Abstract
There are many different cells in the immune system. To mount an effective immune response, they need to communicate with each other. One way in which this is done is by the formation of immunological synapses between cells. Recent developments show that the immune synapse serves as a focal point for exocytosis and endocytosis, directed by centrosomal docking at the plasma membrane. In this respect, formation of the immunological synapse bears striking similarities to cilia formation and cytokinesis. These intriguing observations suggest that the centrosome may play a conserved role in designating a specialized area of membrane for localized endocytosis and exocytosis.
Cells of the immune system communicate both by direct interactions via membrane-bound receptors and via secreted mediators from one cell to another. Although the membrane-bound antigen receptors specifically recognize their target cells, this is not necessarily true for secreted proteins such as cytokines. The problem of bestowing specificity on secreted proteins seems to have been overcome by the formation of what has become known as the immunological synapse. The immunological synapse has created a frenzy of research since the term was first coined by Bill Paul in 1994 to describe the directed secretion of cytokines into a “small space between the two interacting cells,” based on the work of Poo et al. (1988), Kupfer et al. (1991), and Kupfer et al. (1994). Synapses are formed between both T cells (Dustin et al., 1998; Monks et al., 1998; Grakoui et al., 1999) and B cells (Batista et al., 2001) with their cognate antigen-presenting cells (APCs), and both are characterized by a dramatic reorganization of the receptors involved in recognition and adhesion (Fig. 1). Monks et al. (1998) termed these regions the central supramolecular activation complex (SMAC [cSMAC]) and peripheral SMAC (pSMAC), with the cSMAC defining the cluster of T cell receptor (TCR) and associated signaling proteins and the pSMAC a ring of tight adhesion between the cells. The beautiful images generated by Monks et al. (1998) triggered much speculation as to the precise roles of these discrete areas with a great deal of work focused on how the cSMAC controls receptor activation and down-regulation. The picture that is beginning to emerge is that the synapse is a focal point for both exocytosis and endocytosis, both of which are triggered by localized cell signaling at the plasma membrane. Intriguingly, this turns out to be reminiscent of other areas of focused secretion and endocytosis, which form during cytokinesis and cilia formation in other cell types.
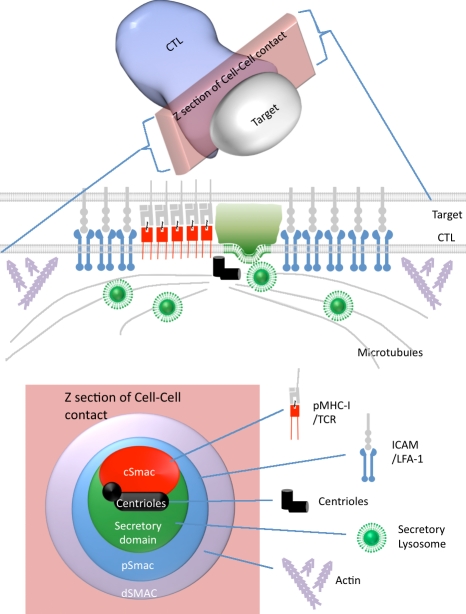
Figure 1.
The immunological synapse. Cartoon summary of the organization of receptors showing the relative positions of the cSMAC, pSMAC, dSMAC, centrioles, actin and microtubule cytoskeletons, and secretory lysosomes at the immunological synapse in cross section and across the area of cell contact.
Several events occur at the immune synapse that are critical in focusing both exocytic and endocytic events to this site. Signaling triggers massive reorganization of both the actin and microtubule cytoskeletons, with the centrosome (the microtubule-organizing center [MTOC] in T cells) moving right up to the plasma membrane at the cSMAC (Fig. 1). Both the Golgi and recycling endosomes polarize to the synapse. This directs both secretion and endocytosis to a small cleft sealed off from the external environment by the tightly apposed membranes in the pSMAC.
Signaling at the synapse
The precise site of signaling events leading to immune synapse formation has been controversial (for review see Dustin, 2009). Early signaling events have been shown to occur in peripheral microclusters in the pSMAC that coalesce into the cSMAC in both T and B cell synapses (Campi et al., 2005; Yokosuka et al., 2005; Varma et al., 2006; Depoil et al., 2008), and active tyrosine kinases were only detected early in CD4 synapse formation (Lee et al., 2002). These findings raised a question over whether the cSMAC served to sustain TCR signaling or rather simply to terminate signaling by becoming a site for TCR internalization and degradation (Liu et al., 2000; Lee et al., 2002). More recent experiments have shown active tyrosine kinases within the cSMAC of CD4 synapses at later time points and lower antigen doses (Cemerski et al., 2008), and these authors suggest that the quality of the peptide–major histocompatibility complex (pMHC) ligand might determine whether signaling is enhanced in the cSMAC or down-regulated by internalization of the receptor.
Most of these initial studies (see previous paragraph) were performed in CD4 T cells, which make long-lived synapses (hours) with their APCs. More recent data suggests that signaling in the cSMAC of CD8 cells may differ from CD4 cells. CD8 T cells form much more transient synapses than CD4 cells, lasting only minutes, as the targets are killed. Activated Src kinases were detected in the cSMAC of CD8 (Beal et al., 2009; Jenkins et al., 2009) but not CD4 cells at early time points (Beal et al., 2009). Upon disruption of CD8 interactions, Src kinase activation was no longer seen in the cSMAC but only in peripheral microclusters. Use of cytotoxic T lymphocytes (CTLs) from OT-1 transgenic mice, in which the strength of the TCR signal is easily adjusted by using variant peptides, revealed that signaling does occur within the cSMAC of CD8 T cells in response to both strong and weak pMHC ligands (Jenkins et al., 2009). However, signaling in response to weak pMHC ligands was rapidly down-regulated by phosphatases and could only be detected in the presence of a tyrosine phosphatase inhibitor. Collectively, these results indicate that the cSMAC serves as a focal zone of signaling but that these events can be regulated on several levels.
