Abstract
S1P1 receptor expression is required for the egress of newly formed T cells from the thymus and exit of mature T and B cells from secondary lymphoid organs. In this study, we deleted the expression of the S1P1 receptor gene (S1pr1) in developing B cells in the bone marrow. Although B cell maturation within the bone marrow was largely normal in the B cell–specific S1pr1 knockout (B-S1pr1KO) mice, their newly generated immature B cells appeared in the blood at abnormally low numbers as compared with control mice. In the bone marrow of B-S1pr1KO mice, immature B cells in contact with the vascular compartment displayed increased apoptosis as compared with control mice. Forced expression of CD69, a negative regulator of S1P1 receptor expression, in developing bone marrow B cells also reduced the number of immature B cells in the blood. Attenuation of CXCR4 signaling, which is required for the proper retention of developing B cells in bone marrow, did not release immature B cells into the blood of B-S1pr1KO mice as effectively as in control mice. Our results indicate that the S1P1 receptor provides a signal necessary for the efficient transfer of newly generated immature B cells from the bone marrow to the blood.
The S1P1 receptor, which is among the most highly and ubiquitously expressed members of the large family of G protein–coupled receptors (Regard et al., 2008), provides basic cell functions that include survival, migration, adhesion, and proliferation (Sanchez and Hla, 2004; Alvarez et al., 2007; Kono et al., 2008). The receptor binds the lysophospholipid sphingosine-1-phosphate (S1P), triggering signaling cascades coupled to the Gi pathway.
The S1P1 receptor is one of five S1P receptors. Within the immune system, S1P receptor signaling has several roles in the regulation of innate and adaptive immune responses (Cyster, 2005; Rosen and Goetzl, 2005; Goetzl et al., 2008; Rivera et al., 2008). The S1P1 receptor has been shown to control lymphocyte egress from the thymus (Matloubian et al., 2004; Allende et al., 2008), spleen (Cinamon et al., 2004; Matloubian et al., 2004), and lymph nodes (Matloubian et al., 2004). The importance of the S1P1 receptor in this process was initially recognized by the demonstration that immunosuppressant compounds that trapped lymphocytes in lymph nodes and the thymus were ligands for multiple S1P receptors (Brinkmann et al., 2002; Mandala et al., 2002). It was later shown that S1P1 receptor–deficient lymphocytes were not competent to exit the thymus and lymph nodes, demonstrating the importance of the S1P1 receptor in mediating lymphocyte egress from these lymphoid tissues (Matloubian et al., 2004; Allende et al., 2008).
The lipid ligand S1P is ubiquitously synthesized but largely degraded in most tissues, resulting in low ligand levels in lymphoid tissues and higher concentrations in blood and lymph (Schwab et al., 2005). The S1P concentration differential is believed to drive lymphocyte egress from lymphoid tissues to blood and lymph via stimulation of the lymphocyte S1P1 receptor (Lo et al., 2005; Schwab et al., 2005; Pappu et al., 2007).
Although the S1P1 receptor has been shown to provide a mandatory egress signal for lymphocyte emigration from the thymus, lymph nodes, and spleen, a similar role for the S1P1 receptor in the transfer of newly formed, immature B cells from the bone marrow into the periphery has not been described, nor is this emigration process well understood. However, it is known that newly formed immature B cells transit from the bone marrow parenchyma through the endothelium and into the sinusoids, where they are temporarily retained until their release into the general circulation (Osmond and Batten, 1984; Nagasawa, 2006; Pereira et al., 2009). In this paper, we show that these immature B cells require the S1P1 receptor for their efficient appearance in the bone marrow vascular compartment and peripheral blood. Furthermore, the expression on bone marrow B cells of CD69, an S1P1 receptor–interacting protein that modifies thymic and lymph node egress of lymphocytes by lowering S1P1 receptor levels on their cell surface (Shiow et al., 2006), also negatively modulates the appearance of immature B cells in peripheral blood. These results provide new details about the process of immature B cell release from the bone marrow by identifying the S1P1 receptor as a key regulator of this process.
RESULTS
Expression of S1P receptors by bone marrow B cells
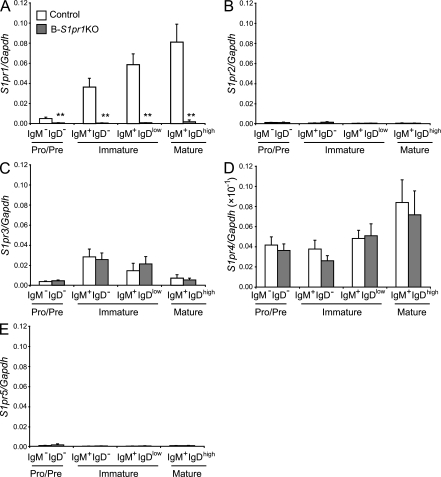
We determined the expression of the five members of the S1P receptor family in bone marrow B cells by real-time quantitative PCR (RT-qPCR). Bone marrow B cells were sorted into four populations: (1) B220+ IgM− IgD− (pro–/pre–); (2) B220+ IgM+ IgD− and (3) B220+ IgM+ IgDlow (immature); and (4) B220+ IgM+ IgDhigh (mature, recirculating) B cells. S1pr1 expression increased as differentiation progressed in bone marrow B cells, showing that S1pr1 is regulated during the development of bone marrow B cells (Fig. 1 A, control). S1pr3 and S1pr4 transcripts were also found on developing bone marrow B cells (Fig. 1, C and D), whereas S1pr2 and S1pr5 were expressed at very low levels (Fig. 1, B and E).
Figure 1.
Expression of S1P receptors in bone marrow B cell subpopulations. (A–E) mRNA expression for S1pr1 (A), S1pr2 (B), S1pr3 (C), S1pr4 (D), and S1pr5 (E) in sorted B cell subpopulations from control and B-S1pr1KO bone marrow was determined by real-time PCR. Bars represent mean values ± SD from three experiments (n = 3 mice per genotype). **, P < 0.01 (Student's t test).
Generation and characterization of B cell–specific S1pr1 KO (B-S1pr1KO) mice
To study the role of the S1P1 receptor in B cells, we deleted the S1pr1 gene specifically in B cells using the Cre/loxP system. We bred mice bearing the loxP-flanked S1pr1 allele (Allende et al., 2003) with mice carrying the Cre recombinase gene under the transcriptional control of the endogenous CD19 promoter (Rickert et al., 1997) to obtain mice homozygous for the floxed S1pr1 allele and carrying the Cre transgene, termed B-S1pr1KO mice. In the CD19-Cre mice, the Cre recombinase expression has been detected in pre–, immature, and mature B cells in bone marrow and the spleen (Schwenk et al., 1997). To determine the magnitude and specificity of the S1pr1 gene deletion in the B-S1pr1KO mice, we measured the levels of S1pr1 mRNA by RT-qPCR. Splenic B cells isolated from the B-S1pr1KO mice showed a substantial reduction of S1pr1 mRNA expression compared with those from control mice, whereas mRNA levels in the thymus and brain were unchanged, confirming the B cell specificity of the Cre-mediated deletion (unpublished data). To verify the onset of S1P1 receptor deletion during B cell development, we analyzed the mRNA levels in sorted bone marrow pro–/pre– (B220+ IgM− IgD−), immature (B220+ IgM+ IgD− and B220+ IgM+ IgDlow), and mature (B220+ IgM+ IgDhigh) B cell populations and found that S1pr1 expression was highly reduced in pro–/pre–, immature, and mature B cells in the B-S1pr1KO mice compared with control mice (Fig. 1 A), consistent with Cre-mediated S1pr1 deletion occurring by the pre–B cell stage in the B-S1pr1KO mice. There were no compensatory increases of S1pr2, 3, 4, or 5 transcript levels in the B-S1pr1KO bone marrow B cell populations (Fig. 1, B–E).
