Abstract
The γc-cytokines are critical regulators of immunity and possess both overlapping and distinctive functions. However, comparative studies of their pleiotropic effects on human T cell–mediated tumor rejection are lacking. In a xenogeneic adoptive transfer model, we have compared the therapeutic potency of CD19-specific human primary T cells that constitutively express interleukin-2 (IL-2), IL-7, IL-15, or IL-21. We demonstrate that each cytokine enhanced the eradication of systemic CD19+ B-cell malignancies in nonobese diabetic/severe combined immunodeficient (NOD/SCID)/γcnull mice with markedly different efficacies and through singularly distinct mechanisms. IL-7– and IL-21–transduced T cells were most efficacious in vivo, although their effector functions were not as enhanced as IL-2– and IL-15–transduced T cells. IL-7 best sustained in vitro T-cell accumulation in response to repeated antigenic stimulation, but did not promote long-term T-cell persistence in vivo. Both IL-15 and IL-21 overexpression supported long-term T-cell persistence in treated mice, however, the memory T cells found 100 days after adoptive transfer were phenotypically dissimilar, resembling central memory and effector memory T cells, respectively. These results support the use of γc-cytokines in cancer immunotherapy, and establish that there exists more than 1 human T-cell memory phenotype associated with long-term tumor immunity.
Introduction
The immune system can recognize and eliminate some cancers in mice and human subjects.1 Within the tumor microenvironment, however, tumor-infiltrating lymphocytes may be rendered incapable of rejecting tumor cells and/or remain insufficient in number. Several tumor escape mechanisms, including down-regulation of immunogenic tumor antigen, major histocompatibility complex class I, or antigen-processing machinery, as well as expression of a variety of immunosuppressive factors, such as interleukin-10 (IL-10), tumor growth factor-β, and programmed death ligand-1 (PD-L1), have been documented.2 Furthermore, suppressive immune cells including arginase- and indoleamine-2,3-deoxygenase–expressing myeloid suppressor cells3 and CD4+CD25+Foxp3+ regulatory T cells4 can be recruited to, or induced by, the tumor microenvironment. Molecules that sustain or enhance the function of tumor-reactive T cells, or that counteract inhibitory factors, are therefore likely to be beneficial for T cell–mediated tumor rejection.
Numerous cytokines profoundly affect T-cell development, differentiation, and homeostasis. IL-2, IL-7, IL-15, and IL-21 are members of a cytokine family whose heteromeric receptors share the common γ chain (γc). Each cytokine has been described as a T-cell growth factor5 and each has been used to augment the T-cell antitumor immune response,6–10 most notably IL-2.11,12 At a finer level, however, each cytokine possesses nonredundant functions that differentially shape T-cell responses: IL-2 plays a crucial role in the development and maintenance of regulatory T cells, a function not shared among other γc-cytokines13; IL-7 mediates homeostasis of naive and memory CD4+ and CD8+ T cells; and IL-15 is essential for maintenance of the CD8+ memory T-cell subset.14 The role of IL-21 in T cell–mediated tumor immunity is less defined, with reports demonstrating its antitumor efficacy as a single agent,9 or only in synergistic combination with IL-15.10
Despite extensive preclinical studies, and growing clinical data, a direct, multiparameter investigational comparison of the in vivo contributions of γc-cytokines to antitumor human T-cell biology has not been reported. Adoptive transfer approaches are well suited to compare the function and potency of a constant number of T cells exposed to different γc-cytokines.
In the following studies, we have taken a genetic approach to analyze and compare the antitumor properties of IL-2, IL-7, IL-15, and IL-21. Using bicistronic retroviral vectors, we engineered human primary T cells to recognize the CD19 antigen and overexpress a γc-cytokine, and compared their ability to eradicate disseminated lymphomas in nonobese diabetic/severe combined immunodeficient (NOD/SCID)/γcnull mice. We analyzed the contribution of each cytokine to T-cell proliferation, survival, effector function, and phenotype. Our studies reveal that all γc-cytokines augment tumor rejection, but through singularly divergent mechanisms.
Methods
Retroviral vectors and viral production
Plasmids encoding the SFG oncoretroviral vector were prepared using standard molecular biology techniques. Synthesis of 19z1, Pz1, and ΔLNGFR have been described.7,15,16 Human IL-2, IL-15, and IL-21 cDNA was prepared from phytohemagglutinin (PHA)–stimulated human peripheral blood leukocytes (PBLs); human IL-7 cDNA was obtained from Invivogen. To increase IL-15 secretion, the N-terminal signal peptide of IL-15 was replaced with the human IL-2 signal peptide and a FLAG tag was added to the C-terminus.17,18 The P2A bicistronic element was prepared from purified oligodeoxynucleotides (Sigma-Genosys).19 PG13 oncoretroviral vector producer cell lines were prepared from plasmid-transfected H29 cell supernatant.
Cell lines
Construction of Raji-eGFP/Firefly Luciferase (Raji-GL), EL4-CD19, EL4-PSMA, and the artificial antigen-presenting cells (AAPCs) NIH3T3-CD19-CD80 and NIH3T3-PSMA-CD80 have been described.7,15
PBL collection and retroviral transduction
Peripheral blood was obtained from healthy donors after informed consent under a protocol approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) institutional review board in accordance with the Declaration of Helsinki. PBLs were isolated by density gradient centrifugation and activated with PHA for 48 hours. Activated T cells were then isolated by negative selection using a pan T-cell isolation kit (Miltenyi Biotec) and were transduced on 2 consecutive days by centrifugation in retronectin-coated (Takara), oncoretroviral vector–bound plates. Seven days after PHA stimulation, T-cell cultures were exposed to irradiated AAPCs. Exogenous cytokine of any type was not administered at any time unless explicitly stated.
Flow cytometry
Flow cytometry was performed on a BD LSRII. Anti–human granzyme A (CB9), interferon-γ (IFN-γ; B27), tumor necrosis factor-α (TNF-α; MAb11), IL-2 (MQ1-17H12), CD27 (L128), Bcl-2 (6C8), CCR7 (3D12), CCR7 isotype (R35-95), and CD19 (SJ25C1) were obtained from BD; anti–human CD3 (S4.1), CD4 (S3.5), CD8 (3B5), CD28 (10F3), CD45RA (MEM-56), LIVE/DEAD violet, 4′,6-diamidino-2-phenylindole, and goat anti–mouse immunoglobulin G (used for detection of 19z1 and Pz1) were obtained from Caltag/Invitrogen; anti–human CD45RO (UCHL1) was obtained from Beckman Coulter; anti–human CD8 (RPA-T8), CD62L (DREG-56), Foxp3 (236A/E7), and Foxp3 isotype were obtained from eBioscience.
Mouse tumor model and quantitative bioluminescence
Mice were cared for in accordance with the institutional guidelines of MSKCC. Male 6- to 12-week-old NOD/SCID/γcnull mice (The Jackson Laboratory) were inoculated intravenously with 5 × 105 Raji-GL. Six days later, 2 × 105 19z1+CD8+ T cells were infused intravenously; cell dose was based on the percentage CD3h+19z1+CD8+CD4−. T cells were injected 6 days after AAPC stimulation. To quantify tumor burden (measured in photons/s/cm2/steradian), a constant region of interest was made that encompassed the entire mouse except for the tail. Living Image software (Xenogen) was used to acquire and quantify bioluminescent imaging (BLI) datasets as described.20 Mice were considered at the experimental end point if they developed hind-limb paralysis, were otherwise moribund, or had a tumor burden greater than 2 × 107 photons/s/cm2/sr.
