Abstract
Dendritic cells (DCs) play an important role in CD4+ T helper (Th) cell differentiation and in the initiation of both protective and pathogenic immunity. Granulocyte/macrophage colony-stimulating factor (GM-CSF) is a DC growth factor critical for the induction of experimental autoimmune encephalomyelitis (EAE) and other autoimmune diseases, yet its mechanism of action in vivo is not fully defined. We show that GM-CSF is directly required for the accumulation of radiosensitive dermal-derived langerin+CD103+ DCs in the skin and peripheral lymph nodes under steady-state and inflammatory conditions. Langerin+CD103+ DCs stimulated naive myelin-reactive T cells to proliferate and produce IFN-γ and IL-17. They were superior to other DC subsets in inducing expression of T-bet and promoting Th1 cell differentiation. Ablation of this subset in vivo conferred resistance to EAE. The current report reveals a previously unidentified role for GM-CSF in DC ontogeny and identifies langerin+CD103+ DCs as an important subset in CD4+ T cell–mediated autoimmune disease.
Myeloid DC subsets play specialized roles in tolerance induction during homeostasis and in protective immunity during infection. Several recent studies have focused on DCs in the dermis, intestines, lung, liver, kidney, and pancreas that express the integrin αEβ7 (CD103; Ginhoux, et al., 2009). In the dermis, lung, liver, and kidney, these cells coexpress the C type lectin langerin, and are CD11b low or negative. Dermal langerin+CD103+CD11blo-neg DCs have been implicated in CD4+ and CD8+ T cell priming after epicutaneous immunization (Bursch et al., 2007; Ginhoux et al., 2007; Shklovskaya et al., 2008; Wang et al., 2008; Bedoui et al., 2009). Pulmonary langerin+CD103+ DCs are required for optimal clearance of influenza virus (GeurtsvanKessel et al., 2008). The recognition that the langerin+CD103+ DC subset might be particularly adept at inducing certain forms of T cell immunity has stimulated interest in its developmental lineage and biological properties.
GM-CSF is a growth factor that promotes the differentiation and mobilization of myeloid cells in vivo (Hamilton and Anderson, 2004; King et al., 2009). It is widely used in vitro to stimulate the development of DCs from bone marrow precursors (Inaba et al., 1992). Studies with GM-CSF–deficient mice and WT mice treated with anti–GM-CSF neutralizing antibodies have established a nonredundant role of this cytokine in the generation of protective immunity against a range of microbes, as well as pathological immunity against self-antigens. Hence, GM-CSF−/− mice succumb to infection with Mycobacteria and Streptococcus and are resistant to the induction of experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis, and autoimmune myocarditis (LeVine et al., 1999; Cook et al., 2001; McQualter et al., 2001; Sonderegger et al., 2008; Szeliga et al., 2008).
In the these studies, GM-CSF deficiency was associated with impaired antigen-specific CD4+ T cell responses (McQualter et al., 2001; Sonderegger et al., 2008). For example, GM-CSF−/− mice actively immunized with an encephalitogenic peptide of myelin oligodendrocyte glycoprotein (MOG35-55) mount relatively meager antigen-specific IL-2 and IFN-γ recall responses (McQualter et al., 2001). Because GM-CSF primarily acts on myeloid cells, it has been widely assumed that such T cell defects are an indirect consequence of abnormalities in the development of APCs, and DCs in particular. (Rosas et al., 2007) .
Historically, GM-CSF was thought to be dispensable for steady-state DC differentiation (Vremec et al., 1997). However, two recent studies have demonstrated that GM-CSF supports the accumulation of CD11c+CD103+CD11b+ DCs in the lamina propria in the absence of conspicuous infection (Bogunovic et al., 2009; Varol et al., 2009). We questioned whether GM-CSF−/− and βc−/− (deficient in the common β subunit of the GM-CSF, IL-3, and IL-5 receptors) mice also have subtle defects in cutaneous DC subsets that were overlooked in past papers. Furthermore, in the earlier studies, mice were examined under homoeostatic conditions (Vremec et al., 1997); hence, the role of GM-CSF in de novo differentiation of DCs during inflammation was not addressed.
In this paper, we show that GM-CSF−/− and βc−/− mice selectively lack a subset of radiosensitive migratory dermal DCs that coexpress langerin and CD103. Depletion of radiosensitive langerin-expressing DCs suppressed IFN-γ and IL-17 responses in vivo and conferred resistance to EAE. Collectively, our data suggest that GM-CSF–dependent langerin+CD103+ dermal DCs promote CD4+ effector Th cell differentiation and play a defining role in a classical model of autoimmune pathogenesis.