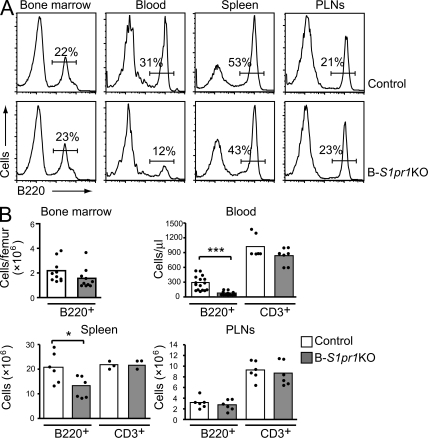
B220+ B cells were quantified in the bone marrow, blood, spleen, and peripheral LNs (PLNs) of the B-S1pr1KO mice relative to controls (Fig. 2, A and B). Overall, total B220+ cell numbers in B-S1pr1KO mice were normal in the bone marrow and PLNs but were significantly decreased in the spleen and peripheral blood. In blood, spleen, and PLNs, the number of CD3+ T lymphocytes was unchanged (Fig. 2 B).
Figure 2.
Reduced numbers of B cells in the blood and spleens of B-S1pr1KO mice. (A and B) Flow cytometry analysis of B220+ B cells and CD3+ T cells in peripheral tissues from 8–12-wk-old B-S1pr1KO and control mice. Lymphocytes from the bone marrow, peripheral blood, spleens, and PLNs from control and B-S1pr1KO mice were analyzed by flow cytometry using FITC-conjugated anti-B220 and PE-conjugated anti-CD3 antibodies. Results are shown as histograms (A) and the absolute number of cells counted (B) in each organ or per microliter of peripheral blood. In B, bars represent mean values of pooled data, and the closed circles are individual mice. Data are pooled from two to four experiments. *, P < 0.05; ***, P < 0.005 (Student's t test).
Numbers of immature and mature B cells are reduced in the blood and spleen of B-S1pr1KO mice
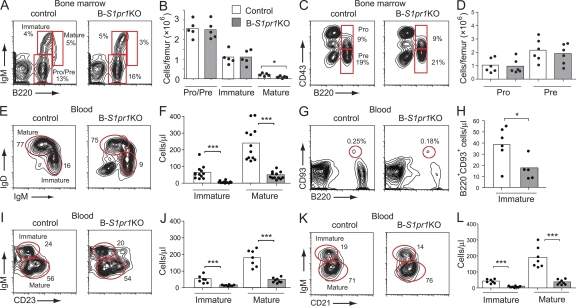
Analysis of the bone marrow lineage B cells in B-S1pr1KO mice revealed normal numbers of pro–/pre– and immature B cells, defined by expression of B220, IgM, and CD43, with a significant deficiency in the small population of mature, recirculating B cells (B220high IgM+; Fig. 3, A–D).
Figure 3.
Reduced numbers of mature and immature B cells in the blood of B-S1pr1KO mice. (A–D) Bone marrow B cell subpopulations from control and B-S1pr1KO mice were analyzed by flow cytometry using FITC-conjugated anti-B220, APC-conjugated anti-IgM, and PE-conjugated anti-CD43 antibodies. Pro–/pre–B cells were identified as B220low IgM−, immature B cells were identified as B220low IgM+, and mature B cells were identified as B220high IgM+. Pro–B cells were identified as B220low IgM− CD43+ and pre–B cells were identified as B220low IgM− CD43−. Results are shown as density plots (A and C) and as the absolute number of cells counted per femur (B and D). The percentage of cells in each gate is indicated on the plots. Bars represent mean values of pooled data from two experiments, and the closed circles are individual mice. *, P < 0.05 (Mann-Whitney test). (E–L) B cells from the peripheral blood of control and B-S1pr1KO mice were analyzed by flow cytometry. Immature B cells were identified as B220+ IgDlow IgMhigh (E and F), B220+ CD93+ (G and H), B220+ CD23low IgMhigh (I and J), and B220+ CD21low IgMhigh (K and L). Mature B cells were identified as B220+ IgDhigh IgMlow (E and F), B220+ CD23high IgMlow (I and J), and B220+ CD21high IgMlow (K and L). Results are expressed as a density plot (E, G, I, and K) and the absolute number of cells counted per microliter of peripheral blood (F, H, J, and L). *, P < 0.05; ***, P < 0.005 (Mann-Whitney test). Data in F, H, J, and L are mean values representative of three independent experiments.
The peripheral blood, which normally contains immature B cells (B220+ IgMhigh IgDlow) recently released from the bone marrow along with recirculating mature B cells (B220+ IgMlow IgDhigh), was significantly deficient in both of these B cell populations in B-S1pr1KO mice (Fig. 3, E and F). Similar results were obtained with B cell maturation markers CD93, CD23, and CD21 (Fig. 3, G–L). Immature B cell numbers in the blood of S1pr1loxP/+ mice were similar irrespective of the presence of the CD19-Cre transgene (Fig. S1), showing that Cre expression alone did not reduce the appearance of immature B cells in the blood.
In the spleen, the numbers of immature (B220+ IgMhigh IgDlow) and mature (B220+ IgMlow IgDhigh) B cells as defined by surface Ig markers were both reduced in B-S1pr1KO mice compared with controls (Fig. S2, A and B). Follicular B cells, which are characterized by B220+ CD21int CD23high expression (Fig. S2, C and D), were also found to be present in lower numbers in the spleens of B-S1pr1KO mice compared with controls. Immunostained frozen sections of spleen tissue confirmed the reduction of B cells relative to T cells in the follicles of B-S1pr1KO mice compared with controls (Fig. S2 E). The numbers of marginal zone B cells, defined as B220+ CD21high CD23low, were slightly but significantly reduced in the B-S1pr1KO mice compared with controls (Fig. S2 D). By histological evaluation, their absence was noted in the marginal zone regions (Fig. S2 F). Earlier studies using chimeras reconstituted with fetal liver from S1pr1-deficient embryos have shown that the S1P1 receptor on B cells was required for their proper positioning within the marginal zone, but did not note a decrease in total marginal zone B cell numbers (Cinamon et al., 2004). In agreement with the flow cytometric data (Fig. 2), the B cell compartment appeared relatively normal in PLNs from B-S1pr1KO mice by histological analysis (Fig. S2 G).
Apoptosis is elevated in S1pr1-deficient immature B cells in the bone marrow
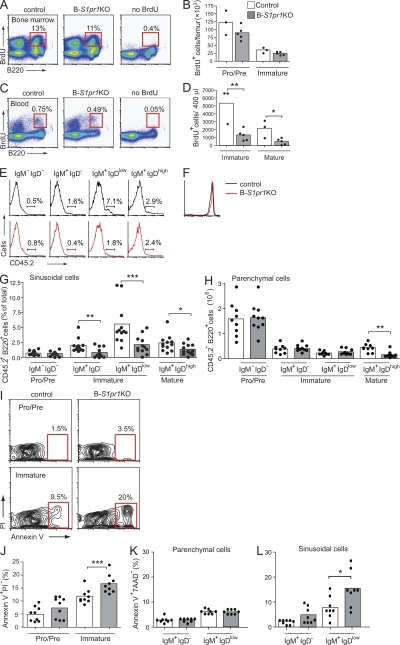
The deficiency of immature B cells in the blood and spleen could be explained by the inefficient release of newly formed B cells into the peripheral blood from their developmental niche in the bone marrow. To further evaluate this possibility, we labeled rapidly proliferating B cells with a pulse of BrdU and, after 48 h, determined the numbers of BrdU-labeled B cells in the bone marrow and peripheral blood (Fig. 4, A–D). Within the bone marrow, the fraction of B cells labeled with BrdU was similar in the B-S1pr1KO and control mice, with no significant labeling differences observed between pro–/pre– (B220low IgM−) and immature B cell populations (B220low IgM+; Fig. 4, A and B). However, the total number of BrdU+ B220+ cells in the blood from the B-S1pr1KO mice was significantly lower than in control mice, with lower numbers of BrdU+ immature (B220+ IgMhigh IgDlow) and mature (B220+ IgMlow IgDhigh) B cells observed (Fig. 4, C and D).
