Abstract
Mouse natural killer T (NKT) cells with an invariant Vα14-Jα18 rearrangement (Vα14 invariant [Vα14i] NKT cells) are either CD4+CD8− or CD4−CD8−. Because transgenic mice with forced CD8 expression in all T cells exhibited a profound NKT cell deficit, the absence of CD8 has been attributed to negative selection. We now present evidence that CD8 does not serve as a coreceptor for CD1d recognition and that the defect in development in CD8 transgene homozygous mice is the result of a reduction in secondary T cell receptor α rearrangements. Thymocytes from mice hemizygous for the CD8 transgene have a less severe rearrangement defect and have functional CD8+ Vα14i NKT cells. Furthermore, we demonstrate that the transcription factor Th, Poxviruses and Zinc finger, and Krüppel family (Th-POK) is expressed by Vα14i NKT cells throughout their differentiation and is necessary both to silence CD8 expression and for the functional maturity of Vα14i NKT cells. We therefore suggest that Th-POK expression is required for the normal development of Vα14i NKT cells and that the absence of CD8 expression by these cells is a by-product of such expression, as opposed to the result of negative selection of CD8-expressing Vα14i NKT cells.
Vα14 invariant (Vα14i) NKT cells are a subset of mouse T cells that have been implicated in many immune and inflammatory responses, including the regulation of antimicrobial responses, the balance between tolerance and autoimmunity, and even inflammatory processes such as intestinal adhesions after viral infection, ischemia reperfusion injury, and the formation of atherosclerotic plaques (Godfrey et al., 2004; Lappas et al., 2006; Tupin et al., 2007; Kosaka et al., 2008). As the name implies, Vα14i NKT cells express a limited TCR repertoire, featuring an invariant Vα14-Jα18 TCR α chain usually paired with diverse Vβ8.2, Vβ7, or Vβ2 chains. These cells recognize lipid antigens presented by CD1d, a class I–like antigen-presenting molecule, and they can therefore be precisely identified by tetramers of CD1d loaded with α galactosyl ceramide (αGalCer), a potent glycolipid agonist. A homologous population of cells also exists in humans, which mostly express the human orthologues of the Vα14i/Vβ8.2 TCR, namely an invariant Vα24 with a more diverse Vβ11. Moreover, invariant NKT cells from humans and mice exhibit interspecies cross-reactivity, in that mouse Vα14i NKT cells can respond to glycolipids presented by human CD1d, and vice versa, indicating conservation of this specificity (Brossay et al., 1998a; Kjer-Nielsen et al., 2006).
Vα14i NKT cells are further characterized by their innate-like behavior. They constitutively express cell surface proteins also found on NK cells and activated or memory T cell populations, such as NK1.1 and CD69, and they rapidly secrete both Th1 and Th2 cytokines in response to antigen without priming. The activated phenotype of these cells is imprinted during thymic differentiation, suggesting that they could be selected and/or expanded in the thymus by self-agonists (Bezbradica et al., 2006). It is therefore not surprising that the thymic selection of Vα14i NKT cells exhibits several unique requirements. Notable among these is the need for the adaptor SAP acting downstream of SLAM family receptors and positive selection mediated by double-positive (DP) thymocytes (Kronenberg and Engel, 2007). Upon the initiation of the Vα14i NKT cell developmental program, CD8 is down-regulated, and later during their maturation a fraction of these cells lose CD4 expression as well, such that mature Vα14i NKT cells are either CD4 single positive (SP) or double negative (DN) but never CD8 SP. They share this pattern of expression with other unconventional TCR αβ+ lymphocytes, including CD1d-reactive cells with more diverse TCRs, and cells not reactive to CD1d, such as MAIT (mucosal-associated invariant T cell). In contrast, a fraction of human Vα24i NKT cells express CD8; however, this is predominantly CD8αα, and the acquisition of CD8 expression occurs largely after maturation in the thymus is complete (Loza et al., 2002; Godfrey et al., 2004; Berzins et al., 2005).
The mechanisms underlying the absence of CD8 SP lymphocytes in Vα14i NKT cells and other unconventional TCR αβ+ lymphocytes are not known. Pioneering studies found that mice with a transgene that enforced the expression of CD8αβ throughout the T cell lineage lacked NKT cells, defined in part in those early studies as cells coexpressing NK1.1 and TCR-αβ (Lantz and Bendelac, 1994; Bendelac, 1995). Furthermore, the Vβ repertoire of NKT cells from CD8-deficient mice was subtly altered, and CD8 SP T cells from Vα14i transgenic mice exhibited a selective depletion of the Vβ8 and Vβ7 chains most commonly expressed by mouse Vα14i NKT cells (Bendelac et al., 1996). These data led to the conclusion that CD8+ Vα14i NKT cells were eliminated via negative selection. This hypothesis, together with subsequent studies demonstrating that Vα14i NKT cells were self-reactive to CD1d, suggested that CD8 might interact with CD1d as a coreceptor, which is similar to its interaction with classical MHC class I molecules (Bendelac et al., 1995). Indirect evidence using cells overexpressing CD1d was also consistent with a CD1d–CD8 interaction (Teitell et al., 1997). Therefore, according to this view, the positive selection of Vα14i NKT cells by self-agonists may put them close to the threshold of negative selection, with the coengagement of CD8 pushing them over the line. Co-receptor expression by Vα14i NKT cells and selection by self-agonists fit nicely with hypotheses attributing CD4 expression in conventional T cells to enhanced lck signal strength or prolonged kinetics of signaling (Singer et al., 2008), although the selection of Vα14i NKT cells by self-agonists remains unproven.
In this paper, we report on experiments that examine the mechanisms by which CD8 expression is excluded from the Vα14i NKT lineage. We conclude that CD8+ Vα14i NKT cells are not eliminated by negative selection. Instead, we find roles for the transcription factor Th, Poxviruses and Zinc-finger (POZ), and Krüppel family (Th-POK) in enforcing the expression of CD4, as opposed to CD8 on Vα14i NKT cells, as well as for the full functional response of this cell subset. Collectively, our data suggest that CD8 expression is excluded from the Vα14i NKT cells as a by-product of the expression of factors required for the developmental program of this population.
RESULTS
CD8 expression does not enhance Vα14i NKT cell responses
We previously performed surface plasmon resonance studies to identify an interaction between CD8 and the thymus leukemia antigen, a nonclassical class I molecule (Fisher et al., 1985; Leishman et al., 2001). Experiments performed in parallel failed to convincingly demonstrate binding of CD8 even to CD1d tetramers (unpublished data). However, because there could be biologically meaningful interactions close to or below the level of detection, we focused our efforts on in vitro and in vivo functional assays to determine if the expression of CD8 affects the antigen response of Vα14i NKT cells. Initially, retroviruses were used to direct the expression of CD8α and CD8β chains in three mouse Vα14i NKT cell hybridomas. The 1.2 and 3.C3 cells express a TCR with Vβ8.2 β chains having different CDR3 regions, whereas the 1.4 TCR utilizes an atypical Vβ10 polypeptide (Burdin et al., 1998). Cells expressing near physiological levels of CD8αβ were selected by magnetic and flow cytometric cell sorting (Fig. 1 A and not depicted). We also established control hybridoma sublines transduced with empty retrovirus vector, and we compared the CD8-expressing Vα14i NKT cell hybridomas with the controls for IL-2 production in response to thymocytes pulsed with the synthetic antigen αGalCer, a potent agonist related to the antigens found in Sphingomonas bacteria. Antigen-pulsed CD1d transfectants of the B cell lymphoma A20 and bone marrow–derived DCs were also tested as APCs. Furthermore, to allow for the possibility that CD8αβ expressed by the hybridomas might bind to classical class I molecules on the APC, providing an adhesion function separate from a true coreceptor function, we also tested the hybridomas in an APC-free system with αGalCer presented by plate-bound CD1d protein. If CD8 acted as a TCR coreceptor, we would expect a shift in the antigen dose response curve as a result of enhanced antigen sensitivity mediated by CD8. However, we did not find a consistent difference between the CD8-expressing hybridomas and empty vector control transductants in their antigen response (Fig. 1 B and not depicted), even at αGalCer concentrations as low as 2 ng/ml. Similar experiments using the Sphingomonas-derived glycolipid GalA-GSL, a less potent agonist, also failed to find any effect of CD8 expression on the antigen response (unpublished data).
Figure 1.
