Abstract
The notion that brown adipose tissue (BAT) in mice or humans maintains energy balance by burning off excess calories seems incompatible with evolutionary biology. Studies in obese rats and mice lacking UCP1 indicate that diet-induced thermogenesis by BAT is unlikely.
Introduction
The concept that brown adipose tissue (BAT) is a thermogenic tissue initially came from morphological changes in the tissue when animals were exposed to the cold (Smith and Horwitz, 1969). The physiological basis for the enormous capacity of BAT for heat production during cold exposure awaited the ingenious application of radiolabeled microspheres to measure blood flow in BAT and other tissues (Foster and Frydman, 1978). That production of heat occurred by uncoupling mitochondria was firmly established with the elegant experiments of Nicholls on physiology of BAT mitochondria and Ricquier’s identification of a unique cold-inducible protein in the mitochondria of BAT (Nicholls and Locke, 1984). The presence of large amounts of differentiated BAT in human neonates and species like sheep that require active thermogenesis at birth to protect the newborn from cold exposure on a snow-covered pasture in spring argues that BAT evolved primarily to function as a thermogenic system to protect body temperature (Casteilla et al., 1989).
What is a matter for debate is whether BAT thermogenesis burns off excess calories in a state of positive energy balance to maintain energy homeostasis as a major physiological function. This concept emerged from experiments in the 1970s when Rothwell and Stock observed that rats fed a cafeteria diet (composed of junk foods high in fats and sugars) gained less weight than expected from caloric intake, and they proposed that excess unaccounted calories were being burned off by the induction of BAT thermogenesis (Rothwell and Stock, 1979). Accordingly, BAT thermogenesis was proposed as a mechanism not only for protecting body temperature, but also to protect against obesity and the development of insulin resistance.
The data for diet-induced thermogenesis dovetailed with evidence that cold sensitivity and obesity phenotypes in ob/ob mice were associated with defective BAT nonshivering thermogenesis (Trayhurn et al., 1977). This idea that obesity was caused by a defective BAT set in motion a major effort by clinicians to find obese individuals with a slow metabolism. Prentice has commented on the depth of frustration experienced by clinicians when they failed to find obese humans with slow metabolism (Prentice and Jebb, 2004). Prentice blamed research investigating energy expenditure in the ob/ob mouse for this fruitless phase of obesity research in humans, concluding that the fundamental phenotypes of obesity and body weight regulation in ob/ob mice and humans were different. It is now well established that energy expenditure increases as a function of body mass in humans, and accordingly severe obesity is associated with an increase in energy expenditure (Leibel et al., 1995). Moreover, in the interim it became clear that ob/ob mice have neither reduced energy expenditure nor defective BAT per se. Energy expenditure in ob/ob mice was underestimated from the erroneous calculation of energy expenditure in studies comparing lean and obese mice, a problem that persists with a frustratingly high frequency to this day (Butler and Kozak, 2010). Perceived defects in brown fat did not come from intrinsic defects in Ucp1 induction, but from secondary problems related to excessive white fat in morbidly obese ob/ob mice and regulatory problems arising from downregulation of β1- and β3-adrenergic receptors in ob/ob mice and other models of obesity that attenuate induction of Ucp1 and lipolysis (Robidoux et al., 2004). That none of the phenotypes of energy balance in ob/ob mice are due to modulation of Ucp1 expression was established with experiments showing that phenotypes of energy balance including food intake, adiposity, and oxygen consumption do not vary between ob/ob and Lep−/−.Ucp−/− mice under basal and leptin-stimulated conditions (Ukropec et al., 2006). Accordingly, the basic phenotypes relating to energy expenditure and obesity in mouse and human models of leptin null mutations are in agreement (Farooqi et al., 1999), and the data do not implicate BAT.