In vitro T-cell assays
Enzyme-linked immunosorbent assay (ELISA) kits were used to quantify cytokine production: IL-2 and IL-7 (R&D), IL-15 (BD), and IL-21 (eBioscience). For blocking assays, anti–human IL-2Rα (20 μg/mL, clone 22722; R&D) and anti–human IL-7Rα (10 μg/mL, clone R34-34; IMGENEX) were added to cultures. For accumulation experiments involving cytokine titration, 19z1-ΔLNGFR cells were treated with IL-2 (Chiron), IL-7 (BD), IL-15 (R&D), or IL-21 (Biosource) as indicated. Fold increase in T cells was calculated using viable cell number (Guava) and percentage of 19z1+ T cells. Chromium-release assays were performed as described.15 For proliferation and effector cytokine production assays, 19z1+ T cells 7 days after AAPC stimulation were purified by positive selection with the anti-19z1 monoclonal antibody 19E3-phycoerythrin,21 a generous gift from Dr I. Rivière (MSKCC), and anti-phycoerythrin microbeads (Miltenyi Biotec). After purification, T cells were more than 99% 19z1+ (data not shown). For proliferation assays, purified 19z1+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) prior to stimulation with OKT3 (eBioscience) and anti-CD28 (BD). For effector cytokine production assays, purified 19z1+ T cells were likewise stimulated, but in the presence of brefeldin A (GolgiPlug; BD) and monensin (GolgiStop; BD).
Statistical methods
The paired t test was used to analyze normalized Bcl-2 data. Tumor burden at specific time points was analyzed using the Wilcoxon rank sum test. Survival data were analyzed using the log-rank test. The unpaired t test was used to analyze all other data.
Results
Efficient expression of γc-cytokine transgenes in human CD19-targeted primary T cells
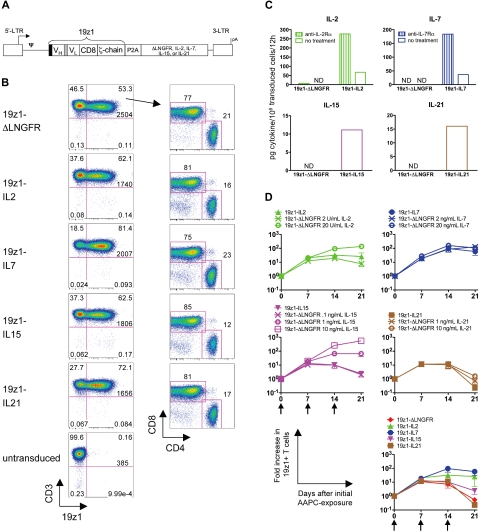
To establish conditions whereby human primary T cells would be continuously exposed to γc-cytokines in vitro and in vivo, we took a genetic approach based on the transduction of human peripheral blood T lymphocytes. Rapid generation of antitumor cytokine-secreting CD19-reactive human primary T cells was accomplished using bicistronic γ-retroviral vectors (Figure 1A) encoding 19z1, an major histocompatibility complex–independent chimeric antigen receptor (CAR) specific for CD19,7 and either IL-2, IL-7, IL-15, or IL-21. A doubly mutated human low-affinity nerve growth factor receptor (termed ΔLNGFR) served as control. Gene transfer into human primary T cells with the cytokine-encoding vectors was efficient (> 60%) and comparable between vectors (Figure 1B and Table 1). Significantly, the mean fluorescence intensity (MFI) of 19z1 in transduced cells was also similar between groups (Figure 1B), and T-cell cultures were more than 99% CD3+ (Table 1). Furthermore, although there was a trend in the IL-2– and IL-15–transduced groups toward an increase in the CD8+/CD4+ ratio, the difference was not significant, nor was it significantly different in the IL-7– and IL-21–transduced groups (Table 1).
Figure 1.
Retroviral vector design and expression of 19z1 and cytokine transgenes in human primary T cells. (A) Schematic diagram of bicistronic oncoretroviral vectors used for 19z1 and cytokine overexpression. 19z1 indicates human CD19-specific chimeric antigen receptor; black box, CD8 leader sequence; VH, variable heavy chain; gray box, (Gly3Ser)4 linker; VL, variable light chain; CD8, CD8 hinge and transmembrane domains; ζ-chain, TCR ζ-chain cytoplasmic domain; P2A, porcine teschovirus-1–derived 2A peptide; ΔLNGFR, doubly mutated human low-affinity nerve growth factor receptor; LTR, long terminal repeat; ψ, packaging signal; and pA, polyadenylation signal. (B) Cell surface expression of 19z1, CD3, CD4, and CD8 by transduced T cells 6 days after AAPC stimulation. Numbers at the bottom of the upper right quadrant represent the 19z1 MFI of the CD3+19z1+ subset. CD4/CD8 dot plots have been gated on the CD3+19z1+ subset. Dot plots are representative of 8 to 10 experiments using 6 different donors. (C) Secretion of IL-2, IL-7, IL-15, or IL-21 by transduced T cells. Equivalent numbers of 19z1+ T cells were washed and cultured in the presence or absence of an antireceptor blocking antibody. Culture supernatants were assayed for cytokine content by ELISA 12 hours later. Data shown are the average of 2 donors. ND indicates below limit of detection. (D) Overall accumulation of transduced T cells in response to tumor antigen exposure. T cells were transduced and exposed weekly to AAPCs. In parallel, control 19z1-ΔLNGFR T cells were cultured with titrating amounts of exogenously added IL-2, IL-7, IL-15, or IL-21. The number of viable 19z1+ T cells was assessed at the indicated time points. Arrows denote AAPC restimulation. The bottom right graph represents an overlay of the accumulation of cytokine-transduced T cells. Data are average (± SEM) of 3 donors.
Table 1.
Cell surface phenotype of cytokine-transduced T-cell cultures 6 days after AAPC stimulation
| Vector | No. | CD3+ |
CD3+ 19z1+ |
19z1+CD8+/% 19z1+CD4+ |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average % | SD | Range | Average % | SD | Range | Average ratio* | SD | Range | ||
| 19z1-ΔLNGFR | 10 | 99.7 | 0.3 | 99.1-99.9 | 62.5 | 9.8 | 45.5-77.5 | 4.3 | 3.2 | 2.0-13.0 |
| 19z1-IL2 | 8 | 99.7 | 0.3 | 99.1-99.9 | 62.9 | 6.9 | 50.6-72.2 | 6.6 | 4.3 | 1.8-14.4 |
| 19z1-IL7 | 8 | 99.8 | 0.1 | 99.7-99.9 | 79.7 | 7.7 | 67.9-91.7 | 3.7 | 2.4 | 0.99-8.6 |
| 19z1-IL15 | 8 | 99.5 | 0.3 | 99.0-99.8 | 60.6 | 8.8 | 46.6-72.3 | 7.9 | 4.0 | 2.3-14.0 |
| 19z1-IL21 | 9 | 99.8 | 0.2 | 99.5-99.9 | 64.1 | 14.5 | 37.1-80.1 | 5.4 | 3.4 | 2.3-13.9 |
SD indicates standard deviation.