Unaltered response to αGalCer by CD8 expressing Vα14i NKT cell hybridomas. (A) CD8 expression in Vα14i NKT cell hybridomas 1.2 (left) and 1.4 (right) after transduction with CD8α and CD8β retroviral constructs and selection for expression of CD8 and TCR. Top, staining with anti-CD8α; bottom, staining with anti-CD8β. Solid black lines, hybridoma transduced with CD8α and CD8β retroviral constructs; gray lines, TCR-β+ CD8SP splenocytes; dotted lines, hybridoma transduced with empty vector retroviral construct. (B–D) Hybridomas were cultured in the presence of thymocytes (B) or A20.CD1d cells (C) pulsed with 2, 20, or 200 ng/ml of αGalCer, or plates coated with recombinant mouse CD1d with 2, 20, or 200 ng/well of αGalCer (D), and supernatants collected after overnight culture were assayed for IL-2 by ELISA. The amounts of IL-2 produced by hybridomas stimulated by APCs or recombinant CD1d not incubated with αGalCer were below the detectable levels in these experiments (not depicted). Data are representative of at least two independent experiments.
We also measured the effect of CD8 expression on the avidity of the 1.2 and 1.4 hybridoma lines for αGalCer-loaded CD1d (αGalCer-CD1d) tetramers by staining the CD8-expressing and control lines with serial dilutions of tetramers and determining the fluorescence intensity via flow cytometry. The resulting tetramer isotherm analyses revealed that CD8 expression had little or no effect on αGalCer-CD1d tetramer avidity (Fig. S1). Similar experiments using CD1d tetramers loaded with the less potent αGalCer analogue α glucosyl ceramide also did not reveal any effect of CD8 expression on antigen avidity (unpublished data).
Effect of CD8 deficiency on the Vβ repertoire
We examined if CD8 normally acts to modulate Vα14i NKT cell differentiation. CD8-null mice were previously reported to contain elevated fractions of Vβ7+ NKT cells, (Bendelac et al., 1994), suggesting that CD8 plays a role in shaping the Vβ repertoire of Vα14i NKT cells. Subsequent results from other studies suggested that Vα14i TCRs containing Vβ7 have a higher affinity for endogenous ligands presented by CD1d and, therefore, the increase in Vβ7-expressing cells in the absence of CD8 is consistent with a partial release from negative selection (Chun et al., 2003; Schümann et al., 2006; Wei et al., 2006). Precise identification of Vα14i NKT cells was difficult at the time these earlier studies were performed, however, because CD1d tetramers were not available.
We stained thymocytes from CD8-null and WT mice with αGalCer-CD1d tetramers together with antibodies against Vβ2, Vβ7, and Vβ8.1/8.2. We observed no significant difference between the Vβ repertoires of Vα14i NKT thymocytes from CD8-deficient and WT mice (Table I), suggesting that CD8 expression normally does not modulate the selection of Vβ segments by Vα14i NKT cells.
Table I.
Effect of CD8 deletion on Vα14i NKT thymocyte and Vβ repertoire
| Strain | Vα14i NKT cells that express Vβ2 | Vα14i NKT cells that express Vβ7 | Vα14i NKT cells that express Vβ8.1/8.2 |
| % | % | % | |
| C57BL/6 (n = 6) | 14 ± 4 | 15 ± 4 | 70 ± 2 |
| Cd8a−/− (n = 6) | 11 ± 1 | 16 ± 3 | 67 ± 5 |
| Fold change in KO versus WT | 0.8 | 1.1 | 1.0 |
Expression of CD8 by Vα14i NKT cells in transgenic mice
The observations in the previous sections led us to reexamine if the development of Vα14i NKT cells is affected by enforced expression of CD8 in the T cell lineage. We therefore obtained and analyzed the same CD8 transgenic line that was examined in earlier studies (Robey et al., 1991; Bendelac et al., 1994). In these mice, CD8α and β expression is controlled by enhancer and promoter sequences derived from the CD2 gene (Robey et al., 1991). Thymus, spleen, and liver cell suspensions prepared from CD8 transgenic and WT mice were stained with αGalCer-CD1d tetramers together with a mix of antibodies to define Vα14i NKT cells and other subsets. In agreement with the original study, we found that mice homozygous for the CD8 transgene (CD8 Tg/Tg) exhibited markedly reduced (>10-fold) numbers of Vα14i NKT cells in both the thymus and periphery (Fig. 2). Surprisingly, mice that were hemizygous for the CD8 transgene (CD8 Tg/+) had significant populations of Vα14i NKT cells, although the numbers were approximately two- to threefold lower than those in WT littermates (Fig. 2). Some of the Vα14i NKT cells that expressed CD8 also coexpressed CD4, although the percentage of DN Vα14i NKT cells was increased compared with WT mice (Fig. 2 A).
Figure 2.
Vα14i NKT cells in CD8 transgenic mice. (A) Flow cytometric analysis of thymocytes from (top) WT mice, mice homozygous for the CD8 transgene (CD8 Tg/Tg; middle), and CD8 Tg hemizygous mice (CD8 Tg/+; bottom). The first column shows anti–TCR-β and αGalCer-CD1d tetramer staining, the second column shows Vα14i NKT cells, defined as gated in the left column, stained for CD4 and CD8α, the third column shows CD4 and CD8α staining of total thymocytes, and the fourth column shows the staining of Vα14i NKT thymocytes for NK1.1 and CD44, which was used to determine the percentages of cells within the stage 1 (CD44low, NK1.1low, bottom left box), stage 2 (CD44high, NK1.1low, top left box) and stage 3 (CD44high, NK1.1high, right box) Vα14i NKT developmental subsets. Data are representative of at least nine independent experiments. (B) Flow cytometric analysis of liver mononuclear cells (LMCs) obtained from the indicated mice stained with αGalCer-CD1d tetramers together with antibodies against TCR-β and CD8α. The left column shows staining with αGalCer-CD1d tetramers and anti–TCR-β to define the Vα14i NKT and conventional T subsets, with CD8α staining depicted for Vα14i NKT cells (middle column) and conventional T cells (right column). Data are representative of more than six independent experiments. (C) Scatter plot of the number of Vα14i NKT cells observed in thymus, spleen, and livers of CD8 Tg/+ and WT littermate mice. Matching symbol styles represent numbers determined from littermates on the same day. Paired Student's t test p-values are shown, with significant differences indicated by asterisks (**, 0.001 ≥ P ≤ 0.01; ***, P < 0.001). Error bars show SD and horizontal bars show the arithmetic mean of the samples in each column.
Vα14i NKT development in the thymus is characterized by the successive induction of high levels of CD44 followed by NK1.1 (Benlagha et al., 2002). Mutations that disrupt NKT development could cause a block in the transition from CD44low NK1.1low (stage 1) to CD44high NK1.1low (stage 2) or from stage 2 to CD44high NK1.1high (stage 3), and hence result in higher percentages of stage 1 or stage 2 NKT cells. To determine whether enforced CD8 expression arrested NKT development at either stage 1 or 2, we assessed the expression of CD44 and NK1.1 on Vα14i NKT thymocytes from CD8 transgenic and nontransgenic littermates. The fractions of NKT cells in stages 1, 2, and 3 in either CD8 hemizygous or homozygous transgenic mice were similar to those of nontransgenic littermates, suggesting that expression of CD8 did not affect the developmental transition from stage 1–3 (Fig. 2 A).
The influence of CD8 transgene copy number on Vα14i NKT cell differentiation suggested the possibility that there was a threshold level of CD8 beyond which Vα14i NKT cell development was nearly completely inhibited. Thus, it was important to compare the levels of CD8 expressed in CD8 Tg/+ mice to those expressed by WT CD8+ SP thymocytes and mature T cells. We measured the anti-CD8 median fluorescence intensity (MFI) observed in WT SP CD8+ thymocytes, mature CD8+ T cells, and Vα14i NKT cells and conventional T lymphocytes from CD8 transgenic mice. We determined the number of anti-CD8 binding sites by interpolation of the MFI from each population against a standard curve prepared by staining beads with defined numbers of antibody binding sites. An analogous approach was also used to measure the numbers of anti-CD4 binding sites in CD4+ Vα14i NKT cells and conventional CD4+ T lymphocytes. We found that the numbers of both anti-CD8α and anti-CD8β binding sites on mature (TCRhigh) CD4+ thymocytes and CD4+ peripheral T cells in CD8 Tg/+ mice were similar to those observed in WT CD8+ SP thymocytes and T cells (Fig. S2 and not depicted). Vα14i NKT cells from CD8 Tg/+ mice were found to express levels of both CD8α and CD8β that were approximately half of those observed in WT CD8+ SP thymocytes and T cells (Fig. S2 and not depicted). However, a similar reduction in the amount of CD4 was also observed in CD4+ Vα14i NKT cells from either WT or CD8 transgenic animals as compared with WT CD4+ SP thymocytes and T cells (Fig. S2). These data demonstrate that the CD8 transgene construct drove expression levels in transgene hemizygous T cells that appeared physiologically appropriate. Furthermore, they suggest that the lower CD8 amounts observed in Vα14i NKT cells from CD8 Tg/+ mice might be attributable to general mechanisms that normally act to down-regulate CD4, and perhaps also expression of the TCR, in Vα14i NKT cells.
