β-Arrestin functions as a scaffold for CaMKII and the Rap guanine nucleotide exchange factor Epac to regulate signaling from β1-ARs.
Abstract
Ca2+/calmodulin kinase II (CaMKII) plays an important role in cardiac contractility and the development of heart failure. Although stimulation of β1–adrenergic receptors (ARs) leads to an increase in CaMKII activity, the molecular mechanism by which β1-ARs activate CaMKII is not completely understood. In this study, we show the requirement for the β1-AR regulatory protein β-arrestin as a scaffold for both CaMKII and Epac (exchange protein directly activated by cAMP). Stimulation of β1-ARs induces the formation of a β-arrestin–CaMKII–Epac1 complex, allowing its recruitment to the plasma membrane, whereby interaction with cAMP leads to CaMKII activation. β-Arrestin binding to the carboxyl-terminal tail of β1-ARs promotes a conformational change within β-arrestin that allows CaMKII and Epac to remain in a stable complex with the receptor. The essential role for β-arrestin and identification of the molecular mechanism by which only β1-ARs and not β2-ARs activate CaMKII significantly advances our understanding of this important cellular pathway.
Introduction
β–Adrenergic receptors (ARs) are G protein–coupled receptors that are powerful regulators of cardiac function (Rockman et al., 2002). Acute stimulation of β-ARs physiologically augments cardiac contraction by increasing intracellular [Ca2+]i, whereas chronic β-AR stimulation promotes myocardial hypertrophy and heart failure (Koch et al., 2000; Lohse et al., 2003). An important β-AR–stimulated signaling pathway implicated in the development of heart failure involves the multifunctional protein kinase Ca2+/calmodulin kinase II (CaMKII; Zhang and Brown, 2004; Anderson, 2009). Consistent with a pathogenic role, CaMKII activity and expression are increased in animal models of structural heart disease (Zhang et al., 2005; Yoo et al., 2009) and after chronic β-AR stimulation (Zhu et al., 2003).
The mechanism of CaMKII activation after β-AR stimulation appears to be mediated through a pathway that involves the protein Epac (exchange protein directly activated by cAMP; Bos, 2006; Oestreich et al., 2007, 2009). Epac is a cAMP-dependent guanine nucleotide exchange factor for the small GTPases Rap1 and -2, which mediates cellular cAMP signaling independent of PKA (Bos, 2006; Ponsioen et al., 2009). Increasing intracellular concentration of cAMP by β-AR stimulation or with a cAMP analogue causes the Epac-mediated increase in CaMKII-dependent Ca2+ cycling, ryanodine receptor phosphorylation (Pereira et al., 2007), and diastolic sarcoplasmic reticulum (SR) Ca2+ leak (Curran et al., 2007). Interestingly, despite similar levels of cAMP generation by both β1- and β2-AR subtypes, only β1-AR stimulation increases CaMKII activity (Wang et al., 2004; Zhu et al., 2003; Yoo et al., 2009).
Although both β1- and β2-ARs stimulate adenylyl cyclase (AC) and generate the second messenger cAMP, considerable differences exist in the ability of these subtypes to activate downstream signaling pathways. For instance, CaMKII-dependent induction of fetal genes and apoptotic pathways in cardiac myocyte is mediated by β1-ARs but not β2-ARs (Zhu et al., 2003; Sucharov et al., 2006). In contrast, stimulation of β2-ARs activates cell survival signals, whereas β1-ARs trigger cardiomyocyte apoptotic pathways (Communal et al., 1999). Although the molecular mechanism for the β-AR subtype specificity in activating CaMKII has not been fully elucidated, amino acid sequence differences in the carboxyl-terminal tail (C-tail) between the β1- and β2-AR may be important. Indeed, studies have shown that β1- and β2-ARs have different PDZ (PSD-95/Dlg/ZO-1 homology domain)-binding motifs within their C-tail, leading to the recruitment of unique regulatory proteins with agonist stimulation. For example, the NHERF (Na+/H+ exchanger regulatory factor) binds to a DSLL motif in the C-tail of β2-ARs to stimulate Na+/H+ exchange (Hall et al., 1998), whereas N-methyl-d-aspartate (NMDA) receptor binds to an ESKV motif within the C-tail of β1-ARs and promotes receptor internalization (Hu et al., 2000). Interestingly, the C-tail of both β-AR subtypes is critical for the recruitment of β-arrestin, which is a molecule known to be an important receptor regulatory protein.
β-Arrestins are key regulators of β-AR endocytosis and trafficking. β-Arrestins interact with agonist-occupied and phosphorylated β-ARs and inhibit further G protein activation (Ferguson et al., 1996). Beyond their classical function, β-arrestins also function as scaffold proteins linking receptors to several downstream effectors such as the MAPK cascade (ERK [extracellular signal-regulated kinase] and JNK), Src, and the ubiquitin ligase Mdm2 (Lefkowitz and Shenoy, 2005; Lefkowitz et al., 2006). β-Arrestins are also known to bind to calmodulin (Wu et al., 2006) and to CaMKII-δ (Xiao et al., 2007). Whether β-arrestin is required for β1-AR–mediated CaMKII activation is not known. Therefore, we tested the hypothesis that β-arrestin regulates activation of the CaMKII signaling pathway and provides specificity for CaMKII signal transduction through its recruitment to the β1-AR. We show that β-arrestin scaffolds CaMKII and Epac, triggering translocation of this multimeric complex to agonist-occupied β1-ARs on the plasma membrane. Binding of β-arrestin to the C-tail of the β1-AR induces a β-arrestin conformation that stabilizes the β-arrestin–CaMKII–Epac interaction, thereby permitting the activation of Epac by cAMP and subsequent CaMKII signaling.
Results
Isoproterenol (ISO)- and 8-pCPT-2′-O-Me-cAMP (8-CPT)–mediated CaMKII activation is β-arrestin dependent
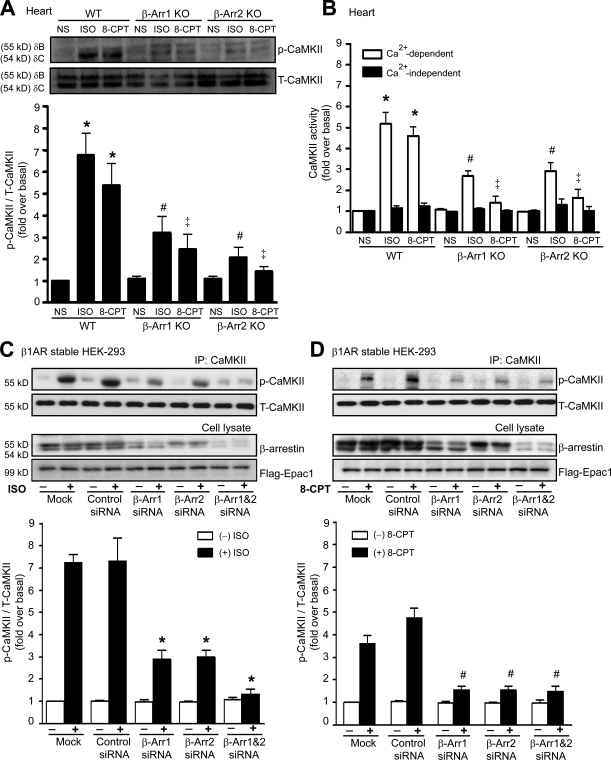
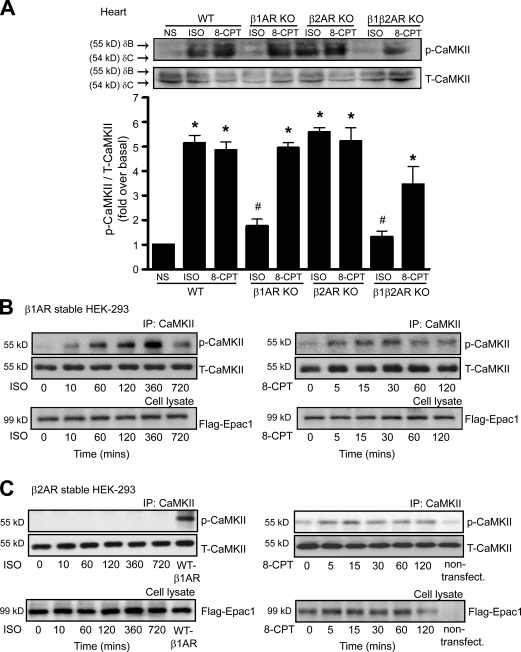
To test our hypothesis that activation of CaMKII is β-arrestin dependent, we administered ISO or 8-CPT to wild-type (WT) and β-arrestin knockout (KO) mice (Conner et al., 1997; Bohn et al., 1999). After ISO administration, the levels of phosphorylated CaMKII (pCaMKII; the active form of CaMKII) markedly increased in hearts of WT mice, whereas ISO-mediated CaMKII phosphorylation was significantly inhibited in hearts from either β-arrestin1 or -2 KO mice (Fig. 1 A). Infusion with 8-CPT, a cAMP analogue which selectively activates Epac, resulted in a significant increase in pCaMKII in hearts of WT mice. In contrast, 8-CPT showed little effect on pCaMKII in either β-arrestin1 or -2 KO mice. We also measured CaMKII activity using an in vitro kinase assay in heart extracts from WT and β-arrestin KO mice. For both ISO and 8-CPT, CaMKII activity was significantly inhibited in the β-arrestin KO mice compared with WT mice (Fig. 1 B).
Figure 1.
