risk of losing the remaining wild type BRCA1 allele and developing breast/ovarian cancer (1–9). BRCA1 haploin sufficiency alone, a feature of sporadic breast/ovarian cancer, is sufficient to compromise genome stability by triggering spontaneous recombination events that are likely to account for the loss of the remaining wild type BRCA1 allele and increased cancer risk (9). The genomic instability observed in BRCA1 and BRCA2 mutation carriers is associated with a down-regulation of nuclear BRCA1 protein accumulation in the dot like structures (10). BRCA1 is mutated in about 50% of all hereditary breast cancer cases, and its expression is frequently decreased in sporadic breast cancer (5–8).
Recently, WT-BRCA1 was shown to downregulate cellular levels of reactive oxygen species (ROS) (11). Oxidative stress due to various environmental and hormonal factors in breast results in production of ROS, a risk factor in carcinogenesis (12, 13). Normal cellular metabolism is associated with the production of ROS (14). Common forms of ROS include superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radicals, and nitric oxide. Under nonphysiologic conditions, increases in ROS levels can cause damage to DNA, proteins and lipids in cells (15, 16). BRCA1 participates in various cellular processes in response to DNA damage in order to maintain genomic integrity (17–24). BRCA1 is considered as one of the DNA damage sensors and a platform to coordinate different activities during stress responses (23–27). Absence of full-length Brca1 sensitized mice to oxidative stress induced lethality (25). In addition, BRCA1 can protect cells from oxidative stress by inducing expression of antioxidants (26), suggesting that BRCA1 is activated in response to oxidative stress. BRCA1 exists as a RING heterodimer with BARD1 to provide ubiquitin E3 ligase activity that is required for its tumor suppressor function (28, 29).
β-catenin is a multifunctional protein that forms an adhesion complex with E-cadherin, α-catenin and actin, and also plays a central role in Wnt signaling through its nuclear translocation and association with T-cell factor (TCF) and lymphoid enhancer factor (LEF) (30). Cytosolic β-catenin is tightly regulated by interactions with several proteins, such as APC, GSK-3β, and axin (31–34). GSK-3β phosphorylates β-catenin at threonine 41, serine 37, and serine 33 residues, thereby facilitating its ubiquitination and proteosomal degradation (35). Recently, a ROS-dependent interaction between FOXO and β-catenin that appears to be evolutionary conserved was reported (36, 37). Under normal cellular conditions β-catenin, through Wnt signaling, is involved in cell proliferation and differentiation but under changed ROS conditions, its function can shift to regulate transcription factors that support cell survival through increased stress resistance and ROS clearance (30). β-catenin-mediated response is strongly dependent on the stimulus and the subsequent interaction with other downstream targets and specific transcription factors (30).
The role of β-catenin in breast cancer is not clear. Decreased β-catenin staining in the membrane of breast cancer cells was reported (38). In addition, lack of nuclear β-catenin was also reported in breast cancer specimens (39–41) except for one report that showed that the nuclear expression of β-catenin was associated with the outcome of breast cancer (42). β-catenin activity is precisely regulated by modulating its degradation and nuclear translocation. The non-phosphorylated β-catenin at the GSK-3β-phosphorylation site was identified as a transcriptionally active form of β-catenin in the nucleus (37), also known as the active form of β-catenin or ABC form in the nucleus, which can be specifically detected by anti-ABC antibody (43, 44).
Both β-catenin and BRCA1 proteins were demonstrated to be necessary for normal mammary gland development, while their aberrant activation and inactivation were shown to be involved in carcinogenesis. This prompted us to address whether inactivation of BRCA1 by germ-line mutations has any effect on β-catenin. In this study, we hypothesized that BRCA1 regulates the active nuclear form of β-catenin (ABC), during oxidative stress responses. Our results show that the expression of the active nuclear form of β-catenin was lower or absent in most of BRCA1 familial breast cancer tissues. We found that WT-BRCA1, but not mutated BRCA1, interacted with β-catenin and that WT-BRCA1 protected the nuclear active form of β-catenin during oxidative stress responses.
Materials and Methods
Cell culture
HEK293, HEK293T, MCF-7, MDA-MB-231, and SW480 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). BRCA1-mutant HCC1937 breast cancer cells and wild type BRCA1-reconstituted HCC1937 cells (HCCBRCA1) were kindly provided by Dr. Chen. All cell lines were maintained in RPMI 1640 supplemented with 10% FBS, 2 mM Glutamine, and 100 U of penicillin and 100 μg of streptomycin per ml of medium. MCF 10A immortalized mammalian breast epithelial cell line was kindly provided by Dr. Harikrishna Nakshatri (School of medicine, Indiana University).
Antibodies and chemicals
Anti-HA monoclonal antibody (HA.11) and anti-Flag monoclonal antibody (M2) were purchased from Covance (Princeton, NJ) and Sigma (St. Louis, MO), respectively. The antibodies purchased from Santa Cruz (Santa Cruz, CA) are anti-Myc monoclonal antibody (9E10), anti-β-catenin polyclonal antibody (H102), anti-BRCA1 polyclonal antibody (I-20), anti-Lamin B1 polyclonal antibody, anti-GST antibody, and anti-CSK polyclonal antibody. Anti-β-catenin monoclonal antibody (C19220) (BD Biosciences, Franklin Lakes, NJ), the anti-active form (Ser37/Thr41-non-phosphorylated) of β-catenin antibody (05-665) (Upstate, Charlottesville, VA) were used for Western blotting, immunoprecipitation, and immunostaining as indicated. Anti-BRCA1 polyclonal antibody (9010), and anti-BRCA1 C-terminal monoclonal antibody (Ab-3) were purchased from Cell Signaling (Danvers, MA) and Calbiochem (San Diego, CA), respectively. An anti-BRCA1 polyclonal antibody against a 428-683aa fragment of BRCA1 was generated in our laboratory for immunostaining. Anti-Actin monoclonal antibody was purchased from Chemicon (Temecula, CA). Anti-GAPDH monoclonal antibody was purchased from Abcam (Cambridge, MA). H2O2 was purchased from Sigma (St. Louis, MO).
Plasmid constructs
Wild type and mutants of BRCA1, and GST-BRCA1 plasmids were previously described (48). The BARD1 construct was described before (46). The HA-BRCA1 (1181-1863aa) construct was generated by inserting an amplified PCR product of 1181-1863aa into a pcDNA3-HA vector (48). Myc-β-catenin plasmid was kindly provided by Dr. Hecht. Flag-β-catenin (1-133aa), (134-671aa) and (672-781aa) were created by inserting amplified PCR products into a pCMV Flag-4N vector (Invitrogen, Carlsbad, CA). Newly generated constructs were confirmed by sequencing. Wild type and dominant-negative GSK-3β plasmids were previously described (56). Flag-IκBα and constitutively active HA-IKKβ (SE) were kindly provided by Dr. Fuchs (57).
Transfection, immunoprecipitation and Western blot analysis
LipofectAMINE plus reagent (Invitrogen) was used for the transfection experiments in mammalian cells. Immunoprecipitation and Western blotting were carried out as described (48). Briefly, cells were lysed in lysis buffer (50 mM Tris pH 7.5, 1% Triton X-100, 0.5% deoxycholate, 10 mM EDTA, 150 mM NaCl, 50 mM NaF, 1 mM sodium vanadate, 10% glycerol) containing a protease inhibitor cocktail (Roche, Basel, Switzerland). The lysates were pre-cleared by incubation with protein-G Sepharose beads for 1 hour. The supernatants were incubated with the indicated antibody for 2–3 hours followed by additional incubation with protein-G Sepharose beads for 1 hour. The immunocomplexes were washed three times with the cell lysis buffer, followed by elution in sample buffer (125 mM Tris-HCl pH 6.8, 4% SDS, and 20% glycerol). The samples were separated by SDS-PAGE and transferred onto PVDF membrane (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat dried milk in PBS-0.1% Tween 20, followed by incubation with primary antibodies overnight at 4°C. The horseradish peroxidase-conjugated secondary antibodies (Amersham, Buckinghamshire, UK) were incubated for 1 hour at room temperature followed by enhanced chemiluminescence treatment (PerkinElmer, Waltham, MA).
Western blot analysis for protein detection was carried out using total cell extracts lysed in the sample buffer (125 mM Tris-HCl pH 6.8, 4% SDS, and 20% glycerol).
GST pull-down assays
The GST pull-down assays were performed with HEK293T cell lysates transfected with Myc-β-catenin and bacterially-purified GST-BRCA1 protein fragments, as described (48).