Cold Exposure and Adrenergic Receptor Agonists Regulate Adiposity
We are now entering another heady era for research that seeks to relate directly the function of BAT thermogenesis to the regulation of body weight. The basis of this renewed interest is the realization that PET/CT images of active glucose uptake in humans are sites of active BAT. The function of BAT as a thermogenic mechanism for the regulation of body temperature is well established and indisputable. In the key experiment, Foster and Frydman showed that following norepinephrine administration to cold-acclimated rats, blood flow through BAT accounted for 33% of cardiac output even though BAT mass constitutes only 1.3% of total body mass. In the same animals, skeletal muscle constituted 38% of body mass, yet accounted for only 8.4% of cardiac output (Foster and Frydman, 1978). The effects of this thermogenic system for maintaining body temperature on energy balance are correspondingly impressive. For example, when diet-induced obese C57BL/6J mice were exposed to an ambient temperature of 5°C for 7 days, food intake is increased by 50%, yet they lose 55% of body fat mass (Nikonova et al., 2008). Excessive depletion of white fat does not occur during the high demand for heat as long as sufficient food is available, underscoring the stringent regulatory relationships between heat production, adiposity, and food intake (Nikonova et al., 2008).
It is not necessary to expose an animal to cold in order to utilize BAT thermogenesis to reduce obesity and bring body composition into balance. Several studies with Zucker rats, dogs, and mice with obesity showed robust reductions in adiposity when treated with a β3-adrenergic agonist (Robidoux et al., 2004), although in humans, β3-adrenergic agonists have not been successful. This ineffectual response in humans may be related to the low number of β3-adrenergic receptors and/or inducible brown adipocytes in human white fat. With the proper drug, there is no obvious reason why adrenergic stimulation cannot be an effective weight-reducing strategy, just as exercise is used for this purpose, even though its primary function is not to regulate body composition.
If the response of humans to the weight-reducing effects of cold exposure or β3-adrenergic agonist treatment has a genetic component, then the genetic studies in mice can provide insights into how BAT may function in humans (Xue et al., 2007). As reviewed by Spiegelman in this issue, brown adipocytes in adult mice have two origins; they can be formed in the fetus from common BAT-muscle progenitor cells into discrete depots of brown adipocytes in the interscapular region (iBAT) or from undefined progenitors into diffuse areas of brown adipocytes in the traditional white fat depots (wBAT) of 21-day-old mice (Xue et al., 2007). The amount of iBAT among mouse strains is genetically invariant, whereas the amount of wBAT is a genetically variable trait involving up to eight quantitative trait loci (Xue et al., 2007). The development of obesity in an obesogenic environment is not influenced by whether or not the mouse can induce BAT (Guerra et al., 1998). In contrast, the capacity of an obese mouse to reduce obesity in response to adrenergic stimulation by cold or β3-adrenergic stimulation very much depends upon genetic variability in the induction of wBAT (Guerra et al., 1998).
BAT and Diet-Induced Thermogenesis
It has been argued that the maintenance of body weight over decades must be determined by a system able to precisely and unconsciously match food intake with energy expenditure. While there has been significant progress in understanding how food intake is regulated, the identification of the energy expenditure arm has been slow, and for lack of plausible alternative thermogenic systems, much of the focus has been on BAT. Is it necessary to have an unconsciously controlled thermogenic mechanism to closely control body weight? Premodern humans spent an enormous amount of energy either running to catch their meal or to avoid becoming a meal themselves; consequently, obesity was essentially nonexistent (Hayes et al., 2005). Therefore, the idea that a major function of BAT is to maintain energy balance by burning off excess calories, which they rarely had, seems incompatible with evolutionary biology. To posit that brown fat evolved to maintain a lean body composition is tantamount to saying that the ability to run evolved to prevent obesity. Nevertheless, a cafeteria diet fed to rats was shown to increase hyperphagia, BAT mass, noradrenaline turnover, oxygen consumption, respiratory enzyme activity, and UCP1 protein. Furthermore, since these phenotypes could be blocked by the β-adrenergic antagonist, propranolol, it was proposed that induction of brown fat thermogenesis reduced the increase in obesity expected from the level of energy intake (Rothwell and Stock, 1979). However, Foster and colleagues noted that a direct quantitative assessment of the contribution of BAT to the increase in metabolic rate in rats fed a cafeteria diet had not been determined (Ma et al., 1988). Using microspheres to measure blood flow and arteriovenous oxygen differences across iBAT, they determined the contribution of BAT to whole-animal energy expenditure in conscious rats fed control and cafeteria diets. The results clearly showed that at ambient temperatures of either 24°C or 28°C, rats fed a cafeteria diet, although showing an increase in whole-body resting oxygen consumption attributable to diet-induced thermogenesis, had levels of oxygen consumption by iBAT no greater than in rats fed the control diet. Thus, diet-induced thermogenesis, as evidenced by the increase in oxygen consumption in rats fed the cafeteria diet, was not determined by increased oxygen consumption by BAT. This conclusion has been corroborated in a study showing that oxygen consumption increases comparably in Ucp1+/+ and Ucp1−/− mice when the diet is switched from chow to high fat/sucrose (Anunciado-Koza et al., 2008), indicating that diet-induced thermogenesis is independent of Ucp1 expression.