ISO and 8-CPT activate β-arrestin–dependent CaMKII activity. (A and B) WT, β-arrestin1 (β-Arr1) KO and β-arrestin2 KO mice were administered with saline (NS), 5 mg/kg ISO, or 2.5 mg/kg 8-CPT by i.v. infusion for 5 min. (A) LV homogenates were immunoblotted with anti-pCaMKII and anti-CaMKII antibodies. The CaMKII activation was quantified, expressed as fold increase over NS-treated WT mice, and shown as mean ± SEM (n = 5). (B) CaMKII kinase activity was determined under Ca2+-dependent and -independent conditions. The CaMKII activity was quantified, expressed as fold increase over NS-treated WT mice, and shown as mean ± SEM (n = 5). (A and B) *, P < 0.01 versus NS-treated WT mice; #, P < 0.01 versus ISO-treated WT mice; ‡, P < 0.01 versus 8-CPT–treated WT mice. (C and D) HEK-293 cells stably expressing WT–β1-AR were transfected with CaMKII-δC and Flag-Epac1 alone (Mock) or with siRNAs targeting β-arrestin1, β-arrestin2, β-arrestin1/2, or control siRNA. Serum-starved cells were stimulated at 37°C with 10 µM ISO (C) or 5 µM 8-CPT (D). Cell lysates were then immunoprecipitated with anti-CaMKII antibody before blotting with anti-pCaMKII and anti-CaMKII antibodies. The CaMKII activation was quantified, expressed as fold increase over nonstimulated mock cells, and shown as mean ± SEM (n = 5). Inhibited ISO- and 8-CPT–mediated CaMKII activation were observed in cells transfected with β-arrestin siRNA. *, P < 0.01 versus ISO-stimulated control siRNA; #, P < 0.05 versus 8-CPT–stimulated control siRNA. IP, immunoprecipitation; T-CaMKII, total CaMKII.
Next, we used siRNA to specifically target β-arrestin1 or -2 or both and examined the contribution of each β-arrestin isoform on β-AR–mediated CaMKII activation. HEK-293 cells stably expressing WT–β1-ARs were transfected with the β-arrestin siRNAs, along with CaMKII-δC and Flag-Epac1. Stimulation of β1-AR with ISO markedly elevated the level of pCaMKII in mock- and control siRNA–transfected cells (Fig. 1 C). In contrast, CaMKII activation was reduced in the presence of siRNA targeting either β-arrestin1 or -2 and was completely inhibited when both β-arrestin isoforms were knocked down (Fig. 1 C). Similar results were obtained with stimulation by 8-CPT (Fig. 1 D).
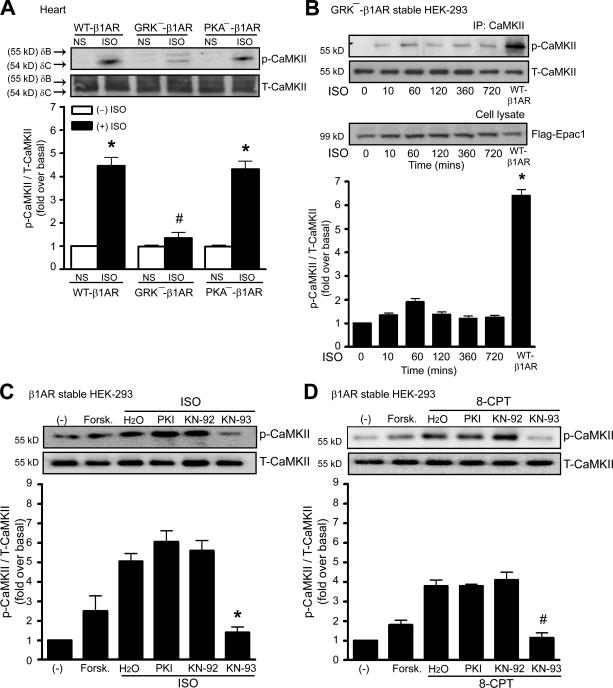
To further demonstrate the requirement of β-arrestin for β-AR–mediated CaMKII activation in the heart, we used transgenic (Tg) mice with cardiac-specific overexpression of a mutant β1-AR that is unable to recruit β-arrestin with agonist stimulation because it lacks G protein–coupled receptor kinase (GRK) phosphorylation sites (GRK−–β1-AR; Rapacciuolo et al., 2003; Noma et al., 2007). ISO-mediated CaMKII activation was significantly increased in hearts of Tg mice overexpressing WT–β1-ARs but was unable to increase pCaMKII levels in hearts of Tg mice overexpressing GRK−–β1-ARs (Fig. 2 A) or in HEK-293 cells with stable expression of GRK−–β1-ARs (Fig. 2 B). Collectively, these data demonstrate that β-arrestin is required for agonist activation of CaMKII in vivo and in vitro and that this process uses both β-arrestin1 and -2 isoforms.
Figure 2.
Stimulation of β1-AR activates CaMKII in a β-arrestin–dependent and PKA-independent manner. (A) Tg mice overexpressing mouse WT–β1-AR, GRK−–β1-AR, or PKA−–β1-AR were administered with saline (NS) or 5 mg/kg ISO by i.v. infusion for 5 min. LV homogenates were immunoblotted with anti-pCaMKII and anti-CaMKII antibodies. The CaMKII activation was quantified, expressed as fold increase over NS-treated WT mice, and shown as mean ± SEM (n = 5). Increased ISO-mediated CaMKII was observed in WT–β1-AR and PKA−–β1-AR Tg mice. *, P < 0.01 versus nonstimulation; #, P < 0.01 versus ISO-treated WT–β1-AR Tg mice. (B) HEK-293 cells stably expressing GRK−–β1-AR were transfected with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with 10 µM ISO at 37°C. The CaMKII activation was quantified, expressed as fold increase over nonstimulated cells, and shown as mean ± SEM (n = 5). ISO stimulation of WT–β1-AR is a positive control. *, P < 0.01 versus ISO stimulation of GRK−–β1-AR. IP, immunoprecipitation. (C and D) HEK-293 cells stably expressing WT–β1-AR were transfected with CaMKII-δC and Flag-Epac1. Serum-starved cells were pretreated without or with PKI or KN-92 or -93 before stimulation with 10 µM ISO (C) or 5 µM 8-CPT (D) at 37°C. The CaMKII activation was quantified, expressed as fold increase over nonstimulated cells, and shown as mean ± SEM (n = 5). Inhibited ISO- and 8-CPT–mediated CaMKII activation were observed in cells pretreated with KN-93, a specific CaMKII inhibitor, but not in cells pretreated with PKI. *, P < 0.01 versus ISO stimulation; #, P < 0.01 versus 8-CPT stimulation. Forsk., forskolin; T-CaMKII, total CaMKII.
Stimulation of β1-AR stimulates AC activity via the G protein Gs, leading to the generation of intracellular cAMP that directly activates Epac and CaMKII (de Rooij et al., 1998; Bos, 2006; Pereira et al., 2007). Consistent with these studies, we show that the activation of CaMKII in HEK-293 cells stably expressing WT–β1-ARs can be mediated by forskolin (an activator of AC) and inhibited by CaMKII inhibitor KN-93. A specific PKA inhibitor (PKI) has no effect on either ISO- or 8-CPT–mediated CaMKII activation (Fig. 2, C and D), confirming that β1-AR activates CaMKII in an Epac-dependent, PKA-independent manner (Zhu et al., 2003; Oestreich et al., 2007).
β-Arrestin forms a complex and colocalizes with CaMKII and Epac
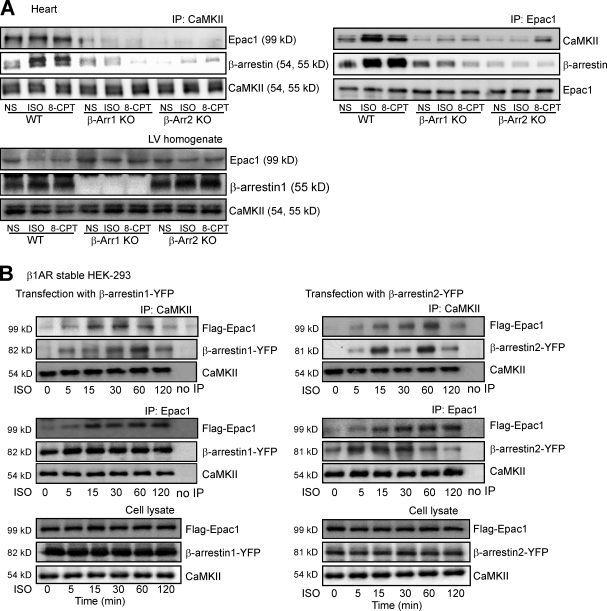
To determine whether β-arrestin serves as a scaffold for both CaMKII and Epac, we immunoprecipitated either CaMKII or Epac1 from heart homogenates of WT and β-arrestin KO mice and immunoblotted for CaMKII, Epac1, and β-arrestin. Under basal conditions, CaMKII was in complex with Epac1 and β-arrestin in hearts of WT mice, which was enhanced after stimulation with the agonists ISO or 8-CPT (Fig. 3 A). Loss of either β-arrestin1 or -2 prevented the formation of a CaMKII–β-arrestin–Epac1 complex in either unstimulated or agonist-stimulated conditions. β-Arrestin could still associate with Epac1 in the absence of CaMKII-δ (Fig. S1).
Figure 3.
Interaction of CaMKII and Epac1 with β-arrestins. (A) WT, β-arrestin1 (β-Arr1) KO, and β-arrestin2 KO mice were administered with saline (NS), 5 mg/kg ISO, or 2.5 mg/kg 8-CPT by i.v. infusion for 5 min. LV homogenates were immunoprecipitated with either anti-CaMKII or anti-Epac1 antibodies. Immunoprecipitated proteins were analyzed by Western blotting using the specific antibodies (n = 5). (B) HEK-293 cells stably expressing WT–β1-AR were transiently transfected with CaMKII-δC and Flag-Epac1, along with β-arrestin1– (left) or β-arrestin2–YFP (right). Serum-starved cells were stimulated with 10 µM ISO for the indicated times at 37°C. Cell lysates were immunoprecipitated with either anti-CaMKII or anti-Epac1 antibodies. Immunoprecipitated proteins were analyzed by Western blotting using the specific antibodies (n = 5). ISO stimulation resulted in a time-dependent increase in the association of CaMKII and Epac1 with β-arrestins. IP, immunoprecipitation.