All cytokine-transduced groups versus 19z1-ΔLNGFR, P > .05.
To quantify vector-encoded cytokine output, supernatants of cytokine-transduced T cells were analyzed by ELISA (Figure 1C). IL-2–, IL-7–, IL-15–, and IL-21–transduced T cells accumulated their respective cytokine to 68, 37, 11, and 16 pg/106 transduced cells/12 hours, respectively. The higher amount of vector-encoded IL-2, in comparison with IL-15, has been previously noted.22 Addition of anti–IL-2Rα to IL-2–transduced cultures and anti–IL-7Rα to IL-7–transduced cultures increased cytokine accumulation (277 and 184 pg/106 transduced cells/12 hours, respectively, Figure 1C), suggesting the presence of receptor-mediated cytokine uptake. Although blocking antibodies for IL-15Rα and IL-21R were not commercially available, it is possible that a similar phenomenon occurred in IL-15– and IL-21–transduced cultures, accounting for the smaller amount of cytokine observed. Knowing that it was not technically feasible to equalize the molar output for every cytokine, we sought to compare the effects of ectopic overexpression of each cytokine expressed from the same promoter.
To compare vector-encoded cytokine expression with exogenous cytokine treatment, 19z1-ΔLNGFR–transduced T cells were treated with titrated amounts of human IL-2, IL-7, IL-15, and IL-21, and the fold increase in 19z1+ T cells in response to weekly AAPC stimulation was monitored (Figure 1D). IL-2–transduced T cells accumulated to a level similar to ΔLNGFR-transduced T cells treated with 2 to 20 U/mL IL-2. IL-7–transduced T cells accumulated to a similar extent as ΔLNGFR-transduced T cells treated with either 2 ng/mL or 20 ng/mL IL-7, suggesting that in our system a threshold had been reached by both exogenous cytokine doses and IL-7 overexpression by which the accumulation capacity had been met. IL-15–transduced T cells accumulated to a similar extent as ΔLNGFR-transduced T cells treated with 0.1 ng/mL IL-15. Finally, IL-21–transduced T cells accumulated to a lesser extent than ΔLNGFR-transduced T cells treated with 1 ng/mL IL-21.
Comparative analysis of in vivo efficacy of CD19-targeted cytokine-overexpressing human primary T cells
We compared the antitumor potency of γc-cytokine–transduced T cells in a xenogeneic adoptive transfer model in immunodeficient mice bearing systemic, established Raji human B-cell lymphoma. Previous animal studies from our laboratory used the SCID/bg mouse,7,23,24 a strain devoid of T cells and B cells but retaining modest NK-cell activity. Because of the potential for T cell–derived γc-cytokines, particularly IL-15, to augment host NK cell function, we developed a mouse model using the more severely immunodeficient NOD/SCID/γcnull mouse. In a direct comparison, both Raji cells and 19z1+ T cells persisted to a greater extent in NOD/SCID/γcnull mice than in SCID/bg mice (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In NOD/SCID/γcnull mice, the Raji tumor cells extensively infiltrated the bone marrow, lymph nodes, liver, spinal cord, and pituitary gland (supplemental Figure 1B-F). Importantly, treatment of Raji cells with exogenous cytokine had no effect on tumor growth kinetics (supplemental Figure 2A).
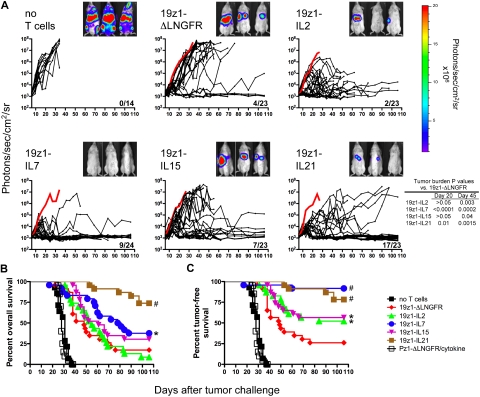
To determine the in vivo efficacy of γc-cytokine–transduced T cells, NOD/SCID/γcnull mice bearing established, systemic Raji tumors received 2 × 105 19z1+CD8+ T cells of each vector type. Tumor burden in individual mice was monitored biweekly using in vivo bioluminescent imaging (BLI) in the control groups and 5 treatment groups (Figure 2A). Control mice were treated with T cells transduced with analogous cytokine-encoded vectors, but with a CAR specific for prostate-specific membrane antigen (PSMA), termed Pz1 (supplemental Figure 3). In mice receiving no treatment or any PSMA-targeted T-cell treatment, all mice succumbed to disease with a median survival of 30 and 28 days, respectively (Figure 2B). In contrast, treatment with CD19-specific T cells coexpressing any or none of the 4 γc-cytokines significantly delayed tumor progression and induced more frequent long-term remissions. 19z1-IL7 and 19z1-IL21 mice had an overall superior survival benefit compared with 19z1-ΔLNGFR-mice, whereas 19z1-IL2– and 19z1-IL15–treated mice did not.
Figure 2.
Differential in vivo antitumor potency of tumor-specific human primary T cells overexpressing IL-2, IL-7, IL-15, or IL-21. NOD/SCID/γcnull mice were inoculated with 5 × 105 Raji-GL tumor cells and 6 days later received adoptive transfer of 2 × 105 19z1+CD8+ T cells transduced with 19z1-ΔLNGFR (n = 23), 19z1-IL2 (n = 23), 19z1-IL7 (n = 24), 19z1-IL15 (n = 23), 19z1-IL21 (n = 23), or no T cells (n = 14). As nonspecific controls, mice received T cells transduced with Pz1-ΔLNGFR, Pz1-IL2, Pz1-IL7, Pz1-IL15, or Pz1-IL21 vectors (n = 4 each). The percentage of 19z1+ T cells for each of the 3 experiments at the time of adoptive transfer was as follows: 19z1-ΔLNGFR: 53, 71, 61 (mean, 62); 19z1-IL2: 62, 64, 72 (mean, 66); 19z1-IL7: 81, 92, 85 (mean, 86); 19z1-IL15: 63, 68, 72 (mean, 68); and 19z1-IL21: 72, 78, 76 (mean, 75). (A) Quantified tumor burden measured by in vivo bioluminescent imaging. Immediately prior to T-cell injection, biweekly until day 55, and weekly thereafter, mice were anesthetized and administered intraperitoneal d-luciferin (150 mg/kg), and ventral tumor burden was assessed by the Xenogen 100 in vivo imaging system. Mice were imaged up to 3 at a time, separated by opaque shields. Saturated luminescent signals were imaged for shorter durations, down to 2 seconds. Each black line represents 1 animal; dots along the line indicate an imaging time point. The thick red line represents the average tumor burden of mice that received PSMA-specific T cells. Fractions in the lower right corner of each graph represent the proportion of 19z1-treated surviving mice at day 106. Bioluminescent images from 3 representative mice from 1 experiment at day 27 after tumor challenge are shown. 19z1 data are from 3 pooled experiments (n = 5-10), each testing T cells from a different donor. (B-C) Efficacy of tumor immunotherapy with cytokine-transduced T cells. Kaplan-Meier survival curves depicting overall survival (B), and tumor-free survival (C) in which mice were considered tumor free if their tumor burden was < 104 photons/s/cm2/sr. Pz1-ΔLNGFR/cytokine refers to the combined survival of all mice treated with PSMA-specific T cells. *P < .05, #P < .005 versus 19z1-ΔLNGFR.