Endocytosis at the synapse
The cSMAC not only plays a role in signaling but also in receptor recycling. This arises from the fact that, as the synapse forms, the recycling endosomal compartment polarizes to the point immediately beneath the cSMAC of the immunological synapse (Das et al., 2004). Das et al. (2004) demonstrated the continuous recycling of TCR from the cell surface to the recycling compartment, where it colocalizes with transferrin receptor, to the plasma membrane at the cSMAC. Upon engagement by pMHC ligands, the rate of endocytosis of TCR is unchanged, but the receptors are down-regulated as they fail to recycle to the plasma membrane and are instead sent to the lysosome for degradation (Liu et al., 2000). TCR signaling is initiated by Lck, which associates with CD4 or CD8 chains of the TCR complex. Lck delivery to the plasma membrane appears to be controlled by activation of Rab11 on recycling endosomes, suggesting that Lck delivery to the membrane may also be controlled via this organelle (Gorska et al., 2009). Rab35 also plays a key role in regulating recycling and the formation of the immunological synapse. Experiments identifying the Rab GTPase-activating protein for Rab35, EP164C, revealed a central role in formation of the immunological synapse (Patino-Lopez et al., 2008). Rab35 was first described as blocking traffic from the recycling compartment to the plasma membrane in HeLa cells, where it was also shown to play a role in cytokinesis (Kouranti et al., 2006). A more recent study in Jurkat T cells has shown that Rab35 colocalizes with TCR-ζ in the recycling endosome and concentrates at the synapse (Patino-Lopez et al., 2008). Overexpression of the dominant-negative form (which locks Rab35 in its GDP-bound state) blocks delivery of transferrin receptor to the plasma membrane and reduces conjugate formation. This is consistent with reduced exit from the recycling endosome to the plasma membrane, which would include TCR. In this way, both endocytosis and exocytosis carefully control the level of TCR available within the cSMAC and thus the strength of the signals that can be generated. These studies highlight the role of the cSMAC not only in signaling but also in exocytic and endocytic events.
How does the recycling endosome come to lie underneath the cSMAC? Kupfer et al. (1987) and Kupfer and Singer (1989) showed that during synapse formation, there is a dramatic polarization of both the actin and microtubule cytoskeletons that was antigen dependent (i.e., dependent on antigen receptor recognition). What has emerged from a great many subsequent studies is that receptor activation triggers an initial accumulation of actin across the synapse (Ryser et al., 1982; Kupfer et al., 1994), which rapidly clears to form an outer ring around the synapse (Stinchcombe et al., 2001, 2006; for review see Stinchcombe and Griffiths, 2007), known as the distal SMAC (dSMAC), which lies outside the pSMAC (Fig. 1; Freiberg et al., 2002). Actin clearance is coordinated with the movement of the MTOC, which polarizes toward the synapse (Stinchcombe et al., 2006). Because the Golgi stacks and the recycling endosome are closely associated with the MTOC by means of motor protein attachments (Allan and Kreis, 1986; Allan et al., 2002), these organelles move together with the MTOC, bringing them to the immunological synapse.
Polarization and secretion at the synapse
Polarized secretion at the immunological synapse has been particularly well studied in CD8 CTLs (Fig. 1). These cells destroy virally infected and tumor cells by release of specialized secretory lysosomes containing the lytic pore-forming protein perforin, which enables a series of granzymes to enter the cytoplasm of the target cell and rapidly trigger apoptosis (for review see Stinchcombe and Griffiths, 2007). In these cells, the centrosome, which is the only MTOC of T cells, has been shown to polarize right up to the plasma membrane, contacting the synapse at the cSMAC (Stinchcombe et al., 2006). The secretory lysosomes (also known as lytic granules) are delivered to a specialized secretory domain within the synapse by moving along microtubules in a minus end direction toward the centrosome. Granule contents are then released into a small cleft between the two cells that appears sealed by the tightly apposed membranes.
Centrosome movement is triggered by TCR signals and correlates with the clearance of actin from its initial aggregation across the synapse to form the dSMAC (for review see Stinchcombe and Griffiths, 2007). The actin-binding protein IQGAP1 colocalizes with actin, relocating to the dSMAC as the synapse forms (Fig. 1). Because IQGAP1 is known to bind and stabilize the plus ends of microtubules and tether them to the actin cytoskeleton, this observation is consistent with the idea that the forces generated by actin clearance might propel the centrosome forward. Dynein is also located in the dSMAC, recruited there by ADAP (adhesion- and degranulation-promoting adapter protein) or Fyb (Fyn-binding protein), which is able to bind to the actin cytoskeleton. In this way, dynein recruitment is linked to TCR activation and synapse formation. Once recruited, dynein would be able to generate tension on the microtubules and effectively reel in the centrosome (Combs et al., 2006) to the center of the synapse, where the initiating signals occurred. The centrosome always docks at the cSMAC within the synapse (Stinchcombe et al., 2006; Jenkins et al., 2009). Whether this is simply because the centrosome is pulled in as the actin reorganization radiates out from the signals generated at the cSMAC or whether there are additional proteins recruited to the cSMAC that influence the point of docking of the centrosome is not yet clear.