To determine the time course for this multimeric protein interaction, we used HEK-293 cells stably expressing WT–β1-AR transiently transfected with either β-arrestin1– (Fig. 3 B, left) or β-arrestin2–YFP (Fig. 3 B, right) along with CaMKII-δC and Flag-Epac1. Consistent with our in vivo experiments, ISO stimulation resulted in a time-dependent increase in the association of Epac1, CaMKII, and β-arrestin.
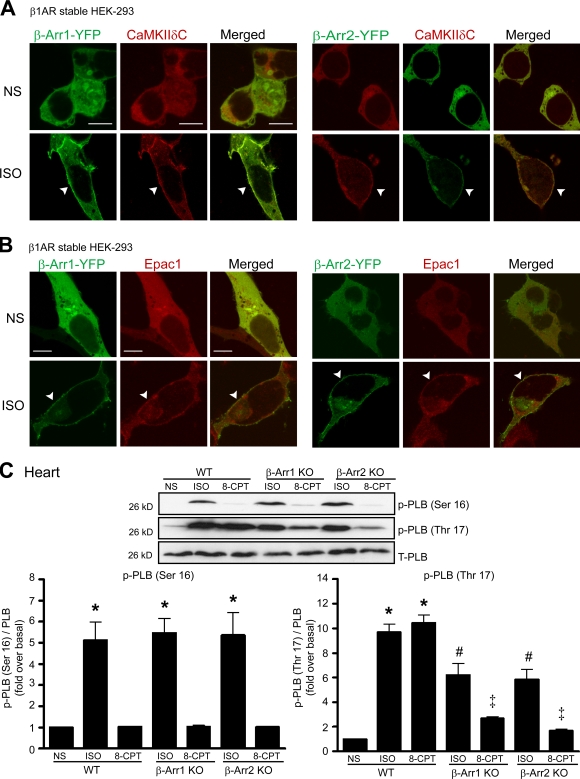
We next used confocal microscopy to determine whether β-arrestin colocalizes with CaMKII and Epac1 and whether they traffic together in response to agonist. In the absence of ISO, CaMKII-δC and β-arrestin–YFP were colocalized in the cytosol, which redistributed to the plasma membrane after ISO stimulation (Fig. 4 A, arrowheads). Similarly, Epac1 was uniformly distributed in the cytoplasm along with β-arrestin, which translocated to the plasma membrane after ISO stimulation (Fig. 4 B, arrowheads). These data are in concordance with a recent study showing the rapid recruitment of Epac1 to the plasma membrane after ISO stimulation (Ponsioen et al., 2009). Collectively, these results are consistent with our immunoprecipitation data showing that both β-arrestin1 and -2 interact with CaMKII and Epac1 and traffic together in an agonist-dependent fashion.
Figure 4.
ISO induced the translocation of CaMKII-δC and Epac1 to plasma membrane and the phosphorylation of PLB at Thr17 site. (A and B) HEK-293 cells stably expressing WT–β1-AR were transiently transfected with CaMKII-δC (A) or Flag-Epac1 (B), along with β-arrestin1 (β-Arr1)– (left) or β-arrestin2–YFP (right). Serum-starved cells were stimulated with 10 µM ISO at 37°C. Dual labeling experiments were performed wherein cells were stained for CaMKII-δC and Flag-Epac1 with Texas red, and β-arrestin was visualized by YFP fluorescence. ISO stimulation resulted in marked redistribution of β-arrestin–YFP together with CaMKII-δC (A) or Flag-Epac1 (B) to the membrane (arrowheads). (C) WT, β-arrestin1 KO, and β-arrestin2 KO mice were administered with saline (NS), 5 mg/kg ISO, or 2.5 mg/kg 8-CPT by i.v infusion for 5 min. LV homogenates were immunoblotted with anti–phospho-PLB (p-PLB) at Ser16, anti–phospho-PLB at Thr17, and anti-PLB antibodies. The phosphorylation of PLB at Ser16 (bottom left) and Thr17 (bottom right) was quantified, expressed as fold increase over NS-treated WT mice, and shown as mean ± SEM (n = 5). KO of either β-arrestin1 or -2 inhibited ISO- or 8-CPT–mediated PLB phosphorylation at Thr17. *, P < 0.01 versus NS-treated WT mice; #, P < 0.01 versus ISO-treated WT mice; ‡, P < 0.01 versus 8-CPT–treated WT mice. T-PLB, total PLB. Bars, 10 µm.
β-Arrestin is required for CaMKII-mediated phospholamban (PLB) phosphorylation
PLB serves as an important downstream effector of CaMKII in the heart (Bers, 2002). Therefore, we tested whether β-arrestin is required for CaMKII-mediated phosphorylation of PLB in hearts of WT and β-arrestin KO mice. CaMKII selectively phosphorylates PLB at Thr17, whereas PKA phosphorylates PLB at Ser16. After β-AR stimulation with ISO, the level of PLB phosphorylation at Ser16 (PKA site) significantly increased in hearts of WT and β-arrestin KO mice (Fig. 4 C, bottom left). 8-CPT, a cAMP analogue, is a selective activator of Epac and not PKA. It activates Epac1 with an EC50 = 2.2 µM, whereas the EC50 for PKA is >20 µM; Enserink et al., 2002). Consistent with this selective activity, we found that 8-CPT activated Epac to mediate CaMKII activation and phosphorylation of PLB at Thr17 (Fig. 4 C, bottom right) but had little activity in promoting PKA-mediated phosphorylation of PLB at Ser16 (Fig. 4 C, bottom left). In contrast, PLB phosphorylation at Thr17 (CaMKII site) was significantly blunted in the β-arrestin1 and -2 KO mice after ISO or 8-CPT stimulation (Fig. 4 C, bottom right), indicating that β-arrestin is necessary for downstream CaMKII signaling.
Stimulation of β1-ARs but not β2-ARs activates CaMKII signaling
Although it has recently been shown that stimulation of β1-ARs induces CaMKII activation in the heart (Zhu et al., 2003; Yoo et al., 2009), the mechanism by which only β1-ARs and not β2-ARs mediates CaMKII signaling is unknown. With ISO stimulation, pCaMKII significantly increased in hearts of WT and β2-AR KO mice, whereas ISO had no effect on CaMKII activation in hearts from β1-AR KO or β1-, β2-AR double KO mice (Fig. 5 A). In contrast, direct stimulation of Epac with 8-CPT induced CaMKII activation equally in hearts from β1-AR KO, β2-AR KO, and β1-, β2-AR double KO mice. Similar to the in vivo heart experiments, HEK-293 cells stably expressing WT–β1-ARs showed a time-dependent increase in pCaMKII with ISO stimulation (Fig. 5 B), whereas cells stably expressing β2-ARs were unresponsive to ISO (Fig. 5 C). 8-CPT–mediated CaMKII phosphorylation occurred in both β1- and β2-AR stable HEK-293 cells (Fig. 5, B and C). These data show the subtype specificity in CaMKII activation solely through stimulation of β1-ARs. This observation is particularly puzzling because agonist stimulation of both β-AR subtypes robustly activates AC to generate cAMP and should be able to activate Epac and CaMKII equally.
Figure 5.
Activation of CaMKII is mediated by β1-AR stimulation. (A) WT, β1-AR KO, β2-AR KO, and β1-, β2-AR double KO mice were administered with saline (NS), 5 mg/kg ISO, or 2.5 mg/kg 8-CPT by i.v. infusion for 5 min. LV homogenates were immunoblotted with anti-pCaMKII and anti-CaMKII antibodies. The CaMKII activation was quantified, expressed as fold increase over NS-treated WT mice, and shown as mean ± SEM (n = 5). Stimulation of β1-AR but not β2-AR activated CaMKII. *, P < 0.01 versus NS-treated WT mice; #, P < 0.01 versus ISO-treated WT mice. (B and C) HEK-293 cells stably expressing WT–β1-AR (B) or WT–β2-AR (C) were transiently transfected with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with either 10 µM ISO or 5 µM 8-CPT for the indicated times at 37°C (n = 5). Stimulation of β1-AR by ISO markedly increased CaMKII activity over a period of time, whereas β2-AR stimulation had no effect on CaMKII activation. IP, immunoprecipitation; T-CaMKII, total CaMKII.