We examined the response of CD8 Tg/+ mice to αGalCer administered in vivo to determine if CD8 expression affected Vα14i NKT cell function. Spleen and liver cell suspensions, together with serum samples, were prepared 90 min after αGalCer injection. The cell suspensions were stained to identify Vα14i NKT cells, followed by intracellular staining for IFN-γ, IL-4, and TNF. We observed no difference in the response of CD8+ and WT Vα14i NKT cells to antigen administered in vivo, as measured by intracellular cytokine staining (Fig. 3 and not depicted). Thus the expression of CD8 had no apparent effect on the in vivo antigen response of Vα14i NKT cells.
Figure 3.
Response of CD8 Tg Vα14i NKT cells to antigen in vivo. CD8 Tg/+ and WT littermate mice were injected i.p. with 2 µg αGalCer. Mice were killed 90 min later, and liver and spleen cell suspensions were prepared and stained with αGalCer-CD1d tetramers and additional antibodies to define Vα14i NKT cells. Depicted is the intracellular staining of Vα14i NKT cells from αGalCer-challenged mice with anti-IFNγ (A) and anti–IL-4 (B) mAbs as compared with the staining of Vα14i NKT cells from mock-challenged mice. Data are representative of four independent experiments.
Effect of CD8 on Vα14i NKT cell TCR avidity
If CD8 acted as a coreceptor when expressed by Vα14i NKT cells, it would be predicted to increase the avidity and to decrease the TCR off rate for cognate antigen (Garcia et al., 1996). We therefore examined if CD8αβ increased Vα14i NKT cell antigen avidity by comparing CD8 transgenic and WT Vα14i NKT cells in two different CD1d tetramer binding assays. An equilibrium binding or tetramer binding isotherm analysis was performed by mixing WT and CD8 Tg/+ thymocytes and by staining aliquots with serial dilutions of αGalCer-CD1d tetramers, followed by staining with antibodies against CD8 and other T cell surface markers that permit unambiguous identification of Vα14i NKT cells. We also measured the tetramer off rates from WT and CD8 transgenic Vα14i NKT cells by determining the decrease in αGalCer-CD1d tetramer mean fluorescence intensity after incubation of stained aliquots at 37°C. We could not detect any effect of CD8 expression on Vα14i NKT cell antigen avidity or the off rate (Fig. S3).
Effects of the CD8 Tg on Vα14i NKT T cell Vβ gene repertoire and CD1d expression
The modulation of CD1d expression by thymocytes can affect NKT cell Vβ repertoire, with changes in CD1d levels correlating inversely with the fraction of Vα14i NKT cells expressing Vβ7 (Chun et al., 2003; Schümann et al., 2006; Wei et al., 2006). These data suggest that Vβ7 usage is enhanced under conditions of reduced antigen avidity. We thus examined the fraction of cells expressing Vβ7 and Vβ8.1/8.2 in CD8+ Vα14i NKT cells from CD8 Tg/+ mice. The CD8+ Vα14i NKT cell populations in the thymus contained an increased fraction of cells expressing Vβ7 (Fig. S4), which would be the opposite of expectation if CD8 expression enhanced the avidity of Vα14i NKT cells for endogenous ligands. Breeding the CD8 transgene onto a Kb and Db-null background had no influence on the increased Vβ7 expression by Vα14i NKT thymocytes from CD8 Tg mice (unpublished data; Vugmeyster et al., 1998). These findings indicate that the CD8 transgene could modulate Vα14i NKT cell selection through a mechanism independent of its ability to interact with classical MHC class I antigen-presenting molecules but in a manner apparently inconsistent with CD8 coreceptor function.
The effect of the CD8 transgene on the Vα14i NKT cell TCR Vβ repertoire was similar to that observed in mice with reduced levels of thymocyte CD1d expression (Schümann et al., 2006; Wei et al., 2006). These observations led us to examine CD1d expression by cells from CD8 Tg mice. We found no differences in the amount of CD1d between CD8 Tg/+ and WT mice in mononuclear cells obtained from the spleen or liver (not depicted) or in mature TCRhigh thymocytes (Fig. S5 A). In contrast, we found that CD1d expression in TCRlow DP thymocytes from CD8 Tg hemizygotes was reduced, with the MFI obtained by anti-CD1d staining only ∼60% of that observed in nontransgenic littermates (Fig. S5). The CD1d staining intensity of the TCRlow DP thymocyte subset in CD8 Tg/Tg mice was reduced even further to ∼30% of the level of WT (Fig. S5 B and not depicted). The amount of CD1d expression by the CD8 Tg/+ mice was not much greater than that expressed by Cd1d+/− mice, whereas the CD8 Tg homozygotes expressed less CD1d than either strain (unpublished data). These data implied that the effect of the CD8 Tg on the Vα14i NKT TCR Vβ repertoire was, at least in part, the result of an unanticipated reduction in CD1d expression by DP thymocytes in these mice, and they suggest that the effect of increased levels of CD8 in the Tg homozygous mice had an additive effect on CD1d expression.
Decreased thymocyte survival in CD8 Tg mice
The CD8 Tg mouse line used in this and the earlier study (Bendelac et al., 1994) is known to exhibit defects in thymocyte maturation (Wack et al., 2000). We therefore performed experiments to test if the deficit in Vα14i NKT cells in CD8 Tg homozygotes could be related to the more general defects in thymocyte development, as opposed to self-ligand–mediated negative selection. As previously reported (Wack et al., 2000), the transition from the DN to the DP stages is partially inhibited in thymocytes from the CD8 Tg line, which was reflected both by increases in the fractions of DN and immature SP thymocytes and by decreases in the fraction of DP thymocytes and overall thymocyte cellularity (Fig. 4, A and B; and not depicted). Furthermore, we found that the severity of these maturation defects was much more pronounced in mice that were homozygous for the CD8 transgene (Fig. 4, A and B; and not depicted).
Figure 4.
Effect of the CD8 transgene on thymocyte differentiation. (A) Decreased total thymocytes. Scatter plot depicting total thymocytes from six independent littermate groups, each containing one nontransgenic, one CD8 Tg/+, and one or two CD8 Tg/Tg mice. Littermates analyzed on the same day are represented by identical symbols. Paired Student's t test p-values are shown, with significant differences indicated by asterisks (***, P < 0.001). (B) Decreased DP thymocytes. CD4 and CD8 staining of thymocytes from two CD8 Tg/Tg mice along with littermates that were either CD8 Tg/+ or nontransgenic littermate controls (NLCs). The antibody used against CD8β.2 detects only the endogenous CD8β allele. (C) Decreased distal Jα rearrangements. Genomic DNA prepared from thymocytes from a CD8 Tg/+ mouse, a littermate control (NLC), and two CD8 Tg/Tg mice, as well as Rag−/− splenic DNA, was amplified in separate reactions with primers specific for Vα14 and four different Jα segments from either the proximal or distal region of the Jα locus. Samples of each reaction were subjected to agarose gel electrophoresis and analyzed by Southern blotting. Rearrangement products were identified by hybridization with a 32P-labeled Vα14 probe. Numbers designate bands resulting from rearrangement of Vα14 to the indicated Jα elements, and the proximity of the Jα segments allows the detection of several bands with one primer. All data in this figure are representative of at least three independent experiments.
Thymus hypocellularity can be attributed to defects either in thymocyte expansion or survival. To determine if thymocyte survival is perturbed in CD8 Tg mice, we used ex vivo cultures. DP thymocytes from CD8 Tg/Tg mice exhibited a markedly reduced viability in culture compared with either Tg/+ or WT littermates (Fig. S6 A). These data suggest that the lifespan of DP thymocytes in vivo in CD8 Tg/Tg mice was likely to be compromised, which could account for the more than fivefold decrease in total thymocyte number. Additionally, we observed that CD8 Tg/Tg DP thymocytes had lower TCR expression and higher forward light scatter than hemizygous and nontransgenic littermates (Fig. S6, B and C). These phenotypes suggest that CD8 Tg/Tg DP thymocytes had a higher fraction of relatively immature cells, and they are consistent with, although they do not formally demonstrate, a survival defect within the more mature DP subset.