Northern analysis
TRIzol Reagent (Invitrogen, Carlsbad, CA) was used to isolate total RNA. 10 μg of RNA was separated by 1% agarose gel and transferred onto a Hybond-N+ Nylon membrane (Amersham, Buckinghamshire, UK). The Prime-It II Random Primer Labeling Kit (Stratagene, La Jolla, CA) was used to label the β-catenin cDNA fragment digested by EcoRI and XhoI.
siRNA transfection
Small interfering (si)-GL2, si-βTrcp1, and siRNA SMARTpool BRCA1 (Upstate Biotechnology, Charlottesville, VA) was transfected by OligofectAMINE reagent (Invitrogen, Carlsbad, CA). 48 hrs after transfection, the NER Nuclear and Cytoplasmic Extraction Kit (Pierce Inc.) was used to fractionate the nuclear and cytoplasmic proteins. The cytoplasmic and nuclear fractions were subjected to SDS-PAGE and Western blotting.
35S-labeling of β-catenin and pulse-chase assay
The 35S-labeling and pulse-chase assay were performed as described (58). Briefly, Myc-β-catenin plasmid was co-transfected with or without the indicated BRCA1 plasmids into HEK293 cells. 16 hrs after transfection, cells were labeled with 35S-Met/Cys (ICN, Costa Mesa, CA) followed by pulse-chase analysis for the indicated times. Equal amounts of total cell extracts were immunoprecipitated with anti-Myc monoclonal antibody followed by SDS-PAGE.
Confocal assays
Cells were fixed in 3% paraformaldehyde supplemented with 2% sucrose or methanol for 10 min and permeabilized in 0.5% Triton X-100 for 5 min. After blocking with 10% goat serum in phosphate-buffered saline for 30 min, slides were incubated sequentially with antibodies against BRCA1 (I-20, Santa Cruz, 1:100) and the active form of β-catenin (1:100). The FITC and Texas Red conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were diluted at 1:1000. Images were analyzed using a Zeiss LSM 5 image examiner at the Harvard Center for Neurodegeneration and Repair.
Immunohistochemical analysis
The immunohistochemical studies were performed on 5μm sections of breast tumors and normal breast tissues placed on poly-lysine-coated slides. After deparaffinization in xylene and rehydration through graded alcohol, each section was treated with 0.03% hydrogen peroxide for 5 min. The slides were incubated overnight at 4°C in a closed chamber, followed by immunostaining with anti-β-catenin monoclonal antibody (C19220, BD Biosciences), the anti-active form of β-catenin antibody and anti-BRCA1 monoclonal antibody (Ab-3, Calbiochem) (59). The streptoavidin-biotin-peroxidase complex technique (EnVision+System-HRP, DakoCytomation, Glostrup, Denmark) was applied for immunohistochemical staining.
Statistical Analysis
For comparison of the means of two groups, the Student’s t-test was used. For comparison of the means of multiple groups, analysis of variance (ANOVA) was performed.
Results
The active nuclear form of β-catenin protein is absent or expressed weakly in BRCA1 familial breast tumors. The Ser37/Thr41-non-phosphorylated β-catenin, at the site of the GSK-3β-mediated phosphorylation, has been identified as a transcriptionally active form of β-catenin in the nucleus (called the active form of β-catenin in the nucleus or ABC) (37). This nuclear form of β-catenin can be specifically detected by ABC antibody (clone 8E7, Upstate Biotech) (36, 43). To understand the effects of BRCA1 on this active form of β-catenin protein in the nucleus, we stained 38 breast tissue samples, including 14 sporadic breast cancer cases, 17 BRCA1 familial breast cancer cases, and 7 normal breast tissues with the active form of β-catenin antibody (α-ABC). The α-ABC antibody immunostained the Ser37/Thr41-non-phosphorylated β-catenin protein in the nucleus in 5 out of 7 normal breast tissues and 11 out of 14 sporadic breast cancer tissues (Table 1 and Supplemental Figures 1–3). Surprisingly, in 15 out of 17 BRCA1 familial breast tumors (15/17=88.2%), the active form of β-catenin protein was absent or weakly stained in the nucleus and cytoplasm (Table 1). These results are statistically significant (p=0.0025; Fisher’s test) between the sporadic breast tumors and the BRCA1 familial breast cancer samples. BRCA1 protein status in the BRCA1 familial breast tumors was confirmed by immunostaining with anti-BRCA1 monoclonal antibody (Table 1). These data suggest that the Ser37/Thr41-non-phosphorylated β-catenin protein, the active form of β-catenin protein in the nucleus, is down-regulated in BRCA1 familial breast cancer samples.
Table 1.
Immunohistostaining of β-catenin and BRCA1 antibody
| Sample No. | BRCA1 | Total β-catenin | Active form of β-catenin |
|---|---|---|---|
| Sporadic breast cancer (N=14) | |||
| 7803A2 | Nucleus | Cytoplasm | Both |
| 7277A2 | Nucleus | Cytoplasm | Both |
| 6750A1 | Nucleus | Cytoplasm | Both |
| 6579A2 | Nucleus | Cytoplasm | Both |
| 6966A1 | Nucleus | Cytoplasm | Both |
| 5613A3 | Nucleus | Cytoplasm | Both |
| 6109A2 | Abscent | Cytoplasm | Both |
| 7984B3 | Nucleus | Cytoplasm | Nucleus |
| 7251A1 | Nucleus | Cytoplasm | Nucleus |
| 7430A1 | Nucleus | Cytoplasm | Nucleus |
| 7409A6 | Nucleus | Cytoplasm | Nucleus |
| 7861A2 | Abscent | Cytoplasm | Abscent |
| 6897A2 | Abscent | Cytoplasm | Abscent |
| 7174A2 | Abscent | Cytoplasm | Abscent |
| BRCA1 mutant breast cancer (N=17) | |||
| 96-13274E | Abscent | Cytoplasm | Abscent |
| 97-33047D | Abscent | Cytoplasm | Abscent |
| 98-40452F | Abscent | Cytoplasm | Abscent |
| 98-39396C | Abscent | Cytoplasm | Abscent |
| 99-34824J | Abscent | Cytoplasm | Abscent |
| 99-54775B | Abscent | Cytoplasm | Abscent |
| 99-46137J | Abscent | Cytoplasm | Abscent |
| 00-22507P | Abscent | Cytoplasm | Abscent |
| 00-33584E | Abscent | Cytoplasm | Abscent |
| 01-840H | Abscent | Cytoplasm | Abscent |
| 02-5845F | Abscent | Cytoplasm | Abscent |
| 99-50810E | Nucleus | Cytoplasm | Abscent |
| 00-34056J | Nucleus | Cytoplasm | Abscent |
| 02-32869C | Nucleus | Cytoplasm | Abscent |
| 99-33943L | Both | Cytoplasm | Abscent |
| 04-7124K | Abscent | Cytoplasm | Both |
| 96-16383J | Nucleus | Cytoplasm | Both |
| Normal mammary gland (N=7) | |||
| 7827A3 | Nucleus | Cytoplasm | Both |
| 7561A1 | Nucleus | Cytoplasm | Both |
| 7690A2 | Nucleus | Cytoplasm | Both |
| 7643A1 | Nucleus | Cytoplasm | Both |
| 7518A1 | Nucleus | Cytoplasm | Both |
| 6486A1 | Nucleus | Cytoplasm | Cytoplasm |
| 7649A2 | Nucleus | Cytoplasm | Cytoplasm |
Both: Nucleus and Cytoplasm
In all samples, we detected cytoplasmic and cell membranous staining with total b-catenin antibody. As regards the expression of active b-catenin protein, no staining was observed in 15 out of 17 BRCA1 mutant cases (15/17=88.2%). On the other hand, in 3 out of 14 sporadic cases, no staining was detected (3/14=21.4%). This difference was statistically significant (p=0.00025: Fisher’s exact test). Furthermore, no BRCA1 staining was observed in 3 sporadic breast cancers without the expression of active b-catenin protein.
indicates the absence of active b-catenin expression in the samples.