RESULTS AND DISCUSSION
Seeding of the dermis by langerin+CD103+ DCs is GM-CSF dependent
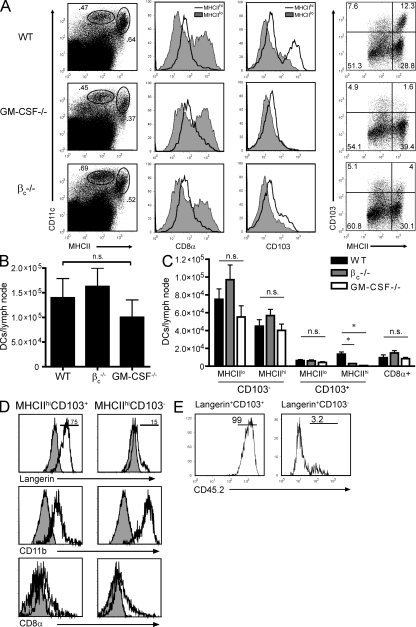
To investigate the role of GM-CSF in the accumulation of DCs in the skin, we analyzed MHCII+ cells in the epidermis and dermis of WT and GM-CSF−/− mice by flow cytometry. Three types of DCs have been identified in the skin of immunocompetent mice (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). Langerhans cells (langerin+CD103−CD11b+) originate in the epidermis and migrate to the cutaneous lymph nodes both during homeostasis and inflammation. DCs that reside in the dermis include langerin−CD103−CD11b+ and langerin+CD103+CD11blo subsets, the respective roles of which remain to be elucidated in detail. We found that a small percentage (∼5%) of MHCII+ cells harvested from the dermis of WT mice express langerin (Fig. 1 A). Approximately half of these langerin+ cells were CD103−CD11bhi, likely representing migrating Langerhans cells, and half were CD103+CD11bneg/lo (Fig. 1 A). Although MHCII+ dermal cells from GM-CSF−/− mice also contained a langerin+ population, it was predominantly composed of CD103−CD11bhi DCs (Fig. 1 A). In contrast, epidermal MHCII+ cells in both WT and GM-CSF−/− mice were uniformly langerin+CD103−CD11bhi, consistent with the cell-surface phenotype of Langerhans cells (Fig. 1 B; Bursch et al., 2007). There were no differences between WT and GM-CSF−/− mice in the frequency of dermal langerin−CD11b+ DCs (not depicted). Based on these results, we concluded that GM-CSF−/− mice are selectively deficient in the langerin+CD103+CD11bneg/lo subset of dermal DCs.
Figure 1.
Langerin+CD103+MHCIIhi dermal DCs are GM-CSF dependent. (A) FACS analysis of dermal mononuclear cells from naive WT and GM-CSF−/− mice. Dot plots are gated on total MHCII+ (left) or langerin+MHCII+ cells (right). (B) Epidermal mononuclear cells from naive WT and GM-CSF−/− mice. Histograms are gated on MHCII+ cells. Shaded histograms indicate isotype control staining. Dot plots (right) are gated on langerin+ epidermal cells. (C) Immunofluorescent staining for langerin (green) and CD103 (red) in ear skin sections from WT and GM-CSF−/− mice. The epidermis lies above the dermis in each image. (D) CD45.2 expression on CD11b+CD103−MHCII+ (left) and CD11b−CD103+MHCII+ (right) dermal cells from mixed bone marrow chimeric mice reconstituted with a 1:1 mixture of CD45.2βc−/− and CD45.1 WT bone marrow cells. (E) Percentage of TRITC+ cells among the langerin+CD11c+CD103+ (left) and CD103− (right) subsets of auricular lymph node cells in WT and GM-CSF−/− mice on days 2, 4, and 7 after ear painting (*, P < 0.05; error bars represent SEM). All data shown are representative of two to four experiments with at least three mice per group. Percentages are shown in A, B, and D. Bars, 10 µm.
Immunofluorescent staining of ear skin sections corroborated our flow cytometric data. Langerin+ cells were readily identified in the dermis of both WT and GM-CSF−/− mice. However, GM-CSF−/− dermis only contained langerin+CD103− cells, whereas doubly labeled langerin+CD103+ cells were present in WT dermis (Fig. 1 C). As expected, epidermal Langerhans cells in both groups failed to stain with the anti-CD103 antibody.
Langerhans cells and langerin+CD103+ dermal DCs migrate to draining lymph nodes after skin sensitization (Bursch et al., 2007). To assess the impact of GM-CSF on the DC composition of inflamed lymph nodes, we painted the ears of WT and GM-CSF−/− mice with tetramethyl rhodamine isothiocyanate (TRITC). At serial time points, draining auricular lymph node cells were analyzed for the accumulation of TRITC+langerin+ DCs (our gating strategy is shown in Fig. S1). TRITC+langerin+CD103+ cells appeared in the auricular lymph nodes of WT mice within 24 h after priming, peaked at 48 h, and slowly declined thereafter (Fig. 1 E, left; and not depicted). In contrast, TRITC+langerin+CD103+ DCs failed to accumulate in the auricular lymph nodes of GM-CSF−/− mice beyond baseline levels. We detected TRITC+langerin+CD103− cells in the draining lymph nodes of GM-CSF−/− mice within 48 h, although at slightly lower frequencies than in their WT counterparts (Fig. 1 E, right). TRITC+langerin+ DCs were not detected in the nondraining lymph nodes of either WT or GM-CSF−/− mice at any time point (unpublished data). Collectively, these observations indicate that GM-CSF is specifically required for the accumulation of langerin+CD103+CD11bneg/lo DCs and/or their precursors in the dermis during homeostasis. After skin sensitization, these cells migrate to draining lymph nodes, retaining their cell surface phenotype.