Preferential Jα rearrangement in CD8 Tg mice
Mutations that restrict DP thymocyte lifespan are known to cause severe reductions in the development of Vα14i NKT cells (Bezbradica et al., 2005; Egawa et al., 2005). This has been attributed to the fact that gene rearrangement within the TCR-α locus proceeds in an ordered fashion, with recombination beginning at the 5′ or Vα proximal end of the Jα cluster, and then progressing in a 3′ or distal direction via secondary α rearrangements that occur during thymocyte maturation (Yannoutsos et al., 2001). Because the canonical Vα14-Jα18 rearrangement utilizes a Jα element near the 3′ end of the Jα locus, Vα14i NKT cell development is highly dependent on these secondary rearrangements that are decreased when DP thymocyte survival is impaired. We therefore performed multiplex PCR analysis to assess the relative frequency of rearrangement between Vα14 and both proximal and distal Jα elements. Rearrangement events between Vα14 and Jα elements near the proximal end of the Jα locus were readily detectable in all of the thymus samples regardless of the presence of CD8 transgene (Fig. 4 C). The canonical Vα14-Jα18 rearrangement was dramatically reduced in CD8 Tg/Tg (Fig. 4 C), but not in CD8 Tg/+ thymocyte DNA, which fits with the low frequency of Vα14i NKT thymocytes in homozygotes and near normal numbers in CD8 Tg/+ mice. However, CD8 Tg thymocytes exhibited lower frequencies of rearrangement events between Vα14 and all distal Jα elements assessed. Although this was particularly evident in thymocytes from mice homozygous for the CD8 transgene, reductions in the very distal Jα gene segments were observed even in CD8 Tg/+ mice (Fig. 4 C). These data demonstrate that the CD8αβ transgene induced a general defect in rearrangement to the distal end of the Jα locus, and they imply that the inhibition of Vα14i NKT cell development in CD8 Tg/Tg mice is not a result of antigen receptor specificity but rather of a reduction in the frequency of rearrangements necessary for the expression of the Vα14i TCR.
A Vα14i transgene rescues NKT development in CD8 Tg homozygous mice
To confirm that the decrease in Vα14i NKT cells in CD8 Tg/Tg mice is the result of a Jα rearrangement defect, we circumvented the putative gene rearrangement road block by generating mice that were homozygous for the CD8 transgene and also carried a transgene that enforced expression of a rearranged Vα14i TCR-α chain in all T cells (Bendelac et al., 1996). Analyses of these Vα14i Tg + CD8 Tg/Tg mice demonstrated that the introduction of the Vα14i transgene resulted in the generation of large numbers of αGalCer-CD1d tetramer-binding T cells and that a substantial portion of these cells exhibited a CD44high NK1.1high surface antigen phenotype that is characteristic of mature WT Vα14i NKT cells (Fig. 5 A). Furthermore, Vα14i NKT cells from Vα14i Tg + CD8 Tg/Tg double transgenic mice made a robust response to αGalCer stimulation in vivo, with equivalent IFN-γ synthesis and only slightly decreased IL-4 synthesis compared with Vα14i Tg mice hemizygous for the CD8 Tg (Fig. 5 B). These data confirm that thymocytes in CD8 Tg homozygotes expressing the Vα14i NKT TCR are not subject to negative selection and, furthermore, that the reduced amount of CD1d expressed by CD8 Tg/Tg thymocytes is sufficient to promote Vα14i NKT cell differentiation.
Figure 5.
A Vα14i transgene rescues the development of Vα14i NKT cells in CD8 Tg/Tg mice. (A) Flow cytometric analysis of the indicated electronically gated populations from the spleens of littermate controls, Vα14i Tg, CD8 Tg/Tg, and Vα14i Tg + CD8 Tg/Tg double transgenic mice depicting the staining with αGalCer-CD1d tetramers and anti–TCR-β, as well as the staining of cells within the Vα14i NKT cell gate for CD44 and NK1.1. Data are representative of two separate experiments involving five Vα14i Tg + CD8 Tg/Tg double transgenic mice and similar numbers of controls. (B) Flow cytometric analysis of intracellular cytokine expression by liver CD44high Vα14i NKT cells from Vα14i Tg + CD8 Tg/Tg double transgenic, Vα14i Tg, and control WT mice analyzed ex vivo 90 min after injection of αGalCer. Data are representative of three Vα14i Tg + CD8 Tg/Tg double transgenic Vα14i mice.
Th-POK is required for the differentiation of CD4+ Vα14i NKT cells
To determine why Vα14i NKT cells do not normally assume a CD8 SP fate, we explored the possibility that differentiation of the Vα14i NKT cell lineage might involve the expression of factors that inhibit CD8 expression. The Zbtb7b gene directs the expression of the Th-POK or c-krox protein, which is a member of the BTB-POZ family of Zn finger transcriptional repressors which is required for the development of conventional CD4 SP T cells (He et al., 2005). Mice that lack expression of a functional Th-POK protein lack mature CD4 SP T cells, and Th-POK–null thymocytes expressing TCRs that would normally promote positive selection to a CD4 SP lineage are instead redirected to a CD8 SP fate (Keefe et al., 1999; He et al., 2005). Th-POK has also been shown to repress CD8 expression by conventional CD4 peripheral T cells (Wang et al., 2008). Moreover, enforced expression of Th-POK changes the fate of thymocytes selected by classical MHC class I molecules from CD8 SP to CD4 SP (He et al., 2005; Sun et al., 2005). There is no evidence, however, that Th-POK has effects on the negative selection of T lymphocytes.
We examined whether a deficiency in Th-POK affected Vα14i NKT cells by phenotypic analysis of mice homozygous for the spontaneous helper deficient (hd) mutation in the Th-POK gene (Dave et al., 1998; He et al., 2005). We found that the numbers and percentages of Vα14i NKT cells in hd/hd mice were similar to those in WT or hd/+ littermates (Fig. 6, A and B). Interestingly, the mature Vα14i NKT cells from hd/hd mice were either CD8 SP or DN, with the ratio of CD8 SP to DN similar to the CD4/DN ratio observed in mice WT for Th-POK (Fig. 6 C). These data demonstrate that Th-POK is required for the differentiation of both CD4 SP Vα14i NKT and conventional T cells but not for the generation of DN Vα14i NKT cells, although there is evidence that these T lymphocytes are derived from a CD4+ precursor (Benlagha et al., 2005).
Figure 6.
Th-POK regulates CD4 and CD8 expression in Vα14i NKT cells. (A) Staining of thymocytes and electronically gated B220− splenocytes and liver mononuclear cells from hd/hd and +/+ littermates with αGalcer-CD1d tetramers and anti–TCR-β. The percentages of Vα14i NKT cells are indicated next to the gates. Data are representative of at least five individual experiments. (B) Scatter plots depicting the numbers of Vα14i NKT cells observed in the thymus (n = 6), spleen (n = 4), and liver (n = 3) of 6–10-wk-old hd/hd and hd/+ littermates. Different symbols are used to represent separate littermate pairs. Paired Student's t test p-values are shown, with significant differences indicated by asterisks (*, 0.05 > P < 0.01). Error bars show SD and horizontal bars show the arithmetic mean of the samples in each column. (C) CD4 and CD8 expression in T cells from mice carrying the hd mutation in the Th-POK gene. Liver mononuclear cells from WT (+/+, left) and hd/hd mice (right) were stained with αGalCer-CD1d tetramers together with antibodies against CD4, CD8α, and TCR-β. Plots shown depict the CD8 and CD4 expression in Vα14i NKT and conventional T cell populations (Conv T cells). Data are representative of at least six independent experiments.
Th-POK is required for Vα14i NKT cell function
Although Vα14i NKT cells from hd/hd mice exhibited no defects in the expression of surface antigens such as CD44 and NK1.1, we did find that they expressed abnormally low levels of the activation marker CD69 (Fig. 7 A). These data suggested that hd/hd Vα14i NKT cells might have a maturation or activation defect. We therefore subjected hd/hd and WT control mice to in vivo challenge with αGalCer. As assessed by intracellular cytokine staining and measurement of serum IL-4 levels, we found that hd/hd mice exhibited a markedly reduced in vivo response to αGalCer (Fig. 7, B and C). The decrease was strongest for IL-4 production but also involved TNF synthesis and IFN-γ to a lesser extent. These findings demonstrate that Th-POK is required for full Vα14i NKT cell function, although normal numbers of Vα14i NKT cells are generated in the hd mutant mice. Furthermore, the reduced response was not a direct consequence of CD8 expression by the mature cells because DN Vα14i NKT cells from hd/hd mice exhibited intracellular cytokine levels that were the same as or lower than those of the CD8+ subset (Fig. S7). However, it remained possible that very low levels of CD8 expression were sufficient to render Vα14i NKT cells hyporesponsive or that prior expression of CD8 partially anergized DN Vα14i NKT cells. We thus crossed hd mutants with mice carrying a targeted deletion of the CD8α gene and measured the responses of hd/hd CD8α−/− and hd/hd CD8α+/− Vα14i NKT cells to in vivo αGalCer challenge by intracellular cytokine staining (Fung-Leung et al., 1991). The response of hd/hd NKT cells was not affected by their CD8α genotype (Fig. 7 D). These data indicate that the effects of Th-POK deficiency on the function of Vα14i NKT cells are independent of its role in repressing CD8 expression in these cells.
