Abstract
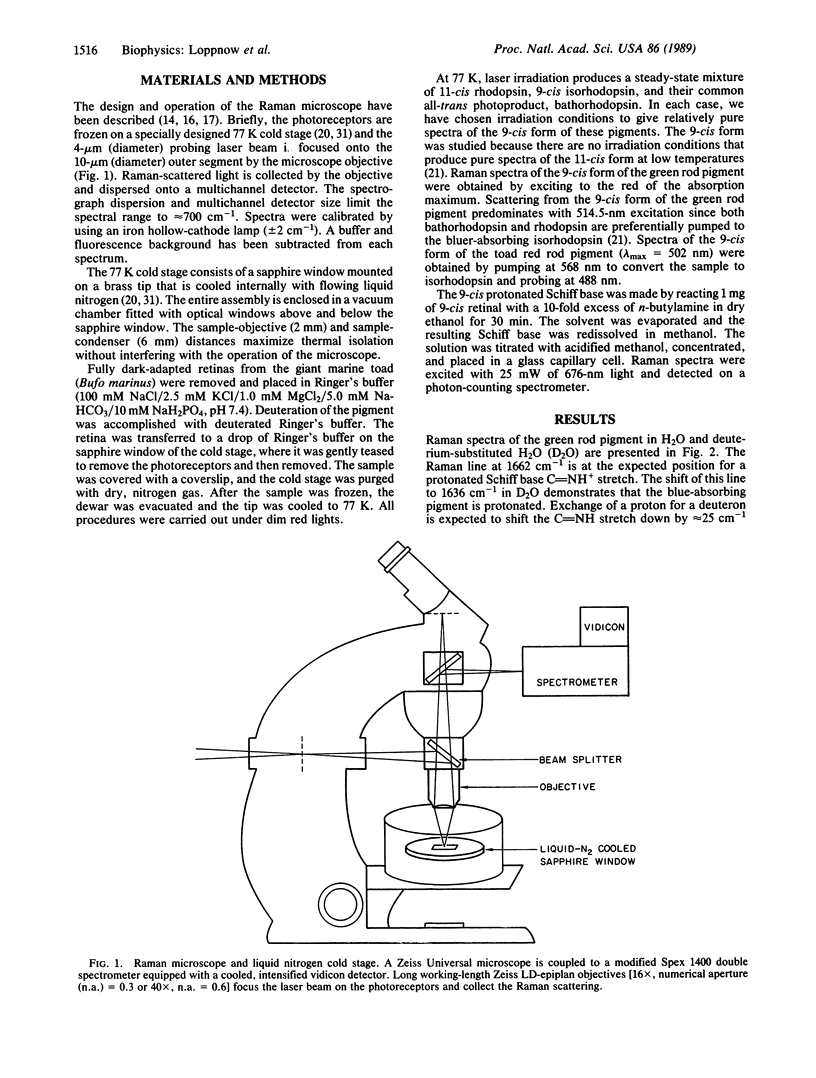
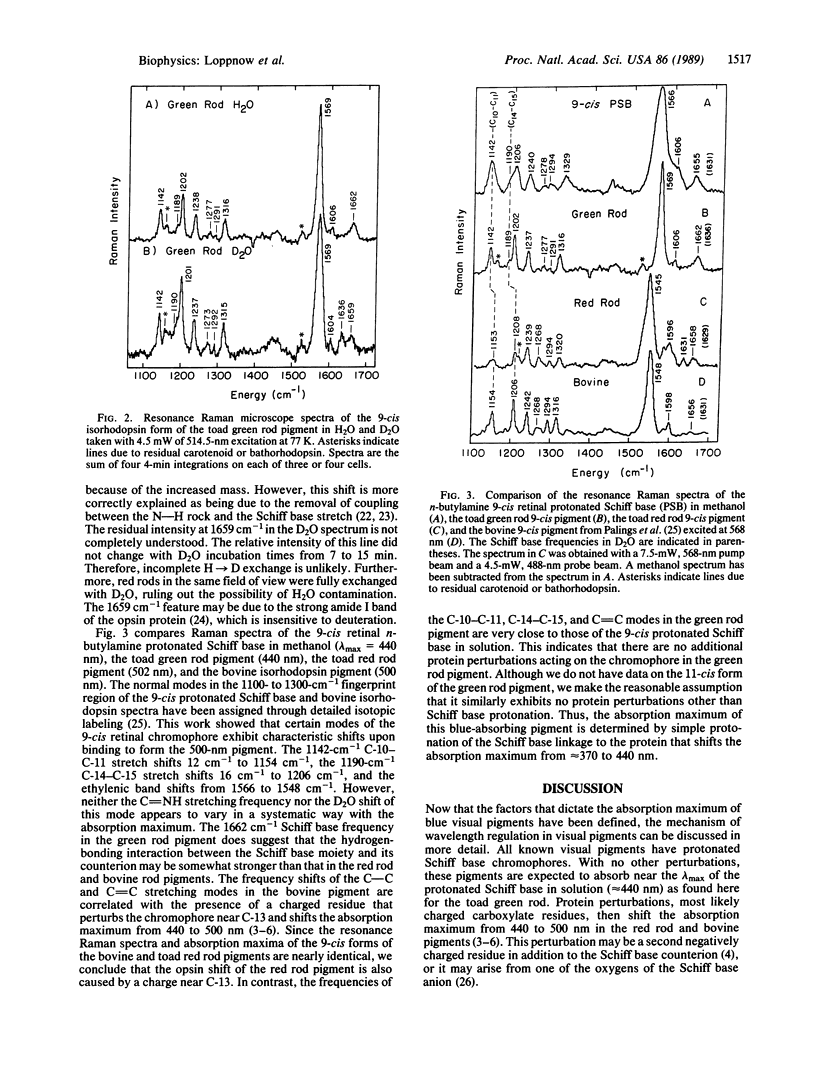
A resonance Raman microscope has been developed to study the structure of the retinal prosthetic group in the visual pigments of individual photoreceptor cells. Raman vibrational spectra are obtained by focusing the probe laser on intact photoreceptors frozen on a 77 K cold stage. To elucidate the mechanism of wavelength regulation in blue visual pigments, we have used this apparatus to study the structure of the chromophore in the 440-nm absorbing pigment found in "green rods" of the toad (Bufo marinus). The 9-cis isorhodopsin form of the green rod pigment exhibits a 1662-cm-1 C = NH+ Schiff base stretching mode that shifts to 1636 cm-1 in deuterium-substituted H2O. This demonstrates that the Schiff base linkage to the protein is protonated. Protonation of the Schiff base is sufficient to explain the 440-nm absorption maximum of this pigment without invoking any additional protein-chromophore interactions. The absence of additional perturbations is supported by the observation that the ethylenic band and the perturbation-sensitive C-10-C-11 and C-14-C-15 stretching modes have the same frequency as those of the 9-cis protonated retinal Schiff base in solution. Our demonstration that a blue visual pigment contains an unperturbed protonated Schiff base provides experimental evidence that the protein charge perturbation responsible for the opsin shift in the 500-nm absorbing pigments is removed in the opsins of blue pigments, as suggested by the sequence data.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. L., Etz E. S. Molecular microanalysis of pathological specimens in situ with a laser-Raman microprobe. Science. 1979 Nov 9;206(4419):716–718. doi: 10.1126/science.493979. [DOI] [PubMed] [Google Scholar]
- Barry B., Mathies R. A., Pardoen J. A., Lugtenburg J. Raman microscope and quantum yield studies on the primary photochemistry of A2-visual pigments. Biophys J. 1987 Oct;52(4):603–610. doi: 10.1016/S0006-3495(87)83250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry B., Mathies R. A. Raman microscope studies on the primary photochemistry of vertebrate visual pigments with absorption maxima from 430 to 502 nm. Biochemistry. 1987 Jan 13;26(1):59–64. doi: 10.1021/bi00375a009. [DOI] [PubMed] [Google Scholar]