The initial polarization of the centrosome is influenced by the accumulation of DAG at the center of the synapse (Spitaler et al., 2006; Quann et al., 2009). DAG is a lipid second messenger generated upon TCR activation and serves as a substrate for PKD, which also focuses in the center of the synapse, binding to DAG. Blocking DAG production or impairing its localization prevented MTOC movement from the rear of the cell toward the synapse (Quann et al., 2009). It seems likely that signaling events might control the recruitment of polarity proteins such as the partitioning defective (Par) proteins first discovered in Caenorhabditis elegans. Par3 has been observed, recruited at the synapse (Ludford-Menting et al., 2005), and more recent data support a functional role for Par1b (Lin et al., 2009). The model that emerges from the study by Lin et al. (2009) is that Par1b is associated with the plasma membrane in resting T cells but that upon activation, PKC-ζ phosphorylates Par1b, allowing it to dissociate from the membrane and bind to 14-3-3 proteins, which trigger the removal of Par1b to the cytoplasm (clustered beneath the immune synapse). The authors propose that this allows Par3 to then accumulate at the contact site. Most dramatically, overexpression of a dominant-negative form of Par1b, which would block all three isoforms of Par1 at the plasma membrane, impaired the relocation of the MTOC to the synapse, supporting a functional role for Par proteins in synapse formation.
A recent study has examined how the strength of TCR signal influences the polarization of the centrosome to the plasma membrane (Jenkins et al., 2009). Using CTLs from OT-1 TCR transgenic mice, our laboratory compared the polarization of the centrosome in response to the strong ovalbumin peptide, which triggers high levels of target cell death, with that of the weaker peptide G4, which elicits virtually no target cell killing. The striking finding was that the centrosome polarized readily to both the strong and the weak signal. But only the stronger ovalbumin signal also elicited granule polarization and secretion at the synapse (Jenkins et al., 2009). These results are consistent with the ability of the centrosome to oscillate rapidly between two targets (Kuhn and Poenie, 2002) and to kill only the target triggering the stronger signal (Wiedemann et al., 2006). The nature of the additional signal required to recruit the granules is not entirely clear. One study suggests a possible role for PKC-δ, as CTLs deficient in PKC-δ fail to polarize their granules upon contact with targets (Ma et al., 2008). Another study has suggested roles for ERK (extracellular signal-regulated kinase) and PI3K (phosphatidylinositol 3-kinase; Robertson et al., 2005), and it is very likely that several signaling pathways contribute.
Exocytosis of both lytic granule contents, delivering regulated secretory cargo, and constitutively secreted cytokines become focused into the synapse. Images of the Golgi at the synapse show that the stacks contact the plasma membrane (Stinchcombe et al., 2006), which would lead to very focused secretion of cytokines. Interleukin 2 (IL2), IL4, IL5, and interferon-γ are all secreted in a polarized manner toward the target APC (Poo et al., 1988; Kupfer et al., 1991). More recently, it has been found that not only are the cytokines recruited to the synapse in a focused fashion but that their receptors are also recruited to the synapse in a selective manner, dependent on the signals received, that can then influence cellular differentiation (Maldonado et al., 2009).
Exocytosis
Exocytosis requires fusion of secretory vesicles at the immunological synapse, and several studies have implicated several SNARE proteins in this process (Das et al., 2004; zur Stadt et al., 2005; Ménager et al., 2007; Marcet-Palacios et al., 2008; Loo et al., 2009). However, pinpointing the SNARE complex that is required for the final fusion of secretory granules at the synapse is proving complicated. To mediate membrane fusion, a functional SNARE complex requires the formation of a four-helix bundle with a VAMP (vesicle-associated membrane protein; also known as synaptobrevin) associating with a syntaxin and SNAP, which are both associated with the membrane to which the vesicle is fusing (for review see Südhof and Rothman, 2009). Functional studies in CTLs and natural killer (NK) cells have shown that both VAMP7 knockdown as well as VAMP8 gene disruption cause a reduction but not a complete loss of target cell killing (Marcet-Palacios et al., 2008; Loo et al., 2009). It is possible that this reflects some functional redundancy. However, another possible explanation for these results is that VAMP proteins control membrane fusion events at various points along the biosynthetic pathway. Because it has been shown that the lytic granule proteins perforin, granzymes A and B, and Fas ligand can all be secreted at the synapse not only via the granule pathway but also directly from the Golgi as new synthesis takes place upon TCR triggering (Isaaz et al., 1995; Haddad et al., 2001; Makedonas et al., 2009), this could explain why it is so difficult to identify events of the granule pathway only.
Syntaxin 11 seemed a likely candidate for a SNARE component mediating granule fusion as loss of syntaxin 11 gives rise to FHL4 (familial hemophagocytic lymphohistiocytosis type 4), a genetic disease characterized by loss of cytotoxic function (zur Stadt et al., 2005). However, syntaxin 11 has been shown to localize to Golgi membranes (Valdez et al., 1999), suggesting that this protein may well be acting earlier in the secretory pathway. Furthermore, FHL4 CTLs and NK cells can overcome their secretory defect upon incubation with IL2 (Bryceson et al., 2007), which up-regulates synthesis of the granule contents.
Lytic granule release is associated not only with the release of the content but also the appearance of lysosomal membrane proteins, including LAMP-1 and -2, at the plasma membrane. These lysosomal membrane proteins are known to be rapidly endocytosed upon degranulation, and this uptake is used as a measure of degranulation (Betts and Koup, 2004). This bidirectional traffic of LAMP-1 has been found to be controlled by integrin engagement in the cytolytic synapses formed by NK cells (Liu et al., 2009). Using lipid bilayers to reconstitute selective receptors for triggering NK cells, Liu et al. (2009) examined the localization of exocytosed LAMP-1 from NK cells when the integrin LFA-1 was or was not engaged by ICAM-1. When ICAM-1 was triggered, LAMP-1 did not escape and diffuse all over the cell surface, whereas in the absence of ICAM-1, LAMP-1 distributed over the periphery of the cell in microclusters. Because the overall levels of surface and endocytosed LAMP-1 remained the same regardless of ICAM-1, this suggests that ICAM-1–LFA-1 engagement in the pSMAC acts to restrict and focus endocytic and exocytic events to the center of the synapse.