BRCA1 interacts with β-catenin in vitro and in vivo
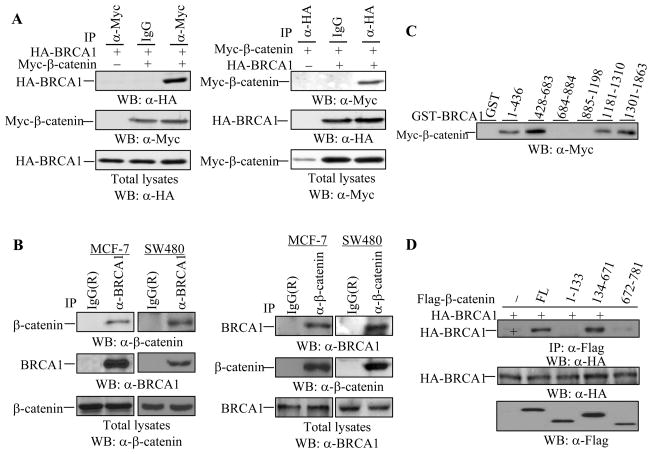
To understand the potential link between BRCA1 and β-catenin in breast cancer, we investigated the possible physical interaction between the two proteins. HA-BRCA1 and Myc-β-catenin were co-transfected into HEK293T cells, followed by immunoprecipitation and Western blotting. An association between HA-BRCA1 and Myc-β-catenin was observed, as demonstrated by reciprocal immunoprecipitations (Figure 1A). In an examination of the Myc-β-catenin protein expression, we noticed that the levels of Myc-β-catenin protein expression were increased in the presence of wild type BRCA1 (Figure 1A). To confirm an interaction between the endogenous BRCA1 and endogenous β-catenin, we employed MCF-7 breast cancer cells and SW480 colon cancer cells in which β-catenin accumulates in the cytoplasm and large amounts of β-catenin reside in the nucleus due to an adenomatous polyposis coli (APC) mutation. An interaction between endogenous BRCA1 and endogenous β-catenin was observed in both cell lines (Figure 1B), suggesting that an interaction between BRCA1 and β-catenin occurs in vivo.
Figure 1. Interaction of BRCA1 with β-catenin in vitro and in vivo.
(A) BRCA1 interacted with β-catenin in HEK293T cells. HA-BRCA1 and Myc-β-catenin were co-expressed in HEK293T cells followed by immunoprecipitation as indicated. Anti-mouse monoclonal IgG was used as a control. BRCA1 and β-catenin expression were detected in 10 μg of total lysates.
(B) Endogenous BRCA1 and β-catenin formed a complex in MCF-7 and SW480 cells. Immunoprecipitation with anti-BRCA1 antibody or anti-β-catenin antibody and subsequent Western blotting were carried out in MCF-7 and SW480 cells as indicated. The membranes were re-probed with the indicated antibodies to monitor the immunoprecipitations. The same amounts of total lysates were applied to detect BRCA1 and β-catenin expression.
(C) BRCA1 bound to β-catenin via two regions, the N-terminus (1-683aa) and the C-terminus (1181-1863aa). A GST pull-down assay was performed with total extracts of HEK293T cells over-expressing β-catenin and 10 μg of various GST-BRCA1 protein fragments purified from E. coli.
(D) β-catenin bound to BRCA1 via the armadillo repeats. Full-length (FL) and deleted forms of Flag-β-catenin, including 1-133aa, 134-671aa, and 672-781aa, were co-expressed with HA-BRCA1 in HEK293T cells, followed by immunoprecipitation and Western blotting with the indicated antibodies. 10 μg of total lysates were applied to monitor HA-BRCA1 and Flag-β-catenin protein(s) expression.
To identify β-catenin binding sites on BRCA1, a GST pull-down assay was performed using various GST-BRCA1 protein fragments and HEK293T cell lysates transfected with Myc-β-catenin plasmid. Four fragments of BRCA1, including 1-436aa, 428-683aa, 1181-1310aa, and 1301-1863aa, were shown to interact with β-catenin protein (Figure 1C), suggesting that the N-terminal region covering the RING finger domain and the C-terminal region covering two tandem BRCT repeats mediated the BRCA1 association with β-catenin. The N-terminal fragment (1-683aa) of BRCA1 and the C-terminal fragment (1181-1863aa) of BRCA1 were confirmed to interact with β-catenin in HEK293T cells (data not shown). The BRCA1 binding site on β-catenin was identified using various Flag-β-catenin proteins, including full-length (FL), 1-133aa, 134-671aa, and 672-781aa of β-catenin. 134-671aa of β-catenin, covering the armadillo repeats, but not 1-133aa or 672-781aa of β-catenin, interacted with HA-BRCA1 (Figure 1D), suggesting that BRCA1 binds to the armadillo repeats in β-catenin, which is also the binding site for β-Trcp1 (45).
WT-BRCA1 increases the levels of β-catenin protein expression in vitro
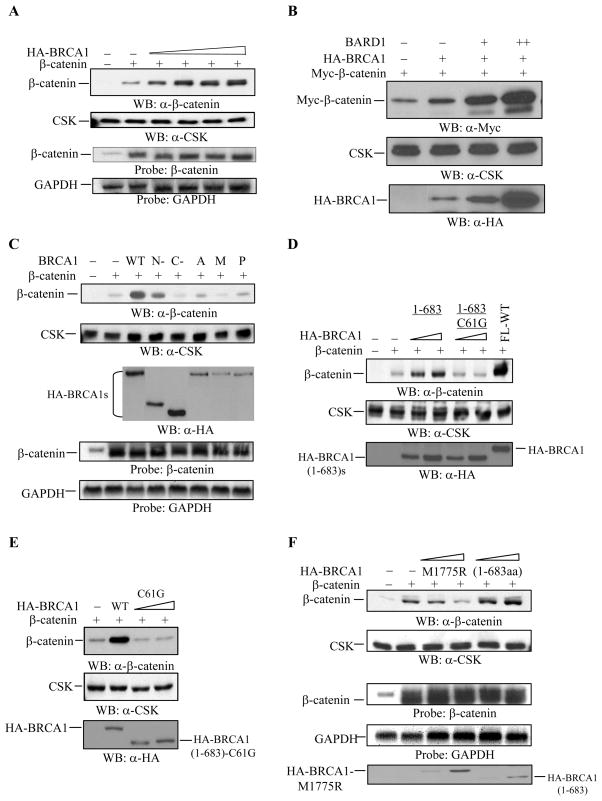
We hypothesized that physical interaction between BRCA1 and β-catenin contributes to an increase in the levels of β-catenin protein expression in vitro. To further demonstrate the BRCA1-dependent increase in the expression levels of β-catenin protein, we analyzed β-catenin protein expression in the presence of increasing amounts of WT-BRCA1 in HEK293 cells. The expression levels of β-catenin protein were increased by wild type BRCA1 in a dose-dependent manner (Figure 2A), independent of β-catenin plasmid DNA concentrations (data not shown). However, β-catenin itself did not affect the expression levels of WT-BRCA1 protein (data not shown). To examine if the increase in the expression levels of β-catenin protein resulted from an increase in the transcription of transfected β-catenin plasmid by BRCA1, total RNA was isolated in a parallel experiment and then subjected to Northern blot analysis with β-catenin cDNA as a probe. The expression levels of the transfected β-catenin mRNA, which is slightly shorter in size than the endogenous β-catenin mRNA, were not affected by WT-BRCA1 (Figure 2A). These data suggest that the expression levels of β-catenin protein are regulated by WT-BRCA1 post-translationally. The BRCA1-binding partner, BARD1, is known to interact with BRCA1 protein (46, 47). BARD1 alone did not increase the expression levels of β-catenin protein (data not shown); whereas BARD1 largely increased the expression levels of β-catenin protein in the presence of WT-BRCA1, in a dose-dependent manner (Figure 2B). The levels of WT-BRCA1 were also increased by BARD1 in a dose-dependent manner (Figure 2B). These data demonstrate a BRCA1-dependent increase in the expression levels of β-catenin protein in vitro.
Figure 2. WT-BRCA1 increases the expression levels of β-catenin protein.
(A) WT-BRCA1 increased β-catenin protein expression in a dose dependent manner. 5 μg of total lysates from HEK293 cells expressing 0.375 μg of β-catenin and increasing amounts of wild type BRCA1 (0.1, 0.25, 0.375, and 0.5 μg) were applied for Western blotting with anti-β-catenin antibody. The membrane was blotted with anti-CSK antibody to monitor equal loading. In the fourth panel, 10 μg of total RNA from a parallel experiment was subjected to Northern blotting analysis with full-length β-catenin cDNA as a probe. Full-length GAPDH cDNA was used to monitor equal loading.
(B) BARD1 increased β-catenin protein expression in the presence of wild type BRCA1. 0.25 μg of Myc-β-catenin, 0.1 μg of wild type BRCA1, and 0.1 μg (+) or 0.25 μg (++) of BARD1 were co-transfected into HEK293 cells as indicated. 5 μg of total lysates were applied for Western blotting to detect Myc-β-catenin protein. Anti-CSK antibody was applied to monitor equal loading. 10 μg of total lysates were applied to detect HA-BRCA1 protein expression.
