Abstract
Spines are sites of excitatory synapse formation in central neurons. Alterations in spine structure and function are widely believed to actively contribute to the cellular mechanisms of learning and memory. In this issue, Mendez et al. (2010. J. Cell Biol. doi:10.1083/jcb.201003007) demonstrate a pivotal role for the cell adhesion molecule N-cadherin in activity-mediated spine stabilization, offering a new mechanism for how spine dynamics and stability are regulated by activity in central neurons.
Dendritic spines are protrusions that arise on neuronal dendrites. At their enlarged heads, they bear synapses, which are the sites of excitatory synaptic transmission. During development, spines are dynamic and undergo active formation and elimination, thereby contributing to the sculpting of neural networks. In the adult, spines tend to be less dynamic as the spines stabilize and spine elimination is reduced. Spines can undergo structural remodeling and, to a lesser extent, changes in numbers in response to various physiological stimuli and synaptic activity, thereby contributing to the refinement of neural networks (Majewska et al., 2006). Long-term potentiation induces spine head enlargement accompanied by selective recruitment of proteins (Lee et al., 2009) and reorganization of the actin cytoskeleton. More recently, it has been suggested that stability of spines tightly correlates with certain forms of learning and memory (Xu et al., 2009; Yang et al., 2009; Roberts et al., 2010). Moreover, abnormal spine and synapse structures are often observed in several human diseases, including mental retardations and autisms (Chapleau et al., 2009), with underlying deficits in learning and memory.
The molecular mechanisms that underlie the ability of selective spines to persist or retain stability have remained unclear. Cell adhesion molecules are potential candidates to mediate this functional role because of their ability to mediate adhesive roles and signal through a variety of intracellular pathways (Ko et al., 2009; Woo et al., 2009). In this issue, Mendez et al. provide support for this idea by showing that N-cadherin, a transsynaptic cell adhesion molecule, regulates activity-mediated stabilization of spines in hippocampal neurons. N-cadherin possesses a single transmembrane domain with an intracellular tail region through which it is associated with a family of proteins, the catenins, that enable it to function in multiple signaling pathways (Arikkath and Reichardt, 2008).
Using long-term imaging of transfected hippocampal neurons, Mendez et al. (2010) show that overexpression of N-cadherin augments spine stability. Furthermore, knockdown of N-cadherin or expression of a dominant-negative construct of N-cadherin that is deficient in mediating adhesion and can compete for endogenous intracellular N-cadherin–mediated signaling decreases spine stability. The stability of spines that are newly formed is affected. Interestingly, the dominant-negative mutant also prevents the activity-dependent stabilization of new spines and accumulation of PSD-95, a postsynaptic marker, at synapses. The authors then examined the role of N-cadherin in maintaining the stability of preexisting spines using a similar approach and demonstrate that N-cadherin function is required for spines to persist over long periods of time.
The authors further demonstrate that the expression of EGFP–N-cadherin is restricted to some spines and that this expression correlates with the stability of spines. Furthermore, N-cadherin is preferentially recruited to activated synapses. This also raises the interesting question of a role for N-cadherin in synaptic tagging, as has been recently proposed for Homer-1a (Okada et al., 2009). A synaptic tag is a marker that is associated with synapses that have been activated.
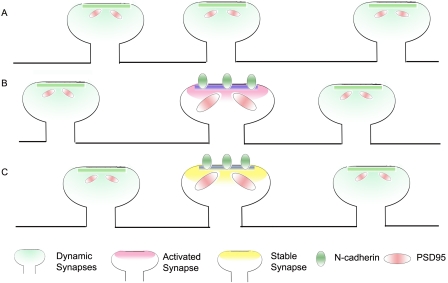
At the level of individual synapses, the induction of long-term potentiation promotes the enlargement of the spine head, accompanied by an increase in the stability of spines (Yang et al., 2008; Lee et al., 2009). The expression of the dominant-negative construct of N-cadherin prevents this activity-dependent enlargement of the spine head. Interestingly, this effect is also prevented by the expression of the full-length N-cadherin, suggesting that a physiological level of N-cadherin is critical for its ability to regulate the spine head width. More importantly, it provides evidence for a key role for N-cadherin in activity-dependent spine stabilization (Fig. 1). Thus, Mendez et al. (2010) have identified a protein marker that is recruited to synapses in an activity-dependent manner and promotes the stability of activated synapses.
Figure 1.
N-cadherin promotes dendritic spine stability. (A) Spines are dynamic and undergo formation and elimination, leading to spine turnover. These spines have relatively small postsynaptic densities and PSD-95 puncta. (B) Synaptic activity promotes the localization of N-cadherin to activated synapses. (C) Activated synapses expressing N-cadherin are stabilized, have large postsynaptic densities, and express large PSD-95 puncta.
The work of Mendez et al. (2010) is clearly an important step forward in our understanding of the molecular mechanisms that underlie activity-dependent spine stability and persistence and should provide interesting leads to understand the molecular mechanisms of learning and memory. Several interesting questions remain. What are the intracellular effectors that allow N-cadherin to mediate its functional role? How does synaptic activity regulate the expression and localization of N-cadherin? Can N-cadherin function as a tag for synapses that persist during the lifetime of the animal? Is selective stabilization of N-cadherin–expressing synapses associated with certain forms of memory? It would be interesting to examine how this functional role of N-cadherin contributes to neural network function and learning and memory. The availability of a mouse model and advanced imaging technologies would surely facilitate progress in this exciting area.
References
- Arikkath J., Reichardt L.F. 2008. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 31:487–494 10.1016/j.tins.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau C.A., Calfa G.D., Lane M.C., Albertson A.J., Larimore J.L., Kudo S., Armstrong D.L., Percy A.K., Pozzo-Miller L. 2009. Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol. Dis. 35:219–233 10.1016/j.nbd.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J., Fuccillo M.V., Malenka R.C., Südhof T.C. 2009. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 64:791–798 10.1016/j.neuron.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Escobedo-Lozoya Y., Szatmari E.M., Yasuda R. 2009. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 458:299–304 10.1038/nature07842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska A.K., Newton J.R., Sur M. 2006. Remodeling of synaptic structure in sensory cortical areas in vivo. J. Neurosci. 26:3021–3029 10.1523/JNEUROSCI.4454-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P., De Roo M., Poglia L., Klauser P., Muller D. 2010. N-cadherin mediates plasticity-induced long-term spine stabilization. J. Cell Biol. 189:589–600 10.1083/jcb.201003007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada D., Ozawa F., Inokuchi K. 2009. Input-specific spine entry of soma-derived Vesl-1S protein conforms to synaptic tagging. Science. 324:904–909 10.1126/science.1171498 [DOI] [PubMed] [Google Scholar]
- Roberts T.F., Tschida K.A., Klein M.E., Mooney R. 2010. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 463:948–952 10.1038/nature08759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J., Kwon S.K., Choi S., Kim S., Lee J.R., Dunah A.W., Sheng M., Kim E. 2009. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat. Neurosci. 12:428–437 10.1038/nn.2279 [DOI] [PubMed] [Google Scholar]
- Xu T., Yu X., Perlik A.J., Tobin W.F., Zweig J.A., Tennant K., Jones T., Zuo Y. 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 462:915–919 10.1038/nature08389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Pan F., Gan W.B. 2009. Stably maintained dendritic spines are associated with lifelong memories. Nature. 462:920–924 10.1038/nature08577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang X.B., Frerking M., Zhou Q. 2008. Spine expansion and stabilization associated with long-term potentiation. J. Neurosci. 28:5740–5751 10.1523/JNEUROSCI.3998-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]