- Barry B., Mathies R. Resonance Raman microscopy of rod and cone photoreceptors. J Cell Biol. 1982 Aug;94(2):479–482. doi: 10.1083/jcb.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge R. R., Einterz C. M., Knapp H. M., Murray L. P. The nature of the primary photochemical events in rhodopsin and isorhodopsin. Biophys J. 1988 Mar;53(3):367–385. doi: 10.1016/S0006-3495(88)83114-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson H. S., Honig B. H., Croteau A., Zarrilli G., Nakanishi K. Analysis of the factors that influence the C=N stretching frequency of polyene Schiff bases. Implications for bacteriorhodopsin and rhodopsin. Biophys J. 1988 Feb;53(2):261–269. doi: 10.1016/S0006-3495(88)83087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Herzfeld J., Griffin R. G. Solid-state nitrogen-15 nuclear magnetic resonance study of the Schiff base in bacteriorhodopsin. Biochemistry. 1983 Jan 4;22(1):1–4. doi: 10.1021/bi00270a600. [DOI] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Courtin J. M., Lugtenburg J., Herzfeld J., Mathies R. A., Griffin R. G. Solid-state 13C NMR detection of a perturbed 6-s-trans chromophore in bacteriorhodopsin. Biochemistry. 1985 Nov 19;24(24):6955–6962. doi: 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., McDowell J. H., Curtis D. R., Wang J. K., Juszczak E., Fong S. L., Rao J. K., Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- Kakitani H., Kakitani T., Rodman H., Honig B. On the mechanism of wavelength regulation in visual pigments. Photochem Photobiol. 1985 Apr;41(4):471–479. doi: 10.1111/j.1751-1097.1985.tb03514.x. [DOI] [PubMed] [Google Scholar]
- Kosower E. M. Assignment of groups responsible for the "opsin shift" and light absorptions of rhodopsin and red, green, and blue iodopsins (cone pigments). Proc Natl Acad Sci U S A. 1988 Feb;85(4):1076–1080. doi: 10.1073/pnas.85.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenburg J., Mathies R. A., Griffin R. G., Herzfeld J. Structure and function of rhodopsins from solid state NMR and resonance Raman spectroscopy of isotopic retinal derivatives. Trends Biochem Sci. 1988 Oct;13(10):388–393. doi: 10.1016/0968-0004(88)90181-8. [DOI] [PubMed] [Google Scholar]