(C) WT-BRCA1, but not the BRCA1 mutants increased β-catenin protein expression. 0.375 μg of β-catenin plasmid were co-expressed with 0.375 μg of the wild type or mutant forms of BRCA1 in HEK293 cells. 5 μg of total lysates were applied for Western blotting with anti-β-catenin antibody. Anti-CSK antibody was used to monitor equal loading. 10 μg of total lysates were applied to monitor various HA-BRCA1 proteins expression. 10 μg of total RNA obtained from the parallel experiment was applied for Northern blotting analysis, and full-length β-catenin cDNA was used as a probe. Full-length GAPDH cDNA was used to monitor equal loading. WT: wild type BRCA1; N-: BRCA1 (1-683aa); C-: BRCA1 (1301-1863aa); A, M, P, and Y: BRCA1-A1708E, BRCA1-M1775R, BRCA1-P1749R, and BRCA1-Y1853x, respectively.
(D) BRCA1 (1-683aa)-C61G failed to increase β-catenin expression. 0.375 μg of β-catenin plasmid was co-transfected with 0.1 μg and 0.375 μg of HA-BRCA1 (1-683aa) and HA-BRCA1 (1-683aa)-C61G plasmids into HEK293 cells. 5 μg of total lysates were applied to detect β-catenin and BRCA1 protein expression levels. Anti-CSK antibody was used to monitor equal loading.
(E) Full-length BRCA1 with the C61G mutation, (FL)-BRCA1-C61G, failed to increase β-catenin protein expression. 0.375 μg of β-catenin was co-transfected with 0.1 μg and 0.375 μg of (FL)-BRCA1-C61G or with 0.375 μg of WT-BRCA1 in HEK293 cells. 5 μg of total cell extract was applied for the Western blotting. Anti-CSK antibody was used to monitor equal loading. In addition, 5 μg of total cell lysates were applied to monitor the levels of WT-BRCA1 and HA-BRCA1 (1-683aa)-C61G protein expression as indicated.
(F) The BRCA1-M1775R mutant failed to increase β-catenin protein expression, while BRCA1 (1-683aa) slightly increased β-catenin protein expression. 0.375 μg of β-catenin plasmid was co-transfected with or without 0.1 μg and 0.375 μg of BRCA1-M1775R or BRCA1 (1-683aa) plasmid as indicated. 5 μg of total protein extract was applied to detect β-catenin protein expression by Western blotting. Anti-CSK antibody was used to monitor equal loading. In a parallel experiment, 10μg of total RNA was applied for Northern blot analysis. Full-length GAPDH cDNA was used as a probe to monitor equal loading. 5 μg of total cell lysates were applied to monitor the levels of HA-BRCA1-M1775R and HA-BRCA1 (1-683aa) protein expression as indicated.
BRCA1 mutants could not increase the expression levels of β-catenin protein
To understand the effects of BRCA1 mutations on β-catenin protein expression, various BRCA1 mutants including BRCA1-A1708E, BRCA1-M1775R, BRCA1-P1749R, and BRCA1-Y1853x, and two truncated forms of BRCA1 [BRCA1 (1-683aa) and BRCA1 (1301-1863aa) (48) were used to examine the expression levels of β-catenin protein in HEK293 cells. As shown in Figure 2C, the expression levels of β-catenin protein were increased by WT-BRCA1 (more than 6-fold) and by BRCA1 (1-683aa) (approximately 3-fold), but not by BRCA1-A1708E, BRCA1-M1775R, and BRCA1-P1749R. There were slight variations in the expression levels of WT or mutants of HA-BRCA1 protein, but the highly expressing HA-BRCA1 (1301-1863aa) protein did not increase the expression levels of β-catenin protein, compared to the mutants of HA-BRCA1. In addition, β-catenin did not affect the protein levels of either WT-BRCA1 or BRCA1 mutants (data not shown). To examine whether the transcripts of transfected β-catenin plasmid were affected by various BRCA1 constructs, total RNA was isolated in a parallel experiment and then subjected to Northern blot analysis with β-catenin cDNA as a probe. Co-transfection with or without WT or mutants of BRCA1 rarely affected the expression levels of transfected β-catenin mRNA (Figure 2C). Taken together, these data suggest that mutations in BRCA1 result in a loss of BRCA1 activity in regulating β-catenin protein expression.
Given that the BRCA1 domain (1-683aa) protein exhibited a low capacity to increase the expression levels of β-catenin protein, we examined the effects of point mutations in the RING finger domain of BRCA1 on β-catenin protein expression. In addition, we looked at the effects of increasing doses of BRCA1 mutants plasmids on the levels of β-catenin protein expression, in order to understand whether the low expression levels of BRCA1 mutants contribute to the lower levels of β-catenin protein expression. The expression levels of β-catenin protein were increased by HA-BRCA1 (1-683aa) (approximately 2.5- to 3.5-fold) in a dose-dependent manner, which was abolished upon disruption of Cys61 (replaced by Gly) in the RING finger domain of HA-BRCA1 (1-683aa) (Figure 2D). Disruption of Cys61 by Gly in the RING finger domain of full-length BRCA1 resulted in a failure to increase the expression levels of β-catenin protein (Figure 2E). These data suggest the significance of the RING finger domain of BRCA1 in regulating β-catenin protein expression. In addition, as a representative of the BRCT-mutants of BRCA1, the HA-BRCA1-M1775R mutant failed to induce a dose-dependent increase in the expression levels of β-catenin protein, although the transcripts of exogenously expressing β-catenin mRNA were rarely affected by a variety of mutants or doses of the HA-BRCA1 constructs (Figure 2F), suggesting that the BRCT region in BRCA1 also participates in regulating β-catenin protein expression.
WT-BRCA1 specifically regulates the expression levels of β-catenin protein
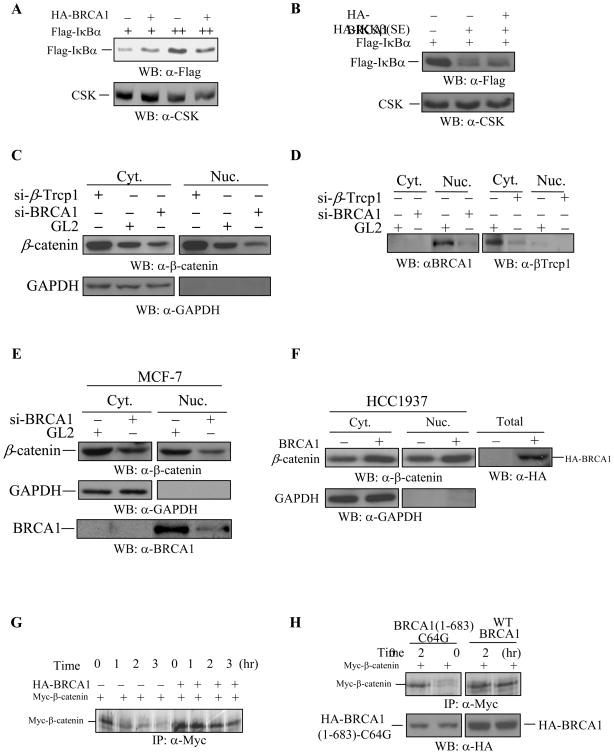
Like β-catenin protein, IκBα protein is also a substrate of the SCF-E3 ubiquitin ligase complex for protein degradation (49). To analyze the specificity of the WT-BRCA1-dependent increase in β-catenin protein expression, we investigated the effects of WT-BRCA1 on IκBα protein expression. WT-BRCA1 could not increase the levels of Flag-IκBα protein expression in HEK293 cells (Figure 3A). The constitutively active IKKβ (SE) largely decreased the expression levels of Flag-IκBα protein in HEK293 cells (Figure 3B), resulting from IKKβ (SE)-mediated degradation as reported (50) WT-BRCA1 failed to inhibit the IKKβ (SE)-dependent degradation of Flag-IκBα protein (Figure 3B), suggesting that IκBα protein expression is not regulated by BRCA1.
Figure 3. The specificity of BRCA1 in regulating β-catenin protein expression.
(A) WT-BRCA1 did not increase the levels of IκBα protein expression. 0.25 μg and 0.375 μg of Flag-tagged IκBα was co-transfected with 0.375 μg of WT-BRCA1 into HEK293 cells. 5 μg of total protein extract was subjected to Western blotting with anti-Flag antibody (M2) to detect the levels of IκBα protein expression. Anti-CSK antibody was used to monitor equal loading.
(B) Constitutively active IKKβ(SE) decreased the levels of IκBα protein expression, which was not affected by WT-BRCA1. 0.375 μg of Flag-IκBα plasmid was co-transfected with 0.1 μg and 0.25 μg of the constitutively-active HA-IKKβ(SE), with or without 0.375 μg of the WT-BRCA1 into HEK293 cells. 5 μg of total protein was applied to detect the levels of IκBα protein expression. Anti-CSK antibody was used to monitor equal loading.