Figure 7.
Th-POK is necessary for the normal functional response of Vα14i NKT cells. (A) Reduced CD69 expression. Histograms depicting the expression of CD44, NK1.1, and CD69 on Vα14i NKT cells from the livers and spleens of hd/hd (red) and +/+ (blue) littermates, with the staining of WT CD8 SP T cells (green) overlain for comparison. Results are representative of at least six independent experiments. (B) Reduced IL-4 production. Intracellular cytokine staining of Vα14i NKT cells from hd/hd and WT mice two h after injection of αGalCer or vehicle. Liver and spleen cell suspensions were stained with αGalCer-CD1d tetramers and anti–TCR-β, after which cells were fixed, permeabilized, and stained with antibodies against IL-4 and IFN-γ. Data are representative of six independent experiments. (C) ELISA was used to measure IL-4 in blood drawn from the indicated strains of mice injected 2 h previously with αGalCer. Similar results were obtained in separate assays of two other sets of hd/hd and +/+ or hd/+ littermates challenged with αGalCer (not depicted). (D) The functional effect of the hd mutation in Vα14i NKT cells is independent of CD8 expression. Staining for IL-4, IFN-γ, and TNF in permeabilized liver Vα14i NKT cells from mice with the indicated genotypes heterozygous or homozygous for the hd mutation and a disrupted CD8α allele. Cells were harvested 90 min after αGalCer challenge. Similar responses were observed in a second pair of hd/hd, CD8α−/−, and hd/hd CD8α+/− littermates.
Th-POK–mediated transcriptional regulation of cytokines in Vα14i NKT cells
Our data suggested that Th-POK might play a role in the induction of cytokine transcription upon antigen recognition by Vα14i NKT cells. We therefore prepared liver mononuclear cell RNA from mice with or without WT Th-POK function that had been challenged by αGalCer and measured the relative amounts of Il4 and Ifng transcripts by quantitative real-time PCR analysis of cDNA generated from these preparations. In agreement with the intracellular cytokine staining data, the induction of Il4 transcripts by αGalCer was dramatically reduced in Th-POK mutants, whereas the effect of Th-POK deficiency on Ifng transcript accumulation was more modest (Fig. 8).
Figure 8.
Effect of Th-POK deficiency on cytokine gene transcription in Vα14i NKT cells. (A and B) RNA analyzed was obtained from either liver mononuclear cells from the indicated mice that had been injected with 2 µg αGalCer (A) or mock-injected (not depicted) or from liver mononuclear cells from unstimulated mice from the same strains that had been highly enriched for Vα14i NKT cells by magnetic selection (B). cDNA was amplified with primers specific for the Il4 and Ifng loci using a real-time thermocycler. Relative transcript quantities were normalized to L32 and the fraction of Vα14i NKT cells in each preparation, as determined by flow cytometry. The bar graphs show data normalized to the amount from WT samples. Error bars indicate variation between duplicate or triplicate samples in one experiment. Data are representative of at least two experiments testing independently collected samples. Injection of αGalCer resulted in a >100-fold increase in Il4 and Ifng transcripts (not depicted).
Vα14i NKT cells are distinguished by the constitutive expression of detectable amounts of both Il4 and Ifng RNA in the absence of exogenous antigen stimulation (Stetson et al., 2003). The presence of these transcripts is likely to be a reflection of the epigenetic structure of the Il4 and Ifng gene loci, which may contribute to the ability of these cells to produce cytokines rapidly upon exposure to antigen. To determine if Th-POK contributed to the expression of basal cytokine transcripts, we analyzed RNA from liver mononuclear cells from unstimulated mice that were highly enriched for Vα14i NKT cells. We found that Il4 transcripts were dramatically reduced in Th-POK mutant Vα14i NKT cells (Fig. 8). Basal Ifng transcripts, in contrast, were only modestly affected by Th-POK mutation (Fig. 8).
Expression of Th-POK by Vα14i NKT cells
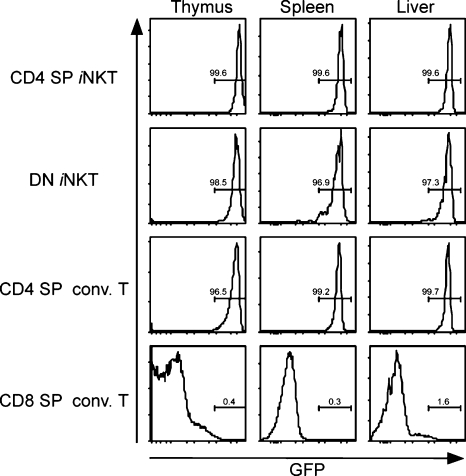
Our data suggest that Vα14i NKT cells normally express Th-POK, which might be required not only for the repression of CD8 expression but for their functional maturation. To examine Th-POK gene expression in Vα14i NKT cells, we analyzed Th-POKGFP/+ mice, in which the coding region of one allele of the Th-POK gene was replaced with sequence encoding GFP (Fig. 9; Setoguchi et al., 2008). Analysis of both the thymus and periphery of Th-POKGFP/+ mice revealed that the intensity of GFP expression in the vast majority of Vα14i NKT cells was similar to that of conventional CD4 SP T cells, regardless of whether the Vα14i NKT cells were CD4 SP or DN (Fig. 9). Qualitatively similar results were obtained when we analyzed CD4 SP and DN Vα14i NKT cells from F2F3 strain mice containing a Th-POK-GFP transgene (unpublished data; He et al., 2008). Altogether, these data indicate that Th-POK is expressed by essentially all Vα14i NKT cells and that it is necessary for normal Vα14i NKT cell basal cytokine gene transcription and secretion by antigen-stimulated cells.
Figure 9.
Th-POK is expressed throughout Vα14i NKT cell differentiation. Histograms depicting GFP reporter expression in Vα14i NKT and conventional T cell populations from the thymus, spleen, and liver of Th-POKGFP/+ mice. Similar data were obtained in at least four independent experiments on at least six mice.
DISCUSSION
The regulation of CD4 and CD8αβ expression during thymocyte maturation is largely dictated by the TCR coreceptor function of these molecules, as they participate in the recognition of self-ligands presented by MHC class I or class II molecules, respectively. The roles, if any, of these coreceptors in the function of those TCR-αβ+ T cell populations not selected by classical MHC class I or class II molecules are not as well understood. The initial characterizations of Vα14i NKT cells reported that they were absent from mice in which CD8 expression was enforced in T cells and, furthermore, that their Vβ repertoire was altered in CD8-deficient mice (Bendelac et al., 1994; Lantz and Bendelac, 1994). These data led to the conclusion that differentiating NKT cells in the thymus that expressed CD8 were negatively selected, and they implied that CD8 acts as a CD1d coreceptor to increase the avidity of these cells for targets expressing the selecting glycolipid antigens. According to this view, the thymic self-ligands for Vα14i NKT cells are of sufficient avidity that they are true TCR agonists or close to that level of affinity. Therefore, they can positively select Vα14i NKT cells in the absence of a coreceptor, with CD8 coreceptor expression in fact causing their negative selection.
In this manuscript, however, we report that Vα14i NKT cells can develop and function in the context of enforced CD8 expression, and we demonstrate that CD8 has no apparent effect on Vα14i NKT cell avidity for cognate antigen presented by CD1d. The negative effect of the CD8 Tg on the thymic differentiation of Vα14i NKT cells is not a result of negative selection, but is related to the effect of the transgene on the normal developmental progression of all thymocytes. The mouse line used in our studies was in fact previously shown to exhibit a reduced efficiency of early thymocyte maturation from the DN to DP stage (Wack et al., 2000), which we have also observed (unpublished data). The previous paper did not report any effects of the CD8αβ transgene on DP lifespan, however, and in fact demonstrated that a CD8 transgene construct that was not expressed until the DP stage had no effect on thymic cellularity. It is important to note, however, that all of the transgenic animals examined in the previous study were hemizygous for the CD8αβ transgenes. We also observed a much less dramatic phenotype in hemizygotes as compared with CD8 Tg homozygotes, in terms of the DP lifespan during in vitro culture, the accumulation of DP thymocytes in vivo and overall thymus cellularity, and the frequency of distal Jα rearrangements. Thus, our data collectively indicate that the CD8αβ transgene can affect at least two stages of thymocyte development but the effects of the transgene on DP thymocytes are much more apparent in homozygous animals. The mechanism for the developmental effect of the CD8 transgene on DP cell number is not known, and because the transgene overexpression is not physiological, we have not sought to determine the mechanism in this study of Vα14i NKT cell differentiation. We speculate, however, that the overexpression of CD8αβ could lead to the sequestration of critical signaling molecules, such as the src family tyrosine kinase lck, away from TCR signaling complexes. Aberrant TCR signaling in mice homozygous for the CD8 transgenes would then lead to a reduction in the DP thymocyte lifespan, with effects on rearrangement within the TCR-α locus that are the likely proximal cause of their Vα14i NKT cell deficiency.