Cilia formation and cytokinesis
The immunological synapse bears striking similarities not only to neurological synapses but also to other structures in which an area of the plasma membrane becomes a focal zone for endocytosis and exocytosis, in particular during cilia formation and cytokinesis. Immunological synapses, sites of cilia formation, and cytokinesis all share the unusual property of forming around the site of centrosomal docking at the plasma membrane. This is best characterized in primary cilia formation, where physical links are formed between the appendages of the mother centriole and the plasma membrane (Fig. 2 A). Some of the proteins involved have now been identified, and disruption of these appendage proteins such as Odf2 and CEP164 prevent primary cilia formation (Ishikawa et al., 2005; Graser et al., 2007). During cytokinesis, the centrosome also contacts the plasma membrane: after the two centrioles separate, the mother centriole repositions to the bridge formed by the midbody. Final abscission occurs only after the mother centriole moves back to the center of the cell (Piel et al., 2001). At the immunological synapse, both centrioles migrate to the synapse, and no clear preference for mother versus daughter contacting the plasma membrane has yet emerged (Stinchcombe et al., 2006). Interestingly, lymphocytes have been noted for being unable to form primary cilia (Wheatley, 1995; Hildebrandt and Otto, 2005), and the finding that they nevertheless form a very similar structure at the immunological synapse raises the possibility that the synapse represents a frustrated cilium.
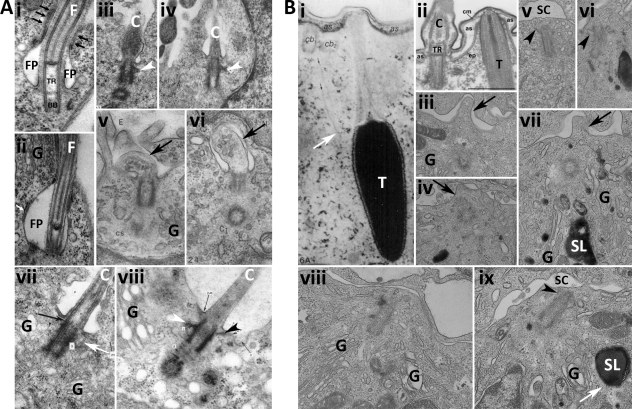
Figure 2.
Electron micrographs comparing flagella, primary cilia, trichocyst secretion, and the immunological synapse. (A) EM images of flagella (i and ii) and primary cilia (C; iii–viii) showing basal bodies (BB), flagella (F), flagella pocket (FP), transition region (TR), Golgi (G), distal (black arrowheads) and subdistal (white arrowheads) appendages of the mother centriole, membrane protrusions formed during ciliogenesis (black arrows in v and vi), and microtubules linking the centriole to the membrane (white arrow in vii). An area of tight contact between the flagella pocket and flagella (black arrows in i) and a flat compartment close to the flagella pocket (white arrow in ii) are also shown. Basal body fibers and cilia fibers are contiguous (black arrow in vii). Images were reproduced from Absalon et al. (2008; i and ii; with permission from the Journal of Cell Science), Archer and Wheatley (1971; iii; with permission from Wiley-Blackwell), Wheatley (1971; iv and vii; with permission from Wiley-Blackwell), Sorokin (1962; v and vi), and Wheatley (1967; viii; with permission from Wiley-Blackwell). (B) EM images of trichocyst (T) secretion from paramecia (i and ii) showing cilia (C), ciliary basal bodies (cb), alveolar sacs (as), cell membrane (cm), epiplasm (ep), and transition region (TR) and immune synapses formed between CTLs (lower cell) and targets (upper cell; iii–ix) showing Golgi (G), secretory lysosomes (SL), synaptic cleft (SC), membrane bulges (black arrows), mother centriole appendages (black arrowheads), and microtubules associated with microtubules (white arrows). Images were reproduced from Glas-Albrecht et al. (1991; i; with permission from the Journal of Cell Science), Plattner (2002; ii; with permission from Wiley-VCH Verlag GmbH & Co. KGaA and the author). CTL images are from J.C. Stinchcombe.
Morphologically, primary cilia and immune synapses bear striking similarities (Fig. 2). Primary cilia form from the mother centriole, which docks at the plasma membrane (becoming the basal body; Fig. 2 A, iii–viii). The cilium extends, pushing out the plasma membrane as it forms. Similar protrusions at the plasma membrane are also seen at the immune synapse, with a small bump in the plasma membrane at the point of centriole contact with the plasma membrane (Fig. 2 B, iii, iv, and vii, arrows). Although it is not clear that it is always the mother centriole that contacts the plasma membrane at the immune synapse, some images show appendages on the docking centriole, similar to those in cilia formation (Fig. 2 B, v, vi, and ix, arrowheads). Other similarities are also apparent from these images. The Golgi apparatus, which polarizes at the immunological synapse, is also focused near the plasma membrane at the point of cilia formation. The Golgi is seen close to the point of primary cilia formation, as well as next to the flagella pocket of Trypanosoma brucei (Fig. 2 A, i and ii). Other studies have revealed that the recycling compartment for endocytosis is also focused both at the immunological synapse (Das et al., 2004) and next to the flagella pocket of T. brucei (Jeffries et al., 2001).
A similar focus of endocytosis and exocytosis is also seen at the point of abscission during cytokinesis, and this also seems to be directed by movement of the centrosome (Fig. 3). Centriolin has been shown to deliver exocyst and SNARE proteins involved in the secretory events during abscission (Gromley et al., 2005), and the Rab11 recycling endosome localizes to the cleavage furrow (Fielding et al., 2005; Wilson et al., 2005), as do post-Golgi elements (Goss and Toomre, 2008). The ESCRT (endosomal-sorting complex required for transport) and Alix proteins involved in endocytosis and down-regulation via the ubiquitin pathway are also recruited to the cleavage furrow (Carlton and Martin-Serrano, 2007).