GM-CSF is required for the accumulation of langerin+CD103+ DCs in the cutaneous lymph nodes after subcutaneous immunization and during homeostasis
Previous studies have indicated a role for DCs in the differentiation of Th1 and Th17 effector cells and in the pathogenesis of autoimmune disease (Macatonia et al., 1995; Banchereau and Steinman, 1998). We and others have previously found that GM-CSF−/− mice on a C57BL/6 background are resistant to EAE induced by immunization with an immunodominant MOG peptide (MOG35-55) in CFA (McQualter et al., 2001; King et al., 2009). C57BL/6 βc−/− mice are also resistant to disease induction (Fig. S2 A). Consistent with previous reports, draining lymph node cells from MOG-immunized GM-CSF−/− and βc−/− mice contained lower frequencies of antigen-specific IFN-γ and IL-17 producers than WT controls (Fig. S2 B; McQualter et al., 2001).
Next, we performed flow cytometry on draining lymph node cells from MOG-immunized WT, GM-CSF−/−, and βc−/− mice to determine whether reduced IFN-γ and IL-17 responses correlate with a paucity of langerin+CD103+ DCs. The frequencies and absolute numbers of total CD11c+MHCII+ DCs were comparable across the three groups on day 7 after immunization, a time point when MOG-specific cytokine responses had clearly diverged between the knockout and WT mice (Fig. 2, A and B; Fig. S2 B; and not depicted). In addition, there were no significant differences in CD8α+ DCs, which predominantly expressed intermediate to low levels of MHCII (Fig. 2, A and C). However, both groups of knockout mice were deficient in CD11c+CD103+ lymph node cells (Fig. 2, A and C). The CD11c+CD103+ lymph node cells in immunized WT mice were langerin+CD11bint/loCD8αlo/− (Fig. 2 D), and expressed the maturation markers CD40, CD86, and DEC-205 (Fig. S3 A), consistent with the phenotype of CD103+ dermal DCs as reported by other investigators (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007; Allenspach et al., 2008; Shklovskaya et al., 2008; Wang et al., 2008; Bedoui et al., 2009; Ginhoux et al., 2009). In CD45 congenic bone marrow chimeras, langerin+CD103+ lymph node DCs were exclusively donor derived, indicating that they are radiosensitive and rapidly replaced by hematopoietic precursors (Fig. 2 E). This characteristic is also consistent with previous descriptions of CD103+ dermal DCs and distinguishes them from conventional langerin+CD103− Langerhans cells, which are radioresistant and GM-CSF independent (Fig. 2 E; and Fig. S3, B and C; Merad et al., 2002; Bursch et al., 2007). The frequency and number of MHCIIhiCD103+ DCs were reduced in cutaneous lymph nodes from naive as well as immunized βc−/− and GM-CSF−/− mice, indicating that GM-CSF is required for their accumulation under both homeostatic and inflammatory conditions (Fig. 3, A and B).
Figure 2.
Langerin+CD103+MHCIIhi DCs require GM-CSF signaling to accumulate in the peripheral lymph nodes of MOG-immunized mice. (A) Percentages of CD11c+ subsets in draining lymph nodes of WT, GM-CSF−/−, or βc−/− mice on day 7 after immunization with MOG/CFA. Histograms are based on the gates illustrated in the dot plots (left). (far right) Dot plots are gated on all CD11c+MHCII+ cells. (B and C) Absolute number of total CD11c+MHCII+ cells (B) and DC subsets (C) in draining lymph nodes on day 7 after immunization with MOG/CFA (*, P < 0.05; n.s., not statistically significant; error bars represent SD). (D) Cell-surface profiles of CD103+ and CD103−MHCIIhi lymph node DCs from MOG-immunized WT mice. Shaded histograms indicate background staining. (E) CD45.2 expression on langerin+ DC subsets in cutaneous lymph nodes of CD45.2+→CD45.1+ bone marrow chimeras. Percentages are shown in D and E. Data are representative of three independent experiments with three or more mice per group.
Figure 3.
Functional GM-CSF receptor expression is necessary for accumulation of langerin+CD103+MHCIIhi DCs in the cutaneous lymph nodes during homeostasis. (A) CD103 and MHCII expression on DCs in lymph nodes from unimmunized WT, βc−/−, and GM-CSF−/− mice, gating on CD11c+MHCII+ cells. (B) Total number of DC subsets in cutaneous lymph nodes of naive mice (*, P < 0.05; **, P < 0.01; error bars represent SEM). (C) CD45.1/2 expression on langerin+CD103+MHCIIhi lymph node DCs in unimmunized mixed bone marrow chimeric mice. Irradiated CD45.1+ WT hosts were reconstituted with a 1:1 mixture of either CD45.2+ WT and CD45.1+ WT cells (left) or CD45.2+βc−/− and CD45.1+ WT cells (right). Percentages are shown in A and C. (D) Percentage of cells derived from WT versus βc−/− donors within CD11c+ lymph node DC subsets from mixed bone marrow chimeras (error bars represent SEM). All data in A–D are representative of three separate experiments with at least three mice per group.