The Vα14i NKT cells that are found in CD8 Tg/+ mice are functionally normal, as measured by intracellular cytokine expression after antigen challenge in vivo. In addition, we have found no differences in the expression of CD69, NK1.1, or other NK and activation markers between Vα14i NKT cells from CD8 Tg/+ mice and nontransgenic littermates (unpublished data). In contrast, the CD8 transgene does appear to affect the TCR repertoire of Vα14i NKT cells, in that Vα14i NKT thymocytes from CD8 Tg hemizygous mice have an elevated fraction of Vβ7+ cells. These data might suggest that the expression of CD8 directly affects the avidity of Vα14i NKT cells for selecting determinants, but contrary to the conclusions drawn from some previous studies, they imply that Vβ7-expressing Vα14i NKT cells are more dependent, rather than less dependent, on the added avidity putatively provided by CD8. However, a second unexpected finding from the CD8 Tg mice, in addition to a reduced DP lifespan, is decreased CD1d expression by thymocytes. We consider it likely that the changes in the Vβ repertoire of Vα14i NKT cells observed in these mice are the result of reduced CD1d expression rather than CD8 mediating a coreceptor function or enhanced cell–cell interactions. This interpretation is consistent with studies showing that the fraction of Vβ7+ Vα14i NKT cells correlated inversely with CD1d expression levels (Chun et al., 2003; Schümann et al., 2006; Wei et al., 2006). Although CD1d expression is further reduced in CD8 Tg homozygotes as compared with CD8 Tg hemizygotes or CD1d+/− mice, the fact that introduction of a Vα14i transgene restores NKT development and function demonstrates that these low CD1d levels are nevertheless sufficient to drive the differentiation of Vα14i NKT cells, and they point to the rearrangement defect as the principal cause of the NKT deficit in the CD8 Tg/Tg background.
Vα14i NKT cell development is uniquely dependent on a variety of receptors, signaling molecules, and transcription factors (Matsuda and Gapin, 2005). Recent studies of mice deficient for the nuclear high mobility group protein Tox have shown that this factor is required for the development of both conventional CD4+ T cells and the Vα14i NKT cell subsets (Aliahmad and Kaye, 2008), demonstrating a common genetic requirement for these two T lymphocyte subtypes. Our data reveal that the BTB-POZ zinc finger transcription factor Th-POK also has important roles in directing the differentiation of both conventional CD4+ and Vα14i NKT cells. In Th-POK mutant mice, the differentiation of all CD4 SP cells, including conventional and Vα14i NKT cells, is redirected toward a CD8 SP fate (He et al., 2005; Keefe et al., 1999). Interestingly, in addition to CD8+ cells, a population of DN Vα14i NKT cells remains in these mice. Additionally, our data indicate that Th-POK is expressed in all mature Vα14i NKT cells, whether they are CD4 SP or DN. Th-POK–deficient Vα14i NKT cells are hyporesponsive to antigenic stimulation. In WT mice, DN Vα14i NKT cells are highly responsive to stimulation with αGalCer (Kronenberg and Gapin, 2002), and Vα14i NKT cells are also normal in CD4-deficient mice (Bendelac et al., 1994). In this manuscript, we demonstrate that DN Vα14i NKT cells in the Th-POK mutant mice are as hyporesponsive as their CD8-expressing counterparts and that deletion of the CD8α gene has no effect on the response of Th-POK–deficient Vα14i NKT cells to αGalCer. Collectively, these data suggest that the functional defect in immune responses by Vα14i NKT cells in the Th-POK mutant mice is not a direct consequence of either the absence of CD4 expression or the presence of CD8. Our data further suggest that factors such as Th-POK are expressed as a consequence of the activation of the Vα14i NKT cell developmental program. Because Th-POK is capable of repressing CD8, this implies that Vα14i NKT cells down-regulate CD8 at least in part as a consequence of Th-POK expression (Wang et al., 2008). Furthermore, unexpectedly Th-POK is also required for the full functional maturation of these cells. Interestingly, another BTB-POZ family member, PLZF, has been recently shown to play a major role in specifying the function of Vα14i NKT cells (Kovalovsky et al., 2008; Savage et al., 2008). However, PLZF-deficient Vα14i NKT cells exhibit changes in surface antigen expression, tissue distribution, and antigen response that are distinct from those in Th-POK mutants (Kovalovsky et al., 2008; Savage et al., 2008). Thus, it remains to be determined whether the structural similarities between Th-POK and PLZF bear any relation to the mechanisms underlying their diverse effects on Vα14i NKT development and function.
In conclusion, we provide strong evidence that the development of CD8+ Vα14i NKT cells is not prevented by negative selection and that when CD8 expression is either forced or removed by genetic manipulation there is little effect on Vα14i NKT cell repertoire or function. Furthermore, our data suggest that the transcription factor Th-POK, expressed essentially throughout the Vα14i NKT developmental program, is required for the normal function of these cells. As a by-product of this expression, they are CD8 negative and directed to either a CD4 SP or DN fate.
MATERIALS AND METHODS
Mice.
The CD8αβ Tg mouse line was obtained from cryopreserved storage at The Jackson Laboratory. Mice with targeted mutations in CD1d (Mendiratta et al., 1997) were provided by L. van Kaer (Vanderbilt University, Nashville, TN). CD8α-deficient mice were purchased from The Jackson Laboratory (Fung-Leung et al., 1991). The hd/hd and Th-POKGFP mouse lines have been previously described (Keefe et al., 1999; Setoguchi et al., 2008). Mice with mutations in H-2Kb and H-2Db (Vugmeyster et al., 1998) were gifts from A. Sette (La Jolla Institute for Allergy and Immunology, La Jolla, CA). Vα14i transgenic mice (Bendelac et al., 1996) were obtained from A. Bendelac (University of Chicago, Chicago, IL). All mice, except for the Th-POKGFP line, had been backcrossed extensively onto a C57BL/6 background. Mice were housed under specific pathogen-free conditions and the experiments were in accordance with animal protocols approved by the Institutional Animal Care and Use Committees at the La Jolla Institute for Allergy and Immunology or the Fox Chase Cancer Center.
Retroviral-mediated CD8 expression.
Full-length cDNAs encoding mouse CD8α and CD8β were subcloned into the retroviral vector MigRI (Pear et al., 1998). Transfection of the ϕNX ecotropic retroviral packaging cell line and transduction of iNKT cell hybridomas were performed as previously described (Kinsella and Nolan, 1996; Engel and Murre, 1999). Transduced lines were enriched for CD8β+ cells by labeling with magnetic µ beads and passage over LS columns (Miltenyi Biotec) and selected for expression of near-physiological levels of CD8 by cell sorting with a FACSVantage SE (BD). TCR expression on transduced hybridoma lines was examined by flow cytometry before each αGalCer stimulation assay, and magnetic enrichment or cell sorting was used when necessary to equalize TCR expression levels between CD8-expressing and empty vector–transduced lines.
Antigen stimulation.
Thymocytes (5 × 106/ml) or A20.CD1d cells (106/ml; Brossay et al., 1998b) were incubated with various concentrations of αGalCer for 4 h at 37°C and then washed, resuspended at the same cell concentration, and added to 96-well flat-bottom tissue culture plates in a volume of 0.1 ml/well. Hybridomas (5 × 104/well) were added to wells in a volume of 0.1 ml, and cells were incubated overnight at 37°C. Stimulation with αGalCer-loaded purified CD1d and ELISA assays were performed as previously described (Sidobre et al., 2002). In vivo antigen challenge was performed by injecting mice i.p. or i.v. with 2 µg αGalCer dissolved in 0.2 ml of PBS. Mice were killed 90–120 min after injection, at which time blood was drawn for serum cytokine analysis and spleen and liver mononuclear cell suspensions were prepared.
Antibodies, cell staining, and flow cytometry.
R-phycoerythrin–conjugated and allophycocyanin streptavidin-CD1d tetramers loaded with αGalCer were produced as previously described (Sidobre et al., 2002). Allophycocyanin-conjugated streptavidin-CD1d tetramers loaded with the αGalCer analogue PBS57 were supplied by the National Institutes of Health Core Tetramer Facility. All antibodies were purchased from BD, eBioscience, or BioLegend. Flow cytometry was done using either a FACSCalibur or LSRII (BD), and data was analyzed using FlowJo software (Tree Star, Inc.). Intracellular cytokine staining was performed using a kit and protocol purchased from BD. Primary mouse cell suspensions analyzed were obtained from mice between 6 and 10 wk of age.