- Nathans J., Piantanida T. P., Eddy R. L., Shows T. B., Hogness D. S. Molecular genetics of inherited variation in human color vision. Science. 1986 Apr 11;232(4747):203–210. doi: 10.1126/science.3485310. [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., Hogness D. S. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986 Apr 11;232(4747):193–202. doi: 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA Rhodopsin and bacteriorhodopsin: structure-function relationships. FEBS Lett. 1982 Nov 8;148(2):179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- Palings I., Pardoen J. A., van den Berg E., Winkel C., Lugtenburg J., Mathies R. A. Assignment of fingerprint vibrations in the resonance Raman spectra of rhodopsin, isorhodopsin, and bathorhodopsin: implications for chromophore structure and environment. Biochemistry. 1987 May 5;26(9):2544–2556. doi: 10.1021/bi00383a021. [DOI] [PubMed] [Google Scholar]
- Pande C., Deng H., Rath P., Callender R. H., Schwemer J. Resonance raman spectroscopy of an ultraviolet-sensitive insect rhodopsin. Biochemistry. 1987 Nov 17;26(23):7426–7430. doi: 10.1021/bi00397a034. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Andrew J. R., De Grip W. J., Stanley H. E. Opsin structure probed by raman spectroscopy of photoreceptor membranes. Science. 1976 Mar 19;191(4232):1176–1178. doi: 10.1126/science.1257742. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Mathies R. A., Pardoen J. A., Winkel C., van den Berg E. M., Lugtenburg J. Vibrational analysis of the all-trans retinal protonated Schiff base. Biophys J. 1985 May;47(5):653–664. doi: 10.1016/S0006-3495(85)83961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., Palings I., Copié V., Raleigh D. P., Courtin J., Pardoen J. A., Lugtenburg J., Mathies R. A., Griffin R. G. Low-temperature solid-state 13C NMR studies of the retinal chromophore in rhodopsin. Biochemistry. 1987 Mar 24;26(6):1606–1611. doi: 10.1021/bi00380a018. [DOI] [PubMed] [Google Scholar]
- Spudich J. L., McCain D. A., Nakanishi K., Okabe M., Shimizu N., Rodman H., Honig B., Bogomolni R. A. Chromophore/protein interaction in bacterial sensory rhodopsin and bacteriorhodopsin. Biophys J. 1986 Feb;49(2):479–483. doi: 10.1016/S0006-3495(86)83657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