(C–D) Depletion of BRCA1 reduces the levels of β-catenin in the cytoplasm and nucleus. HeLa cells were transfected with siRNA against GL2 (control), β-Trcp1, and BRCA1. After 48 hours, the cells were fractionated and 5μg of cytosolic extract and 10 μg of nuclear extract were subjected to SDS-PAGE and Western blotting. Depletion of β-Trcp1 resulted in an accumulation of β-catenin, while depletion of BRCA1 reduced the levels of β-catenin. GAPDH was used to monitor the efficiency of cell fractionation and equal loading. The depletion of BRCA1 in the nucleus and β-Trcp1 in the cytoplasm was shown in Figure D.
(E) Depletion of BRCA1 reduced the levels of β-catenin in MCF-7 cells. MCF-7 cells were transfected with siRNA against GL2 (control) and BRCA1. After 48 hours, the cells were fractionated and 5μg of cytosolic extract and 10 μg of nuclear extract were subjected to SDS-PAGE and Western blotting. Depletion of BRCA1 reduced the levels of β-catenin. GAPDH was used to monitor the efficiency of cell fractionation and equal loading. Reduction of BRCA1 levels were shown in the bottom panel.
(F) Over-expression of WT-BRCA1 accumulated the levels of β-catenin in HCC1937 cells. HCC1937 cells were transiently transfected with wild type BRCA1. After 48 hours of transfection, the cells were fractionated and 10 μg of cytosolic extract and 20μg of nuclear extract were subjected to SDS-PAGE and Western blotting as indicated. Over-expression of WT-BRCA1 accumulated β-catenin in the cytoplasm and nucleus. GAPDH was used to monitor the efficiency of cell fractionation and equal loading. The expression of HA-BRCA1 protein was detected in total cell lysates.
(G) WT-BRCA1 delayed β-catenin protein turnover. Myc-β-catenin plasmid was co-transfected with or without wild type BRCA1 plasmid into HEK293 cells. 16 hrs after transfection, cells were labeled with 35S-Met/Cys followed by pulse-chase analysis for the indicated times. Equal amounts of total cell extracts were immunoprecipitated with anti-Myc monoclonal antibody followed by SDS-PAGE.
(H) The BRCA1 (1-683aa)-C64G mutant failed to delay β-catenin protein turnover. Myc-β-catenin plasmid was co-transfected with WT-BRCA1 or BRCA1 (1-683aa)-C64G plasmid into HEK293 cells. 16 hrs after transfection, cells were labeled with 35S-Met/Cys followed by pulse-chase analysis. Equal amounts of total cell extracts were immunoprecipitated with anti-Myc monoclonal antibody followed by SDS-PAGE. The levels of HA-BRCA1 (1-683aa)-C64G and WT-HA-BRCA1 protein expression were also determined by Western blot analysis using 5 μg of total cell lysates.
BRCA1 regulation of β-catenin in vivo
We further demonstrated BRCA1 regulation on the levels of β-catenin protein by depleting endogenous BRCA1 and by over-expressing WT-BRCA1 in mutant BRCA1-containing HCC1937 breast cancer cells. To demonstrate that BRCA1 regulates the levels of β-catenin, cells were transfected with siRNAs against GL2 (control), β-Trcp1 (positive control) and BRCA1. It is known that β-Trcp1 specifically recognizes GSK-3β-phosphorylated β-catenin resulting in β-catenin ubiquitination and degradation. Reduction of β-Trcp1 protein resulted in an accumulation of β-catenin in the cytoplasm and nucleus (Fig. 3C), suggesting that siRNA-mediated reduction of β-Trcp1 protein caused an accumulation of β-catenin protein. Upon depletion of BRCA1, the levels of β-catenin were reduced in the cytoplasm and nucleus (compared to the transfectant with siRNA-GL2) (Fig. 3C), suggesting that BRCA1 regulates the levels of β-catenin protein. Similarly, depletion of BRCA1 in MCF-7 cells caused a reduction of β-catenin in the cytoplasm and nucleus, compared to the levels of β-catenin in siRNA-GL2-transfectants (Fig. 3E). Further, by over-expressing WT-BRCA1 in HCC1937 breast cancer cells, we observed that WT-BRCA1 increased the levels of β-catenin protein (Fig. 3F). These results support our notion that BRCA1 regulates the levels of β-catenin protein.
WT-BRCA1, but not BRCA1 mutants, enhanced the half-life of β-catenin protein
To examine the BRCA1-dependent increase in β-catenin protein expression, we investigated the half-life of β-catenin protein in the absence or presence of WT-BRCA1. The Myc-β-catenin protein was expressed in the HEK293 cells and then labeled with 35S-Met/Cys, followed by pulse-chase analysis. The half-life of Myc-β-catenin protein was approximately 1.5 hours in the absence of WT-BRCA1 (Figure 3G, left four lanes); co-expression of WT-BRCA1 extended the half-life of Myc-β-catenin protein more than 2-fold (Figure 3G, right four lanes). The BRCA1 (1-683aa)-C64G mutant also failed to prolong the half-life of Myc-β-catenin protein (Figure 3H). These data suggest that WT-BRCA1 extends the half-life of β-catenin protein, which may contribute to the WT-BRCA1-dependent increase in β-catenin protein expression.
WT-BRCA1 regulates the Ser37/Thr41-non-phosphorylated β-catenin
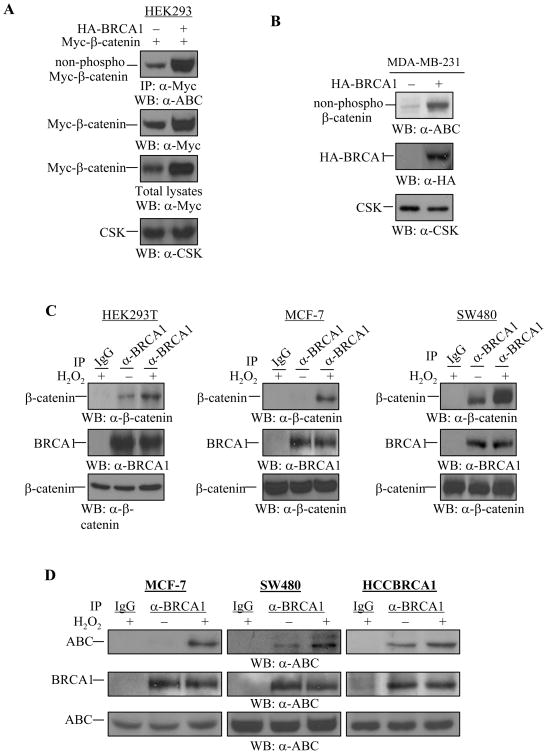
The expression levels of Ser37/Thr41-non-phosphorylated β-catenin protein were also increased in the presence of WT-BRCA1 in HEK293 cells, as demonstrated by the fact that immunoprecipitated Myc-β-catenin protein was detected by the anti-ABC antibody (Figure 4A). Over-expression of WT-BRCA1 in MDA-MB-231 breast cancer cells also resulted in an accumulation of endogenous Ser37/Thr41-non-phosphorylated β-catenin protein (Figure 4B), suggesting that over-expression of WT-BRCA1 increases the levels of the Ser37/Thr41-non-phosphorylated β-catenin protein.
Figure 4. Regulation of Ser37/Thr41-non-phosphorylated β-catenin by WT-BRCA1 and H2O2.
(A) WT-BRCA1 increased the Ser37/Thr41-non-phosphorylated β-catenin protein expression in HEK293 cells. 0.375 μg of Myc-β-catenin plasmid was co-transfected with 0.375 μg of wild type BRCA1 into HEK293 cells. Myc-β-catenin protein was immunoprecipitated by anti-Myc antibody followed by Western blotting with anti-Ser37/Thr41-non-phosphorylated β-catenin (a-ABC) antibody. The membrane was reprobed with anti-Myc antibody to detect total Myc-β-catenin expression. 5 μg of total lysates were applied to detect Myc-β-catenin protein (total) expression, and anti-CSK antibody was used to monitor equal loading.
(B) WT-BRCA1 increased the expression of Ser37/Thr41-non-phosphorylated β-catenin in MDA-MB-231 cells. MDA-MB-231 cells were transfected with WT-BRCA1 or pcDNA3 vector (1.0 μg). 48 hrs after transfection, 20 μg of total cell lysates were applied for Western blotting to detect the Ser37/Thr41-non-phosphorylated β-catenin expression. Anti-CSK antibody was used to monitor equal loading. 20 μg of total lysates were used to confirm expression of the exogenous HA-BRCA1 protein by using anti-HA monoclonal antibody. The expression levels of ABC in HCC1937 cells and wild type BRCA1-reintroduced HCC1937 (HCC1937-BRCA1, Cl3.4). 25 μg of total lysates from HCC1937 and HCC1937-BRCA1, Cl3.4 were subjected to Western blotting with anti-ABC antibody. The same membrane was used to monitor equal loading with anti-Actin antibody. The levels of ABC in HCC1937 cells were lower than in HCC1937-BRCA1, Cl3.4.