GM-CSF stimulates langerin+CD103+ DCs to accumulate in cutaneous lymph nodes by a direct pathway
The GM-CSF receptor is expressed on a wide range of hematopoietic cell types (Rosas et al., 2007). Therefore, GM-CSF could induce the accumulation of langerin+CD103+ DCs in the dermis and skin-draining lymph nodes by either a direct or indirect pathway. To distinguish between those possibilities, we constructed mixed bone marrow chimeras by injecting lethally irradiated CD45.1+ hosts with a combination of bone marrow cells from CD45.1+βc+/+ and CD45.2+βc−/− donors at a 1:1 ratio. Bone marrow chimeras constructed with a mixture of CD45.1+βc+/+ and CD45.2+βc+/+ donors were used as a control. After reconstitution, cutaneous lymph node cells were harvested and flow cytometric analysis was performed to determine the contribution of each donor source to various DC subsets.
The vast majority of langerin+CD103+ DCs were derived from βc+/+ precursors (Fig. 3, C and D). In contrast, βc+/+ and βc−/− genotypes were equally represented among all other radiosensitive DC subsets investigated (Fig. 3 D). A similar requirement for GM-CSF signaling was observed in CD103+, but not CD103−, DCs from dermal cell preparations (Fig. 1 D). These data demonstrate that the requirement of GM-CSF signaling for the accumulation of langerin+CD103+ DCs in the dermis and skin-draining lymph nodes is cell intrinsic.
Deletion of radiosensitive langerin+ DCs in vivo inhibits Th1/Th17 responses and confers resistance to EAE
Recently, transgenic mice were created that express the human diphtheria toxin receptor (DTR) downstream of the langerin promoter (langerin-DTR mice; Kissenpfennig et al., 2005). To directly assess the functional role of langerin+CD11b−CD103+ cells in the development of encephalitogenic Th1 and Th17 cells, we generated bone marrow chimeras in which lethally irradiated CD45.1+ hosts were reconstituted with bone marrow from CD45.2+ langerin-DTR mice. Administration of DT to these mice eliminates radiosensitive langerin+CD103+ dermal DCs while sparing radioresistant Langerhans cells (Fig. 4 A and not depicted).
Figure 4.
Radiosensitive langerin+CD103+ DCs promote encephalitogenic Th1/Th17 responses and induction of EAE. (A, top) Langerin-DTR→WT chimeric mice were treated with vehicle (left) or DT (right) for 2 d before analysis of cutaneous lymph node cells by flow cytometry. Dot plots are gated on CD11c+MHCII+ cells. (bottom) Gating on langerin+MHCIIhi lymph node cells from MOG-immunized chimeric mice. Percentages are shown. (B) Clinical course of EAE in chimeric mice injected with DT or vehicle alone. Data shown are combined from two separate experiments with five or more mice per group (*, P < 0.05; **, P < 0.002; N.S., not statistically significant; error bars represent SEM). (C) ELISPOT analysis of draining lymph nodes 6 d after immunization of DT-treated chimeric or WT mice and vehicle-treated chimeric mice (*, P < 0.05; **, P < 0.01; error bars represent SEM). Data are representative of two independent experiments with five mice per group.
Chimeric mice were actively immunized with MOG35-55 in CFA the day after systemic injection of DT or vehicle. DT-treated chimeras experienced a relatively delayed and milder course of EAE (Fig. 4 B, left). In contrast, administration of DT had no significant effect on the course of EAE in WT mice (Fig. 4 B, right). Increased resistance of DT-treated chimeric mice to EAE was associated with a significant reduction in the number of MOG-specific IFN-γ– and IL-17–producing lymph node cells by comparison to chimeric mice pretreated with vehicle alone or WT mice pretreated with DT (Fig. 4 C).
Langerin+CD103+ DCs acquire myelin antigens in vivo and promote Th cell differentiation
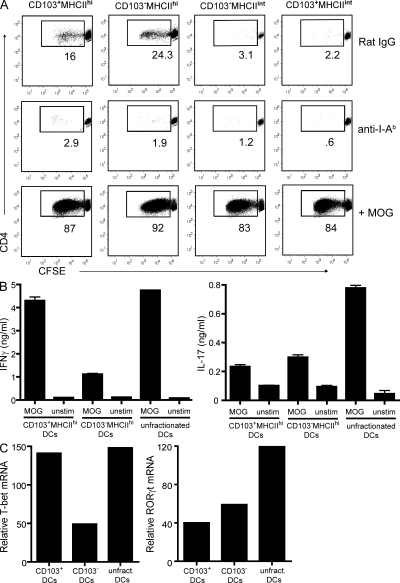
These results suggest that langerin+CD103+ cells are particularly efficient APCs for the generation of encephalitogenic T cells. Therefore, we compared their professional antigen-presenting capabilities to those of other cutaneous lymph node DC subsets in vitro. Lymph node cells were harvested from WT mice 20 h after immunization with MOG35-55 in CFA, and CD11c+ cells were sorted into four subsets based on CD103 and MHCII expression (Fig. 3 A). Each subset was cultured with naive CD4+ T cells that bear a transgenic TCR specific for MOG35-55 (2D2 cells) with or without exogenous MOG peptide. CD103+ and CD103−MHCIIhi, but not MHCIIint/lo, DCs stimulated the proliferation of CFSE-labeled 2D2 cells directly ex vivo in the absence of exogenous MOG peptide (Fig. 5 A). All DC subsets induced 2D2 expansion after they were pulsed with MOG35-55.