Genomic rearrangement analysis.
A multiplex PCR strategy to detect TCR-α rearrangements has already been described (Pasqual et al., 2002). Genomic DNA was isolated from thymocytes or RAG-deficient splenocytes using the DNeasy Blood and Tissue kit (QIAGEN) and was amplified using an upstream primer specific for the Vα14 gene segments paired in separate reactions with primers specific for Jα 2, 9, 16, 48, and 56. Each primer pair amplified the specific Vα14-Jα (x) rearrangement, as well as rearrangements to Jα gene segments within up to ∼5 kb upstream of the targeted Jα. DNA samples were also amplified with Cα primers as a positive control. PCR primers used in this study have been described previously (Mancini et al., 2001; Pasqual et al., 2002; Hager et al., 2007). Amplifications were performed with Expand High-Fidelity Polymerase (Roche Applied Science) or Platinum Taq DNA Polymerase High Fidelity (Invitrogen) kits. Thermocycling conditions used were as previously described (Hager et al., 2007), except that the extension temperature was lowered to 68°C when the enzyme and kit were used (Invitrogen). PCR products were resolved on 1.4% agarose gels and blotted onto positively charged nylon membranes under alkaline conditions. A Vα14-specific probe was generated by PCR amplification of plasmid DNA containing a Vα14i NKT TCR construct using the following primers: 5′-GGGAGAGAACTGCGTCCTTCAATGTA-3′ and 5′-AGATGTAGGTGGCAGTGTCATCCAG-3′. The resulting PCR product was gel purified and labeled with 32P-dCTP by random oligonucleotide priming, according to the DNA labeling systems kit and protocol (GE Healthcare). The resulting radiolabeled probe was used for Southern blot hybridization as previously described (Budowle and Baechtel, 1990).
Real-time PCR.
For Vα14i NKT cell preparation, liver mononuclear cells were first incubated using biotinylated antibodies against CD19, CD45R, CD62L, TER-119, CD11b, CD24, F4/80, and Ly6G/Gr-1 (2 µg/ml each), and antibody-bound cells were removed using the EasySep system (Stem Cell Technologies). The remaining cells were then incubated with PE-CD1d tetramers loaded with αGalCer (1:100 dilution) together with 1 µg/ml of free streptavidin, and tetramer-binding cells were isolated using anti-PE microbeads and LS columns (Miltenyi Biotec). The cells were kept at 4°C after tetramer incubation until final isolation, at which point they were stored at −80°C. Purity as determined by flow cytometry was routinely 70% or greater. RNA was prepared using the RNeasy kit and protocol (QIAGEN). cDNA was prepared using the iScript kit and protocol (Bio-Rad Laboratories), and PCR reactions were performed using the Light SYBR 480 Master Mix (Roche). Cytokine gene transcript levels were normalized either to L32 transcript levels and the percentage of Vα14i NKT cells in each cell preparation or to Vα14-Jα18 transcript levels. Similar results were observed using either method of normalization. PCR primer sequences were as follows: Il4 forward, 5′-ACAGGAGAAGGGACGCCAT-3′; Il4 reverse, 5′-GAAGCCCTACAGACGAGCTCA-3′; Ifng forward, 5′-GGATGCATTCATGAGTATTGC-3′; Ifng reverse, 5′-CCTTTTCCGCTTCCTGAGG-3′; L32 forward, 5′-GAAACTGGCGGAAACCCA-3′; L32 reverse, 5′-GGATCTGGCCCTTGAACCTT-3′; Vα14 forward, 5′-CTAAGCACAGCACGCTGCACA-3′; and Jα18 reverse, 5′-CAGGTATGACAATCAGCTGAGTCC-3′.
Statistical analyses.
All calculations of statistical significance were determined using a paired Student's t test. When multiple samples of the same genotype were determined in the same experiment, the arithmetic mean of those samples was used for the paired t test.
Online supplemental material.
Fig. S1 shows that CD8 expression has no effect on the avidity of Vα14i NKT hybridomas for CD1d tetramers. Fig. S2 shows CD8 and CD4 expression levels by Vα14i NKT cells and conventional T lymphocytes from the thymus and liver of CD8 Tg/+ and WT littermates. Fig. S3 shows CD1d tetramer binding by Vα14i NKT thymocytes from CD8 Tg and nontransgenic littermates. Fig. S4 depicts the effect of the CD8 transgene on Vα14i NKT cell Vβ usage. Fig. S5 shows that the CD8 transgene acts to lower CD1d expression in DP thymocytes. Fig. S6 shows that CD8 Tg thymocytes have reduced survival in vitro and an altered phenotype. Fig. S7 shows that the reduced response of hd/hd Vα14i NKT cells to αGalCer is not restricted to the CD8-expressing subset.
Acknowledgments
We thank Archana Khurana for preparation of CD1d tetramers, Laurent Gapin for advice on rearrangement analysis, and Jr-Wen Shui for help with Southern blotting and hybridization.
This work was supported by National Institutes of Health grants R37 AI71922 (M. Kronenberg) and R01 AI42915 (D. Kappes), and a NHMRC Australia CJ Martin Fellowship #237029 (K. Hammond).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- αGalCer
- α galactosyl ceramide
- DN
- double negative
- DP
- double positive
- hd
- helper deficient
- MFI
- median fluorescence intensity
- POZ
- Poxviruses and Zinc-finger
- SP
- single positive
- Th-POK
- Th, POZ, and Krüppel family
- Vα14i
- Vα14 invariant
References
- Aliahmad P., Kaye J. 2008. Development of all CD4 T lineages requires nuclear factor TOX. J. Exp. Med. 205:245–256 10.1084/jem.20071944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A. 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182:2091–2096 10.1084/jem.182.6.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A., Killeen N., Littman D.R., Schwartz R.H. 1994. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 263:1774–1778 10.1126/science.7907820 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Lantz O., Quimby M.E., Yewdell J.W., Bennink J.R., Brutkiewicz R.R. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science. 268:863–865 10.1126/science.7538697 [DOI] [PubMed] [Google Scholar]
- Bendelac A., Hunziker R.D., Lantz O. 1996. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J. Exp. Med. 184:1285–1293 10.1084/jem.184.4.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlagha K., Kyin T., Beavis A., Teyton L., Bendelac A. 2002. A thymic precursor to the NK T cell lineage. Science. 296:553–555 10.1126/science.1069017 [DOI] [PubMed] [Google Scholar]
- Benlagha K., Wei D.G., Veiga J., Teyton L., Bendelac A. 2005. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202:485–492 10.1084/jem.20050456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins S.P., Cochrane A.D., Pellicci D.G., Smyth M.J., Godfrey D.I. 2005. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur. J. Immunol. 35:1399–1407 10.1002/eji.200425958 [DOI] [PubMed] [Google Scholar]
- Bezbradica J.S., Hill T., Stanic A.K., Van Kaer L., Joyce S. 2005. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc. Natl. Acad. Sci. USA. 102:5114–5119 10.1073/pnas.0408449102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezbradica J.S., Gordy L.E., Stanic A.K., Dragovic S., Hill T., Hawiger J., Unutmaz D., Van Kaer L., Joyce S. 2006. Granulocyte-macrophage colony-stimulating factor regulates effector differentiation of invariant natural killer T cells during thymic ontogeny. Immunity. 25:487–497 10.1016/j.immuni.2006.06.017 [DOI] [PubMed] [Google Scholar]
- Brossay L., Naidenko O., Burdin N., Matsuda J., Sakai T., Kronenberg M. 1998a. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J. Immunol. 161:5124–5128 [PubMed] [Google Scholar]
- Brossay L., Tangri S., Bix M., Cardell S., Locksley R., Kronenberg M. 1998b. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J. Immunol. 160:3681–3688 [PubMed] [Google Scholar]
- Budowle B., Baechtel F.S. 1990. Modifications to improve the effectiveness of restriction fragment length polymorphism typing. Appl. Theor. Electrophor. 1:181–187 [PubMed] [Google Scholar]
- Burdin N., Brossay L., Koezuka Y., Smiley S.T., Grusby M.J., Gui M., Taniguchi M., Hayakawa K., Kronenberg M. 1998. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J. Immunol. 161:3271–3281 [PubMed] [Google Scholar]