(C) H2O2 induces BRCA1 and β-catenin protein association in vivo. HEK293T, MCF-7, and SW480 cells were untreated or treated with 100 μM of H2O2 for 1 hr. Equal amounts of total cell lysates were subjected to immunoprecipitation with anti-BRCA1 antibody, followed by Western blotting with anti-β-catenin antibody. The membrane was probed again with anti-BRCA1 polyclonal antibody. 5 μg of total lysates were used to monitor β-catenin protein expression.
(D) H2O2 induces BRCA1 and the active form of β-catenin protein association. MCF-7, SW480, and HCCBRCA1 cells were untreated or treated with 100 μM of H2O2 for 1 hr. Equal amounts of total cell lysates were subjected to immunoprecipitation with anti-BRCA1 antibody, followed by Western blotting with anti-ABC antibody. The membrane was probed again with anti-BRCA1 polyclonal antibody. 15 μg of total lysates were used to monitor ABC protein expression.
Hydrogen peroxide induces BRCA1 and β-catenin association in vivo
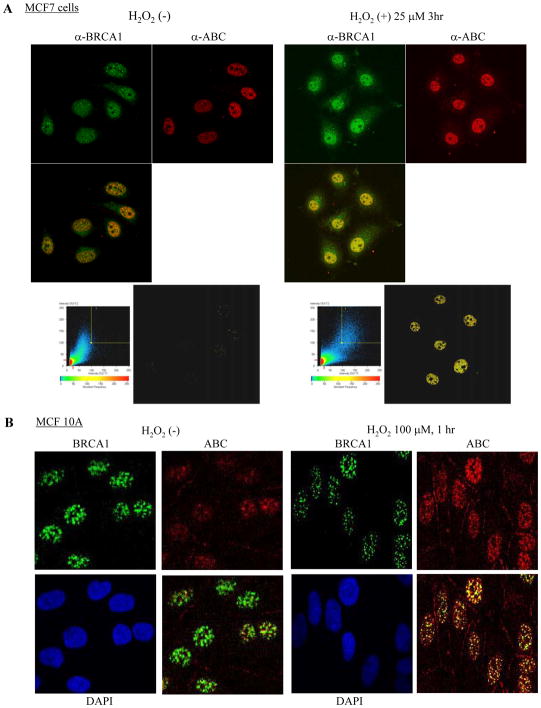
β-catenin protein is implicated in FOXO family-mediated oxidative stress responses (37, 44). We therefore asked whether BRCA1 and β-catenin converge onto the oxidative stress signaling pathway. We assessed an association between BRCA1 and β-catenin in the presence of ROS, represented by hydrogen peroxide (H2O2). Endogenous β-catenin protein increasingly interacted with endogenous BRCA1 protein upon H2O2 treatment in HEK293T, MCF-7, and SW480 cells, as demonstrated by immunoprecipitation assays (Figure 4C). In particular, an association between BRCA1 and β-catenin was significantly induced by H2O2 in SW480 cells, indicating that possibly large amounts of β-catenin protein in the nucleus are available for interacting with endogenous BRCA1 protein. These data suggest that oxidative stress signals induce BRCA1 and β-catenin interaction in vivo. Inducible interaction between endogenous BRCA1 and ABC form of β-catenin by H2O2 was demonstrated by immunoprecipitation-Western blotting in MCF-7 and SW480 cells (Figure 4D) and confocol assay in MCF-7, MCF 10A cells (Figure 5A and 5B), and SW480 cells (data not shown), suggesting that oxidative stress signals induce an interaction between BRCA1 and Ser37/Thr41-non-phosphorylated β-catenin in vivo.
Figure 5.
WT-BRCA1 increasingly colocalized with the ABC of β-catenin upon H2O2 treatment in MCF-7 cells (A) and MCF 10A cells (B). Cells were untreated or treated with 25 μM of H2O2 for 3 hours (A) or 100 μM of H2O2 for 1 hr (B), followed by immunostaining with anti-BRCA1 polyclonal antibody (I-20)) and α-ABC. The colocalization of the proteins was evaluated based on the density of yellow color observed under the same viewing conditions.
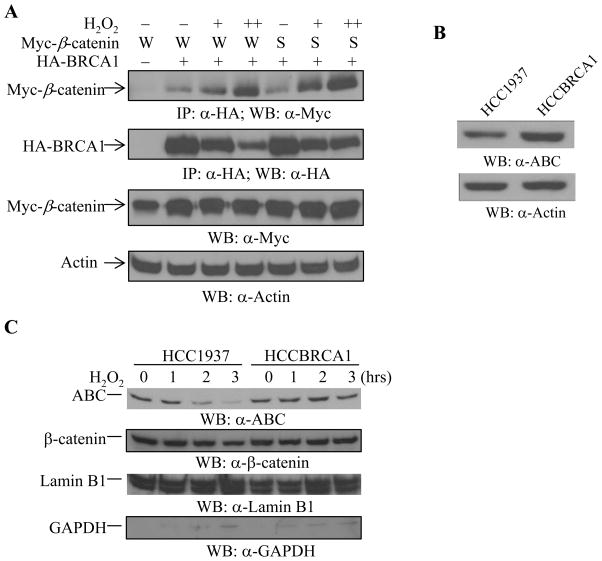
We further demonstrated an inducible interaction between BRCA1 and β-catenin by H2O2 in a dose-dependent manner, in HEK293T cells. We co-expressed Myc-β-catenin including wild type and stable form (S33A), with HA-BRCA1, followed by H2O2 treatment for 1 hour. The total lysates were then subjected to immunoprecipitation with anti-HA antibody to detect BRCA1-bound Myc-β-catenin protein. As shown in Figure 6A, H2O2 treatment increased the association of BRCA1 and β-catenin in a dose dependent manner. The stable form of β-catenin (S33A) also associated with BRCA1 in response to increasing doses of H2O2 (Figure 6A).
Figure 6. H2O2 regulates BRCA1 and β-catenin association and β-catenin levels in the nucleus.
(A) H2O2 treatment increases BRCA1 and β-catenin association. Wild type (W) or a stable form (S33A, shown as S) of Myc-β-catenin was co-transfected with wild type BRCA. The cells were treated with 100 μM (+) and 200 μM (++) of H2O2 for 1 hour, followed by immunoprecipitation and Western blotting as indicated. H2O2 increased BRCA1 and β-catenin association in a dose-dependent manner. The same membrane was reprobed with anti-HA antibody to detect the levels of HA-BRCA1 protein expression. 5 μg of total cell lysates were used to detect the levels of Myc-β-catenin protein. Actin was used to monitor equal loading.
(B–C) The non-phospho-β-catenin protein in the nucleus was reduced in HCC1937 cells and down-regulated by H2O2 in HCC1937 cells but not in HCCBRCA1 cells. (B) The levels of ABC expression in HCC1937 and HCCBRCA1 cells were determined from 15 μf of total cell lysates. The membrane was reprobed with anti-Actin antibody to monitor equal loading. (C) HCC1937 and HCCBRCA1 cells were untreated or treated with 100 μM of H2O2 as indicated, followed by the cytoplasmic and nuclear fractionation. 10 μg of the cytoplasmic extract and nuclear extract were separated by SDS-PAGE, followed by Western blotting with the anti-active form of β-catenin antibody. The membranes were stripped and re-blotted with anti-β-catenin antibody. The same membranes were also used to detect Lamin B1 and GAPDH protein expression levels for equal loading and fractionation efficiency.
The nuclear active form of β-catenin protein is sensitive to hydrogen peroxide in BRCA1-deficient HCC1937 breast cancer cells. Next, we asked whether loss of BRCA1 affects the levels of the Ser37/Thr41-non-phosphorylated β-catenin in the nucleus during oxidative stress responses. BRCA1-deficient HCC1937 breast cancer cells have reduced expression of the ABC form of β-catenin (Figure 6B), as compared to WT-BRCA1-reconstituted HCC1937 (HCCBRCA1) cells. When the cells were treated with 100μM H2O2 for the time period as indicated, and the nuclear proteins were fractionated and subjected to Western blotting with the anti-active form of β-catenin antibody, H2O2 treatment decreased the levels of Ser37/Thr41-non-phosphorylated β-catenin protein in the nucleus of HCC1937 cells in a time-dependent manner (Figure 6C, left four lanes in top panel) but not in the nucleus of the WT-BRCA1-reconstituted HCC1937 cells (Figure 6C, right four lanes in top panel), suggesting that the Ser37/Thr41-non-phosphorylated β-catenin protein is sensitive to H2O2 treatment in the absence of WT-BRCA1. Taken together, these data suggest that BRCA1 plays an important role in regulating the responses of the active form of β-catenin in the nucleus during oxidative stress.