The C-tail of the β1-AR is required for ISO-mediated CaMKII activation
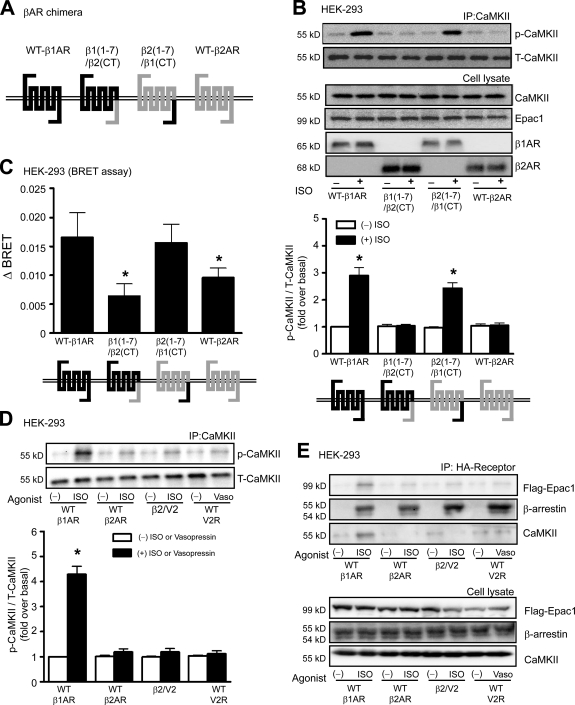
We reasoned that the selective activation of CaMKII by β1-ARs might be the result of its ability to recruit β-arrestin and induce a unique β1-AR–β-arrestin conformation that promotes scaffolding of the Epac–CaMKII complex at the plasma membrane. Because β-arrestin is recruited to the C-tail of β-ARs, we postulated that unique amino acid residues within the C-tail of the β1-ARs account for the β-AR subtype specificity. To test this hypothesis, we used β-AR chimeras in which the C-tail of the β1-AR was exchanged with the C-tail of the β2-AR (Fig. 6 A; Shiina et al., 2000). Only β-ARs that contained the β1-AR C-tail (i.e., WT–β1-AR or β2(1–7)/β1(CT)) were able to activate CaMKII signaling (Fig. 6 B), indicating the critical nature for amino acid residues within the C-tail of the β1-AR in CaMKII activation.
Figure 6.
The carboxyl terminus of β1-AR but not β2-AR is necessary for CaMKII activation and conformational changes of β-arrestin. (A) Structure of chimeric β1/β2-ARs. Dark and light lines indicate the peptide sequences derived from β1- and β2-AR, respectively. The β1(1–7)/β2(CT) represents β1-AR containing the carboxyl terminus of β2-AR, whereas β2(1–7)/β1(CT) represents β2-AR containing the carboxyl terminus of β1-AR. (B) HEK-293 cells were transiently transfected with WT–β1-AR, β1(1–7)/β2(CT), β2(1–7)/β1(CT), or WT–β2-AR, along with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with 10 µM ISO at 37°C. The CaMKII activation was quantified, expressed as fold increase over nonstimulated WT–β1-AR, and shown as mean ± SEM (n = 5). Increased ISO-mediated CaMKII activation was observed in cells expressing WT–β1-AR and β2(1–7)/β1(CT) chimeric receptors. *, P < 0.01 versus nonstimulation. (C) HEK-293 cells were transiently transfected with WT–β1-AR, β1(1–7)/β2(CT), β2(1–7)/β1(CT), or WT–β2-AR with β-arrestin2 double brilliance biosensor (Luc–β-arrestin2–YFP). The BRET ratio, which indicated the conformational change of β-arrestin2, was determined. ΔBRET was defined as the ISO stimulated ‒ nonstimulated BRET ratio, and data are shown as mean ± SEM (n = 5). *, P < 0.05 versus WT–β1-AR. (D) HEK-293 cells were transiently transfected with WT–β1-AR, WT–β2-AR, β2/V2, or V2R, along with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with 10 µM ISO or vasopressin at 37°C. The CaMKII activation was quantified, expressed as fold increase over nonstimulated WT–β1-AR, and shown as mean ± SEM (n = 5). *, P < 0.01 versus nonstimulation. (E) HEK-293 cells were transiently transfected with CaMKII-δC and Flag-Epac1, along with HA–β1-AR, HA–β2-AR, HA-β2/V2, or HA-V2R. Serum-starved cells were stimulated with either 10 µM ISO or vasopressin at 37°C. β-ARs were immunoprecipitated with anti-HA beads and immunoblotted for associated Epac1, β-arrestin, and CaMKII (n = 5). IP, immunoprecipitation; T-CaMKII, total CaMKII.
We next tested whether binding of β-arrestin to the C-tail of the β1-AR induces β-arrestin to adopt a unique conformation such that it can subserve CaMKII signaling. We used a bioluminescence resonance energy transfer (BRET)–based biosensor of β-arrestin2 that detects structural changes of β-arrestin2 in real time (Shukla et al., 2008). HEK-293 cells were transiently transfected with either WT or chimeric β-ARs, along with the YFP–β-arrestin2–Luc biosensor, and BRET was measured before and after ISO stimulation. Stimulation of either WT–β1-AR or β2(1–7)/β1(CT) chimeric receptors resulted in a threefold greater increase in ΔBRET compared with that of WT–β2-AR and β1(1–7)/β2(CT) (Fig. 6 C). To exclude the possibility that the difference in ΔBRET was caused by the amount of β-arrestin bound to agonist-occupied receptors, we performed coimmunoprecipitation experiments and showed that the level of β-arrestin bound to receptor was similar for each of the WT β-ARs and the tail swap chimeric receptors (Fig. S2).
A possible explanation for the β1-AR–selective activation of CaMKII is that β1-ARs simply bind β-arrestin with higher affinity than β2-ARs. To test this notion, we used a chimeric β2-AR containing the C-tail of the vasopressin V2 receptor (V2R), which is known to have very high affinity and prolonged binding for β-arrestin (Oakley et al., 1999). Although CaMKII activation occurred in cells expressing WT–β1-ARs, stimulation of the β2-AR/V2 C-tail chimeric receptor with ISO or the WT V2R with vasopressin was unable to activate CaMKII (Fig. 6 D), despite robust recruitment of β-arrestin to either agonist-occupied receptor (Fig. 6 E). Indeed, only β1-ARs could promote the agonist-induced scaffolding of Epac1 and CaMKII with β-arrestin (Fig. 6 E). These data support our hypothesis that the C-tail of β1-AR mediates a unique conformation of β-arrestin that allows for scaffolding of Epac and CaMKII to form a stable complex, leading to the subsequent cAMP/Epac-mediated activation of CaMKII.
Interaction of CaMKII, Epac1, and β-arrestins with β1-ARs
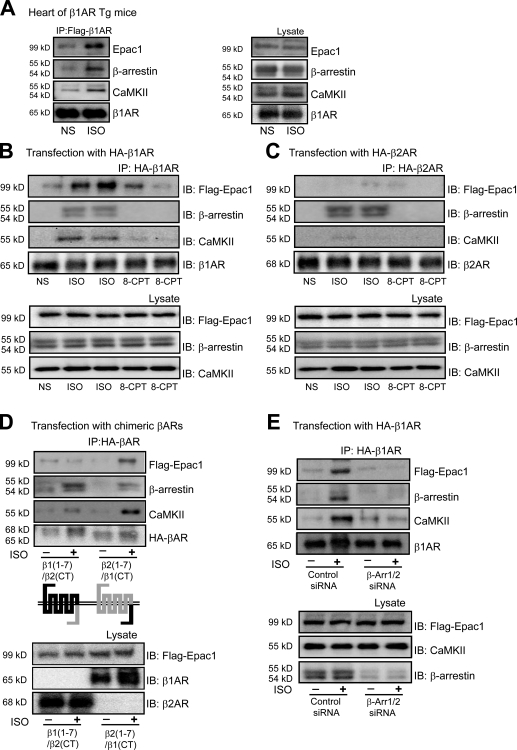
Our data suggest that the binding of β-arrestin to the C-tail of β1-ARs may trigger a unique β-arrestin conformation that stabilizes its binding of CaMKII and Epac as a multimeric complex. To test this hypothesis, we used Tg mice overexpressing Flag-tagged WT–β1-ARs (Noma et al., 2007) and measured the amount of CaMKII, Epac1, and β-arrestin bound to immunoprecipitated β1-ARs before and after ISO simulation. Stimulation of β1-AR by ISO induced the recruitment of Epac, β-arrestin, and CaMKII to agonist-occupied β1-ARs in heart tissue (Fig. 7 A).
Figure 7.
CaMKII and Epac1 interact with the carboxyl terminus of β1-ARs. (A) Tg mice with cardiac-specific overexpression of mouse WT–β1-AR (WT–β1-AR Tg) were administered with saline (NS) or 5 mg/kg ISO by i.v. infusion for 5 min. LV homogenates were immunoprecipitated with anti-Flag antibody. Immunoprecipitated proteins were analyzed by Western blotting using the specific antibodies. Stimulation with ISO increased the amount of CaMKII, Epac1, and β-arrestin binding with β1-AR in hearts. (B and C) HEK-293 cells were transiently transfected with CaMKII-δC and Flag-Epac1, along with either HA–β1-AR (B) or HA–β2-AR (C). Serum-starved cells were stimulated with either 10 µM ISO or 5 µM 8-CPT for 10 min at 37°C. β-ARs were immunoprecipitated with anti-HA beads and immunoblotted for associated Epac1, β-arrestin, and CaMKII. ISO stimulation resulted in significant recruitment of Epac1, β-arrestin, and CaMKII to β1-AR, whereas only β-arrestin recruited to the β2-AR complex. (D) HEK-293 cells were transiently transfected with either β1(1–7)/β2(CT) or β2(1–7)/β1(CT) chimeric receptors, along with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with 10 µM ISO for 10 min at 37°C. The receptor chimeras were immunoprecipitated with anti-HA beads and immunoblotted for associated Epac1, β-arrestin, and CaMKII. The C-tail of β1-AR rather than β2-AR interacted with CaMKII and Epac1 after ISO stimulation. (E) HEK-293 cells were transfected with either control or β-arrestin1/2 siRNA. HEK-293 cells were then transfected with HA–β1-AR along with CaMKII-δC and Flag-Epac1. Serum-starved cells were stimulated with 10 µM ISO for 10 min at 37°C. β1-ARs were immunoprecipitated with anti-HA beads and immunoblotted for associated Epac1, β-arrestin, and CaMKII. Depletion of β-arrestin1/2 inhibited ISO-induced recruitment of CaMKII and Epac1 to the β1-AR complex. Data illustrated in A–E are representative of five independent experiments. IB, immunoblot; IP, immunoprecipitation.