Figure 4.
Immature B cells in the bone marrow do not efficiently enter blood and have elevated apoptosis in the absence of S1P1 receptor. (A–D) Mice were pulsed with BrdU, and B cells from the bone marrow (A and B) and peripheral blood (C and D) of control and B-S1pr1KO mice were analyzed by flow cytometry using anti-B220, anti-IgD, and anti-IgM antibodies in combination with BrdU detection methodology, as described in Materials and methods. Results are shown as dot plots (A and C), and as absolute numbers of BrdU+ B220low IgM− (pro–/pre–) and B220low IgM+ (immature) B cells per femur (B) and BrdU+ B220+ IgDlow IgMhigh (immature) and B220+ IgDhigh IgMlow (mature) B cells per 400 µl of blood (D). The percentage of cells in each gate is indicated on the plots. Bars represent mean values, and the closed circles are individual mice. Data are representative of three experiments with three to five mice of each genotype per experiment. *, P < 0.05; **, P = 0.01 (Student's t test). (E and G) In vivo staining of bone marrow sinusoidal B cells. Mice were injected intravenously with PE-conjugated anti-CD45.2 antibody. After 2 min, the bone marrow cells and peripheral blood were obtained and stained with PerCP-conjugated anti-B220, APC-conjugated anti-IgM, and FITC-conjugated anti-IgD antibodies. Results are shown as histograms (E) and as the percentage of CD45.2-PE+ (sinusoidal cells; G) for pro–/pre– (B220+ IgD− IgM−), immature (B220+ IgM+ IgD− and B220+ IgM+ IgDlow), and mature (B220+ IgM+ IgDhigh) bone marrow B cells from control and B-S1pr1KO mice. On the histograms (E), the bars show the percentage of PE-CD45.2+ cells for each group. (F) Lymphocytes stained in the peripheral blood by injection of PE-conjugated anti-CD45.2 antibody, showing equal staining in control and B-S1pr1KO mice. (H) Cells that were negative for anti-CD45.2–PE antibody staining were considered as parenchymal cells. Results are shown as mean values of four independent experiments (n = 10–12 mice per genotype). *, P < 0.05; **, P < 0.01; ***, P < 0.005 (Mann-Whitney test). (I and J) Annexin V staining was determined on B cells from the bone marrow of control and B-S1pr1KO mice. Results are shown as density plots (I) and as the percentage (J) of annexin V+ PI− B220low IgM− (pro–/pre–) and B220low IgM+ (immature) B cells per femur. Pooled data are from five experiments (n = 9 for each genotype). ***, P < 0.005 (Mann-Whitney test). (K and L) Annexin V staining on sinusoidal and parenchymal immature B cells. Cells were labeled in vivo with PE-conjugated anti-CD45.2 antibody and gated as in E and H. Annexin V staining was determined on immature bone marrow B cells (B220+ 7-AAD− IgM+ IgD− and B220+ 7-AAD− IgM+ IgDlow) partitioned into parenchymal cells (CD45.2−; K) and sinusoidal cells (CD45.2+; L) from control and B-S1pr1KO mice. Pooled data are from three experiments (n = 8 for each genotype). *, P < 0.05 (Mann-Whitney test).
Upon leaving the bone marrow parenchyma, immature B cells pass through an endothelial barrier and enter the blood sinusoids, where they are retained before finally being released into the peripheral blood. To enumerate the bone marrow parenchymal and sinusoidal B cells, we used in vivo antibody labeling (Galkina et al., 2005; Reutershan et al., 2005; Pereira et al., 2009) that entails intravenous injection of PE-conjugated anti-CD45.2 antibody to selectively label hematopoietic cells in the vascular compartment. By applying this technique to B-S1pr1KO and control mice, we found that the pro–/pre–B cells were minimally labeled, as they were in the parenchyma, and shielded from the circulating antibody (Fig. 4, E–H). In contrast, lymphocytes in the peripheral blood were equally well labeled in both control and B-S1pr1KO mice (Fig. 4 F). A portion of each of the immature and mature bone marrow B cell pools was labeled by the injected anti-CD45.2 antibody, representing cells in the sinusoids. The bone marrow B cells that were not labeled by injected anti-CD45.2 antibody were considered to be parenchymal cells. We found that a significantly lower number of immature B cells (B220+ IgM+ IgD− and B220+ IgM+ IgDlow) was labeled by anti-CD45.2 antibody in the B-S1pr1KO mice compared with control mice, which is consistent with a defect in immature B cell egress from the bone marrow parenchyma into the sinusoids in S1P1 receptor–deficient mice (Fig. 4 G). It might have been anticipated that an inability of immature B cells to egress from the bone marrow into sinusoids would result in an increase in the number of total immature B cells in the parenchyma of the B-S1pr1KO mice. However, we found similar numbers of bone marrow immature B cells (B220+ IgM+ IgDlow) that were CD45.2− in the anti-CD45.2–injected B-S1pr1KO and control mice (Fig. 4 H).
Because we did not detect an accumulation of bone marrow immature cells in the parenchyma of B-S1pr1KO mice, we considered the possibility that cells inappropriately retained in bone marrow because of an exit defect might instead undergo cell death. We used annexin V staining to assess the level of apoptosis in the pro–/pre– and immature B cell populations in the bone marrow of B-S1pr1KO mice. Although the annexin V+ pro–/pre–B cell fraction was similar in B-S1pr1KO and control mice, the immature B cell population showed significantly higher levels of annexin V staining in B-S1pr1KO mice than in control mice, indicating an increased incidence of cell death (Fig. 4, I and J). The numbers of annexin V− immature B cells in total bone marrow and in the parenchymal fraction were similar in the B-S1pr1KO and control mice, showing that there was not a deficiency of viable, potentially egress-competent immature B cells because of the deletion of the S1P1 receptor (Fig. S3, A and B).
We next determined if the increased annexin V+ fraction of immature B cells was in the parenchymal or sinusoidal cell pools by first labeling cells in the vascular compartment by injection of mice with anti-CD45.2 antibody. There was no difference between B-S1pr1KO and control mice in the annexin V staining of the immature cells that were shielded from the anti-CD45.2 antibody (parenchymal cells; Fig. 4 K). However, there was a significantly higher percentage of annexin V+ immature B cells (IgM+ IgDlow) labeled by anti-CD45.2 antibody (sinusoidal cells) in B-S1pr1KO mice compared with control mice (Fig. 4 L). These results indicate that S1pr1-deficient immature bone marrow B cells, in contact with the vascular compartment, undergo elevated apoptosis.
S1P1 receptor deficiency does not alter the migration of bone marrow B cells to S1P
S1P is a chemotactic factor for some B cell types (Cinamon et al., 2004; Rubtsov et al., 2005; Kabashima et al., 2006; Cinamon et al., 2008; Rubtsov et al., 2008) and might directly stimulate migration of immature B cells from bone marrow by its relatively high levels in the blood. This possibility prompted us to determine the potency of S1P as a chemotactic factor for bone marrow B cells from control and B-S1pr1KO mice in an in vitro chemotaxis assay. Of pro–/pre–, immature, and mature B cells from control mice, only immature B cells showed a dose response toward S1P. However, this migration response was not significantly different from that of bone marrow B cell populations from the B-S1pr1KO mice (Fig. S4). SDF-1 stimulated a migration response in bone marrow B cells that was similar in both types of mice (Fig. S4).