Figure 5.
CD103+MHCIIhi DCs prime naive myelin-specific T cells and induce Th effector cell differentiation. (A) DC subsets sorted from cutaneous lymph nodes of WT mice 20 h after immunization with MOG/CFA were cultured with purified, CFSE-stained CD45.1+CD4+ 2D2 T cells in the absence or presence of MOG peptide. Anti–I-Ab antibodies were added to some wells. Plots are gated on CD45.1+ T cells. Percentages are shown. Data are representative of four independent experiments. (B) Sorted naive 2D2 T cells were cultured with sorted lymph node DC subsets or unfractionated lymph node DCs in the presence of MOG peptide for 4 d. Cells were restimulated with anti-CD3/-CD28 for 48 h for detection of IFN-γ or IL-17 in supernatants by ELISA (error bars represent SEM). (C) Cells were prepared as described in B but harvested after 96 h of primary culture for real-time RT-PCR analysis. Data shown in B and C are representative of three separate experiments.
CD103+MHCIIhi DCs stimulated naive 2D2 cells to secrete significantly greater quantities of IFN-γ than they did upon culture with CD103−MHCIIhi DCs (Fig. 5 B). In fact, CD103+MHCIIhi DCs elicited IFN-γ levels comparable to those induced by unfractionated lymph node DCs. Consistent with this finding, the Th1 transcription factor T-bet was expressed at higher levels in 2D2 cultures containing CD103+ DCs than those containing CD103− DCs (Fig. 5 C; Szabo et al., 2000). In contrast, CD103− and CD103+ DC subsets induced comparable IL-17 and GM-CSF production (Fig. 5 B and not depicted). Furthermore, expression of the Th17 transcription factor RORγt did not differ significantly between cultures (Fig. 5 C; Ivanov et al., 2006).
The data in Fig. 4 and Fig. 5 indicate that langerin+CD103+ DCs play a nonredundant role in Th1 cell differentiation after subcutaneous immunization. The mechanism by which they induce Th1 responses remains to be elucidated. Although langerin+CD103+ cells are the major source of IL-12p40 among DCs in the draining lymph nodes of MOG-immunized mice (Fig. S4), we were unable to detect IL-12p70 heterodimer in supernatants of CD103+ dermal DC cultures, irrespective of the activating stimulus (not depicted). It is possible that our assays were not sensitive enough to detect crucial quantities of IL-12p70 released at the immunological synapse. Alternatively, langerin+CD103+ DCs could promote Th1 cell differentiation by an IL-12p70–independent pathway (for example, via CD70–CD27 interactions; Soares et al., 2007).
CD103+ DCs appeared to be less critical for the generation of Th17 cells. They were no better than MHCIIhiCD103− DCs at inducing either RORγt or IL-17 expression in 2D2 cells (Fig. 5, B and C). In vivo depletion of langerin+CD103+ DCs in MOG-immunized mice had a modest impact on antigen-specific IL-17 production (Fig. 4 C). Nevertheless, this partial reduction in the autoreactive Th17 response, along with elimination of the Th1 response, was sufficient to suppress, though not prevent, clinical EAE. We have recently demonstrated that myelin-specific Th1 and Th17 cells are independently capable of inducing EAE, although they use distinct proinflammatory pathways to do so (Kroenke et al., 2008). The data presented in this paper reinforce the concept that multiple pathways can promote initiation of EAE and underscore the complex nature of this disease.
MOG-specific IL-17 responses were more profoundly compromised in GM-CSF–deficient than langerin+CD103+ DC–depleted mice (Fig. 4 C and Fig. S2). This suggests that GM-CSF promotes Th17 cell differentiation by pathways that do not involve CD103+ dermal DCs. In an animal model of autoimmune myocarditis, GM-CSF enhanced secretion of IL-6 and IL-23 by splenic CD11c+ DCs (Sonderegger et al., 2008). No analysis was done to determine which DC subsets were the source of those proinflammatory cytokines. Although we found that CD103+ dermal DCs produce large quantities of IL-6 and IL-23 after CD40 or Toll-like receptor signaling (unpublished data), it is possible that a distinct DC subset, also dependent on GM-CSF, is the major source of these cytokines in vivo. We are currently investigating the role of different APCs in producing IL-23 and polarizing Th17 responses in WT mice with EAE.