We next examined whether the C-tail of β1-ARs is responsible for binding of the CaMKII–β-arrestin–Epac1 complex after agonist stimulation. Immunoprecipitation of only WT–β1-ARs showed ISO-induced recruitment of Epac1, β-arrestin, and CaMKII to the C-tail of agonist-occupied β1-ARs (Fig. 7 B). Importantly, ISO stimulation of β2-ARs still showed robust β-arrestin translocation but failed to retain Epac1 and CaMKII in the immunoprecipitate (Fig. 7 C). Stimulation with 8-CPT was ineffective in recruiting Epac1, β-arrestin, or CaMKII to either β1- or β2-ARs (Fig. 7, B and C). We used β1/β2 chimeric receptors to determine whether the C-tail of the β1-AR instructs β-arrestin to maintain CaMKII and Epac in a multimeric complex with the receptor. After ISO stimulation, CaMKII and Epac1 robustly bound to the β2(1–7)/β1(CT) chimeric β-AR, whereas only β-arrestin was found in complex with the β1(1–7)/β2(CT) chimeric β-AR (Fig. 7 D). Lastly, depleting β-arrestin1/2 by siRNA eliminated the agonist-induced recruitment of all three proteins (CaMKII, Epac1, and β-arrestin) to the β1-AR (Fig. 7 E). These data show that the recruitment of β-arrestin specifically to the C-tail of β1-AR triggers a conformation in β-arrestin that allows it to scaffold both CaMKII and Epac in a stable complex with the agonist-occupied receptor.
Because PDZ-binding motif (ESKV) facilitates the formation of a cellular complex between β1-ARs and NMDA receptors (Hu et al., 2000), we tested whether the PDZ-binding motif is necessary for β1-AR–mediated CaMKII activation. Using β1-ARs containing PDZ-binding motif mutants (S473A, S475A, S475D, or V477A) transfected into HEK-293 cells, we show that disruption of the PDZ-binding domain had no effect on ISO-mediated CaMKII phosphorylation (Fig. S3).
Discussion
In this study, we provide new insights into the molecular mechanism of CaMKII signaling. We demonstrate the requirement of β-arrestin as a scaffold protein for CaMKII and Epac, whereby agonist stimulation results in the recruitment of this multimeric complex to the β1-AR. β-Arrestin binding to the C-tail of β1-AR promotes a conformational change within β-arrestin that allows CaMKII and Epac to remain in a stable complex when bound to agonist-occupied receptors. This unique β-arrestin conformation induced by binding to the C-tail of β1-AR brings Epac and CaMKII in close proximity to the site of cAMP generation by the effector AC, which is required for the activation of CaMKII and downstream signaling. The essential role for β-arrestin and identification of the molecular mechanism by which only the β1-AR subtype activates CaMKII significantly advances our understanding of this important cellular pathway.
The arrestin family consists of four members. Visual arrestins (arrestin1 and -4) are expressed in the retina. The β-arrestin1 and -2 (nonvisual arrestins, also known as arrestin2 and -3, respectively) are ubiquitously expressed and play a role in β-AR internalization and trafficking (Ferguson, 2001). β-AR stimulation leads to GRK-mediated receptor phosphorylation and the recruitment of β-arrestins to phosphorylated β-ARs, whereby β-arrestins sterically inhibit further interaction between receptor and G proteins. Beyond their classical function, β-arrestins also serve as scaffold proteins in receptor endocytosis and signal transduction, linking receptors to several downstream effectors such as MAPK, Src, and the ubiquitin ligase Mdm2 (Lefkowitz and Shenoy, 2005). Previous studies have shown that β-arrestin interacts with CaMKII-δ after AT1aR stimulation (Xiao et al., 2007) and with calmodulin (Wu et al., 2006). We show in this study that β-arrestins play a far greater role in CaMKII signaling by providing three critical functions: (1) to scaffold CaMKII and Epac in the cytoplasm, (2) to translocate this multimeric complex to the plasma membrane after β-AR stimulation and bring Epac and CaMKII in close proximity to the site of cAMP generation, and (3) to adopt a conformation that maintains high affinity binding between β-arrestin and the agonist-occupied β1-AR, CaMKII, and Epac. CaMKII signaling appears to use both β-arrestin1 and -2 because elimination of either isoform in cells by siRNA, or in mice by gene targeting, significantly diminishes CaMKII activation. This dual β-arrestin requirement might reflect distinct roles played by each isoform or perhaps the requirement for heterodimerization of β-arrestin1 and -2 (Lefkowitz et al., 2006).
β-Arrestins can exist as homo- and heterooligomers (Storez et al., 2005; Milano et al., 2006) and raise the possibility that β-arrestin oligomers may be involved in the regulation of their cellular distribution and function. In contrast, only monomeric visual arrestin binds to rhodopsin (Hanson et al., 2007a,b), and when arrestins mobilize MAPKs to a receptor, it is the nonreceptor-binding side of arrestin that is available for interaction with kinases (Gurevich et al., 2008). Our data demonstrating that both β-arrestin1 and -2 are involved in CaMKII activation are consistent with the codependence of both β-arrestins in other receptor systems in which heterooligomerization has been offered as a possible explanation (DeWire et al., 2007). Nonetheless, it remains an unsolved question whether β-arrestins bound to agonist-occupied β-ARs exist as homo- or heterodimers.
Previous studies have identified a family of molecules known as Epacs that directly bind cAMP and exhibit guanine nucleotide exchange factor activity toward Rap1 (de Rooij et al., 1998; Bos, 2006). Translocation of Epac to the plasma membrane by ISO stimulation was recently shown in several cell types, indicating the important spatial control of Epac by cAMP (Ponsioen et al., 2009). There are two subtypes of Epac, Epac1 and -2. Epac1 is mainly expressed in human heart, is increased in heart failure, and promotes CaMKII activity after β-AR stimulation in a PKA-independent fashion (Métrich et al., 2008). Activation of Epac by cAMP or with the selective cAMP analogue 8-CPT leads to CaMKII-dependent stimulation of SR Ca2+ release and diastolic SR Ca2+ leak (Oestreich et al., 2007; Pereira et al., 2007).
The mechanism by which Epac activates CaMKII appears to require the presence of Rap, PLC-ε, and PKC-ε (Oestreich et al., 2007, 2009). Indeed, it has been shown that the Ras family members Rap1 and -2 mediate translocation of PLC-ε to the membrane and enhance PLC-ε activity (Song et al., 2001; Bunney and Katan, 2006). Enzymatic activity of PLC-ε results in effector signaling by producing DAG and inositol 1,4,5-triphosphate as second messengers. Accumulation of DAG also leads to DAG-dependent translocation of PKC-ε to the membrane (Stahelin et al., 2005; Escribá et al., 2007). Although it is likely that Rap, PLC-ε, and PKC-ε are involved in the activation of CaMKII after β1-AR stimulation, it will require further study to determine the precise molecular details of this pathway and whether any of these proteins are assembled within a β-arrestin–Epac–CaMKII complex.
Interestingly, it has been reported that stimulation of β2-ARs can activate a cAMP–Epac–RapGTP–PLC-ε pathway (Schmidt et al., 2001). However, our data clearly show that only β1-ARs and not β2-ARs are able to stimulate CaMKII. In contrast, β-arrestin binding to β2-AR induces a β-arrestin conformation that is unable to maintain Epac and CaMKII in complex with β-arrestin at the plasma membrane. As we show in this study, an important component to this signaling pathway is the assembly of a multimeric complex scaffolded by β-arrestin, which allows for Epac and CaMKII to be held in structural proximity and be brought to the plasma membrane after β-AR stimulation. In the absence of β-arrestin, CaMKII and Epac are unable to maintain this protein–protein interaction and lose the ability to be activated by β-AR agonists.
Although both β1- and β2-ARs can couple to the G protein Gs, there are several differences in their functional properties that lead to subtype-specific activation of signaling pathways (Xiao et al., 2006). Even though β1- and β2-AR share 54% sequence identity, the C-tail of these receptors plays a differential role in receptor endocytosis and signal transduction. For example, the binding of β-arrestin1 and -2 to the third intracellular loop and the C-tail of the β1-AR is lower than that for the β2-AR (Shiina et al., 2000). A chimeric β2-AR containing the C-tail region of β1-AR loses its ability to promote β-arrestin2–mediated ERK nuclear translocation (Kobayashi et al., 2005). Moreover, the PDZ-binding motif of the β2-AR C-tail interacts with NHERF (Hall et al., 1998), whereas NMDA receptors bind to an ESKV motif within the C-tail of the β1-AR (Hu et al., 2000). In this study, we provide new mechanistic information for the role of β1-ARs in CaMKII signaling in response to catecholamine stimulation. Stimulation of β1-ARs augments the association of CaMKII and Epac1 with β-arrestins. This multimeric protein complex, which then translocates to plasma membrane, binds to the β1-AR C-tail and forms a stable protein–protein complex secondary to a conformational change in β-arrestin. Remarkably, despite the strong agonist-dependent binding of β-arrestin to β2-ARs and V2Rs, neither could promote stable association of CaMKII and Epac with β-arrestin at the plasma membrane. Thus, the recruitment of β-arrestins alone to agonist-occupied receptors is not sufficient to promote β-AR–mediated CaMKII activation but requires a unique β1-AR–mediated conformational change in β-arrestin that is triggered by β-arrestin binding to the β1-AR C-tail. We hypothesize that a conformational change in β-arrestin upon recruitment to the β1-AR increases the affinity of CaMKII and Epac1 to β-arrestin, which tethers Epac and CaMKII in a cAMP microdomain, triggering CaMKII activation. Our data are consistent with a recent study showing that the interaction of calmodulin with the proximal region of the serotonin receptor carboxyl terminus is important for arrestin-dependent ERK signaling (Labasque et al., 2008).