Discussion
Although the linkage of BRCA1 mutations with breast cancer development has been well established, the molecular mechanisms for the tissue-specific BRCA1-mediated carcinogenesis remain poorly understood. We demonstrate a novel association between BRCA1 and β-catenin, as well as with the Ser37/Thr41-non-phosphorylated β-catenin. We observed that in most BRCA1 familial breast tumors (15/17=88.2%), the active form of β-catenin in the nucleus was absent or expressed at low levels, compared to its expression in sporadic breast tumors (3/14=21.4%) and normal breast tissue (Table 1). Some sporadic breast tumors (3/14=21.4%), in which BRCA1 was absent from the nucleus, were also found to lack the active form of β-catenin protein in the nucleus (Table 1). We observed that H2O2 treatment down-regulated the active form of β-catenin protein in the nucleus of BRCA1-deficient HCC1937 breast cancer cells in a time-dependent manner, whereas reconstitution of WT-BRCA1 in HCC1937 cells inhibited this H2O2-induced down-regulation. Our data suggest an association between BRCA1 and β-catenin and that loss of BRCA1 may lead to increased sensitivity of the active form of β-catenin protein to H2O2, resulting in decreased expression of the active form of β-catenin.
Our in vitro experiments showed that WT-BRCA1 increases the expression of β-catenin protein, including the Ser37/Thr41-non-phosphorylated β-catenin protein. BRCA1 interacted with β-catenin via the N-terminus containing the RING finger domain and the C-terminus containing two BRCT repeats (Figure 1). Most mutants of BRCA1, including the truncated BRCA1 (1301-1863aa), and mutants such as BRCA1-A1708E, BRCA1-M1775R, BRCA1-P1749R, BRCA1-Y1853x, BRCA1 (1-683aa)-C61G or C64G, and full-length (fl)-BRCA1-C61G, failed to increase the expression levels of β-catenin protein (Figure 2), suggesting that inactivation of BRCA1 results in a failure to increase β-catenin protein expression. Mutations in the RING finger domain and the BRCT repeats in BRCA1 are closely related to inactivation of BRCA1-E3 ubiquitin ligase activity (53). BRCA1 has been shown to regulate the stability and activity of its binding partners, such as BARD1 (46), Nucleophosmin/B23 (NPM) (52), and p53 (54). In addition, BARD1 enhanced the increased levels of β-catenin protein induced by WT-BRCA1 (Figure 2B), supporting the notion that BRCA1/BARD1 might be involved in regulating β-catenin protein expression.
WT-BRCA1 failed to increase the expression of IκBα protein (Figure 3), another substrate for SCF-E3 ubiquitin ligase, indicating that the regulation of BRCA1 on β-catenin is specific. GSK-3β-mediated phosphorylation of β-catenin and its subsequent ubiquitination by SCF-E3 ubiquitin ligase are two crucial steps for β-catenin degradation. Inactivation of GSK-3β blocks β-catenin ubiquitination by SCF-E3 ubiquitin ligase (50, 55). We found that WT-BRCA1 failed to inhibit β-catenin protein phosphorylation at the GSK-3β phosphorylation site in vitro (data not shown), pointing out that BRCA1 does not interfere with β-catenin phosphorylation. Like β-Trcp1, BRCA1 bound to the armadillo repeats in β-catenin (Figure 1D). However, BRCA1 is unlikely to interfere with the complex formation between β-catenin and SCF-E3 ubiquitin ligase, since immunoprecipitation assays failed to show that BRCA1 affects the complex formation between β-Trcp1 and β-catenin or Cul1 and Roc1 (data not shown).
WT-BRCA1 extended the half-life of β-catenin protein (Figure 3G and 3H), suggesting that BRCA1 delays β-catenin turnover. We found that WT-BRCA1 increases β-catenin protein expression, including the Ser37/Thr41-non-phosphorylated β-catenin, which may result from the extended half-life of β-catenin protein in the presence of WT-BRCA1. We demonstrated an increased interaction of BRCA1 and β-catenin in the presence of H2O2 and that oxidative stress down-regulates the Ser37/Thr41-non-phosphorylated β-catenin protein. Therefore, it is possible that oxidative stress signals induce the interaction between BRCA1 and β-catenin, regulating β-catenin signaling pathways. The Ser37/Thr41-non-phosphorylated β-catenin is a transcriptionally active form of β-catenin in the nucleus (36). The Ser31/Ser37/Thr41-phosphorylated β-catenin is prone to degrade in the cytoplasm by 26S proteasome, while the Ser37/Thr41-non-phosphorylated β-catenin is relatively stable, and thereby translocates into the nucleus. We observed that the oxidative stress agent H2O2 down-regulated the Ser37/Thr41-non-phosphorylated β-catenin protein in cultured cells. In APC-mutated SW480 cells, the Ser37/Thr41-non-phosphorylated β-catenin was down-regulated by H2O2 treatment (data not shown), strongly suggesting that H2O2 regulates the Ser37/Thr41-non-phosphorylated β-catenin in an APC-independent manner. Compared to the total β-catenin protein, the Ser37/Thr41-non-phosphorylated β-catenin was significantly down-regulated by H2O2, implying that this form of β-catenin is sensitive to H2O2. The proteasome inhibitor MG132 accumulated the Ser33/Ser37/Thr41-phosphorylated β-catenin but not the Ser37/Thr41-non-phosphorylated β-catenin (data not shown), indicating that the Ser37/Thr41-non-phosphorylated β-catenin is a stable form of β-catenin in the nucleus. Therefore, it is interesting to unveil the molecular mechanisms by which the turnover of the nuclear form of β-catenin is regulated in the nucleus. Our results support the notion that BRCA1 acts as a protector of the active form of β-catenin during oxidative stress responses, based on the following: a) BRCA1 increasingly associated with β-catenin as well as the nuclear active form of β-catenin protein in the presence of H2O2; b) Significantly, reconstitution of WT-BRCA1 in the BRCA1-mutant HCC1937 cells prevented the H2O2-induced down-regulation of the nuclear active form of β-catenin.
Taken together, this study provides for the first time evidence of a novel interaction between BRCA1 and β-catenin, which may be critical for β-catenin mediated survival, increased stress resistance and ROS clearance during oxidative stress responses. It should be interesting to further investigate the effects of BRCA1-deficiency on Wnt/β-catenin canonical pathway and β-catenin/FOXO-mediated oxidative stress pathway. The observations may help us better understand breast and ovarian cancer development and progression.
Supplementary Material
Acknowledgments
This research was supported in part by National Institute of Health CA 096805 (HA) and CA135226 (HA), DOD BC094909 (HA) and Jennifer Randall award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors want to thank Drs. Kazufumi Haina and Kenichi Tanaka for advice and help with immunohistochemistry studies, Makara Men, Dr. Farheen Faizi, and Janet Delahanty for typing and editing of the manuscript.
Grant Support: This work was supported by the National Institutes of Health (Grant CA 096805 and CA13522, H.A.), and DOD breast cancer grant BC094909.