Our observation that GM-CSF−/− and βc−/− mice have a selective deficiency in langerin+CD103+CD11blo DCs while still possessing CD103−CD11b+ DCs supports a growing body of evidence that the former cells diverge from other DC subsets early in ontogeny (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007; Bogunovic et al., 2009; Nagao et al., 2009). In addition to constitutive expression of CD103, langerin+ dermal DCs are sensitive to radiation injury (indicating the potential for rapid renewal), lack expression of epithelial cell adhesion molecule and Dectin-1, and are TGF-β independent (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007; Nagao et al., 2009). Unlike langerin− dermal DCs, they are CD11blow (Bursch et al., 2007). GM-CSF is not compulsory for CD103 expression because lymph nodes in GM-CSF−/− and βc−/− mice contain a subset of langerin−CD103+MHCint DCs at a frequency comparable to WT mice. Furthermore, incubation of langerin+CD103− lymph node or dermal cells from GM-CSF−/− mice with recombinant GM-CSF does not induce surface CD103 expression (unpublished data). Collectively, these data indicate that the langerin+CD103+ phenotype marks a distinct lineage of migratory DCs rather than a transitory activation state. In addition to the dermis and cutaneous lymph nodes, langerin+CD103+CD11blo/− DCs have been identified in the lung, liver, kidney, thymus, and mesenteric lymph nodes (Valladeau et al., 2002; Bursch et al., 2007; Chang et al., 2008). Future studies will address the importance of CD103+ DCs in these tissues for the local generation of Th1/Th17 responses and the development of autoimmune disease provoked by autoantigenic challenge outside of the skin.
The resistance of GM-CSF−/− and βc−/− mice to EAE is more complete than that of langerin+CD103+ DC–depleted mice (Fig. 4 and Fig. S2). This suggests that GM-CSF–driven accumulation of langerin+CD103+ DCs in the dermis and cutaneous lymph nodes reflects only one of several mechanisms by which that cytokine contributes to EAE pathogenesis. In fact, we recently reported that GM-CSF is important for the mobilization of inflammatory monocytes, which eventually give rise to central nervous system (CNS)–infiltrating DCs, from the bone marrow immediately before EAE exacerbations (King et al., 2009). GM-CSF could also stimulate mature myeloid cells within the CNS to up-regulate MHCII and co-stimulatory molecules, and stimulate immature myeloid cells to differentiate into macrophages and DCs in situ (Ponomarev et al., 2007; Mausberg et al., 2009). Finally, it is possible that GM-CSF supports the development and/or survival of an alternate population of APCs (distinct from langerin+CD103+ dermal cells), located in the CNS or another noncutaneous tissue, that is important in EAE pathogenesis. In summary, our report defines a previously unidentified role for GM-CSF in DC differentiation and/or development in vivo and suggests that growth factors such as GM-CSF are putative targets for treatment of organ-specific autoimmune disease.
MATERIALS AND METHODS
Mice.
WT and CD45.1 congenic C57BL/6 mice were purchased from the National Cancer Institute–Frederick. C57BL/6-Tg(Tcra2D2,Tcrb2D2)1Kuch/J mice (transgenic for the TCR Vα3.2 and Vβ11 chains reactive to MOG35–55), commonly known as 2D2 mice, were a gift from V. Kuchroo (Harvard Medical School, Boston, MA; Bettelli et al., 2003). 2D2 mice were backcrossed to CD45.1 congenic C57BL/6 mice in our facility. OT-II mice were purchased from the Jackson Laboratory. GM-CSF−/− and βc−/− mice were gifts of B. Trapnell (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH) and L. Robb (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia), respectively. Animals were housed under specific pathogen–free conditions. All experiments were performed under protocols approved by the University of Rochester Committee on Animal Resources and the University of Michigan Committee on Animal Use and Care of Animals.
Antibodies and flow cytometry.
Fluorochrome-conjugated antibodies to CD11c, I-Ab, CD11b, CD8α, CD4, CD80, CD86, CD40, CD45.1, CD45.2, and CD103, and biotinylated antibodies to langerin, were purchased from eBioscience. Streptavidin-PerCP and streptavidin–PE-Cy7 were obtained from BD and eBioscience, respectively. For detection of intracellular IL-12p40, cells were cultured for 4 h in 10% serum with 10 µg/ml brefeldin A. Cells were then fixed in 4% paraformaldehyde for 20 min and permeabilized with 0.5% saponin (Sigma-Aldrich). Data were collected on a flow cytometer (FACSCanto II; BD) and analyzed with FlowJo software (Tree Star, Inc.).
Induction and evaluation of EAE.
Mice were immunized with 100 µg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK; Biosynthesis) in CFA (5 mg/ml of heat-killed Mycobacterium tuberculosis H37Ra; vol/vol) by s.c. injection at four sites over the flanks. Bordetella pertussis toxin (List Biological Laboratories, Inc.) was injected i.p. (300 ng/mouse) on days 0 and 2. Mice were observed for signs of EAE on a daily basis and graded on a standard scale, as previously described (Carlson et al., 2008).
TRITC painting.
Stock TRITC (10% in DMSO; Invitrogen) was diluted 10-fold in a 1:1 mixture of acetone/dibutylphthalate. 10 µl of TRITC solution was applied to the dorsal and ventral sides of each ear.
Preparation of epidermal and dermal cell suspensions.
The epidermis was separated from ear skin by digestion with collagenase/dispase (Roche) for 1 h at 37°C. Epidermal and dermal tissue was minced into small pieces and digested in 1.84 mg/ml Blendzyme Liberase III (Roche) and collagenase IV (Worthington), respectively, for 30 min at 37°C. 100 mM EDTA was added and tissue was processed into a single-cell suspension by repeated pipetting. Cells were then passed through a 70-µm cell strainer, washed, and stained for flow cytometric analysis.
Generation of bone marrow chimeras.