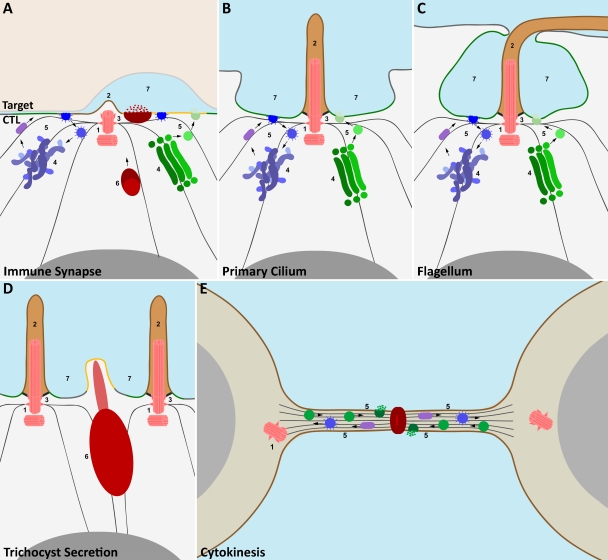
Figure 3.
A common theme in the architecture of diverse cellular structures. Cartoon illustrating comparison of the organization of the immune synapse (A), primary cilium (B), flagella pocket (C), point of trichocyst secretion in paramecia (D), and site of cytokinesis in dividing cells (E), indicating that the site of centrosome polarization is a focal point for exocytosis and endocytosis in different cellular systems. Similarities between some or all of these examples include (1) polarization of the centrosome (pink) to the plasma membrane; (2) formation of membrane protrusions (brown) opposite the point of centrosome docking; polarized movement of (3) the microtubule cytoskeleton and (4) the Golgi complex (green) and endocytic recycling compartments (mauve) away from the nucleus (gray) toward the site of centrosome docking at the plasma membrane; (5) redirection of the biosynthetic (green), endocytic (blue), and recycling (purple) pathways to the plasma membrane; (6) focused polarization of regulated secretory organelles (e.g., trichocysts [crimson] in paramecia and secretory lysosomes [claret] with secretory cores [crimson] in CTLs) to specialized secretory domains at the plasma membrane (orange) by minus end–directed transport along microtubules; and (7) formation of specialized enclosed (e.g., secretory clefts [CTLs] and flagella pockets) or semienclosed (cilia and trichocyst) extracellular spaces to which endocytosis and exocytosis are focused.
The comparison between the synapse and cilia extends beyond the morphological. Both ciliary membranes and those at the synapse are specialized in cell signaling. In the case of the synapse, TCR-mediated signaling controls polarization of the centrosome, secretion, and endocytosis. Cilia act as sensors of the external environment, with receptors for extracellular ligands that can trigger intracellular signaling. The primary cilium plays an important role in signaling pathways, for example Hedgehog (Hh; for review see Singla and Reiter, 2006). Furthermore, mutations in intraflagellar transport (IFT) proteins that prevent cilia formation were also found to block signaling via the Hh pathway (Huangfu et al., 2003). Intriguingly, the receptor required for Hh signaling, Smoothened, is recruited to the cilium upon signaling (Corbit et al., 2005) in a manner analogous to the formation of the cSMAC at the immune synapse.
A recent study has now identified expression of IFT proteins in T lymphocytes (Finetti et al., 2009). These IFT proteins are able to assemble into the same complex of IFT20–IFT88 and IFT57 as they do in cilia and flagella (Rosenbaum and Witman, 2002), and complex formation is enhanced by TCR activation in Jurkat T cells. In ciliated cells, the IFT20 component of this complex localizes to the Golgi (Follit et al., 2006), and a similar Golgi localization is seen in Jurkat T cells with a small amount of additional staining overlapping with other endosomal compartments, and Finetti et al. (2009) suggest that IFT20 also localizes to the recycling endosome in T cells. What is particularly interesting about Finetti et al. (2009) is that when the authors knocked down expression of IFT20, that synapse formation was disrupted. The number of conjugates formed by the T cells was reduced, and clustering of the TCR into a cSMAC was impaired. In addition, a much weaker and more dispersed staining with anti-phosphotyrosine antibodies indicated reduced TCR signaling. When IFT20 expression was knocked down, the transferrin receptor–labeled recycling endosome failed to polarize to the immune synapse, whereas no difference in the MTOC localization was observed between control and knockdown conjugates. To understand the basis of the defect arising in the absence of IFT20, Finetti et al. (2009) triggered a PKC-activated pathway, which leads to TCR phosphorylation and recycling (Cantrell et al., 1985; Krangel, 1987), in both normal Jurkat and IFT20-deficient Jurkat. They found that although TCR was readily recycled in normal cells, TCR accumulated in the recycling compartment of IFT20-deficient cells and failed to undergo recycling to the cell surface. The authors found that the IFT complex associates with TCR upon activation and proposed that this complex might function to regulate the recycling of the TCR from this compartment and thus control synapse formation. It is not yet clear how polarization of the recycling compartment is controlled. These studies reinforce the idea of the synapse as an area of membrane specialized in endocytosis and exocytosis and support the idea that similar molecular mechanisms underlie the formation of cilia and synapses.
A common theme
If there is a common origin to deriving mechanisms that focus endocytosis and exocytosis at the plasma membrane, are there other secretory cells that provide a parallel to the centrosome directing secretion, as seen in CTLs? Studies on trichocyst secretion from Paramecium tetraurelia suggest that there are (Glas-Albrecht et al., 1991; Plattner, 2002). Paramecia are ciliated unicellular protozoa, which contain dense core secretory granules known as trichocysts. There is a regular arrangement of cilia around the surface of paramecia, and secretion of trichocysts occurs between the cilia. Glas-Albrecht et al. (1991) observed the association of trichocysts with microtubules emanating from the basal bodies of the cilia (Fig. 2 B, i and ii) and, using video microscopy, showed the movement of trichocysts along the microtubules toward the basal body. EM revealed that trichocysts often made contact with microtubules originating from neighboring basal bodies. This represents a minus end–directed movement of dense core secretory granules toward a docked centriole (basal body) analogous to that seen more recently in CTLs (Stinchcombe et al., 2006).