However, the imaging studies revealed dramatically distinct patterns in tumor progression. Mice receiving no treatment or unspecific treatment died with large liver and bone marrow tumor burden, as indicated by bioluminescent signal in the abdomen and lower limbs and confirmed by pathology (supplemental Figure 1B-F). In contrast, mice receiving CD19-specific T cells of any type became negative for bioluminescent signal in the limbs, suggesting successful tumor eradication in the bone marrow. Mice that went on to fail CD19-specific T-cell therapy almost uniformly died with progressively accumulating abdominal tumor burden due to failure to eradicate liver disease (Figure 2A and data not shown). Of note, outgrowth of hepatic lymphoma in treated mice was not due to loss of CD19 tumor antigen because tumors removed from mice at end point documented persisting CD19 expression (supplemental Figure 2B). Most importantly, we soon noticed that a substantial number of mice died with a low, near-background bioluminescent signal (< 104 photons/s/cm2/sr), which was confirmed to reflect absence of residual tumor in all mice subjected to necropsy (n = 5, data not shown). This occurred in 10 (43%) of 23 19z1-IL2 mice, 13 (54%) of 24 19z1-IL7 mice, and 6 (26%) of 23 19z1-IL15 mice. In contrast, only 2 (9%) of 23 19z1-ΔLNGFR mice and 1 (4%) of 23 19z1-IL21 mice died with a low tumor burden. Death of these mice with low tumor burden was due to cytokine-exacerbated xenogeneic graft-versus-host disease (XGVHD), as confirmed by clinical and histologic examination (J.C.M. and M.S., unpublished observations).
Because most mice succumb from either massive tumor burden or from XGVHD without evidence of tumor, and because we could differentiate these modes of death by BLI, the antitumor activity of the different T-cell groups is best analyzed by plotting tumor-free survival rather than overall survival. This method assumed that mice dying with a tumor burden of less than 104 photons/s/cm2/sr died of XGVHD, but after successful tumor eradication. As shown in Figure 2C, mice that received any of the 4 γc-cytokine–transduced T cells survived significantly longer than mice receiving 19z1-ΔLNGFR cells, with 19z1-IL7 and 19z1-IL21 inducing greater responses than 19z1-IL2 and 19z1-IL15.
γc-cytokines differentially affect the proliferation, survival, and accumulation of tumor-specific human primary T cells
To elucidate the mechanism underlying the differential in vivo potency of cytokine-transduced T cells, we investigated their preinfusion and postinfusion function and phenotype. The response of transduced T cells to antigenic stimulation was assessed in vitro by monitoring absolute T-cell expansion and accumulation after repeated exposure to CD19+ AAPCs in the absence of added cytokine (Figure 1D). After the first round of stimulation, all groups expanded, more so in the IL-2 and IL-7 groups than the ΔLNGFR, IL-15, and IL-21 groups (19z1-ΔLNGFR, 11-fold expansion; 19z1-IL2, 21-fold; 19z1-IL7, 18-fold; 19z1-IL15, 13-fold; 19z1-IL21, 12-fold). After the second round of CD19+ AAPC stimulation, IL-7–transduced T cells had accumulated to the greatest extent, followed by IL-2 (19z1-ΔLNGFR, 7-fold; 19z1-IL2, 32-fold; 19z1-IL7, 96-fold; 19z1-IL15, 10-fold; 19z1-IL21, 12-fold). This trend continued after the third AAPC stimulation except that 19z1-IL21–transduced T cells became less abundant than the 19z1-ΔLNGFR–transduced T cells, indicating that constitutive IL-21 overexpression may have an antiproliferative or proapoptotic effect on human primary T cells. Overall, these results indicate that IL-2, IL-7, and to a lesser extent, IL-15, but not IL-21, can augment antigen-induced accumulation of specific T cells. The accumulation pattern of CD4+ and CD8+ T cells within these groups paralleled the growth of CD3+ T cells (supplemental Figure 4).
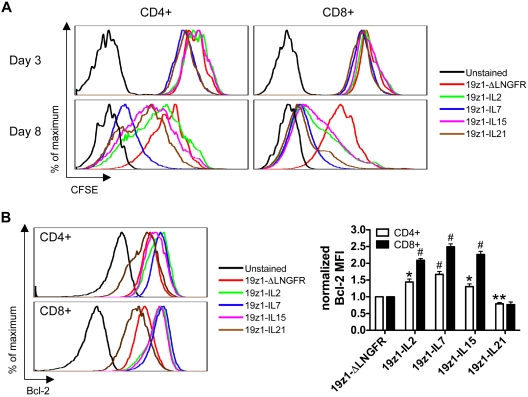
We therefore measured the proliferative capacity of cytokine-transduced T cells. 19z1+ T cells were CFSE-labeled and activated by anti-CD3/anti-CD28 antibodies (Figure 3A). IL-7– and IL-21–transduced CD4+ and CD8+ T cells responded quickest as measured by CFSE dilution on day 3. Near complete CFSE dilution was achieved by day 8 for the IL-7–transduced CD4+ and CD8+ T cells and IL-21–transduced CD8+ T cells. In comparison, the CD4+ subset of the IL-2–, IL-15–, and IL-21–transduced T cells and the CD8+ subset of the IL-2– and IL-15–transduced T cells proliferated less, albeit more than the control 19z1-ΔLNGFR–transduced T cells. These results indicate that each cytokine supported mitogen-induced proliferation, but to different extents.
Figure 3.
Differential effect of IL-2, IL-7, IL-15, and IL-21 gene transfer on tumor-specific human T-cell proliferation and Bcl-2 expression. (A) Proliferation of transduced T cells in response to mitogenic stimulation. On day 7 after AAPC stimulation, 19z1+ T cells were purified, labeled with CFSE (2μM), and stimulated with plate-bound anti-CD3 (10 μg/mL) and soluble anti-CD28 (1 μg/mL) antibodies. At 3 and 8 days later, T cells were analyzed for CFSE dilution of the CD4+ and CD8+ subsets. One of 2 donors is shown with similar results observed in both. (B) Bcl-2 expression by transduced T cells. On day 7 after AAPC stimulation, T cells were assessed for expression of Bcl-2 protein by flow cytometry. Histograms are representative of 4 donors and have been gated on 19z1+CD4+ or 19z1+CD8+ T cells. The bar graph depicts Bcl-2 MFI (± SEM) of 19z1+CD4+ or 19z1+CD8+ cells from 4 donors that has been normalized such that the MFI of Bcl-2 for the 19z1-ΔLNGFR group = 1. *P < .05, **P < .01, #P < .005 versus 19z1-ΔLNGFR.