5 × 106 C57BL/6 WT, CD45.1+ WT, and CD45.2+βc−/− or CD45.2+ langerin-DTR bone marrow cells (provided by K. Hogquist, University of Minnesota, Minneapolis, MN) were injected into the tail veins of lethally irradiated (1,250 rad split into two doses) CD45.1+ congenic hosts. In all cases, the reconstitution efficiency of circulating CD11b+ cells at 6 wk after irradiation exceeded 95%. Langerhans cells were systemically depleted in langerin-DTR chimeras by i.p. injection of 1 µg DT (List Biological Laboratories, Inc.) on days −2 and −1 before immunization.
DC subset sorting and in vitro stimulation.
Cutaneous lymph nodes were incubated with collagenase type IV (Sigma-Aldrich) and DNase (Worthington) for 45 min at 37°C, followed by CD11c+ cell enrichment by MACS (Miltenyi Biotec). CD11c+ cells were sorted into four subsets based on MHCII and CD103 expression (Fig. 2 B) using a FACSAria (BD). 2D2 CD4+ T cells were purified from lymph node cell suspensions over CD4 immunocolumns (Cedarlane). CD4+ cells (>90% pure) were CFSE labeled and incubated with sorted DCs (105 CD4+ T cells/2 × 104 DCs) at 37°C with or without 25 µg/ml MOG35-55. 10 µg/ml anti–I-Ab antibody was added to some wells (eBioscience). CFSE dye dilution was measured after 90 h. For Th cell differentiation assays, sorted naive CD4+CD25−CD44−CD62L+ 2D2 T cells were cultured with various DC subsets for 4 d, rested in media alone for 48 h, and restimulated with platebound anti-CD3 (0.5 µg/ml) and anti-CD28 (0.5 µg/ml). After 48 h, culture supernatants were collected for IFN-γ and IL-17 quantification.
Immunofluorescence.
Whole ears were flash frozen in isopentane (Sigma-Aldrich). 8-µm cryostat sections were collected on poly–l-lysine–coated slides, rehydrated in PBS, and incubated with anti–mouse langerin (clone L31; eBioscience) and biotin anti–mouse CD103 antibodies, followed by anti–rat–Alexa Fluor 488 and streptavidin–Alexa Fluor 594 (Invitrogen). The original magnification was 40×.
Real-time RT-PCR.
Cells were homogenized in TRIzol reagent (Invitrogen). RNA was isolated and cDNA was synthesized using a reverse transcription kit (Quantitect; QIAGEN). Primers and probes were purchased from Applied Biosystems. Samples were analyzed on a PCR machine (iCycler; Bio-Rad Laboratories). All data were normalized to GAPDH and expressed as fold induction over naive.
ELISA and ELISPOT analysis.
Cytokines in 48-h supernatants obtained from T cell–DC cultures were quantified using a sandwich ELISA technique based on noncompeting pairs of antibodies. Capture and detection mAbs were obtained from BD. ELISPOT analysis was performed after whole lymph node cells were cultured for 36 h at 2.5–4 × 105 cells/well.
Statistical analysis.
The unpaired Student’s t test was used for statistical comparisons. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 illustrates the gates used to detect TRITC+langerin+ DCs in draining lymph nodes of mice after ear painting. Fig. S2 shows that GM-CSF−/− and βc−/− mice are resistant to EAE and have impaired MOG-specific Th1/Th17 responses. Fig. S3 A shows the cell surface phenotype of CD103+ and CD103−MHCIIhi lymph node DCs in MOG-immunized mice. Fig. S3 (B and C) shows that langerin+CD103− DCs (Langerhans cells) are present in the cutaneous lymph nodes of GM-CSF−/− mice. Fig. S4 shows that CD103−MHCIIhi DCs are a major source of IL-12p40 in the draining lymph nodes of MOG-immunized mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091844/DC1.
Acknowledgments
We thank B. Moore for assistance with bone marrow chimera generation; T. Dickendesher for assistance with immunohistochemistry; A.M. Des Lauriers and M. Pihalja for flow cytometry expertise; Dr. K. Hogquist for thoughtful discussion and for providing the langerin-DTR bone marrow for chimera generation; and Drs. L. Wang and B. Igyarto for technical assistance and expertise.