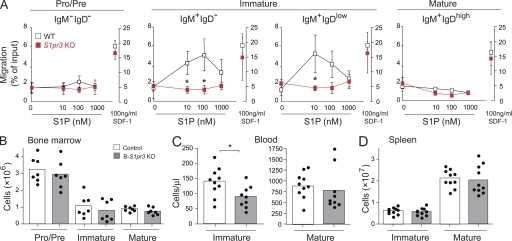
The S1P3 receptor has been shown to play a role in S1P-directed chemotaxis for splenic marginal zone B cells (Cinamon et al., 2004). Thus, we tested the ability of S1P3 receptor–deficient bone marrow B cells to migrate toward S1P. Interestingly, S1pr3 KO bone marrow immature B cells migrated significantly less well than WT cells to increasing S1P concentrations (Fig. 5 A). We next determined the number of immature and mature B cells in the blood and spleen in these KO mice to assess a potential bone marrow exit defect. In blood, there was a significant decrease in immature B cells compared with WT mice (Fig. 5 C), but this decrease was much less profound than that observed in the blood of B-S1pr1KO mice (Fig. 3 F). Bone marrow and splenic B cells in S1pr3 KO mice did not show significant changes compared with WT (Fig. 5, B and D). These data indicate that although the S1P3 receptor is more potent than the S1P1 receptor in mediating an in vitro migration response of immature bone marrow B cells to S1P, the egress defect of immature bone marrow B cells was much more pronounced in B-S1pr1KO than in S1pr3 KO mice.
Figure 5.
Migration of bone marrow B cells toward S1P is mediated by the S1P3 receptor. (A) Total bone marrow cells were added to a Transwell insert and allowed to respond to increasing concentrations of S1P or to 100 ng/ml SDF-1 in the lower well. Percentages of the input that were found in the lower well after a 3-h incubation with S1P (left axis) or with SDF-1 (right axis) were plotted for pro–/pre– (B220+ IgD− IgM−), immature (B220+ IgM+ IgD− and B220+ IgM+ IgDlow), and mature (B220+ IgM+ IgDhigh) B cells. Data are presented as mean values ± SD (n = 3 for each genotype) and are representative of three independent experiments. *, P < 0.05. (B–D) Distribution of B cells in S1pr3 KO mice. B cell subpopulations from the bone marrow (n = 7 per genotype; B), blood (n = 10; C), and spleen (n = 10; D) from WT and S1pr3 KO mice were analyzed by flow cytometry using PE-Cy7–conjugated anti-B220, APC-conjugated anti-IgM, and FITC-conjugated anti-IgD antibodies. Results are pooled data from three independent experiments. *, P < 0.05.
Because adhesion properties of mature B cells can be regulated by S1P1 receptor expression (Halin et al., 2005), we determined the cell-surface expression of CD49d (integrin α4; α subunit of VLA-4), CD49b (integrin α2; α subunit of VLA-2), CD11a (integrin αL; α subunit of LFA-1), and CD62L (L-selectin) on bone marrow B cells, but did not find a difference in the expression of these adhesion molecules between B-S1pr1KO and control mice (unpublished data).
CD69 overexpression reduces the number of immature B cells in blood
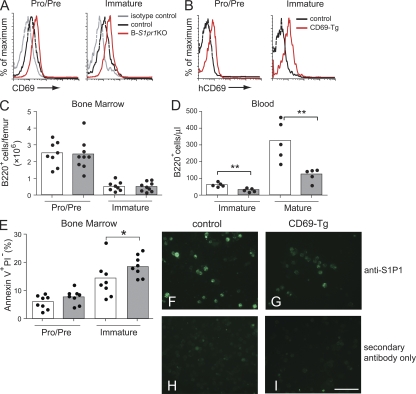
CD69, a C-type lectin, was up-regulated on the surface of pro–/pre– and immature bone marrow B cells in B-S1pr1KO mice compared with control mice (Fig. 6 A), similar to what has previously been described for thymocytes and for mature T and B cells when S1pr1 was deleted (Matloubian et al., 2004). In thymocytes, forced overexpression of CD69 blocked egress of maturing T cells from thymus (Lauzurica et al., 2000; Feng et al., 2002; Nakayama et al., 2002). To determine if CD69 transgenic expression by developing bone marrow B cells also impedes their peripheral blood expression, we established a transgenic mouse line that expressed human CD69 in bone marrow B cells after onset of CD19-Cre expression during B cell development (Fig. 6 B and Fig. S5 A).
Figure 6.
CD69 expression in bone marrow B cells modulates the appearance of immature B cells in peripheral blood. (A) CD69 expression on bone marrow B cells. Results are shown as anti-CD69 fluorescence intensity for cells from control and B-S1pr1KO mice compared with the isotype control. Representative results are from nine independent experiments. (B) Expression of the human CD69 transgene on bone marrow total B cells in control and transgenic (CD69-Tg) mice. B220+ bone marrow cells were analyzed for their expression of human CD69 by flow cytometry. (C and D) Distribution of B220low IgM− (pro–/pre–) and B220low IgM+ (immature) B cells in the bone marrow (C) and of B220+ IgDlow IgM+ (immature) and B220+ IgDhigh IgMlow (mature) B cells in the peripheral blood (D) of control and CD69 transgenic mice. Bars represent mean values, and the closed circles are individual mice. Data represent pooled results from three experiments. **, P < 0.01 (Mann-Whitney test). (E) Annexin V staining on B cells from the bone marrow of control and CD69 transgenic mice. Results are shown as the percentage of annexin V+ PI− B220low IgM− (pro–/pre–) and annexin V+ PI− B220low IgM+ (immature) B cells per femur and represent pooled data from four independent experiments (n = 8). *, P < 0.05 (Mann-Whitney test). (F–I) Expression of S1P1 receptor on IgM+ bone marrow B cells of control and transgenic CD69 mice. IgM+ cells were magnetically sorted from total bone marrow cells, fixed, permeabilized, and stained in suspension using a rabbit anti-S1P1 receptor antibody. After incubation with Alexa Fluor 488–conjugated anti–rabbit IgG, the cells were attached to a slide using a cytocentrifuge and visualized by fluorescence microscopy. Bar, 50 µm.
Transgenic expression of CD69 had no discernible effect on the number of bone marrow lineage B cells (Fig. 6 C). However, these transgenic mice showed a decrease in the number of immature and mature B cells in peripheral blood compared with control mice (Fig. 6 D), which is similar to the observed phenotype for the B-S1pr1KO mice (Fig. 2) and consistent with a bone marrow B cell exit defect. We also detected significantly higher levels of annexin V staining in immature B cell populations in the bone marrow of the CD69 transgenic mice (Fig. 6 E), suggestive of higher levels of cell death in a manner similar to the S1P1 receptor–deficient bone marrow B cells.
Previous studies have shown that CD69 forms a complex with the S1P1 receptor and that overexpression of CD69 can down-modulate the surface expression of the S1P1 receptor, thereby limiting its signaling activity (Shiow et al., 2006). We were unable to determine the S1P1 receptor expression level on the surface of B cells of the CD69 transgenic mice, because there is no antibody available that recognizes an extracellular epitope of the S1P1 receptor on mouse B cells (Lo et al., 2005). However, immunostaining with an antibody that recognizes an intracellular epitope of the S1P1 receptor (Sinha et al., 2009) indicated a reduced level of S1P1 receptor expression on IgM+ bone marrow cells of the CD69 transgenic mice compared with those of control mice (Fig. 6, F and G). To confirm that CD69 overexpression negatively regulates S1P1 receptor expression on the cell surface, we also examined S1P1 receptor cellular localization when it was coexpressed with human CD69 in HEK293 cells. When we expressed CD69 in wild-type HEK293 cells, CD69 was localized intracellularly in vesicles and on the plasma membrane (Fig. S5 B). When HEK293 cells stably expressing S1P1-GFP (Liu et al., 1999) were transfected with CD69, S1P1-GFP expression decreased on the plasma membrane and some colocalized with CD69 in intracellular compartments (Fig. S5 C). These data confirm that S1P1 receptor expression on the plasma membrane is down-modulated by CD69 expression.