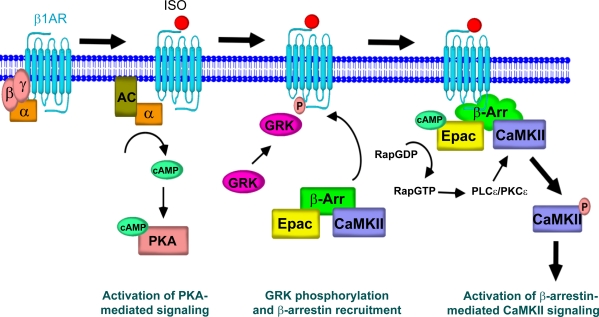
Based on the data from our experiments, we propose an essential role for β-arrestin in mediating CaMKII signaling after β1-AR stimulation (Fig. 8). Agonist binding to β1-ARs leads to G protein–mediated activation of AC and cAMP generation. Promptly thereafter, the stimulation of β1-ARs by agonist leads to GRK-mediated receptor phosphorylation and β-arrestin recruitment. The unique conformational change induced in β-arrestin by binding to β1-ARs allows for a strong interaction with CaMKII and Epac, maintaining these proteins in complex at the plasma membrane. Stimulation of AC activity increases the level of cAMP in the β1-AR–β-arrestin–Epac–CaMKII microdomain. cAMP directly binds to and stimulates Epac, which in turn appears to stimulate Rap–PLC-ε–PKC-ε, leading to the activation of CaMKII. In contrast, β-arrestin binding to β2-ARs induces a β-arrestin conformation that does not promote a stable complex with Epac and CaMKII, and therefore, no CaMKII signaling occurs.
Figure 8.
Schematic diagram representing the essential role of β-arrestin for CaMKII signaling. Agonist binding to β1-ARs leads to G protein coupling, cAMP generation, and activation of PKA signaling. GRK-mediated receptor phosphorylation results in β-arrestin recruitment, which induces a unique conformation in β-arrestin allowing stable interaction with CaMKII and Epac at the plasma membrane. β-Arrestin–mediated translocation of CaMKII and Epac to the β1-AR complex brings these molecules in close proximity to the location of cAMP generation by AC. cAMP directly binds to and stimulates Epac, which leads to CaMKII activation via a Rap–PLC-ε–PKC-ε mechanism, and subsequent phosphorylation of downstream effectors.
CaMKII appears to play an important pathogenic role in the development of heart failure (Anderson, 2009). CaMKII activity is increased in heart failure patients (Gwathmey et al., 1987) and in animal models after myocardial infarction and β-AR stimulation (Yoo et al., 2009). Inhibition of CaMKII activity using a dominant-negative strategy (Zhang et al., 2005) or CaMKII-δ gene ablation (Ling et al., 2009) prevents cardiac dilation and dysfunction after myocardial infarction or pressure overload. Although β-AR stimulation serves as a powerful regulatory mechanism to augment contractility (Rockman et al., 2002), excessive β-AR stimulation causes adverse cardiac remodeling that appears to be predominantly mediated by the β1-AR subtype (Yoo et al., 2009). Our data support a concept whereby excessive β1-AR stimulation promotes a pathological process, in part, through its activation of CaMKII signaling (Zhu et al., 2003, 2007; Wang et al., 2004; Yoo et al., 2009).
In conclusion, we have identified a new signaling mechanism for CaMKII activation regulated by β-arrestins. Activation of CaMKII after β1-AR stimulation requires the scaffolding of CaMKII and Epac by β-arrestin, allowing translocation of this multimeric protein complex to agonist-occupied β1-ARs, which leads to activation of the β-arrestin–mediated CaMKII signaling pathway.
Materials and methods
Reagents and plasmid construction
8-CPT was purchased from Tocris Bioscience. Forskolin, (−)-ISO hydrochloride, and anti-Flag M2 agarose were purchased from Sigma-Aldrich. PKI and KN-92 and -93 were purchased from EMD. γ-[32P]ATP was purchased from PerkinElmer. FuGENE 6 and anti-HA affinity matrix were purchased from Roche. Coelenterazine h was purchased from Promega. Plasmids encoding β-arrestin1–YFP, β-arrestin2–YFP, and β1-AR PDZ-binding motif mutants (S473A, S475A, S475D, and V477A) were obtained from R.J. Lefkowitz (Duke University Medical Center, Durham, NC). Plasmid encoding Luc–β-arrestin2–YFP was obtained from M. Bouvier (Université de Montréal, Montréal, Québec, Canada). Plasmid encoding CaMKII-δC was obtained from G.S. Pitt (Duke University Medical Center). Plasmid encoding Flag-tagged Epac1 was obtained from A.V. Smrcka (University of Rochester, Rochester, NY). Plasmids encoding HA-tagged chimeric β1(1–7)/β2(CT) and β2(1–7)/β1(CT) β-ARs were a gift from H. Kurose (Kyushu University, Fukuoka, Japan).
Cell culture and transient transfection
HEK-293 cells and HEK-293 cells stably expressing WT–β1-AR, GRK−–β1-AR, and WT–β2-AR were cultured as described previously with minor modifications (Naga Prasad et al., 2001). Cells were maintained in either DME or minimal essential medium supplemented with 10% fetal bovine serum and 1:100 penicillin-streptomycin (10,000 U/ml) at 37°C. The cells were seeded at a density of 105 cells for 35-mm confocal dishes or 106 cells for 10-cm dishes before the day of transfection. Appropriate amounts of plasmids (2 µg/35-mm confocal dish, 2 µg/well of 6-well dish, or 4–6 µg/10-cm dish) were used for transient transfection with FuGENE 6 according to the manufacturer’s instructions. Cells were used for assays 2 d after transfection. Under serum starvation conditions, cells were stimulated with 10 µM ISO or 5 µM 8-CPT for the indicated time.
Treatment protocol for mice
The animals in this study were handled according to approved protocols and animal welfare regulations of the authors’ Institutional Review Boards. β-Arrestin KO mice were provided by R.J. Lefkowitz. β-AR KO mice were provided by B.K. Kobilka (Stanford University, Stanford, CA). Mice were administered saline, 5 mg/kg ISO, or 2.5 mg/kg 8-CPT by i.v. infusion. 5 min after ISO or 8-CPT administration, hearts were excised and flash-frozen in liquid N2 for biochemical assays. Hearts were homogenized in lysis buffer containing 20 mM Tris-HCl, pH 7.4, 2 mM EDTA, 1% Nonidet P-40, 10 mM NaF, 1mM Na3VO4, 20% glycerol, 100 µM PMSF, 5 µg/ml aprotinin, and 5 µg/ml leupeptin.
Confocal microscopy
Confocal microscopy was performed as described previously (Mangmool et al., 2006). In brief, HEK-293 cells stably expressing WT–β1-AR transfected with either β-arrestin1– or β-arrestin2–YFP together with either CaMKII-δC or Flag-Epac1 were plated on 35-mm glass-bottomed culture dishes. After stimulation with ISO at 37°C, cells were washed once with PBS, fixed in 4% paraformaldehyde, permeabilized in 0.1% Triton X-100, and stained using Texas red fluorescent–labeled secondary antibodies against either CaMKII-δ or Epac1. Colocalization of β-arrestins with either CaMKII-δC or Epac1 was performed using dual excitation (488 nm for YFP and 543 nm for Texas red) of a laser-scanning microscope (LSM-510M; Carl Zeiss, Inc.) with a Plan-Apochromat 100× NA 1.4 objective lens (Carl Zeiss, Inc.) and observed at room temperature. The image analysis was determined using LSM-510 software (Carl Zeiss, Inc.).
siRNA experiments targeting β-arrestins
To design β-arrestin–specific siRNA duplexes, the mRNA sequences for human β-arrestin1 and -2 were screened for unique 21-nt sequences in the National Center for Biotechnology Information database using the BLAST search algorithm. The siRNA sequences targeting β-arrestin1 (GenBank/EMBL/DDBJ accession no. NM_020251) and β-arrestin2 (GenBank/EMBL/DDBJ accession no. NM_004313) have been previously described (Ahn et al., 2003). A nonsilencing RNA duplex (5′-AAUUCUCCGAACGUGUCACGU-3′) was used as a control. 30–40% confluent HEK-293 cells stably expressing WT–β1-AR were transfected with 100 nM β-arrestin siRNA or control siRNA using GeneSilencer Transfection reagent (Gene Therapy Systems) according to the manufacturer’s instructions. In brief, 5 µl of transfection reagent was added to 45 µl Opti-MEM I (Invitrogen), whereas 100 nM of siRNA duplex was mixed with 45 µl Opti-MEM I. Both solutions were allowed to stand 5–10 min at room temperature and mixed by inversion. After 20 min of incubation, the entire transfection mixture was added to cells in a 10-cm dish containing 4 ml of fresh, serum-free DME. After cells were incubated for 5 h at 37°C and 5% CO2, transfection medium was replaced with the original conditioned medium. All assays were performed at least 3 d after siRNA transfection.
CaMKII activity assay
CaMKII activity of ventricular homogenates was measured with a CaMKII assay kit (Millipore) using a specific peptide substrate (KKALRRQETVDAL) as previously described (Yoo et al., 2009). In brief, left ventricular (LV) homogenate (1 mg protein) was first immunoprecipitated with anti-CaMKII antibody (Santa Cruz Biotechnology, Inc.). Samples were incubated in reaction buffer containing the CaMKII peptide substrate and 0.5 µCi γ-[32P]ATP in a total volume of 50 µl. The reactions were performed at 30°C for 20 min and were terminated by chilling on ice. Equal amounts of reaction products were blotted on membrane and washed with 1% HCl to remove unincorporated isotope. CaMKII activity was analyzed by scintillation counting and normalized to protein concentration.