This work was supported by grants from the National Multiple Sclerosis Society (RG 3866-A-3 and CA 1037A1) and the National Institutes of Health (NS047687-01A1).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- CNS
- central nervous system
- DT
- diphtheria toxin
- EAE
- experimental autoimmune encephalomyelitis
- MOG
- myelin oligodendrocyte glycoprotein
- TRITC
- tetramethyl rhodamine isothiocyanate
References
- Allenspach E.J., Lemos M.P., Porrett P.M., Turka L.A., Laufer T.M. 2008. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 29:795–806 10.1016/j.immuni.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I., Allan R.S., Wojtasiak M., Shortman K., Carbone F.R., et al. 2009. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 10:488–495 10.1038/ni.1724 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. 2003. Myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., et al. 2009. Origin of the lamina propria dendritic cell network. Immunity. 31:513–525 10.1016/j.immuni.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch L.S., Wang L., Igyarto B., Kissenpfennig A., Malissen B., Kaplan D.H., Hogquist K.A. 2007. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204:3147–3156 10.1084/jem.20071966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T., Kroenke M., Rao P., Lane T.E., Segal B. 2008. The Th17−ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J. Exp. Med. 205:811–823 10.1084/jem.20072404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.Y., Cha H.R., Igarashi O., Rennert P.D., Kissenpfennig A., Malissen B., Nanno M., Kiyono H., Kweon M.N. 2008. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J. Immunol. 180:4361–4365 [DOI] [PubMed] [Google Scholar]
- Cook A.D., Braine E.L., Campbell I.K., Rich M.J., Hamilton J.A. 2001. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 3:293–298 10.1186/ar318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel C.H., Willart M.A., van Rijt L.S., Muskens F., Kool M., Baas C., Thielemans K., Bennett C., Clausen B.E., Hoogsteden H.C., et al. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621–1634 10.1084/jem.20071365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Collin M.P., Bogunovic M., Abel M., Leboeuf M., Helft J., Ochando J., Kissenpfennig A., Malissen B., Grisotto M., et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204:3133–3146 10.1084/jem.20071733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. 2009. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206:3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.A., Anderson G.P. 2004. GM-CSF biology. Growth Factors. 22:225–231 10.1080/08977190412331279881 [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- King I.L., Dickendesher T.L., Segal B.M. 2009. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 113:3190–3197 10.1182/blood-2008-07-168575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhé C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D., et al. 2005. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 22:643–654 10.1016/j.immuni.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Kroenke M.A., Carlson T.J., Andjelkovic A.V., Segal B.M. 2008. IL-12– and IL-23–modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 205:1535–1541 10.1084/jem.20080159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine A.M., Reed J.A., Kurak K.E., Cianciolo E., Whitsett J.A. 1999. GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J. Clin. Invest. 103:563–569 10.1172/JCI5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A., Wysocka M., Trinchieri G., Murphy K.M., O’Garra A. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071–5079 [PubMed] [Google Scholar]
- Mausberg A.K., Jander S., Reichmann G. 2009. Intracerebral granulocyte-macrophage colony-stimulating factor induces functionally competent dendritic cells in the mouse brain. Glia. 57:1341–1350 10.1002/glia.20853 [DOI] [PubMed] [Google Scholar]
- McQualter J.L., Darwiche R., Ewing C., Onuki M., Kay T.W., Hamilton J.A., Reid H.H., Bernard C.C. 2001. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J. Exp. Med. 194:873–882 10.1084/jem.194.7.873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I., Weissman I.L., Cyster J.G., Engleman E.G. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135–1141 10.1038/ni852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K., Ginhoux F., Leitner W.W., Motegi S., Bennett C.L., Clausen B.E., Merad M., Udey M.C. 2009. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. USA. 106:3312–3317 10.1073/pnas.0807126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev E.D., Shriver L.P., Maresz K., Pedras-Vasconcelos J., Verthelyi D., Dittel B.N. 2007. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178:39–48 [DOI] [PubMed] [Google Scholar]
- Poulin L.F., Henri S., de Bovis B., Devilard E., Kissenpfennig A., Malissen B. 2007. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 204:3119–3131 10.1084/jem.20071724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas M., Gordon S., Taylor P.R. 2007. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. Eur. J. Immunol. 37:2518–2528 10.1002/eji.200636892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklovskaya E., Roediger B., Fazekas de St Groth B. 2008. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J. Immunol. 181:418–430 [DOI] [PubMed] [Google Scholar]
- Soares H., Waechter H., Glaichenhaus N., Mougneau E., Yagita H., Mizenina O., Dudziak D., Nussenzweig M.C., Steinman R.M. 2007. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12–independent but CD70-dependent mechanism in vivo. J. Exp. Med. 204:1095–1106 10.1084/jem.20070176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger I., Iezzi G., Maier R., Schmitz N., Kurrer M., Kopf M. 2008. GM-CSF mediates autoimmunity by enhancing IL-6–dependent Th17 cell development and survival. J. Exp. Med. 205:2281–2294 10.1084/jem.20071119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Kim S.T., Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669 10.1016/S0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- Szeliga J., Daniel D.S., Yang C.H., Sever-Chroneos Z., Jagannath C., Chroneos Z.C. 2008. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis (Edinb.). 88:7–20 10.1016/j.tube.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J., Clair-Moninot V., Dezutter-Dambuyant C., Pin J.J., Kissenpfennig A., Mattéi M.G., Ait-Yahia S., Bates E.E., Malissen B., Koch F., et al. 2002. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J. Immunol. 168:782–792 [DOI] [PubMed] [Google Scholar]
- Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. 2009. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 31:502–512 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- Vremec D., Lieschke G.J., Dunn A.R., Robb L., Metcalf D., Shortman K. 1997. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27:40–44 10.1002/eji.1830270107 [DOI] [PubMed] [Google Scholar]
- Wang L., Bursch L.S., Kissenpfennig A., Malissen B., Jameson S.C., Hogquist K.A. 2008. Langerin expressing cells promote skin immune responses under defined conditions. J. Immunol. 180:4722–4727 [DOI] [PubMed] [Google Scholar]