CXCR4 antagonism does not stimulate the release of developing B cells from bone marrow into the blood of B-S1pr1KO mice
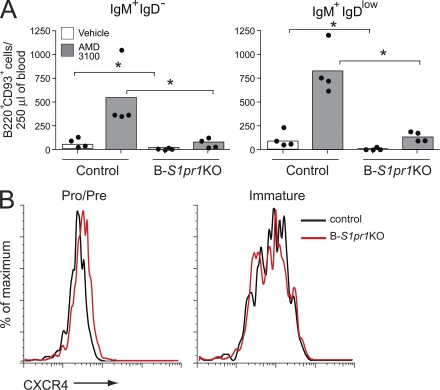
Interaction of the chemokine receptor CXCR4 with its ligand SDF-1 is required for the retention of developing B cells in the bone marrow parenchyma (Nagasawa et al., 1996; Tachibana et al., 1998; Zou et al., 1998; Ma et al., 1999; Nie et al., 2004; Ueda et al., 2004; Pereira et al., 2009). When control mice were treated with AMD3100, a potent CXCR4 antagonist (Broxmeyer et al., 2005), immature bone marrow B cells (B220+ CD93+ IgM+ IgD− and B220+ CD93+ IgM+ IgDlow) were released into peripheral blood (Fig. 7 A). In contrast, the release of immature B cells into blood was significantly lower in B-S1pr1KO mice after antagonist treatment (Fig. 7 A). We did not find an increase of these cells in the spleens in either control or B-S1pr1KO mice after antagonist treatment, ruling out that these immature B cells might be trapped in other lymphoid organs (unpublished data). The cell-surface expression of CXCR4 was similar on bone marrow B cells from control and B-S1pr1KO mice (Fig. 7 B). These results indicate that the S1P1 receptor provides a necessary signal for B cell release from the bone marrow when CXCR4 signaling is attenuated.
Figure 7.
Impaired release of immature bone marrow B cells by CXCR4 antagonism in the absence of S1P1 receptor. (A) Mice were injected with the CXCR4 antagonist AMD3100 or vehicle alone, and bone marrow and blood were collected after 90 min. Immature bone marrow B cells in the blood (B220+ CD93+) were gated as IgM+ IgD− and IgM+ IgDlow, and quantified from control and B-S1pr1KO mice. Results are shown as absolute numbers in 250 µl of blood. Bars represent mean values, and the closed circles are individual mice. Data are representative of three experiments. *, P < 0.05 (Mann-Whitney test). (B) CXCR4 expression on pro–/pre– (B220low IgM−) and immature (B220low IgM+) bone marrow B cells.
DISCUSSION
B cells arise from pluripotent hematopoietic stem cells through a series of developmental stages and selection steps in the bone marrow (Hardy and Hayakawa, 2001; Carsetti et al., 2004). Niches within the parenchymal extravascular spaces in the bone marrow populated by stromal cells supply factors to support the development and retention of the B cell precursors as they differentiate. Ultimately, the immature cells are released into the peripheral blood circulation to gain access to the spleen, where they reach maturity, whereas some immature B cells recirculate back and mature in the bone marrow (Cariappa et al., 2007; Lindsley et al., 2007).
In this paper, we show that S1P1 receptor expression on developing bone marrow B cells is required for their efficient appearance in the sinusoidal blood compartment. Without the signal generated by the S1P1 receptor, immature cells appear to undergo increased apoptosis in the bone marrow while in contact with the vascular compartment. We suggest that the increased cell death observed in S1P1 receptor–deficient mice may be a consequence of the inappropriate retention of the cells within the bone marrow. Developing B cells are known to be exquisitely sensitive to apoptosis (Griffiths et al., 1994). The immature B cells lacking the S1P1 receptor may be unable to completely transmigrate across the endothelium barrier separating the parenchymal and sinusoidal spaces, or else they may not be released from sinusoids after crossing the endothelium. In either case, inappropriately retained, apoptotic B cells would be in contact with the vascular compartment. Their apoptosis precludes a large accumulation of trapped immature cells or, potentially, their further maturation within the bone marrow (Cariappa et al., 2007; Lindsley et al., 2007).
Unlike the effect of the KO of the S1P1 receptor on developing T cells, which caused a nearly complete block in their egress from the thymus and a severe deficiency of mature T cells in the periphery (Allende et al., 2004; Matloubian et al., 2004), in the present study mature B cells were present in the B-S1pr1KO mice, although at reduced numbers in the spleen and at near normal numbers in lymph nodes. Although this finding may have been, in part, the result of incomplete deletion of the S1P1 receptor by the CD19-Cre transgene in developing B cells, chimeras generated by transplantation of embryonic liver from S1pr1-null embryos also exhibited reduced numbers of B cells in the spleen and peripheral blood, and near normal numbers in lymph nodes (Matloubian et al., 2004). These findings suggest that the S1P1 receptor requirement for bone marrow egress of B cells is not an absolute one, and that some S1P1 receptor–deficient B cells may escape the bone marrow. Secondary distortion of the peripheral mature B cell compartment in the B-S1pr1KO mice may be attributable to homeostatic mechanisms, which sense B cell deficiency (Cabatingan et al., 2002), and the trapping of egress-incompetent S1P1 receptor–deficient B cells in lymph nodes and the spleen (Matloubian et al., 2004).
The precise mechanism through which the S1P1 receptor controls egress from lymphoid organs has been difficult to pinpoint. Directed lymphocyte chemotaxis to higher concentrations of S1P in blood and lymph has been invoked as one possible means for the S1P1 receptor dependence of egress (Matloubian et al., 2004); however, we found that in the absence of the S1P1 receptor, bone marrow B cells migrated toward S1P no differently than control cells. Instead, we and others (Donovan et al., 2010) found that the S1P-directed migration response of immature bone marrow B cells appeared to be largely mediated by the S1P3 receptor. Even so, B cell emigration out of bone marrow in S1pr3 KO mice was only slightly affected, based on the number of immature B cells in the blood, when compared with the B-S1pr1KO mice. These data suggest that an S1P-directed chemotaxis mechanism, which can be recaptured by the in vitro migration assay, may not be a critical feature of B cell egress from bone marrow. Similarly, a recent study using in vivo imaging along with in vitro analysis of chemotaxis has argued that B cell egress from lymph nodes occurs by a mechanism that is distinct from S1P-mediated chemotaxis (Sinha et al., 2009). In this study, and in the case of T cells egressing from lymph nodes, a multistep process that includes an obligatory S1P1 receptor–mediated step for egress has been proposed (Pham et al., 2008). In the bone marrow, the S1P1 receptor on immature B cells may be critical for their transmigration through or release from the endothelium.
SDF-1 stimulates the Gi-linked G protein–coupled receptor CXCR4 on developing B cells to mediate their retention within the bone marrow parenchyma (Nie et al., 2004). We found that disruption of the SDF-1–CXCR4 axis by the antagonist AMD3100 elicited the release of B cell precursors from bone marrow in control mice but not in B-S1pr1KO mice, demonstrating that release of cells to the blood after attenuation of the SDF-1–CXCR4 signal is also dependent on the S1P1 receptor. These results suggest the possibility that an interplay between CXCR4 and S1P1 receptor signals may dictate bone marrow retention or release of the immature B cells into the blood. An interaction between CXCR4-mediated retention and S1P1 receptor–mediated blood release signals has been proposed for the regulation of egress of plasma cells from the spleen (Kabashima et al., 2006). Interestingly, the synthetic S1P receptor ligand FTY720 modifies CXCR4 signaling on CD34+ hematopoietic stem and progenitor cells (HSPCs; Kimura et al., 2004; Walter et al., 2007). In addition, a direct interaction between the S1P1 and CXCR4 receptor signaling pathways has been described in HSPCs overexpressing the S1P1 receptor, which inhibits their SDF-1–CXCR4–dependent migration and homing to the bone marrow in vivo by causing down-modulation of CXCR4 expression and signaling (Ryser et al., 2008). In the B-S1pr1KO mice examined in this study, however, we were unable to find that either CXCR4 expression or chemotaxis to SDF-1 was altered in bone marrow B cells.