- Chun T., Page M.J., Gapin L., Matsuda J.L., Xu H., Nguyen H., Kang H.S., Stanic A.K., Joyce S., Koltun W.A., et al. 2003. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J. Exp. Med. 197:907–918 10.1084/jem.20021366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave V.P., Allman D., Keefe R., Hardy R.R., Kappes D.J. 1998. HD mice: a novel mouse mutant with a specific defect in the generation of CD4(+) T cells. Proc. Natl. Acad. Sci. USA. 95:8187–8192 10.1073/pnas.95.14.8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T., Eberl G., Taniuchi I., Benlagha K., Geissmann F., Hennighausen L., Bendelac A., Littman D.R. 2005. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 22:705–716 10.1016/j.immuni.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Engel I., Murre C. 1999. Ectopic expression of E47 or E12 promotes the death of E2A-deficient lymphomas. Proc. Natl. Acad. Sci. USA. 96:996–1001 10.1073/pnas.96.3.996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D.A., Hunt S.W., III, Hood L. 1985. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J. Exp. Med. 162:528–545 10.1084/jem.162.2.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W.P., Schilham M.W., Rahemtulla A., Kündig T.M., Vollenweider M., Potter J., van Ewijk W., Mak T.W. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 65:443–449 10.1016/0092-8674(91)90462-8 [DOI] [PubMed] [Google Scholar]
- Garcia K.C., Scott C.A., Brunmark A., Carbone F.R., Peterson P.A., Wilson I.A., Teyton L. 1996. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 384:577–581 10.1038/384577a0 [DOI] [PubMed] [Google Scholar]
- Godfrey D.I., MacDonald H.R., Kronenberg M., Smyth M.J., Van Kaer L. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231–237 10.1038/nri1309 [DOI] [PubMed] [Google Scholar]
- Hager E., Hawwari A., Matsuda J.L., Krangel M.S., Gapin L. 2007. Multiple constraints at the level of TCRalpha rearrangement impact Valpha14i NKT cell development. J. Immunol. 179:2228–2234 [DOI] [PubMed] [Google Scholar]
- He X., He X., Dave V.P., Zhang Y., Hua X., Nicolas E., Xu W., Roe B.A., Kappes D.J. 2005. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 433:826–833 10.1038/nature03338 [DOI] [PubMed] [Google Scholar]
- He X., Park K., Wang H., He X., Zhang Y., Hua X., Li Y., Kappes D.J. 2008. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 28:346–358 10.1016/j.immuni.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Keefe R., Dave V., Allman D., Wiest D., Kappes D.J. 1999. Regulation of lineage commitment distinct from positive selection. Science. 286:1149–1153 10.1126/science.286.5442.1149 [DOI] [PubMed] [Google Scholar]
- Kinsella T.M., Nolan G.P. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405–1413 10.1089/hum.1996.7.12-1405 [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Borg N.A., Pellicci D.G., Beddoe T., Kostenko L., Clements C.S., Williamson N.A., Smyth M.J., Besra G.S., Reid H.H., et al. 2006. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J. Exp. Med. 203:661–673 10.1084/jem.20051777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Yoshimoto T., Yoshimoto T., Fujimoto J., Nakanishi K. 2008. Interferon-gamma is a therapeutic target molecule for prevention of postoperative adhesion formation. Nat. Med. 14:437–441 10.1038/nm1733 [DOI] [PubMed] [Google Scholar]
- Kovalovsky D., Uche O.U., Eladad S., Hobbs R.M., Yi W., Alonzo E., Chua K., Eidson M., Kim H.J., Im J.S., et al. 2008. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat. Immunol. 9:1055–1064 10.1038/ni.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Engel I. 2007. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr. Opin. Immunol. 19:186–193 10.1016/j.coi.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Gapin L. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557–568 [DOI] [PubMed] [Google Scholar]
- Lantz O., Bendelac A. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097–1106 10.1084/jem.180.3.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas C.M., Day Y.J., Marshall M.A., Engelhard V.H., Linden J. 2006. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J. Exp. Med. 203:2639–2648 10.1084/jem.20061097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman A.J., Naidenko O.V., Attinger A., Koning F., Lena C.J., Xiong Y., Chang H.C., Reinherz E., Kronenberg M., Cheroutre H. 2001. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 294:1936–1939 10.1126/science.1063564 [DOI] [PubMed] [Google Scholar]
- Loza M.J., Metelitsa L.S., Perussia B. 2002. NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur. J. Immunol. 32:3453–3462 [DOI] [PubMed] [Google Scholar]
- Mancini S.J., Candéias S.M., Di Santo J.P., Ferrier P., Marche P.N., Jouvin-Marche E. 2001. TCRA gene rearrangement in immature thymocytes in absence of CD3, pre-TCR, and TCR signaling. J. Immunol. 167:4485–4493 [DOI] [PubMed] [Google Scholar]
- Matsuda J.L., Gapin L. 2005. Developmental program of mouse Valpha14i NKT cells. Curr. Opin. Immunol. 17:122–130 10.1016/j.coi.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Martin W.D., Hong S., Boesteanu A., Joyce S., Van Kaer L. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 6:469–477 10.1016/S1074-7613(00)80290-3 [DOI] [PubMed] [Google Scholar]
- Pasqual N., Gallagher M., Aude-Garcia C., Loiodice M., Thuderoz F., Demongeot J., Ceredig R., Marche P.N., Jouvin-Marche E. 2002. Quantitative and qualitative changes in V-J α rearrangements during mouse thymocytes differentiation: implication for a limited T cell receptor α chain repertoire. J. Exp. Med. 196:1163–1173 10.1084/jem.20021074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W.S., Miller J.P., Xu L., Pui J.C., Soffer B., Quackenbush R.C., Pendergast A.M., Bronson R., Aster J.C., Scott M.L., Baltimore D. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 92:3780–3792 [PubMed] [Google Scholar]
- Robey E.A., Fowlkes B.J., Gordon J.W., Kioussis D., von Boehmer H., Ramsdell F., Axel R. 1991. Thymic selection in CD8 transgenic mice supports an instructive model for commitment to a CD4 or CD8 lineage. Cell. 64:99–107 10.1016/0092-8674(91)90212-H [DOI] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. 2008. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 29:391–403 10.1016/j.immuni.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümann J., Mycko M.P., Dellabona P., Casorati G., MacDonald H.R. 2006. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 176:2064–2068 [DOI] [PubMed] [Google Scholar]
- Setoguchi R., Tachibana M., Naoe Y., Muroi S., Akiyama K., Tezuka C., Okuda T., Taniuchi I. 2008. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 319:822–825 10.1126/science.1151844 [DOI] [PubMed] [Google Scholar]
- Sidobre S., Naidenko O.V., Sim B.C., Gascoigne N.R., Garcia K.C., Kronenberg M. 2002. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 169:1340–1348 [DOI] [PubMed] [Google Scholar]
- Singer A., Adoro S., Park J.H. 2008. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 8:788–801 10.1038/nri2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D.B., Mohrs M., Reinhardt R.L., Baron J.L., Wang Z.E., Gapin L., Kronenberg M., Locksley R.M. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198:1069–1076 10.1084/jem.20030630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G., Liu X., Mercado P., Jenkinson S.R., Kypriotou M., Feigenbaum L., Galéra P., Bosselut R. 2005. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat. Immunol. 6:373–381 10.1038/ni1183 [DOI] [PubMed] [Google Scholar]
- Teitell M., Holcombe H.R., Brossay L., Hagenbaugh A., Jackson M.J., Pond L., Balk S.P., Terhorst C., Peterson P.A., Kronenberg M. 1997. Nonclassical behavior of the mouse CD1 class I-like molecule. J. Immunol. 158:2143–2149 [PubMed] [Google Scholar]
- Tupin E., Kinjo Y., Kronenberg M. 2007. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5:405–417 10.1038/nrmicro1657 [DOI] [PubMed] [Google Scholar]
- Vugmeyster Y., Glas R., Pérarnau B., Lemonnier F.A., Eisen H., Ploegh H. 1998. Major histocompatibility complex (MHC) class I KbDb −/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc. Natl. Acad. Sci. USA. 95:12492–12497 10.1073/pnas.95.21.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wack A., Coles M., Norton T., Hostert A., Kioussis D. 2000. Early onset of CD8 transgene expression inhibits the transition from DN3 to DP thymocytes. J. Immunol. 165:1236–1242 [DOI] [PubMed] [Google Scholar]
- Wang L., Wildt K.F., Castro E., Xiong Y., Feigenbaum L., Tessarollo L., Bosselut R. 2008. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity. 29:876–887 10.1016/j.immuni.2008.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D.G., Curran S.A., Savage P.B., Teyton L., Bendelac A. 2006. Mechanisms imposing the Vβ bias of Vα14 natural killer T cells and consequences for microbial glycolipid recognition. J. Exp. Med. 203:1197–1207 10.1084/jem.20060418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoutsos N., Wilson P., Yu W., Chen H.T., Nussenzweig A., Petrie H., Nussenzweig M.C. 2001. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J. Exp. Med. 194:471–480 10.1084/jem.194.4.471 [DOI] [PMC free article] [PubMed] [Google Scholar]