Conclusions
The parallels between the cilium, the immune synapse, trichocyst secretion, and cytokinesis (Fig. 3) are intriguing. They suggest that the centrosome may play a role in identifying a specialized area of membrane for focal endocytosis and exocytosis. Precisely which signals direct the centrosome to a given point on the membrane in each system are yet to be discovered. This mechanism seems to have been adopted successfully by several specialized cell types, not least of all lymphocytes, when communicating via the immunological synapse.
Acknowledgments
G.M. Griffiths is funded by a Wellcome Trust Principal Research Fellowship.
Footnotes
Abbreviations used in this paper:
- APC
- antigen-presenting cell
- cSMAC
- central SMAC
- CTL
- cytotoxic T lymphocyte
- dSMAC
- distal SMAC
- Hh
- Hedgehog
- IFT
- intraflagellar transport
- MTOC
- microtubule-organizing center
- NK
- natural killer
- pMHC
- peptide–major histocompatibility complex
- pSMAC
- peripheral SMAC
- SMAC
- supramolecular activation complex
- TCR
- T cell receptor
References
- Absalon S., Blisnick T., Bonhivers M., Kohl L., Cayet N., Toutirais G., Buisson J., Robinson D., Bastin P. 2008. Flagellum elongation is required for correct structure, orientation and function of the flagellar pocket in Trypanosoma brucei. J. Cell Sci. 121:3704–3716 10.1242/jcs.035626 [DOI] [PubMed] [Google Scholar]
- Allan V.J., Kreis T.E. 1986. A microtubule-binding protein associated with membranes of the Golgi apparatus. J. Cell Biol. 103:2229–2239 10.1083/jcb.103.6.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan V.J., Thompson H.M., McNiven M.A. 2002. Motoring around the Golgi. Nat. Cell Biol. 4:E236–E242 10.1038/ncb1002-e236 [DOI] [PubMed] [Google Scholar]
- Archer F.L., Wheatley D.N. 1971. Cilia in cell-cultured fibroblasts. II. Incidence in mitotic and post-mitotic BHK 21-C13 fibroblasts. J. Anat. 109:277–292 [PMC free article] [PubMed] [Google Scholar]
- Batista F.D., Iber D., Neuberger M.S. 2001. B cells acquire antigen from target cells after synapse formation. Nature. 411:489–494 10.1038/35078099 [DOI] [PubMed] [Google Scholar]
- Beal A.M., Anikeeva N., Varma R., Cameron T.O., Vasiliver-Shamis G., Norris P.J., Dustin M.L., Sykulev Y. 2009. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 31:632–642 10.1016/j.immuni.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts M.R., Koup R.A. 2004. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 75:497–512 10.1016/S0091-679X(04)75020-7 [DOI] [PubMed] [Google Scholar]
- Bryceson Y.T., Rudd E., Zheng C., Edner J., Ma D., Wood S.M., Bechensteen A.G., Boelens J.J., Celkan T., Farah R.A., et al. 2007. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 110:1906–1915 10.1182/blood-2007-02-074468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi G., Varma R., Dustin M.L. 2005. Actin and agonist MHC–peptide complex–dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202:1031–1036 10.1084/jem.20051182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D.A., Davies A.A., Crumpton M.J. 1985. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc. Natl. Acad. Sci. USA. 82:8158–8162 10.1073/pnas.82.23.8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J.G., Martin-Serrano J. 2007. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 316:1908–1912 10.1126/science.1143422 [DOI] [PubMed] [Google Scholar]
- Cemerski S., Das J., Giurisato E., Markiewicz M.A., Allen P.M., Chakraborty A.K., Shaw A.S. 2008. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 29:414–422 10.1016/j.immuni.2008.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs J., Kim S.J., Tan S., Ligon L.A., Holzbaur E.L., Kuhn J., Poenie M. 2006. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl. Acad. Sci. USA. 103:14883–14888 10.1073/pnas.0600914103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit K.C., Aanstad P., Singla V., Norman A.R., Stainier D.Y., Reiter J.F. 2005. Vertebrate Smoothened functions at the primary cilium. Nature. 437:1018–1021 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Das V., Nal B., Dujeancourt A., Thoulouze M.I., Galli T., Roux P., Dautry-Varsat A., Alcover A. 2004. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity. 20:577–588 10.1016/S1074-7613(04)00106-2 [DOI] [PubMed] [Google Scholar]
- Depoil D., Fleire S., Treanor B.L., Weber M., Harwood N.E., Marchbank K.L., Tybulewicz V.L., Batista F.D. 2008. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol. 9:63–72 10.1038/ni1547 [DOI] [PubMed] [Google Scholar]
- Dustin M.L. 2009. The cellular context of T cell signaling. Immunity. 30:482–492 10.1016/j.immuni.2009.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M.L., Olszowy M.W., Holdorf A.D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P.A., Allen P.M., Shaw A.S. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 94:667–677 10.1016/S0092-8674(00)81608-6 [DOI] [PubMed] [Google Scholar]
- Fielding A.B., Schonteich E., Matheson J., Wilson G., Yu X., Hickson G.R., Srivastava S., Baldwin S.A., Prekeris R., Gould G.W. 2005. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 24:3389–3399 10.1038/sj.emboj.7600803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F., Paccani S.R., Riparbelli M.G., Giacomello E., Perinetti G., Pazour G.J., Rosenbaum J.L., Baldari C.T. 2009. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 11:1332–1339 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J.A., Tuft R.A., Fogarty K.E., Pazour G.J. 2006. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell. 17:3781–3792 10.1091/mbc.E06-02-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg B.A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D.M., Kupfer A. 2002. Staging and resetting T cell activation in SMACs. Nat. Immunol. 3:911–917 10.1038/ni836 [DOI] [PubMed] [Google Scholar]