BAT Function as Revealed by Transgenic and Gene Knockout Models
The Ablation of Brown Adipocytes
The elegant in vivo experiments by Foster and colleagues should have laid to rest the hypothesis that BAT thermogenesis was induced by a cafeteria diet. With the development of technologies to develop transgenic models of BAT expression, the effects of over- and underexpression of BAT could test the conclusions of Foster (Table 1). When Ucp1-DTA transgenic mice fed a regular chow diet became obese and insulin resistant, it was concluded that the ablation of brown adipocytes promoted obesity by loss of a major thermogenic mechanism (Hamann et al., 1996). There are two serious problems with concluding that Ucp1-DTA mice are obese because they have reduced thermogenic capacity from ablation of brown adipocytes. First, no deficiency was observed in the thermogenic capacity of Ucp1-DTA mice (Table 1); that is, Ucp1-DTA mice with 60% of their brown adipocytes ablated were able to maintain a normal body temperature when exposed to an ambient temperature of 4°C for up to 50 hr. Second, Ucp1-DTA mice were sufficiently hyperphagic to account for the development of obesity (Hamann et al., 1996). Since the hyperphagia was variable among three lines and disappeared in a line that recovered their brown adipocytes, it was posited that loss of BAT caused hyperphagia.
Table 1.
Comparison of Energy Balance Phenotypes of Genetic Models of Ucp1/BAT Expression
| Phenotype | B6 Control | Ucp1-DTA | aP2-Ucp1tg/− | aP2-Ucp1tg/tg | Ucp1−/− |
|---|---|---|---|---|---|
| Body temp after 3 hr at 5°C | 35°C | 35°C | 35°C | 27°C | 27°C |
| Food intake | |||||
| Chow | 100% | 131% | 100% | 100% | NA |
| High fat | 100% | 132% | 100% | 100% | 95% |
| Body weight | |||||
| Chow | 29 g | 42 g | 28 g | 27 g | 28 g |
| High fat | 46 g | 52 g | 34 g | 31 g | 37 g |
| Blood glucose | 100% | 130% | 87% | NA | 99% |
Food intake and blood glucose (fasted) are presented as a percent of B6 Control values. Data were taken from Hamann et al., 1996; Stefl et al., 1998; and Liu et al., 2003 and references therein.
A second transgenic model for ablation of BAT unexpectedly occurred when the fat-specific, constitutively regulated aP2 promoter was used to drive expression of Ucp1 in both white and brown fat (Table 1) (Kopecky et al., 1995). The resistance of heterozygous aP2-Ucp1 mice to obesity is consistent with the hypothesis that thermogenesis from elevated expression of Ucp1 reduced adiposity. Unlike a normal mouse, where stimulation of Ucp1 involves a regulated adrenergic response to the need for heat, constant high levels of UCP1 production from the constitutive expression of the aP2 promoter maintains the mitochondria in an uncoupled state and leads to resistance to both diet-induced and genetic obesity (Kopecky et al., 1995).