In this study, we showed that forced expression of CD69 in bone marrow precursor B cells led to an increase in apoptotic frequency of immature bone marrow B cells and decreased numbers of immature B cells in blood, copying the phenotype of the B-S1pr1KO mice. CD69 expression was previously shown to impair egress of maturing T cells from the thymus (Lauzurica et al., 2000; Feng et al., 2002; Nakayama et al., 2002), and B and T cells from lymph nodes (Shiow et al., 2006), by lowering the surface expression of S1P1 receptor through protein–protein complex formation (Shiow et al., 2006). We found that CD69 overexpression decreased S1P1 receptor expression in bone marrow B cells of the CD69 transgenic mice, and that CD69 and S1P1 receptor colocalized in intracellular compartments of HEK293 cells. The cellular trafficking pathways involved in the regulation of the S1P1 receptor by CD69 have not yet been determined.
The S1P1 receptor requirement for bone marrow exit extends to other cell types. Osteoclast precursors, which differentiate from the myeloid lineage in the bone marrow, rely on the S1P1 receptor to move from the bone surface into the peripheral blood (Ishii et al., 2009). An S1P1 receptor requirement has also been suggested for the regulation of HSPC bone marrow retention and release, and their egress from peripheral tissues (Massberg et al., 2007). It will be of interest, and of potential clinical importance, to determine if a dependence on S1P1 receptor signaling extends to other bone marrow cell populations for the control of their emergence in blood as they traffic to peripheral tissues.
MATERIALS AND METHODS
Mice
B-S1pr1KO mice.
We previously generated the S1pr1loxP/loxP mice (Allende et al., 2003; Allende et al., 2004) carrying the S1pr1 gene with loxP sequences flanking exon 2, which contains the entire coding region. To specifically delete the S1P1 receptor from B cells, S1pr1loxP/loxP mice were bred with a CD19-driven Cre transgenic mouse strain (CD19-Cre; Rickert et al., 1997). S1pr1loxP/loxP mice carrying one allele of the CD19-Cre gene (B-S1pr1KO) and littermate S1pr1loxP/loxP mice (used as controls) were genotyped by PCR using Cre-specific primers (cre1, 5′-GCCTGCATTACCGGTCGATGC-3′; cre2, 5′-CAGGGTGTTATAAGCAATCCC-3′). For some experiments, S1pr1loxP/+ mice were used as controls.
B cell–specific CD69 transgenic mice.
The human CD69 cDNA was subcloned downstream of the CAG promoter sequence from the pDRIVE-CAG plasmid (InvivoGen). A cassette containing a loxP-STOP-loxP fragment from pBS302 (Invitrogen) was inserted between the CD69 coding region and the promoter. A linear DNA fragment was prepared and microinjected into the pronucleus of embryos. Potential transgenic mice were screened for the presence of the human CD69 transgene by PCR analysis of DNA from tail biopsies (forward primer, 5′-GTGGACAAGAAAATGATGCCA-3′; reverse primer, 5′-TGTAACGTTGAACCAGTTGTTAAA-3′) and were bred with mice carrying one allele of the CD19-Cre gene. Offspring that were positive for the human CD69 and Cre transgenes were tested for the expression of the human CD69 protein on B cells by flow cytometry using an FITC-conjugated anti–human CD69 antibody (BD). Mice lacking the Cre or the CD69 transgene were used as controls.
S1pr3 KO mice.
S1pr3 KO mice were described previously (Kono et al., 2004) and have been backcrossed seven times to C57BL/6 mice.
All animal procedures were approved by the National Institute of Diabetes and Digestives and Kidney Diseases Animal Care and Use Committee and were performed in accordance with National Institutes of Heath guidelines.
BrdU labeling
For BrdU labeling, mice were injected intraperitoneally once with 200 µl of a 10-mg/ml BrdU solution (BD) and analyzed 48 h later. BrdU+ cells were detected using the FITC-BrdU Flow kit (BD) by flow cytometry.
AMD3100 treatment
B-S1pr1KO mice and littermate controls were injected subcutaneously with 100 µl AMD3100 in PBS (5 mg/kg of body weight; Sigma-Aldrich) or PBS alone. After 90 min, cells from the bone marrow, blood, and spleen were obtained and analyzed by flow cytometry.
Detection of bone marrow sinusoidal and parenchymal B cells
To detect bone marrow sinusoidal and parenchymal B cells, we used an in vivo labeling procedure (Galkina et al., 2005; Reutershan et al., 2005) described by Pereira et al. (2009). B-S1pr1KO mice and littermate controls were injected intravenously with 1 µg of PE-conjugated anti-CD45.2 antibody (clone 104; eBioscience) in 100 µl PBS. After 2 min, the mice were euthanized and the cells were collected from bone marrow and peripheral blood, and then analyzed by flow cytometry.
Lymphocyte preparation
Lymphocytes from the bone marrow, spleen, and PLNs (lingual, axillary, brachial, and inguinal) from mice were dissected and mechanically disaggregated. Single-cell suspensions were obtained using a 40-µm cell strainer. Peripheral blood was obtained by cardiac puncture. Red blood cells were removed from blood samples and splenic single-cell suspensions by NH4Cl lysis. In some experiments, B cells were isolated from the spleen and bone marrow using anti-B220 magnetic microbeads (Miltenyi Biotec) for RNA analysis. Absolute lymphocyte counts were determined by flow cytometry using counting beads (CALTAG; Invitrogen).
Flow cytometry and cell sorting
Cells were diluted in 1% BSA-PBS and incubated with anti-FcγR antibody (BD) to block binding of conjugated antibodies to FcγR. Anti–mouse B220 (FITC and PerCP conjugated), anti-IgM (allophycocyanin [APC] conjugated), anti-IgD (FITC and PE conjugated), anti-CD43 (PE conjugated), anti-CD69 (FITC conjugated), anti-CD1d (FITC conjugated), anti-CD23 (FITC conjugated), anti-CD21 (PE conjugated), anti-CXCR4 (FITC conjugated), anti-CD3 (PE conjugated), anti-CD93 (biotin conjugated; 493 mAb; Rolink et al., 1998), anti-CD49b (FITC conjugated), anti-CD62L (FITC conjugated), anti-CD49d (PE conjugated), and anti-CD11a (PE conjugated) antibodies and PE-conjugated streptavidin were purchased from BD. After cells were labeled with the appropriate antibodies for 30 min on ice and fixed in 1% paraformaldehyde in PBS, they were subjected to flow cytometry on a FACSCalibur or LSR II (both from BD). Data were analyzed using FlowJo software (Tree Star, Inc.).
Bone marrow cells were labeled with anti-B220 (PE-Cy7 conjugated), anti-IgM (APC conjugated), and anti-IgD (FITC conjugated; BD) and sorted into four populations (B220+ IgM− IgD−, B220+ IgM+ IgD−, B220+ IgM+ IgDlow, and B220+ IgM+ IgDhigh) using a cell sorter (FACSAria II; BD).
Annexin V labeling of apoptotic cells
Bone marrow cells were stained with anti-B220 and anti-IgM antibodies, washed, and incubated with propidium iodide (PI) or 7-aminoactinomycin D (7-AAD) and annexin V–FITC (BD) for 15 min at room temperature. Cells were analyzed by flow cytometry, and those found positive for annexin V and negative for PI (or 7-AAD) were quantified.
Immunostaining
12-µm frozen sections of spleens and PLNs were fixed in cold acetone, stained with specific antibodies followed by fluorescently labeled secondary antibodies, and mounted in Vectashield medium (Vector Laboratories). Hamster anti–mouse CD3 and rat anti–mouse B220 antibodies were obtained from BD. FITC-conjugated anti–hamster IgG and Alexa Fluor 594–conjugated anti–rat IgG antibodies (Invitrogen) were used as secondary antibodies. The stained sections were visualized using a fluorescence microscope (DMRXA2; Leica) with 5 and 20× objectives. The images were acquired with a charge-coupled device camera (IEEE1394; Hamamatsu Photonics) using Openlab software (Improvision Inc.)