Because IL-21–transduced T cells exhibited marked early proliferation in response to stimulation, but accumulated very poorly upon repeated antigenic stimulation (Figure 1D), we examined expression of Bcl-2, a key negative regulator of lymphocyte apoptosis. Overexpression of IL-2, IL-7, and IL-15 in CD4+ T cells resulted in a 144%, 167%, and 130% increase in Bcl-2 MFI compared with ΔLNGFR-transduced CD4+ T cells (Figure 3B). A parallel but more extensive Bcl-2 up-regulation was observed for CD8+ T cells transduced with IL-2 (209%), IL-7 (249%), and IL-15 (227%) compared with ΔLNGFR-transduced CD8+ T cells. In contrast, Bcl-2 expression was lower in IL-21–transduced CD4+ T cells (79%); the same trend was observed for CD8+ T cells (77%), although this decrease did not reach statistical significance (P = .06). These data confirm the role of these γc-cytokines in modulating Bcl-2 expression by human T cells.25–27
Transgenic γc-cytokines differentially influence the effector function of tumor-targeted human primary T cells
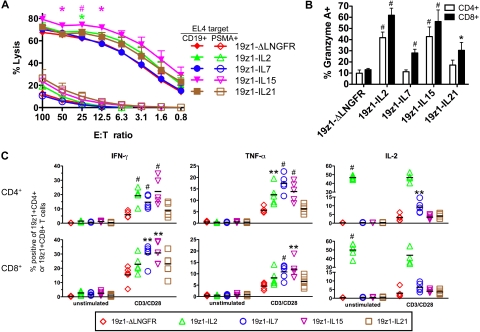
To investigate the influence of ectopic γc-cytokine overexpression on T-cell effector function, we first quantified the cytolytic activity of cytokine-transduced T cells in chromium-release assays (Figure 4A). IL-2– and IL-15–transduced T cells lysed relevant targets significantly more efficiently than ΔLNGFR-transduced T cells at 1 and 3 E/T ratios, respectively, in agreement with a published report.22 This enhancement was not observed in other cytokine-transduced groups.
Figure 4.
Differential effector functions of tumor-specific human primary T cells overexpressing IL-2, IL-7, IL-15, or IL-21. (A) Antigen-specific cytotoxic activity of transduced T cells. On day 6 after AAPC stimulation, T cells were assessed for cytolytic potential in a 4-hour chromium-release assay using EL4-hCD19 and EL4-hPSMA as the relevant and irrelevant targets (T). Effectors (E) were defined as 19z1+CD8+ T cells. Data are average (± SEM) from 4 donors. *P < .05, #P < .005 versus 19z1-ΔLNGFR. (B) Intracellular expression of granzyme A by transduced T cells. On day 7 after AAPC stimulation, T cells were analyzed by flow cytometry for the cytolytic molecule granzyme A. Average percent granzyme A+ T cells (± SEM) of 19z1+CD4+ and 19z1+CD8+ subsets from 7 donors is indicated. (C) IFN-γ, TNF-α, and IL-2 expression by transgenic T cells. On day 7 after AAPC stimulation, 19z1+ T cells were purified and treated with no stimulation (unstimulated) or anti-CD3/anti-CD28 antibodies (CD3/CD28) in the presence of brefeldin A and monensin. After 6 hours, T cells were analyzed for cytokine expression by intracellular flow cytometry. The percentage of CD4+ (top row) or CD8+ (bottom row) T cells that express the indicated effector cytokine from 5 donors is indicated. Horizontal bar indicates average. *P < .05, **P < .01, #P < .005 versus 19z1-ΔLNGFR.
We next determined whether expression of the cytolytic molecule granzyme A was affected by overexpression of γc-cytokines (Figure 4B). Overexpression of each cytokine significantly increased the percentage of granzyme A+ CD8+ T cells over ΔLNGFR-transduced CD8+ T cells. Likewise, IL-2 and IL-15 overexpression significantly increased the percentage of granzyme A+ CD4+ T cells over ΔLNGFR-transduced CD4+ T cells, whereas overexpression of IL-7 and IL-21 did not. The higher percentage of granzyme A+ IL-2– and IL-15–transduced T cells thus paralleled the functional cytolytic activity.
We also assessed the IFN-γ and TNF-α response of transgenic T cells to mitogenic restimulation (Figure 4C). In the CD4+ subset, IFN-γ was expressed in a significantly higher percentage of IL-2–, IL-7–, and IL-15–transduced T cells, but not IL-21–transduced T cells, compared with ΔLNGFR-transduced CD4+ T cells. In the CD8+ subset, a significantly higher percentage of IFN-γ–expressing T cells was found in the IL-7– and IL-15–transduced T cells compared with ΔLNGFR-transduced T cells, but not IL-2– or IL-21–overexpressing groups. The same trend was observed for TNF-α expression in both CD4+ and CD8+ T cells. These data indicate that IL-7 and IL-15 overexpression can significantly up-regulate in vitro IFN-γ and TNF-α expression in both CD4+ and CD8+ tumor-specific T cells.
Influence of γc-cytokine overexpression on the in vitro memory phenotype
The γc-cytokines influence the differentiation state of tumor-specific memory-phenotype (CD45RA−CD45RO+) T cells and their central memory (CCR7+CD62L+, TCM) or effector memory (CCR7−CD62L+/−, TEM) subtypes.8,28,29 To determine whether cytokine overexpression likewise affected T-cell differentiation, we first assayed for CD45RA and CD45RO expression. Seven days after AAPC stimulation, nearly all transduced T cells were CD45RA−CD45RO+ (supplemental Figure 5), indicating that cytokine transduction did not significantly affect in vitro formation of memory-phenotype T cells.
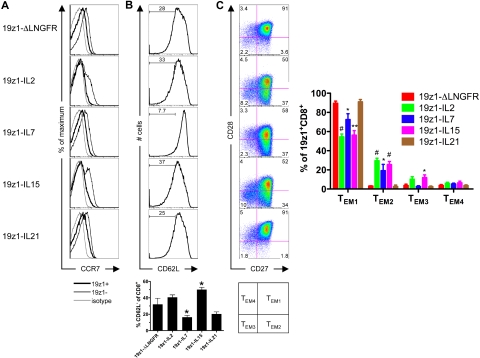
We next assessed the short-term generation of CD8+ TCM and TEM subsets (Figure 5). CCR7 expression was low in CD45RA−CD8+ ΔLNGFR-, IL-7–, IL-15–, and IL-21–transduced groups and negative in the IL-2–transduced group (Figure 5A). Interestingly, CCR7 was more highly expressed in the cocultured, untransduced (19z1−) subset of each group, indicating that the antigen-specific AAPC stimulation was at least partially responsible for CCR7 down-regulation. Compared with ΔLNGFR-transduced T cells, IL-15–transduced memory-phenotype CD8+ T cells lost expression of CD62L, whereas the IL-7–transduced group largely retained CD62L; no significant difference was observed between ΔLNGFR-transduced and IL-2– or IL-21–transduced groups (Figure 5B). Our data indicate that γc-cytokine overexpression did not promote in vitro formation of CD8+ TCM cells; cytokine-transduced CD8+ T cells were phenotypically more similar to TEM cells.
Figure 5.