- Glas-Albrecht R., Kaesberg B., Knoll G., Allmann K., Pape R., Plattner H. 1991. Synchronised secretory organelle docking in Paramecium: saltatory movement along microtubules transiently formed from ciliary basal bodies and selective exclusion of microinjected heterologous organelles. J. Cell Sci. 100:45–54 [Google Scholar]
- Gorska M.M., Liang Q., Karim Z., Alam R. 2009. Uncoordinated 119 protein controls trafficking of Lck via the Rab11 endosome and is critical for immunological synapse formation. J. Immunol. 183:1675–1684 10.4049/jimmunol.0900792 [DOI] [PubMed] [Google Scholar]
- Goss J.W., Toomre D.K. 2008. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J. Cell Biol. 181:1047–1054 10.1083/jcb.200712137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. 1999. The immunological synapse: a molecular machine controlling T cell activation. Science. 285:221–227 10.1126/science.285.5425.221 [DOI] [PubMed] [Google Scholar]
- Graser S., Stierhof Y.D., Lavoie S.B., Gassner O.S., Lamla S., Le Clech M., Nigg E.A. 2007. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 179:321–330 10.1083/jcb.200707181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A., Yeaman C., Rosa J., Redick S., Chen C.T., Mirabelle S., Guha M., Sillibourne J., Doxsey S.J. 2005. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 123:75–87 10.1016/j.cell.2005.07.027 [DOI] [PubMed] [Google Scholar]
- Haddad E.K., Wu X., Hammer J.A., III, Henkart P.A. 2001. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 152:835–842 10.1083/jcb.152.4.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F., Otto E. 2005. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat. Rev. Genet. 6:928–940 10.1038/nrg1727 [DOI] [PubMed] [Google Scholar]
- Huangfu D., Liu A., Rakeman A.S., Murcia N.S., Niswander L., Anderson K.V. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426:83–87 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Isaaz S., Baetz K., Olsen K., Podack E., Griffiths G.M. 1995. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur. J. Immunol. 25:1071–1079 10.1002/eji.1830250432 [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Kubo A., Tsukita S., Tsukita S. 2005. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7:517–524 10.1038/ncb1251 [DOI] [PubMed] [Google Scholar]
- Jeffries T.R., Morgan G.W., Field M.C. 2001. A developmentally regulated rab11 homologue in Trypanosoma brucei is involved in recycling processes. J. Cell Sci. 114:2617–2626 [DOI] [PubMed] [Google Scholar]
- Jenkins M.R., Tsun A., Stinchcombe J.C., Griffiths G.M. 2009. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity. 31:621–631 10.1016/j.immuni.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranti I., Sachse M., Arouche N., Goud B., Echard A. 2006. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 16:1719–1725 10.1016/j.cub.2006.07.020 [DOI] [PubMed] [Google Scholar]
- Krangel M.S. 1987. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J. Exp. Med. 165:1141–1159 10.1084/jem.165.4.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.R., Poenie M. 2002. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 16:111–121 10.1016/S1074-7613(02)00262-5 [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S.J. 1989. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J. Exp. Med. 170:1697–1713 10.1084/jem.170.5.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S.L., Singer S.J. 1987. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J. Exp. Med. 165:1565–1580 10.1084/jem.165.6.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Mosmann T.R., Kupfer H. 1991. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl. Acad. Sci. USA. 88:775–779 10.1073/pnas.88.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer H., Monks C.R., Kupfer A. 1994. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J. Exp. Med. 179:1507–1515 10.1084/jem.179.5.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Holdorf A.D., Dustin M.L., Chan A.C., Allen P.M., Shaw A.S. 2002. T cell receptor signaling precedes immunological synapse formation. Science. 295:1539–1542 10.1126/science.1067710 [DOI] [PubMed] [Google Scholar]
- Lin J., Hou K.K., Piwnica-Worms H., Shaw A.S. 2009. The polarity protein Par1b/EMK/MARK2 regulates T cell receptor-induced microtubule-organizing center polarization. J. Immunol. 183:1215–1221 10.4049/jimmunol.0803887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Bryceson Y.T., Meckel T., Vasiliver-Shamis G., Dustin M.L., Long E.O. 2009. Integrin-dependent organization and bidirectional vesicular traffic at cytotoxic immune synapses. Immunity. 31:99–109 10.1016/j.immuni.2009.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rhodes M., Wiest D.L., Vignali D.A. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 13:665–675 10.1016/S1074-7613(00)00066-2 [DOI] [PubMed] [Google Scholar]
- Loo L.S., Hwang L.A., Ong Y.M., Tay H.S., Wang C.C., Hong W. 2009. A role for endobrevin/VAMP8 in CTL lytic granule exocytosis. Eur. J. Immunol. 39:3520–3528 10.1002/eji.200939378 [DOI] [PubMed] [Google Scholar]
- Ludford-Menting M.J., Oliaro J., Sacirbegovic F., Cheah E.T., Pedersen N., Thomas S.J., Pasam A., Iazzolino R., Dow L.E., Waterhouse N.J., et al. 2005. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 22:737–748 10.1016/j.immuni.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Ma J.S., Haydar T.F., Radoja S. 2008. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 181:4716–4722 [DOI] [PubMed] [Google Scholar]