For immunostaining of the S1P1 receptor in mouse bone marrow cells, IgM+ cells were isolated using autoMACS columns (Miltenyi Biotec). The IgM+ bone marrow cells were fixed in suspension with methanol at −20°C for 20 min, permeabilized with 50 µg/ml digitonin (Sigma-Aldrich) in PBS for 5 min, and incubated with an anti-S1P1 receptor antibody (Santa Cruz Biotechnology, Inc.) diluted in blocking buffer (3% BSA, 0.1% saponin [Sigma-Aldrich] in PBS) for 1 h. Cells were washed with blocking buffer and incubated with a secondary Alexa Fluor 488–conjugated anti–rabbit IgG (Invitrogen). After washing, cells were resuspended in PBS and spun onto a slide using a cytocentrifuge (Shandon Cytospin 3; Thermo Fisher Scientific). Slides were mounted in Vectashield medium and visualized using a fluorescence microscope (DMLD; Leica) with a 40× oil immersion objective. Images were acquired using QCapture software (QImaging Corporation). Cells incubated with only the secondary antibody were used as a negative control.
Chemotaxis assay
The response of bone marrow B cells toward S1P was studied using 6.5-mm Transwell inserts with a 5-µm pore size (Corning). A bone marrow cell suspension from femurs and tibias (100 µl at 107 cells/ml) in RPMI 1640 plus 0.4 mg/ml fatty acid–free (FAF)–BSA (medium + FAF-BSA; Sigma-Aldrich) was added to each insert in a well containing 600 µl of a solution of S1P (Avanti Polar Lipids, Inc.) or SDF-1 (R&D Systems) prepared in medium + FAF-BSA. Wells containing medium + FAF-BSA without S1P or SDF-1 were used as controls. After 2 h at 37°C, cells in the bottom of the wells were harvested, counted, and analyzed by flow cytometry.
RT-qPCR
Total RNA was purified from sorted bone marrow B cells using TRIzol (Invitrogen). The mRNA expression levels of mouse S1pr1, S1pr2, S1pr3, S1pr4, and S1pr5 genes were determined by RT-qPCR using Assay-on-Demand probes and primers (Applied Biosystems) on a Sequence Detection System (ABI Prism 7700; Applied Biosystems). GAPDH mRNA level was used as an internal control.
Confocal microscopy
HEK293 cells stably expressing human S1pr1-GFP (Liu et al., 1999) were obtained from T. Hla (University of Connecticut Health Center, Farmington, CT). A plasmid containing human CD69 driven by a CMV promoter was obtained from Origene.
Transient transfections were performed using the TransIT-293 reagent (Mirus Bio) according to the manufacturer's instructions. Transfected cells were assayed 16–24 h after transfection. For immunofluorescence staining, cells were fixed with methanol for 5 min at room temperature and washed with PBS containing 0.1% Triton X-100. The cells were then incubated with mouse anti–human CD69 (AbD Serotec) antibody followed by Alexa Fluor 594–conjugated goat anti–mouse IgG (Invitrogen) as secondary antibody.
Confocal laser scanning microscopy analysis was performed with a DuoScan system (LSM 5 LIVE; Carl Zeiss, Inc.) using a 63 × 1.4 NA oil immersion objective. Excitation and filters were as follows: GFP, laser 489-nm line for excitation and emission collected with LP 495-nm filter; Alexa Fluor 594, laser 561-nm line for excitation and emission collected with LP 580-nm filter. Images were acquired using ZEN 2007 software (Carl Zeiss, Inc.).
Statistical analysis
Statistical significance was determined using the Mann-Whitney or Student's t test. In all cases, P < 0.05 was considered statistically significant.
Online supplemental material
Fig. S1 shows that CD19-Cre expression alone does not affect the numbers of immature and mature B cells in blood. Fig. S2 compares the numbers of mature and immature B cells in spleens of control and B-S1pr1KO mice. Fig. S3 shows the number of viable bone marrow B cell populations in B-S1pr1KO mice. Fig. S4 examines the migration of bone marrow B cells of control and B-S1pr1KO mice toward S1P. Fig. S5 illustrates that CD69 down-regulates S1P1 receptor surface expression in HEK293 cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092210/DC1.
Acknowledgments
This research was supported by the Intramural Research Program, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 7-AAD
- 7-aminoactinomycin D
- APC
- allophycocyanin
- B-S1pr1KO
- B cell–specific S1pr1 KO
- HSPC
- hematopoietic stem and progenitor cell
- PI
- propidium iodide
- PLN
- peripheral LN
- RT-qPCR
- real-time quantitative PCR
- S1P
- sphingosine-1-phosphate
References
- Allende M.L., Yamashita T., Proia R.L. 2003. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 102:3665–3667 10.1182/blood-2003-02-0460 [DOI] [PubMed] [Google Scholar]
- Allende M.L., Dreier J.L., Mandala S., Proia R.L. 2004. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279:15396–15401 10.1074/jbc.M314291200 [DOI] [PubMed] [Google Scholar]
- Allende M.L., Zhou D., Kalkofen D.N., Benhamed S., Tuymetova G., Borowski C., Bendelac A., Proia R.L. 2008. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. 22:307–315 10.1096/fj.07-9087com [DOI] [PubMed] [Google Scholar]
- Alvarez S.E., Milstien S., Spiegel S. 2007. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18:300–307 10.1016/j.tem.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Davis M.D., Heise C.E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277:21453–21457 10.1074/jbc.C200176200 [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., Hangoc G., Cooper S., Plett P.A., Liles W.C., Li X., Graham-Evans B., Campbell T.B., et al. 2005. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201:1307–1318 10.1084/jem.20041385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabatingan M.S., Schmidt M.R., Sen R., Woodland R.T. 2002. Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J. Immunol. 169:6795–6805 [DOI] [PubMed] [Google Scholar]
- Cariappa A., Chase C., Liu H., Russell P., Pillai S. 2007. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 109:2339–2345 10.1182/blood-2006-05-021089 [DOI] [PubMed] [Google Scholar]
- Carsetti R., Rosado M.M., Wardmann H. 2004. Peripheral development of B cells in mouse and man. Immunol. Rev. 197:179–191 10.1111/j.0105-2896.2004.0109.x [DOI] [PubMed] [Google Scholar]
- Cinamon G., Matloubian M., Lesneski M.J., Xu Y., Low C., Lu T., Proia R.L., Cyster J.G. 2004. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5:713–720 10.1038/ni1083 [DOI] [PubMed] [Google Scholar]
- Cinamon G., Zachariah M.A., Lam O.M., Foss F.W., Jr., Cyster J.G. 2008. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 9:54–62 10.1038/ni1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J.G. 2005. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23:127–159 10.1146/annurev.immunol.23.021704.115628 [DOI] [PubMed] [Google Scholar]
- Donovan E.E., Pelanda R., Torres R.M. 2010. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur. J. Immunol. 40:688–698 10.1002/eji.200939858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Woodside K.J., Vance B.A., El-Khoury D., Canelles M., Lee J., Gress R., Fowlkes B.J., Shores E.W., Love P.E. 2002. A potential role for CD69 in thymocyte emigration. Int. Immunol. 14:535–544 10.1093/intimm/dxf020 [DOI] [PubMed] [Google Scholar]
- Galkina E., Thatte J., Dabak V., Williams M.B., Ley K., Braciale T.J. 2005. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 115:3473–3483 10.1172/JCI24482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E.J., Liao J.J., Huang M.C. 2008. Regulation of the roles of sphingosine 1-phosphate and its type 1 G protein-coupled receptor in T cell immunity and autoimmunity. Biochim. Biophys. Acta. 1781:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S.D., Goodhead D.T., Marsden S.J., Wright E.G., Krajewski S., Reed J.C., Korsmeyer S.J., Greaves M. 1994. Interleukin 7–dependent B lymphocyte precursor cells are ultrasensitive to apoptosis. J. Exp. Med. 179:1789–1797 10.1084/jem.179.6.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halin C., Scimone M.L., Bonasio R., Gauguet J.M., Mempel T.R., Quackenbush E., Proia R.L., Mandala S., von Andrian U.H. 2005. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 106:1314–1322 10.1182/blood-2004-09-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa K. 2001. B cell development pathways. Annu. Rev. Immunol. 19:595–621 10.1146/annurev.immunol.19.1.595 [DOI] [PubMed] [Google Scholar]