Effector memory phenotype of tumor-specific human CD8+ T cells overexpressing IL-2, IL-7, IL-15, or IL-21. (A) CCR7 expression of CD8+ T cells 7 days after AAPC stimulation. Histograms have been gated on the CD8+CD45RA−CD45RO+ subset and are from 1 of 2 donors with similar results observed in both. Thick black line indicates 19z1+; thin black line, 19z1−; and gray line, isotype control. (B) CD62L expression of CD8+ T cells 7 days after AAPC stimulation. Histograms are representative of 8 donors and have been gated on the 19z1+CD8+CD45RA−CD45RO+ subset. The bar graph represents the average percentage (± SEM) of CD62L− T cells within the 19z1+CD8+CD45RA−CD45RO+ subset from 8 donors. Histograms for individual experiments are shown in supplemental Figure 7. (C) CD27 and CD28 expression of CD8+ T cells 7 days after AAPC stimulation. Dot plots are representative of 4 donors and have been gated on the 19z1+CD8+ subset. The bar graph represents the average percentage (± SEM) of CD27+CD28+ (TEM1), CD27+CD28− (TEM2), CD27−CD28− (TEM3), and CD27−CD28+ (TEM4) within the 19z1+CD8+ subset from 4 donors. *P < .05, **P < .01, #P < .005 versus 19z1-ΔLNGFR.
TEM cells are not major producers of IL-2 compared with their TCM counterparts.28 To further verify that the cytokine-transduced T cells in our system are of the TEM phenotype, we assessed their potential to express IL-2 (Figure 4C). Expectedly, most IL-2–transduced CD4+ and CD8+ T cells expressed IL-2 constitutively. Of the remaining groups, only the CD4+ IL-7–transduced T cells contained a higher percentage of IL-2+ T cells compared with ΔLNGFR-transduced T cells. These data help confirm the TEM phenotype of γc-cytokine–overexpressing T cells.
Human CD8+ TEM cells can be further subdivided according to their cell surface expression of the costimulatory molecules CD27 and CD28.30,31 The subsets thus defined (Figure 5C) differ widely in their function, with TEM1 (CD27+CD28+), TEM2 (CD27+CD28−), and TEM3 (CD27−CD28−) cells representing memory-like, intermediate effectors, and late effectors, respectively.31
We thus delineated the TEM subsets of cytokine-transduced memory-phenotype CD8+ T cells by measuring expression of CD27 and CD28 (Figure 5C). In all groups, the majority of 19z1+CD8+ T cells were of the TEM1 subtype. However, a significantly smaller percentage of TEM1 cells existed in IL-2–, IL-7–, and IL-15–transduced groups compared with the ΔLNGFR-transduced group, but not in the IL-21–transduced group. The reduced percentage of TEM1 cells translated into higher percentages of TEM2 and TEM3 populations, indicating a progressive differentiation of these T cells to a late effector phenotype. These data indicate that IL-21 overexpression preserved a limited differentiation CD27+CD28+ TEM1 phenotype.
Of note, overexpression of any γc-cytokine did not influence the generation of CD4+Foxp3+ T cells (supplemental Figure 6).
Long-term in vivo persistence of human T cells in surviving mice
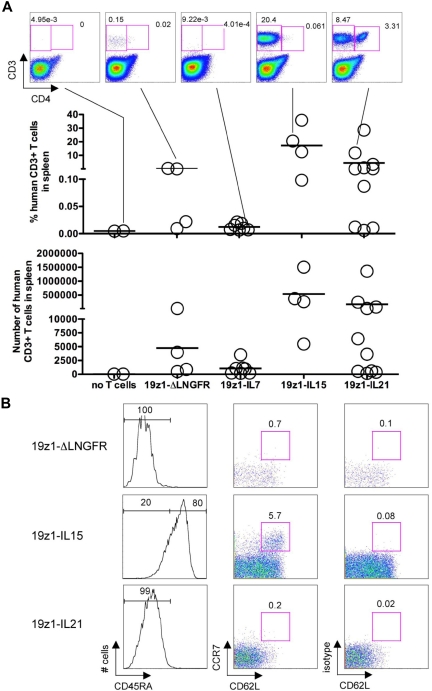
Because in vitro data suggested that we had created T cells with the ability to proliferate and/or survive (Figure 3), we determined whether long-term surviving mice still harbored human T cells and whether their presence and phenotype correlated with efficacy. Surviving mice were killed at or after day 106 after tumor challenge (day 100 after adoptive transfer) and spleens were analyzed for the presence of human T cells (Figure 6). A definitive population of human T cells was detected in the spleens of 1 (25%) of 4 19z1-ΔLNGFR mice, 0 (0%) of 7 19z1-IL7 mice, 3 (75%) of 4 19z1-IL15 mice, and 4 (40%) of 10 19z1-IL21 mice (Figure 6A). Unfortunately, no 19z1-IL2 survivors were available for analysis. Human T cells were detected in the bone marrow in a similar proportion of mice (data not shown). T cells were predominantly or exclusively CD8+ except in the 19z1-IL21 mice in which prominent CD4+ populations existed in both spleen and bone marrow (Figure 6A and data not shown).
Figure 6.
Long-term in vivo persistence of human T cells in surviving mice treated with ΔLNGFR–, IL-7–, IL-15–, or IL-21–overexpressing T cells. (A) Identification of human T cells in the spleen of tumor-free mice on days 106 to 110 after tumor challenge. Dot plots represent the indicated mice. Graphs indicate the percentage and absolute number of human CD3+ cells in the spleen of individual mice. Each circle represents an individual mouse (no T cells, n = 2; 19z1-ΔLNGFR, n = 4; 19z1-IL7, n = 7; 19z1-IL15, n = 4; 19z1-IL21, n = 10). Horizontal bar indicates average. (B) Expression of CD45RA, CCR7, and CD62L by persisting human CD8+ T cells in long-term surviving mice. Histograms are from representative mice and have been gated on the CD3+CD8+ subset (inferred from the CD3+CD4− gate). Dot plots for the 19z1-ΔLNGFR and 19z1-IL21 groups have been gated on the CD3+CD4−CD45RA− cells and dot plots for the 19z1-IL15 group have been gated on the CD3+CD4−CD45RA+ cells.
Splenocytes from mice with prominent human T-cell populations were further characterized. Human CD4+ and CD8+ T cells from mice in the 19z1-ΔLNGFR and 19z1-IL21 groups were predominantly CD45RA− (Figure 6B), consistent with their phenotype at infusion (supplemental Figure 5). By contrast, CD8+ T cells from 19z1-IL15 mice were predominantly CD45RA+CCR7−CD62L+/−, resembling a previously described population of CD45RA+ effector memory T cells (TEMRA).32 In addition, CD8+ T cells recovered from 19z1-IL15 mice contained a CCR7+CD62L+ population, implicating IL-15 in the formation of a CD45RA+ antigen-experienced cell type that possesses cell surface molecules resembling TCM cells (TCMRA).32,33 Long-term persisting CD8+ T cells remained functional, as demonstrated by their ability to produce IFN-γ upon stimulation (data not shown).
Discussion
γc-cytokines exert profound effects on tumor-specific T-cell proliferation, differentiation, and survival, and as such have considerable therapeutic potential. Although much is known about their respective functions in mice, their effect on human cells is less well known, especially in vivo, and comparative studies are lacking. We have therefore taken a genetic approach to study the influence of IL-2, IL-7, IL-15, and IL-21 on tumor-targeted human primary T cells in a xenogeneic tumor model in which a constant dose of CD8+ CD19-targeted T cells was administered to mice with established, systemic lymphoma. In our model, we demonstrate that each of these cytokines potently enhanced tumor eradication, with IL-7 and IL-21 demonstrating superiority to IL-2 and IL-15. Our combined in vitro and in vivo findings, summarized in Table 2, demonstrate distinct mechanisms of action.