Western blotting
After stimulation, cells were washed once with PBS and solubilized in lysis buffer containing 20 mM Tris-HCl, pH 7.4, 0.8% Triton X-100, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 100 µM PMSF, 5 µg/ml aprotinin, and 5 µg/ml leupeptin. Protein concentration of LV homogenates and cell lysates was determined using a protein assay kit (Bio-Rad Laboratories) with bovine serum albumin as standard. Samples were mixed with loading buffer and denatured by heating at 95°C for 5 min before separation by SDS-PAGE. Separated proteins were transferred to polyvinylidene fluoride membrane (Bio-Rad Laboratories) and subjected to immunoblotting with various primary antibodies to CaMKII (1:2,000; Santa Cruz Biotechnology, Inc.), CaMKII-δ (1:2,000; Santa Cruz Biotechnology, Inc.), pCaMKII (Thr286/287; 1:2,000; Cell Signaling Technology), pCaMKII (Thr286/287; 1:2,000; Millipore), PLB (1:2,000; Santa Cruz Biotechnology, Inc.), phospho-PLB (Ser16 or Thr17; 1:2,000; Santa Cruz Biotechnology, Inc.), β-arrestin (1:2,000; BD), β-arrestin (1:3,000; gift from R.J. Lefkowitz), Flag (1:2,000; Sigma-Aldrich), HA (1:2,000; BD), β1-AR (1:2,000; Santa Cruz Biotechnology, Inc.), and β2-AR (1:2,000; Santa Cruz Biotechnology, Inc.). Although both β-arrestin isoforms could be detected in HEK-293 cells, only β-arrestin1 was reliably detected in the heart (Fig. S4). Immunoblots were visualized with an HRP-conjugated secondary antibody and a chemiluminescence detection system (GE Healthcare).
Immunoprecipitation
Protein concentration of LV homogenates and cell lysates was determined using a Bio-Rad Laboratories protein assay kit with bovine serum albumin as standard. Immunoprecipitation was performed by overnight incubation/rotation with 2 µg of rabbit polyclonal anti-Epac (Santa Cruz Biotechnology, Inc.), goat polyclonal anti-CaMKII (Santa Cruz Biotechnology, Inc.), or rabbit polyclonal anti–β-arrestin antibodies (gift from R.J. Lefkowitz). The control (no antibody) was also included for each sample. After incubation, protein A/G plus agarose beads was added to immunoprecipitated samples and rotated for 2 h with rotation. Beads were washed twice with lysis buffer, once with PBS. Bound proteins were eluted by the addition of SDS loading buffer and analyzed by Western blotting using the indicated antibodies.
BRET assay
BRET assays were performed as described previously (Charest et al., 2005; Shukla et al., 2008). In brief, 48 h after transfection, HEK-293 cells coexpressing the WT and chimeric β-ARs and Luc–β-arrestin2–YFP were detached with PBS/EDTA. Approximately 5 × 105 cells per well were plated in a 96-well white microplate. The cells were incubated with 5 µM coelenterazine h for 10 min and subsequently stimulated with 10 µM ISO at 25°C for the indicated time period, and readings were collected immediately by using a microplate reader (Mithras LB940; Berthold Technologies). The BRET signal was determined as the ratio of the light emitted by YFP and the light emitted by Luc. The values were corrected by subtracting the background BRET signals. ΔBRET was defined as the ISO stimulated ‒ nonstimulated BRET ratio.
Statistics
Data are expressed as mean ± SEM. Statistical analyses were determined using one-way analysis of variance. Differences were considered statistically significant at P < 0.05.
Online supplemental material
Fig. S1 demonstrates that β-arrestin associates with Epac1 in CaMKII-δ KO mice. Fig. S2 shows that the level of β-arrestin bound to receptor is similar for each of the WT β-ARs and the tail swap chimeric receptors. Fig. S3 demonstrates that disruption of the PDZ-binding motif of β1-ARs had no influence of ISO-mediated CaMKII phosphorylation. Fig. S4 shows the level of β-arrestin expression in hearts obtained from β-arrestin1 and -2 KO mice. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200911047/DC1.
Acknowledgments
We thank Robert J. Lefkowitz for his intellectual insight and Weili Zou for her excellent technical assistance. We also thank Dr. Ling Zhang for her invaluable help with the preparation of this manuscript.
This work was supported by National Institutes of Health grants HL56687 and HL-75443 to H.A. Rockman.
Footnotes
Abbreviations used in this paper:
- 8-CPT
- 8-pCPT-2′-O-Me-cAMP
- AC
- adenylyl cyclase
- AR
- adrenergic receptor
- BRET
- bioluminescence resonance energy transfer
- CaMKII
- Ca2+/calmodulin kinase II
- C-tail
- carboxyl-terminal tail
- Epac
- exchange protein directly activated by cAMP
- ERK
- extracellular signal-regulated kinase
- GRK
- G protein–coupled receptor kinase
- ISO
- isoproterenol
- KO
- knockout
- LV
- left ventricular
- NMDA
- N-methyl-d-aspartate
- pCaMKII
- phosphorylated CaMKII
- PDZ
- PSD-95/Dlg/ZO-1 homology domain
- PKI
- PKA inhibitor
- PLB
- phospholamban
- SR
- sarcoplasmic reticulum
- Tg
- transgenic
- V2R
- V2 receptor
- WT
- wild type
References
- Ahn S., Nelson C.D., Garrison T.R., Miller W.E., Lefkowitz R.J. 2003. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. USA. 100:1740–1744 10.1073/pnas.262789099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.E. 2009. CaMKII and a failing strategy for growth in heart. J. Clin. Invest. 119:1082–1085 10.1172/JCI39262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D.M. 2002. Cardiac excitation-contraction coupling. Nature. 415:198–205 10.1038/415198a [DOI] [PubMed] [Google Scholar]
- Bohn L.M., Lefkowitz R.J., Gainetdinov R.R., Peppel K., Caron M.G., Lin F.T. 1999. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 286:2495–2498 10.1126/science.286.5449.2495 [DOI] [PubMed] [Google Scholar]
- Bos J.L. 2006. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 31:680–686 10.1016/j.tibs.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Bunney T.D., Katan M. 2006. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 16:640–648 10.1016/j.tcb.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Charest P.G., Terrillon S., Bouvier M. 2005. Monitoring agonist-promoted conformational changes of beta-arrestin in living cells by intramolecular BRET. EMBO Rep. 6:334–340 10.1038/sj.embor.7400373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communal C., Singh K., Sawyer D.B., Colucci W.S. 1999. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 100:2210–2212 [DOI] [PubMed] [Google Scholar]
- Conner D.A., Mathier M.A., Mortensen R.M., Christe M., Vatner S.F., Seidman C.E., Seidman J.G. 1997. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ. Res. 81:1021–1026 [DOI] [PubMed] [Google Scholar]
- Curran J., Hinton M.J., Ríos E., Bers D.M., Shannon T.R. 2007. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ. Res. 100:391–398 10.1161/01.RES.0000258172.74570.e6 [DOI] [PubMed] [Google Scholar]
- de Rooij J., Zwartkruis F.J., Verheijen M.H., Cool R.H., Nijman S.M., Wittinghofer A., Bos J.L. 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 396:474–477 10.1038/24884 [DOI] [PubMed] [Google Scholar]
- DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. 2007. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 69:483–510 10.1146/annurev.physiol.69.022405.154749 [DOI] [PubMed] [Google Scholar]
- Enserink J.M., Christensen A.E., de Rooij J., van Triest M., Schwede F., Genieser H.G., Døskeland S.O., Blank J.L., Bos J.L. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4:901–906 10.1038/ncb874 [DOI] [PubMed] [Google Scholar]
- Escribá P.V., Wedegaertner P.B., Goñi F.M., Vögler O. 2007. Lipid-protein interactions in GPCR-associated signaling. Biochim. Biophys. Acta. 1768:836–852 10.1016/j.bbamem.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Ferguson S.S. 2001. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53:1–24 [PubMed] [Google Scholar]
- Ferguson S.S., Downey W.E., III, Colapietro A.M., Barak L.S., Ménard L., Caron M.G. 1996. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 271:363–366 10.1126/science.271.5247.363 [DOI] [PubMed] [Google Scholar]
- Gurevich V.V., Gurevich E.V., Cleghorn W.M. 2008. Arrestins as multi-functional signaling adaptors. Handb. Exp. Pharmacol. 186:15–37 10.1007/978-3-540-72843-6_2 [DOI] [PubMed] [Google Scholar]
- Gwathmey J.K., Copelas L., MacKinnon R., Schoen F.J., Feldman M.D., Grossman W., Morgan J.P. 1987. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ. Res. 61:70–76 [DOI] [PubMed] [Google Scholar]
- Hall R.A., Premont R.T., Chow C.W., Blitzer J.T., Pitcher J.A., Claing A., Stoffel R.H., Barak L.S., Shenolikar S., Weinman E.J., et al. 1998. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 392:626–630 10.1038/33458 [DOI] [PubMed] [Google Scholar]