- Makedonas G., Banerjee P.P., Pandey R., Hersperger A.R., Sanborn K.B., Hardy G.A., Orange J.S., Betts M.R. 2009. Rapid up-regulation and granule-independent transport of perforin to the immunological synapse define a novel mechanism of antigen-specific CD8+ T cell cytotoxic activity. J. Immunol. 182:5560–5569 10.4049/jimmunol.0803945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R.A., Soriano M.A., Perdomo L.C., Sigrist K., Irvine D.J., Decker T., Glimcher L.H. 2009. Control of T helper cell differentiation through cytokine receptor inclusion in the immunological synapse. J. Exp. Med. 206:877–892 10.1084/jem.20082900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet-Palacios M., Odemuyiwa S.O., Coughlin J.J., Garofoli D., Ewen C., Davidson C.E., Ghaffari M., Kane K.P., Lacy P., Logan M.R., et al. 2008. Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem. Biophys. Res. Commun. 366:617–623 10.1016/j.bbrc.2007.11.079 [DOI] [PubMed] [Google Scholar]
- Ménager M.M., Ménasché G., Romao M., Knapnougel P., Ho C.H., Garfa M., Raposo G., Feldmann J., Fischer A., de Saint Basile G. 2007. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat. Immunol. 8:257–267 10.1038/ni1431 [DOI] [PubMed] [Google Scholar]
- Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. 1998. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 395:82–86 10.1038/25764 [DOI] [PubMed] [Google Scholar]
- Patino-Lopez G., Dong X., Ben-Aissa K., Bernot K.M., Itoh T., Fukuda M., Kruhlak M.J., Samelson L.E., Shaw S. 2008. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J. Biol. Chem. 283:18323–18330 10.1074/jbc.M800056200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel M., Nordberg J., Euteneuer U., Bornens M. 2001. Centrosome-dependent exit of cytokinesis in animal cells. Science. 291:1550–1553 10.1126/science.1057330 [DOI] [PubMed] [Google Scholar]
- Plattner H. 2002. My favorite cell—Paramecium. Bioessays. 24:649–658 10.1002/bies.10112 [DOI] [PubMed] [Google Scholar]
- Poo W.J., Conrad L., Janeway C.A., Jr 1988. Receptor-directed focusing of lymphokine release by helper T cells. Nature. 332:378–380 10.1038/332378a0 [DOI] [PubMed] [Google Scholar]
- Quann E.J., Merino E., Furuta T., Huse M. 2009. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 10:627–635 10.1038/ni.1734 [DOI] [PubMed] [Google Scholar]
- Robertson L.K., Mireau L.R., Ostergaard H.L. 2005. A role for phosphatidylinositol 3-kinase in TCR-stimulated ERK activation leading to paxillin phosphorylation and CTL degranulation. J. Immunol. 175:8138–8145 [DOI] [PubMed] [Google Scholar]
- Rosenbaum J.L., Witman G.B. 2002. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3:813–825 10.1038/nrm952 [DOI] [PubMed] [Google Scholar]
- Ryser J.E., Rungger-Brändle E., Chaponnier C., Gabbiani G., Vassalli P. 1982. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J. Immunol. 128:1159–1162 [PubMed] [Google Scholar]
- Singla V., Reiter J.F. 2006. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 313:629–633 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- Sorokin S. 1962. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15:363–377 10.1083/jcb.15.2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitaler M., Emslie E., Wood C.D., Cantrell D. 2006. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 24:535–546 10.1016/j.immuni.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Griffiths G.M. 2007. Secretory mechanisms in cell-mediated cytotoxicity. Annu. Rev. Cell Dev. Biol. 23:495–517 10.1146/annurev.cellbio.23.090506.123521 [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Barral D.C., Mules E.H., Booth S., Hume A.N., Machesky L.M., Seabra M.C., Griffiths G.M. 2001. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152:825–834 10.1083/jcb.152.4.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J.C., Majorovits E., Bossi G., Fuller S., Griffiths G.M. 2006. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 443:462–465 10.1038/nature05071 [DOI] [PubMed] [Google Scholar]
- Südhof T.C., Rothman J.E. 2009. Membrane fusion: grappling with SNARE and SM proteins. Science. 323:474–477 10.1126/science.1161748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez A.C., Cabaniols J.P., Brown M.J., Roche P.A. 1999. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J. Cell Sci. 112:845–854 [DOI] [PubMed] [Google Scholar]
- Varma R., Campi G., Yokosuka T., Saito T., Dustin M.L. 2006. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 25:117–127 10.1016/j.immuni.2006.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D.N. 1967. Cilia and centrioles of the rat adrenal cortex. J. Anat. 101:223–237 [PMC free article] [PubMed] [Google Scholar]
- Wheatley D.N. 1971. Cilia in cell-cultured fibroblasts. 3. Relationship between mitotic activity and cilium frequency in mouse 3T6 fibroblasts. J. Anat. 110:367–382 [PMC free article] [PubMed] [Google Scholar]
- Wheatley D.N. 1995. Primary cilia in normal and pathological tissues. Pathobiology. 63:222–238 10.1159/000163955 [DOI] [PubMed] [Google Scholar]
- Wiedemann A., Depoil D., Faroudi M., Valitutti S. 2006. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc. Natl. Acad. Sci. USA. 103:10985–10990 10.1073/pnas.0600651103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G.M., Fielding A.B., Simon G.C., Yu X., Andrews P.D., Hames R.S., Frey A.M., Peden A.A., Gould G.W., Prekeris R. 2005. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell. 16:849–860 10.1091/mbc.E04-10-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hashimoto-Tane A., Tokunaga M., Dustin M.L., Saito T. 2005. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6:1253–1262 10.1038/ni1272 [DOI] [PubMed] [Google Scholar]
- zur Stadt U., Schmidt S., Kasper B., Beutel K., Diler A.S., Henter J.I., Kabisch H., Schneppenheim R., Nürnberg P., Janka G., Hennies H.C. 2005. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum. Mol. Genet. 14:827–834 10.1093/hmg/ddi076 [DOI] [PubMed] [Google Scholar]