References
- 1.Jhanwar-Uniyal M. BRCA1 in cancer, cell cycle and genomic stability. Front Biosci. 2003;8:s1107–17. doi: 10.2741/1131. [DOI] [PubMed] [Google Scholar]
- 2.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–76. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 3.Parvin JD. Overview of history and progress in BRCA1 research: the first BRCA1 decade. Cancer Biol Ther. 2004;3:505–8. doi: 10.4161/cbt.3.6.839. [DOI] [PubMed] [Google Scholar]
- 4.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–53. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 5.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 6.Musolino A, Bella MA, Bortesi B, et al. BRCA mutations, molecular markers, and clinical variables in early-onset breast cancer: a population-based study. Breast. 2007;16:280–92. doi: 10.1016/j.breast.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Pinilla SM, Sarrio D, Honrado E, et al. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. Journal of clinical pathology. 2007;60:1006–12. doi: 10.1136/jcp.2006.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanley S, McReynolds K, Ardern-Jones A, et al. Acute chemotherapy-related toxicity is not increased in BRCA1 and BRCA2 mutation carriers treated for breast cancer in the United Kingdom. Clin Cancer Res. 2006;12:7033–8. doi: 10.1158/1078-0432.CCR-06-1246. [DOI] [PubMed] [Google Scholar]
- 9.Cousineau I, Belmaaza A. BRCA1 haploinsufficiency, but not heterozygosity for a BRCA1-truncating mutation, deregulates homologous recombination. Cell cycle (Georgetown, Tex) 2007;6:962–71. doi: 10.4161/cc.6.8.4105. [DOI] [PubMed] [Google Scholar]
- 10.De Brakeleer S, Bogdani M, De Greve J, et al. Loss of nuclear BRCA1 protein staining in normal tissue cells derived from BRCA1 and BRCA2 mutation carriers. Mutat Res. 2007;619:104–12. doi: 10.1016/j.mrfmmm.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Saha T, Rih JK, Rosen EM. BRCA1 down-regulates cellular levels of reactive oxygen species. FEBS letters. 2009;583:1535–43. doi: 10.1016/j.febslet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loft S, Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosone CB. Oxidants and antioxidants in breast cancer. Antioxid Redox Signal. 2000;2:903–17. doi: 10.1089/ars.2000.2.4-903. [DOI] [PubMed] [Google Scholar]
- 14.Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases in redox signaling. Semin Cancer Biol. 2006;16:427–35. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Bowerman B. Cell biology. Oxidative stress and cancer: a beta-catenin convergence. Science. 2005;308:1119–20. doi: 10.1126/science.1113356. [DOI] [PubMed] [Google Scholar]
- 16.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao L, Li W, Kim S, Brodie SG, Deng CX. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 2003;17:201–13. doi: 10.1101/gad.1050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma Y, Hu C, Riegel AT, Fan S, Rosen EM. Growth factor signaling pathways modulate BRCA1 repression of estrogen receptor-alpha activity. Molecular endocrinology (Baltimore, Md) 2007;21:1905–23. doi: 10.1210/me.2006-0397. [DOI] [PubMed] [Google Scholar]
- 19.Cao L, Kim S, Xiao C, et al. ATM-Chk2-p53 activation prevents tumorigenesis at an expense of organ homeostasis upon Brca1 deficiency. EMBO J. 2006;25:2167–77. doi: 10.1038/sj.emboj.7601115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SS, Cao L, Lim SC, et al. Hyperplasia and spontaneous tumor development in the gynecologic system in mice lacking the BRCA1-Delta11 isoform. Mol Cell Biol. 2006;26:6983–92. doi: 10.1128/MCB.00796-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SS, Cao L, Li C, et al. Uterus hyperplasia and increased carcinogen-induced tumorigenesis in mice carrying a targeted mutation of the Chk2 phosphorylation site in Brca1. Mol Cell Biol. 2004;24:9498–507. doi: 10.1128/MCB.24.21.9498-9507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullan PB, Gorski JJ, Harkin DP. BRCA1--a good predictive marker of drug sensitivity in breast cancer treatment? Biochimica et biophysica acta. 2006;1766:205–16. doi: 10.1016/j.bbcan.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraju G, Scully R. Minding the gap: the underground functions of BRCA1 and BRCA2 at stalled replication forks. DNA Repair (Amst) 2007;6:1018–31. doi: 10.1016/j.dnarep.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bochar DA, Wang L, Beniya H, et al. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell. 2000;102:257–65. doi: 10.1016/s0092-8674(00)00030-1. [DOI] [PubMed] [Google Scholar]
- 25.Cao L, Xu X, Cao LL, et al. Absence of full-length Brca1 sensitizes mice to oxidative stress and carcinogen-induced tumorigenesis in the esophagus and forestomach. Carcinogenesis. 2007;28:1401–7. doi: 10.1093/carcin/bgm060. [DOI] [PubMed] [Google Scholar]
- 26.Bae I, Fan S, Meng Q, et al. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004;64:7893–909. doi: 10.1158/0008-5472.CAN-04-1119. [DOI] [PubMed] [Google Scholar]
- 27.Boulton SJ. BRCA1-mediated ubiquitylation. Cell cycle (Georgetown, Tex) 2006;5:1481–6. doi: 10.4161/cc.5.14.2930. [DOI] [PubMed] [Google Scholar]
- 28.Ohta T, Fukuda M. Ubiquitin and breast cancer. Oncogene. 2004;23:2079–88. doi: 10.1038/sj.onc.1207371. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Koike A, Takeshita T, Ohta T. The ubiquitin E3 ligase activity of BRCA1 and its biological functions. Cell Div. 2008;3:1. doi: 10.1186/1747-1028-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogeboom D, Burgering BM. Should I stay or should I go: beta-catenin decides under stress. Biochimica et biophysica acta. 2009 doi: 10.1016/j.bbcan.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3:36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 32.Clevers H. Wnt breakers in colon cancer. Cancer cell. 2004;5:5–6. doi: 10.1016/s1535-6108(03)00339-8. [DOI] [PubMed] [Google Scholar]
- 33.Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. Journal of mammary gland biology and neoplasia. 2003;8:145–58. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- 34.Rowlands TM, Pechenkina IV, Hatsell S, Cowin P. Beta-catenin and cyclin D1: connecting development to breast cancer. Cell cycle (Georgetown, Tex) 2004;3:145–8. [PubMed] [Google Scholar]
- 35.Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 36.Staal FJ, Noort Mv M, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–8. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–4. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- 38.Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–94. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen KB, Nesland JM, Fodstad O, Maelandsmo GM. Expression of S100A4, E-cadherin, alpha- and beta-catenin in breast cancer biopsies. Br J Cancer. 2002;87:1281–6. doi: 10.1038/sj.bjc.6600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Wendy Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–53. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- 41.Gillett CE, Miles DW, Ryder K, et al. Retention of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol. 2001;193:433–41. doi: 10.1002/path.831. [DOI] [PubMed] [Google Scholar]
- 42.Lin SY, Xia W, Wang JC, et al. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4262–6. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Noort M, Weerkamp F, Clevers HC, Staal FJ. Wnt signaling and phosphorylation status of beta-catenin: importance of the correct antibody tools. Blood. 2007;110:2778–9. doi: 10.1182/blood-2007-05-092445. [DOI] [PubMed] [Google Scholar]
- 44.Sedding DG. FoxO transcription factors in oxidative stress response and ageing--a new fork on the way to longevity? Biol Chem. 2008;389:279–83. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]
- 45.Ougolkov A, Zhang B, Yamashita K, et al. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst. 2004;96:1161–70. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 46.Wu-Baer F, Lagrazon K, Yuan W, Baer R. The BRCA1/BARD1 heterodimer assembles polyubiquitin chains through an unconventional linkage involving lysine residue K6 of ubiquitin. J Biol Chem. 2003;278:34743–6. doi: 10.1074/jbc.C300249200. [DOI] [PubMed] [Google Scholar]
- 47.Mallery DL, Vandenberg CJ, Hiom K. Activation of the E3 ligase function of the BRCA1/BARD1 complex by polyubiquitin chains. Embo J. 2002;21:6755–62. doi: 10.1093/emboj/cdf691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Lee TH, Avraham H. A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer. J Biol Chem. 2002;277:20965–73. doi: 10.1074/jbc.M112231200. [DOI] [PubMed] [Google Scholar]
- 49.Amit S, Ben-Neriah Y. NF-kappaB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 50.Fuchs SY. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol Ther. 2002;1:337–41. [PubMed] [Google Scholar]
- 51.Passmore LA, Barford D. Getting into position: the catalytic mechanisms of protein ubiquitylation. Biochem J. 2004;379:513–25. doi: 10.1042/BJ20040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato K, Hayami R, Wu W, et al. Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:30919–22. doi: 10.1074/jbc.C400169200. [DOI] [PubMed] [Google Scholar]
- 53.Brzovic PS, Keeffe JR, Nishikawa H, et al. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5646–51. doi: 10.1073/pnas.0836054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somasundaram K, MacLachlan TK, Burns TF, et al. BRCA1 signals ARF-dependent stabilization and coactivation of p53. Oncogene. 1999;18:6605–14. doi: 10.1038/sj.onc.1203284. [DOI] [PubMed] [Google Scholar]
- 55.Nishikawa H, Ooka S, Sato K, et al. Mass spectrometric and mutational analyses reveal Lys-6-linked polyubiquitin chains catalyzed by BRCA1-BARD1 ubiquitin ligase. J Biol Chem. 2004;279:3916–24. doi: 10.1074/jbc.M308540200. [DOI] [PubMed] [Google Scholar]
- 56.Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 57.Spiegelman VS, Stavropoulos P, Latres E, et al. Induction of beta-transducin repeat-containing protein by JNK signaling and its role in the activation of NF-kappaB. J Biol Chem. 2001;276:27152–8. doi: 10.1074/jbc.M100031200. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–4. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 59.Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–88. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.