Table 2.
Comparison of phenotype and function of tumor-specific human primary T cells overexpressing IL-2, IL-7, IL-15, or IL-21
| Parameter | ΔLNGFR | IL-2 | IL-7 | IL-15 | IL-21 |
|---|---|---|---|---|---|
| Maintenance of T-cell numbers | |||||
| Accumulation | 0 | ++ | ++ | + | − |
| Antigen-induced proliferation | 0 | + | +++ | + | ++ |
| Bcl-2 expression | 0 | + | + | + | − |
| Long-term persistence | 0 | ND | 0 | ++ | + |
| Effector function | |||||
| CTL activity | 0 | + | 0 | ++ | + |
| Granzyme A expression | 0 | ++ | + | ++ | + |
| IFN-γ expression | 0 | + | ++ | ++ | 0 |
| TNF-α expression | 0 | + | ++ | ++ | 0 |
| IL-2 expression | 0 | +++ | + | 0 | 0 |
| Cell surface phenotype | |||||
| CD45RA−CD45RO+ expression | 0 | 0 | 0 | 0 | 0 |
| CCR7 expression | 0 | − | 0 | 0 | 0 |
| CD62L expression | 0 | − | + | − | 0 |
| CD28 expression | 0 | − | − | − | 0 |
| CD27 expression | 0 | − | − | − | 0 |
| TEM1 % | 0 | − | − | − | 0 |
| CD4+Foxp3+ % | 0 | 0 | 0 | 0 | 0 |
| Long-term persistence of TCM-like cells | 0 | ND | 0 | + | 0 |
| Avoidance of XGVHD | 0 | −− | −− | − | 0 |
| Tumor-free survival | 0 | + | ++ | + | ++ |
Parameters were scored using ΔLNGFR-transduced T cells as a baseline. 0 indicates equal to ΔLNGFR-transduced T cells; +, ++, +++, greater than ΔLNGFR-transduced T cells; −, −−, less than ΔLNGFR-transduced T cells; and ND, not determined.
Constitutive expression of IL-7 increased in vitro T-cell accumulation in response to repeated exposure to antigen through a combined increase in proliferation and T-cell survival. However, 19z1-IL7 T cells did not exhibit increased tumor lysis, suggesting that the enhanced antitumor activity was mediated primarily by a quantitative increase in tumor-reactive T cells, consistent with the known ability of IL-7 to maintain T-cell numbers.34 Surprisingly however, no residual T cells were found in long-term survivors, which suggests that IL-7 is unable to sustain memory T cells in this model. This may be explained by the down-regulation of IL-7Rα expression due to chronic IL-7 exposure,35 thus depriving memory T cells of this critical homeostatic signaling pathway.34
IL-21 appeared to exert few if any effects on T-cell expansion in vitro, but displayed unexpected potency in vivo. Similar to IL-7, constitutive expression of IL-21 initially supported extensive proliferation and did not increase the cytolytic potential of T cells. The overall accumulation of cultured 19z1-IL21 T cells, however, was markedly less than observed for 19z1-IL7 T cells, consistent with decreased Bcl-2 expression. Yet, in vivo, 19z1-IL21 T cells were highly efficacious in promoting tumor eradication. In contrast to 19z1-IL7–treated mice, large numbers of T cells with a TEM1 phenotype persisted in the bone marrow and spleen of 19z1-IL21–treated long-term survivors. In aggregate, these findings suggest that enhanced tumor rejection resulted from improved T-cell expansion rather than the up-regulation of effector function.
In contrast to IL-7 and IL-21, constitutive IL-15 expression increased effector functions (eg, higher CTL activity and IFN-γ expression), but did not substantially increase T-cell accumulation in vitro. However, this cytokine sustained a very significant population of long-term surviving T cells with TCM phenotypic properties, which may contribute to their enhanced efficacy in addition to the increased effector function. Interestingly, these CD8+ T cells, which displayed a CD45RA−CCR7−CD62L+/− phenotype at the time of adoptive transfer, reverted to a CD45RA+ phenotype, resembling TEMRA cells, an antigen-experienced CD45RA+CCR7−CD62L− population thought to derive from CD8+ TCM cells.32 A population of CD8+CD45RA+CCR7+CD62L+ T cells was also observed exclusively in 19z1-IL15 mice, which may represent TCMRA cells, a cell type of yet unclear significance.32,33 The in vivo transition of cells with a TEM phenotype to cells resembling TCM-related subtypes parallels a recent study demonstrating that TCM-derived clones, despite displaying a TEM phenotype at the time of transfer, persist and revert to a TCM phenotype in vivo.36 Our data suggest that IL-15 is sufficient to mediate this transition.
Although sharing a common ability to persist in long-term survivors, IL-15– and IL-21–transduced T cells were phenotypically dissimilar, establishing that (1) these closely related cytokines influence the persistence and differentiation of distinct antitumor T-cell subsets and (2) there is more than one T-cell phenotype capable of mediating effective antitumor responses. Whereas these 2 cytokines induced persisting T cells, IL-7 did not, and we were unable to ascertain the effect of IL-2 because of poor overall survival of 19z1-IL2 mice.
Recent data suggest that adoptive transfer of T cells with a less differentiated phenotype correlates with superior antitumor immunity.37–40 Our ability to rapidly redirect polyclonal peripheral blood T cells by retroviral-mediated gene transfer of a CAR allowed us to minimize the culture period (13 days) prior to adoptive transfer and reduce the generation of terminally differentiated, exhausted T cells. This is in contrast to expanded tumor-infiltrating lymphocytes, which, although capable of inducing tumor regression in melanoma patients,41 may display reduced therapeutic potency because of shortened telomeres and loss of CD27 and CD28 expression.40
A proposed mechanism for IL-21 efficacy is the preservation of less differentiated CD27+CD28+ TEM1 cells, a population that has been compared with TCM cells.31 Importantly, TEM1 cells are the least differentiated, have the highest telomerase activity, and have the shortest replicative history,31 which may make them the preferred effector memory subset for adoptive transfer.37 The inability of IL-21 to induce in vitro differentiation of TEM cells is consistent with its previously demonstrated ability to preserve the naive phenotype of CD4+ and CD8+ T cells.38,42 Our data are in agreement with the demonstrated ability of IL-2, IL-7, and IL-15, but not IL-21, to down-regulate CD28 expression on human CD8+ T cells.43,44 Our findings are also in agreement with reports describing the ability of IL-2 and IL-15 to down-regulate CD27 expression to a greater extent than IL-7.25,45,46 IL-21, in the context of IL-2 and IL-7 cotreatment, was recently described to maintain CD27 expression on CD8+ T cells.47 The mechanism by which adoptively transferred T cells that retain a more naive phenotype may provide superior antitumor immunity is ill defined. More differentiated T cells may be less efficacious because of loss of costimulatory receptor expression and tissue homing capacity.37
Our results provide novel insights into the merits of xenogeneic adoptive transfer models to analyze human T-cell memory phenotypes. Although such models are clearly limited in their ability to recapitulate many important facets of human T-cell biology, it is noteworthy that the phenotypes of T cells persisting 100 days after adoptive transfer in NOD/SCID/γcnull mice are consistent with observations made with genetically marked, persisting cells introduced in nonhuman primates36 and consistent with the phenotype of IL-15 and IL-21 transgenic mice, in which memory-phenotype CD8+ T cells selectively accumulate.18,48 Although the role of CD4+ T cells in syngeneic tumor rejection is well established, the role of this population in xenogeneic systems is uncertain.7
Finally, our findings have important implications for the investigation of γc-cytokines in cancer treatment. IL-2 is currently the only Food and Drug Administration–approved γc-cytokine, and comparative clinical studies would be difficult to implement. Our results point to the great promise of IL-7 to support the potency of adoptively transferred T cells. The effect was limited in our model, but restoration of IL-7Rα expression via retroviral-mediated gene transfer may be an option to prolong responsiveness to IL-7 treatment.49 IL-15 and IL-21 seem better suited to promote long-term T-cell persistence under the present conditions, albeit through different mechanisms.