- Hanson S.M., Gurevich E.V., Vishnivetskiy S.A., Ahmed M.R., Song X., Gurevich V.V. 2007a. Each rhodopsin molecule binds its own arrestin. Proc. Natl. Acad. Sci. USA. 104:3125–3128 10.1073/pnas.0610886104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S.M., Van Eps N., Francis D.J., Altenbach C., Vishnivetskiy S.A., Arshavsky V.Y., Klug C.S., Hubbell W.L., Gurevich V.V. 2007b. Structure and function of the visual arrestin oligomer. EMBO J. 26:1726–1736 10.1038/sj.emboj.7601614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.A., Tang Y., Miller W.E., Cong M., Lau A.G., Lefkowitz R.J., Hall R.A. 2000. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J. Biol. Chem. 275:38659–38666 10.1074/jbc.M005938200 [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Narita Y., Nishida M., Kurose H. 2005. Beta-arrestin2 enhances beta2-adrenergic receptor-mediated nuclear translocation of ERK. Cell. Signal. 17:1248–1253 10.1016/j.cellsig.2004.12.014 [DOI] [PubMed] [Google Scholar]
- Koch W.J., Lefkowitz R.J., Rockman H.A. 2000. Functional consequences of altering myocardial adrenergic receptor signaling. Annu. Rev. Physiol. 62:237–260 10.1146/annurev.physiol.62.1.237 [DOI] [PubMed] [Google Scholar]
- Labasque M., Reiter E., Becamel C., Bockaert J., Marin P. 2008. Physical interaction of calmodulin with the 5-hydroxytryptamine2C receptor C-terminus is essential for G protein-independent, arrestin-dependent receptor signaling. Mol. Biol. Cell. 19:4640–4650 10.1091/mbc.E08-04-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R.J., Shenoy S.K. 2005. Transduction of receptor signals by beta-arrestins. Science. 308:512–517 10.1126/science.1109237 [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J., Rajagopal K., Whalen E.J. 2006. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 24:643–652 10.1016/j.molcel.2006.11.007 [DOI] [PubMed] [Google Scholar]
- Ling H., Zhang T., Pereira L., Means C.K., Cheng H., Gu Y., Dalton N.D., Peterson K.L., Chen J., Bers D., Heller Brown J. 2009. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J. Clin. Invest. 119:1230–1240 10.1172/JCI38022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M.J., Engelhardt S., Eschenhagen T. 2003. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 93:896–906 10.1161/01.RES.0000102042.83024.CA [DOI] [PubMed] [Google Scholar]
- Mangmool S., Haga T., Kobayashi H., Kim K.M., Nakata H., Nishida M., Kurose H. 2006. Clathrin required for phosphorylation and internalization of beta2-adrenergic receptor by G protein-coupled receptor kinase 2 (GRK2). J. Biol. Chem. 281:31940–31949 10.1074/jbc.M602832200 [DOI] [PubMed] [Google Scholar]
- Métrich M., Lucas A., Gastineau M., Samuel J.L., Heymes C., Morel E., Lezoualc’h F. 2008. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 102:959–965 10.1161/CIRCRESAHA.107.164947 [DOI] [PubMed] [Google Scholar]
- Milano S.K., Kim Y.M., Stefano F.P., Benovic J.L., Brenner C. 2006. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J. Biol. Chem. 281:9812–9823 10.1074/jbc.M512703200 [DOI] [PubMed] [Google Scholar]
- Naga Prasad S.V., Barak L.S., Rapacciuolo A., Caron M.G., Rockman H.A. 2001. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J. Biol. Chem. 276:18953–18959 10.1074/jbc.M102376200 [DOI] [PubMed] [Google Scholar]
- Noma T., Lemaire A., Naga Prasad S.V., Barki-Harrington L., Tilley D.G., Chen J., Le Corvoisier P., Violin J.D., Wei H., Lefkowitz R.J., Rockman H.A. 2007. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J. Clin. Invest. 117:2445–2458 10.1172/JCI31901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R.H., Laporte S.A., Holt J.A., Barak L.S., Caron M.G. 1999. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem. 274:32248–32257 10.1074/jbc.274.45.32248 [DOI] [PubMed] [Google Scholar]
- Oestreich E.A., Wang H., Malik S., Kaproth-Joslin K.A., Blaxall B.C., Kelley G.G., Dirksen R.T., Smrcka A.V. 2007. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J. Biol. Chem. 282:5488–5495 10.1074/jbc.M608495200 [DOI] [PubMed] [Google Scholar]
- Oestreich E.A., Malik S., Goonasekera S.A., Blaxall B.C., Kelley G.G., Dirksen R.T., Smrcka A.V. 2009. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J. Biol. Chem. 284:1514–1522 10.1074/jbc.M806994200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Métrich M., Fernández-Velasco M., Lucas A., Leroy J., Perrier R., Morel E., Fischmeister R., Richard S., Bénitah J.P., et al. 2007. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J. Physiol. 583:685–694 10.1113/jphysiol.2007.133066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B., Gloerich M., Ritsma L., Rehmann H., Bos J.L., Jalink K. 2009. Direct spatial control of Epac1 by cyclic AMP. Mol. Cell. Biol. 29:2521–2531 10.1128/MCB.01630-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapacciuolo A., Suvarna S., Barki-Harrington L., Luttrell L.M., Cong M., Lefkowitz R.J., Rockman H.A. 2003. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 278:35403–35411 10.1074/jbc.M305675200 [DOI] [PubMed] [Google Scholar]
- Rockman H.A., Koch W.J., Lefkowitz R.J. 2002. Seven-transmembrane-spanning receptors and heart function. Nature. 415:206–212 10.1038/415206a [DOI] [PubMed] [Google Scholar]
- Schmidt M., Evellin S., Weernink P.A., von Dorp F., Rehmann H., Lomasney J.W., Jakobs K.H. 2001. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat. Cell Biol. 3:1020–1024 10.1038/ncb1101-1020 [DOI] [PubMed] [Google Scholar]
- Shiina T., Kawasaki A., Nagao T., Kurose H. 2000. Interaction with beta-arrestin determines the difference in internalization behavior between beta1- and beta2-adrenergic receptors. J. Biol. Chem. 275:29082–29090 10.1074/jbc.M909757199 [DOI] [PubMed] [Google Scholar]
- Shukla A.K., Violin J.D., Whalen E.J., Gesty-Palmer D., Shenoy S.K., Lefkowitz R.J. 2008. Distinct conformational changes in beta-arrestin report biased agonism at seven-transmembrane receptors. Proc. Natl. Acad. Sci. USA. 105:9988–9993 10.1073/pnas.0804246105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Hu C.D., Masago M., Kariyai K., Yamawaki-Kataoka Y., Shibatohge M., Wu D., Satoh T., Kataoka T. 2001. Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J. Biol. Chem. 276:2752–2757 10.1074/jbc.M008324200 [DOI] [PubMed] [Google Scholar]
- Stahelin R.V., Digman M.A., Medkova M., Ananthanarayanan B., Melowic H.R., Rafter J.D., Cho W. 2005. Diacylglycerol-induced membrane targeting and activation of protein kinase Cepsilon: mechanistic differences between protein kinases Cdelta and Cepsilon. J. Biol. Chem. 280:19784–19793 10.1074/jbc.M411285200 [DOI] [PubMed] [Google Scholar]
- Storez H., Scott M.G., Issafras H., Burtey A., Benmerah A., Muntaner O., Piolot T., Tramier M., Coppey-Moisan M., Bouvier M., et al. 2005. Homo- and hetero-oligomerization of beta-arrestins in living cells. J. Biol. Chem. 280:40210–40215 10.1074/jbc.M508001200 [DOI] [PubMed] [Google Scholar]
- Sucharov C.C., Mariner P.D., Nunley K.R., Long C., Leinwand L., Bristow M.R. 2006. A beta1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am. J. Physiol. Heart Circ. Physiol. 291:H1299–H1308 10.1152/ajpheart.00017.2006 [DOI] [PubMed] [Google Scholar]
- Wang W., Zhu W., Wang S., Yang D., Crow M.T., Xiao R.P., Cheng H. 2004. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ. Res. 95:798–806 10.1161/01.RES.0000145361.50017.aa [DOI] [PubMed] [Google Scholar]
- Wu N., Hanson S.M., Francis D.J., Vishnivetskiy S.A., Thibonnier M., Klug C.S., Shoham M., Gurevich V.V. 2006. Arrestin binding to calmodulin: a direct interaction between two ubiquitous signaling proteins. J. Mol. Biol. 364:955–963 10.1016/j.jmb.2006.09.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., McClatchy D.B., Shukla A.K., Zhao Y., Chen M., Shenoy S.K., Yates J.R., III, Lefkowitz R.J. 2007. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. USA. 104:12011–12016 10.1073/pnas.0704849104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R.P., Zhu W., Zheng M., Cao C., Zhang Y., Lakatta E.G., Han Q. 2006. Subtype-specific alpha1- and beta-adrenoceptor signaling in the heart. Trends Pharmacol. Sci. 27:330–337 10.1016/j.tips.2006.04.009 [DOI] [PubMed] [Google Scholar]
- Yoo B., Lemaire A., Mangmool S., Wolf M.J., Curcio A., Mao L., Rockman H.A. 2009. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 297:H1377–H1386 10.1152/ajpheart.00504.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Khoo M.S., Wu Y., Yang Y., Grueter C.E., Ni G., Price E.E., Jr., Thiel W., Guatimosim S., Song L.S., et al. 2005. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 11:409–417 10.1038/nm1215 [DOI] [PubMed] [Google Scholar]
- Zhang T., Brown J.H. 2004. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc. Res. 63:476–486 10.1016/j.cardiores.2004.04.026 [DOI] [PubMed] [Google Scholar]
- Zhu W.Z., Wang S.Q., Chakir K., Yang D., Zhang T., Brown J.H., Devic E., Kobilka B.K., Cheng H., Xiao R.P. 2003. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 111:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Woo A.Y., Yang D., Cheng H., Crow M.T., Xiao R.P. 2007. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J. Biol. Chem. 282:10833–10839 10.1074/jbc.M611507200 [DOI] [PubMed] [Google Scholar]