- Ishii M., Egen J.G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J., Proia R.L., Germain R.N. 2009. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 458:524–528 10.1038/nature07713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K., Haynes N.M., Xu Y., Nutt S.L., Allende M.L., Proia R.L., Cyster J.G. 2006. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J. Exp. Med. 203:2683–2690 10.1084/jem.20061289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Boehmler A.M., Seitz G., Kuçi S., Wiesner T., Brinkmann V., Kanz L., Möhle R. 2004. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 103:4478–4486 10.1182/blood-2003-03-0875 [DOI] [PubMed] [Google Scholar]
- Kono M., Mi Y., Liu Y., Sasaki T., Allende M.L., Wu Y.P., Yamashita T., Proia R.L. 2004. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279:29367–29373 [DOI] [PubMed] [Google Scholar]
- Kono M., Allende M.L., Proia R.L. 2008. Sphingosine-1-phosphate regulation of mammalian development. Biochim. Biophys. Acta. 1781:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzurica P., Sancho D., Torres M., Albella B., Marazuela M., Merino T., Bueren J.A., Martínez-A C., Sánchez-Madrid F. 2000. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood. 95:2312–2320 [PubMed] [Google Scholar]
- Lindsley R.C., Thomas M., Srivastava B., Allman D. 2007. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 109:2521–2528 10.1182/blood-2006-04-018085 [DOI] [PubMed] [Google Scholar]
- Liu C.H., Thangada S., Lee M.J., Van Brocklyn J.R., Spiegel S., Hla T. 1999. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell. 10:1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.G., Xu Y., Proia R.L., Cyster J.G. 2005. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J. Exp. Med. 201:291–301 10.1084/jem.20041509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Jones D., Springer T.A. 1999. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 10:463–471 10.1016/S1074-7613(00)80046-1 [DOI] [PubMed] [Google Scholar]
- Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G.J., Card D., Keohane C., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296:346–349 10.1126/science.1070238 [DOI] [PubMed] [Google Scholar]
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E.A., Huff I.V., Junt T., Wagers A.J., Mazo I.B., von Andrian U.H. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994–1008 10.1016/j.cell.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Lo C.G., Cinamon G., Lesneski M.J., Xu Y., Brinkmann V., Allende M.L., Proia R.L., Cyster J.G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427:355–360 10.1038/nature02284 [DOI] [PubMed] [Google Scholar]
- Nagasawa T. 2006. Microenvironmental niches in the bone marrow required for B-cell development. Nat. Rev. Immunol. 6:107–116 10.1038/nri1780 [DOI] [PubMed] [Google Scholar]
- Nagasawa T., Hirota S., Tachibana K., Takakura N., Nishikawa S., Kitamura Y., Yoshida N., Kikutani H., Kishimoto T. 1996. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 382:635–638 10.1038/382635a0 [DOI] [PubMed] [Google Scholar]
- Nakayama T., Kasprowicz D.J., Yamashita M., Schubert L.A., Gillard G., Kimura M., Didierlaurent A., Koseki H., Ziegler S.F. 2002. The generation of mature, single-positive thymocytes in vivo is dysregulated by CD69 blockade or overexpression. J. Immunol. 168:87–94 [DOI] [PubMed] [Google Scholar]
- Nie Y., Waite J., Brewer F., Sunshine M.J., Littman D.R., Zou Y.R. 2004. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J. Exp. Med. 200:1145–1156 10.1084/jem.20041185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D.G., Batten S.J. 1984. Genesis of B lymphocytes in the bone marrow: extravascular and intravascular localization of surface IgM-bearing cells in mouse bone marrow detected by electron-microscope radioautography after in vivo perfusion of 125I anti-IgM antibody. Am. J. Anat. 170:349–365 10.1002/aja.1001700310 [DOI] [PubMed] [Google Scholar]
- Pappu R., Schwab S.R., Cornelissen I., Pereira J.P., Regard J.B., Xu Y., Camerer E., Zheng Y.W., Huang Y., Cyster J.G., Coughlin S.R. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 316:295–298 10.1126/science.1139221 [DOI] [PubMed] [Google Scholar]
- Pereira J.P., An J., Xu Y., Huang Y., Cyster J.G. 2009. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat. Immunol. 10:403–411 10.1038/ni.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.H., Okada T., Matloubian M., Lo C.G., Cyster J.G. 2008. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 28:122–133 10.1016/j.immuni.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard J.B., Sato I.T., Coughlin S.R. 2008. Anatomical profiling of G protein-coupled receptor expression. Cell. 135:561–571 10.1016/j.cell.2008.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutershan J., Basit A., Galkina E.V., Ley K. 2005. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L807–L815 10.1152/ajplung.00477.2004 [DOI] [PubMed] [Google Scholar]
- Rickert R.C., Roes J., Rajewsky K. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317–1318 10.1093/nar/25.6.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Proia R.L., Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8:753–763 10.1038/nri2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A.G., Andersson J., Melchers F. 1998. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur. J. Immunol. 28:3738–3748 [DOI] [PubMed] [Google Scholar]
- Rosen H., Goetzl E.J. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560–570 10.1038/nri1650 [DOI] [PubMed] [Google Scholar]
- Rubtsov A., Strauch P., Digiacomo A., Hu J., Pelanda R., Torres R.M. 2005. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 23:527–538 10.1016/j.immuni.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Rubtsov A.V., Swanson C.L., Troy S., Strauch P., Pelanda R., Torres R.M. 2008. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J. Immunol. 180:3882–3888 [DOI] [PubMed] [Google Scholar]
- Ryser M.F., Ugarte F., Lehmann R., Bornhäuser M., Brenner S. 2008. S1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homing. Mol. Immunol. 46:166–171 10.1016/j.molimm.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Sanchez T., Hla T. 2004. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 92:913–922 10.1002/jcb.20127 [DOI] [PubMed] [Google Scholar]
- Schwab S.R., Pereira J.P., Matloubian M., Xu Y., Huang Y., Cyster J.G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 309:1735–1739 10.1126/science.1113640 [DOI] [PubMed] [Google Scholar]
- Schwenk F., Sauer B., Kukoc N., Hoess R., Müller W., Kocks C., Kühn R., Rajewsky K. 1997. Generation of Cre recombinase-specific monoclonal antibodies, able to characterize the pattern of Cre expression in cre-transgenic mouse strains. J. Immunol. Methods. 207:203–212 10.1016/S0022-1759(97)00116-6 [DOI] [PubMed] [Google Scholar]
- Shiow L.R., Rosen D.B., Brdicková N., Xu Y., An J., Lanier L.L., Cyster J.G., Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440:540–544 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- Sinha R.K., Park C., Hwang I.Y., Davis M.D., Kehrl J.H. 2009. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 30:434–446 10.1016/j.immuni.2008.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Hirota S., Iizasa H., Yoshida H., Kawabata K., Kataoka Y., Kitamura Y., Matsushima K., Yoshida N., Nishikawa S., et al. 1998. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 393:591–594 10.1038/31261 [DOI] [PubMed] [Google Scholar]
- Ueda Y., Yang K., Foster S.J., Kondo M., Kelsoe G. 2004. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 199:47–58 10.1084/jem.20031104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter D.H., Rochwalsky U., Reinhold J., Seeger F., Aicher A., Urbich C., Spyridopoulos I., Chun J., Brinkmann V., Keul P., et al. 2007. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler. Thromb. Vasc. Biol. 27:275–282 10.1161/01.ATV.0000254669.12675.70 [DOI] [PubMed] [Google Scholar]
- Zou Y.R., Kottmann A.H., Kuroda M., Taniuchi I., Littman D.R. 1998. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 393:595–599 10.1038/31269 [DOI] [PubMed] [Google Scholar]