Our studies did not aim to validate the adoptive transfer of genetically modified T cells that constitutively express cytokines for direct clinical application. However, T cell–mediated local cytokine delivery may avoid, or at least reduce, the severe toxicity that often limits systemic cytokine administration by limiting cytokine delivery to the tumor microenvironment, draining lymph nodes, and other immunologically relevant locations. The clinical use of γc-cytokine overexpression may necessitate a vector-encoded suicide gene, as these T-cell growth factors may increase the risk of T-cell transformation either by themselves or by potentiating insertional mutagenesis.18,25 As an alternative to constitutive expression, transgene-encoded cytokine expression could be made dependent on antigen-specific T-cell activation to restrict cytokine delivery to sites of T-cell activation.50
Acknowledgments
We thank Dr Gabrielle A. Rizzuto for technical assistance and critical reading of the paper; Alberto del Rosario for technical assistance; and the MSKCC Laboratory of Comparative Pathology.
This work was supported by National Institutes of Health (NIH) grant CA59350, Mr William Goodwin and Mrs Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutic Center of MSKCC, NIH MSTP grant GM07739, and a Cancer Research Institute Predoctoral Fellowship (J.C.M.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.C.M. designed experiments, performed research, analyzed data, and wrote the paper; and M.S. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Sadelain, Box 182, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; e-mail: m-sadelain@ski.mskcc.org.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 3.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68(8):2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 4.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 5.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma (c) family cytokines. Nat Rev Immunol. 2009;9(7):480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jicha DL, Mule JJ, Rosenberg SA. Interleukin 7 generates antitumor cytotoxic T lymphocytes against murine sarcomas with efficacy in cellular adoptive immunotherapy. J Exp Med. 1991;174(6):1511–1515. doi: 10.1084/jem.174.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff CA, Finkelstein SE, Surman DR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(7):1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173(2):900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 10.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 12.Heemskerk B, Liu K, Dudley ME, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19(5):496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19(3):320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1(2):123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallardo HF, Tan C, Ory D, Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90(3):952–957. [PubMed] [Google Scholar]
- 17.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160(9):4418–4426. [PubMed] [Google Scholar]
- 18.Fehniger TA, Suzuki K, Ponnappan A, et al. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193(2):219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5(5):627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- 20.Gade TP, Hassen W, Santos E, et al. Targeted elimination of prostate cancer by genetically directed human T lymphocytes. Cancer Res. 2005;65(19):9080–9088. doi: 10.1158/0008-5472.CAN-05-0436. [DOI] [PubMed] [Google Scholar]
- 21.Quintás-Cardama A, Yeh RK, Hollyman D, et al. Multifactorial optimization of gammaretroviral gene transfer into human T lymphocytes for clinical application. Hum Gene Ther. 2007;18(12):1253–1260. doi: 10.1089/hum.2007.088. [DOI] [PubMed] [Google Scholar]
- 22.Quintarelli C, Vera JF, Savoldo B, et al. Co-expression of cytokine and suicide genes to enhance the activity and safety of tumor-specific cytotoxic T lymphocytes. Blood. 2007;110(8):2793–2802. doi: 10.1182/blood-2007-02-072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res. 2007;13(18; pt 1):5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 24.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13(12):1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 25.Hsu C, Hughes MS, Zheng Z, Bray RB, Rosenberg SA, Morgan RA. Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine. J Immunol. 2005;175(11):7226–7234. doi: 10.4049/jimmunol.175.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soares MV, Borthwick NJ, Maini MK, Janossy G, Salmon M, Akbar AN. IL-7-dependent extrathymic expansion of CD45RA+ T cells enables preservation of a naive repertoire. J Immunol. 1998;161(11):5909–5917. [PubMed] [Google Scholar]
- 27.Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J Immunol. 2007;179(6):3596–3603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108(6):871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rufer N, Zippelius A, Batard P, et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood. 2003;102(5):1779–1787. doi: 10.1182/blood-2003-02-0420. [DOI] [PubMed] [Google Scholar]
- 31.Romero P, Zippelius A, Kurth I, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178(7):4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 32.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 33.Walker EB, Haley D, Petrausch U, et al. Phenotype and functional characterization of long-term gp100-specific memory CD8+ T cells in disease-free melanoma patients before and after boosting immunization. Clin Cancer Res. 2008;14(16):5270–5283. doi: 10.1158/1078-0432.CCR-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 35.Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL-7 receptor alpha expression by IL-7 and TCR signaling. J Immunol. 2008;180(8):5201–5210. doi: 10.4049/jimmunol.180.8.5201. [DOI] [PubMed] [Google Scholar]
- 36.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, et al. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med. 2009;206(4):849–866. doi: 10.1084/jem.20081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran KQ, Zhou J, Durflinger KH, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31(8):742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrari-Lacraz S, Chicheportiche R, Schneiter G, Molnarfi N, Villard J, Dayer JM. IL-21 promotes survival and maintains a naive phenotype in human CD4+ T lymphocytes. Int Immunol. 2008;20(8):1009–1018. doi: 10.1093/intimm/dxn059. [DOI] [PubMed] [Google Scholar]
- 43.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12(7):1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175(4):2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Khong HT, Dudley ME, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common gamma-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29(3):284–293. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 47.Kaka AS, Shaffer DR, Hartmeier R, et al. Genetic modification of T cells with IL-21 enhances antigen presentation and generation of central memory tumor-specific cytotoxic T-lymphocytes. J Immunother. 2009;32(7):726–736. doi: 10.1097/CJI.0b013e3181ad4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allard EL, Hardy MP, Leignadier J, et al. Overexpression of IL-21 promotes massive CD8+ memory T cell accumulation. Eur J Immunol. 2007;37(11):3069–3077. doi: 10.1002/eji.200637017. [DOI] [PubMed] [Google Scholar]
- 49.Vera JF, Hoyos V, Savoldo B, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponomarev V, Doubrovin M, Lyddane C, et al. Imaging TCR-dependent NFAT-mediated T-cell activation with positron emission tomography in vivo. Neoplasia. 2001;3(6):480–488. doi: 10.1038/sj.neo.7900204. [DOI] [PMC free article] [PubMed] [Google Scholar]