The consequences of the constitutive high levels of UCP1 generated from the aP2 promoter become more interesting in mice homozygous for the aP2-Ucp1 transgene (Table 1). Similar to heterozygous aP2-Ucp1 mice, homozygous mice were very resistant to diet-induced obesity. In contrast, they were also cold sensitive in a manner indistinguishable from Ucp1−/− gene knockout (Stefl et al., 1998). Accordingly, the excessive overexpression of Ucp1 in BAT of homozygous transgenic mice was cytotoxic to the brown adipocytes, rendering the mice deficient for both BAT and UCP1. Interestingly, the increase in Ucp1 expression by a factor of 2 in homozygous aP2-Ucp1 transgenic mice was sufficient to uncouple brown fat mitochondria to the point that the cells died.
The cytotoxic effects of UCP1 enable us to interpret the phenotype of Ucp1-DTA transgenic mice. A 90% loss of brown adipocytes in homozygous aP2-Ucp1 transgenic mice was not associated with an exacerbated diet-induced obesity or hyperphagia (Stefl et al., 1998). That homozygous aP2-Ucp1 mice had lost their major thermogenic mechanisms for protecting body temperature was evident from the fact that they were as sensitive to the cold as Ucp1−/− mice and were resistant to obesity. In contrast, the Ucp1-DTA mice with a 50% reduction in UCP1/BAT had normal thermoregulation, and they were both obese and hyperphagic (Hamann et al., 1996). It is unlikely that the phenotypes of Ucp1-DTA mice were due to reduced numbers of brown adipocytes. It is known that Ucp1-DTA mice have cataracts from an insertional mutation of the transgene into the γ-crystalline gene on mouse chromosome 16 (B. Chang, N.L. Hawes, M.T. Davisson, and J.R. Hechenlively, personal communication). Since the γ-crystalline gene is expressed in many cells of the CNS, we speculate that the transgenic mutational event also affects mechanisms regulating food intake.
Ucp1−/− Mice and Diet-Induced Thermogenesis
The classic experiments of Foster and colleagues showed that the increase in oxygen consumption by a cafeteria diet at either reduced or thermoneutral ambient temperatures occurred, but it was not due to BAT (Ma et al., 1988). In their experiments, the same increase in brown fat mass and GDP-binding to mitochondria was observed as reported by others (Rothwell and Stock, 1979). This indicated that such morphological and biochemical responses to cafeteria diets were not related to the thermogenic output of BAT. A brown-fat-independent, propranolol-sensitive increase in oxygen consumption occurred in rats fed a cafeteria diet; it must have occurred outside the BAT system, but its location is not known. If the data of Foster et al. indeed indicate that a cafeteria diet does not stimulate thermogenesis from brown fat, then one would predict that Ucp1−/− and Ucp1+/+ mice would be equally sensitive to obesity when fed an obesogenic high-fat diet. Indeed, this is observed, but it depends upon the ambient temperature; that is, at a reduced ambient temperature when thermogenesis must be activated to maintain body temperature, Ucp1−/− mice are more resistant to diet-induced obesity than wild-type mice. As the ambient temperature approaches thermoneutrality, Ucp1−/− and wild-type mice have the same level of adiposity when fed a high-fat diet (Anunciado-Koza et al., 2008). The resistance of UCP1-deficient mice to diet-induced obesity suggests that alternative thermogenic mechanisms are activated/induced to generate sufficient heat to maintain body temperature.
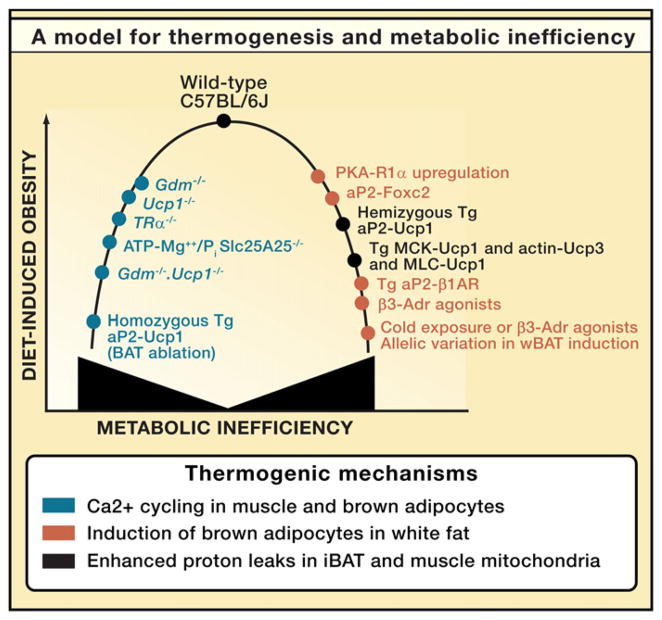
Unlike BAT thermogenesis, alternative biochemical and physiological mechanisms for heat production and maintaining thermal homeostasis appear to be less efficient, requiring expenditure of more calories to maintain body temperature and thereby indirectly reducing adiposity (Anunciado-Koza et al., 2008). Figure 1 presents a general model illustrating how variation in metabolic inefficiency with concomitant effects on obesity can arise from either inactivation of important thermogenic pathways with compensatory effects on metabolism (left-hand slope of the hyperbola) or by expression of transgenes and normal genetic variations that lead to overexpression of brown adipocytes and/or Ucp1 (right-hand slope). The underlying principle is that whenever the capacity for thermogenesis to maintain body temperature is compromised, alternative, less efficient metabolic pathways must be induced to prevent hypothermia.
Figure 1. Model Describing the Relationship between Genetic Models of Metabolic Inefficiency and Sensitivity to DIO in Male C57BL/6J.
On the one hand, metabolic inefficiency can be increased by inactivating major thermogenic pathways, thereby forcing the animal to utilize alternative thermogenic pathways that cost more energetically to maintain body temperature, thus reducing the level of diet-induced obesity (DIO) (left-hand arm of the hyperbola). On the other hand, overexpression and/or ectopic expression of Ucp1 or induction of brown adipocytes in white fat depots enhances the thermogenic capacity, much of which, by being unregulated, increases metabolic inefficiency and reduces DIO (right-hand arm of hyperbola). This is a modification of a model originally published in the Journal of Biological Chemistry (Anunciado-Koza et al., 2008). References documenting the obesity and thermogenic phenotypes are also found in this reference (Anunciado-Koza et al., 2008).
An increase in metabolic inefficiency in Ucp1−/− mice was first deduced from the suppression of adiposity at a reduced ambient temperature. Inactivation of a physiological system as important as thermoregulation causes compensatory responses (Figure 1). An induction in the adrenergically regulated type 2 deiodinase in iBAT and inguinal fat of Ucp1−/− mice suggested a chronic though futile effort to stimulate thermogenesis. Thus, the deficiency in thermogenic capacity in Ucp1−/− mice creates a condition of chronic adrenergic stimulation as the CNS strives to activate BAT thermogenesis. However, chronic adrenergic activity in the context of genetic and diet-induced obesity down-regulates adrenergic receptors and suppresses lipolysis (Robidoux et al., 2004). The resistance of Ucp1−/− mice to an obesity phenotype is controversial. Other groups have found that Ucp1−/− mice are more obese than wild-type (Feldmann et al., 2009; Kontani et al., 2005). In an aging study, Yamashita and colleagues showed that increased late-onset obesity occurs in Ucp1−/− mice fed a high-fat diet that was not explained by differences in food intake (Kontani et al., 2005). Their key mechanistic finding was that the expression of the β3-adrenergic receptor in aged obese Ucp1−/− mice was reduced, and this caused severe suppression of norepinephrine-induced lipolysis in adipose tissues (Kontani et al., 2005). Suppression of adrenergic signaling as observed in the norepinephrine-deficient dopamine β-hydroxylase KO mice does not lead to increased obesity (Ste Marie et al., 2005), whereas mice with inactivated β1-, β2-, and β3-adrenergic receptor genes are severely obese (Lowell and Bachman, 2003). Lowell and colleagues attributed the obese phenotype of the β-less mice entirely to the defect in diet-induced thermogenesis, but they failed to evaluate the effects of β-adrenergic receptor deficiency on lipid metabolism. Considered together, these studies suggest that an imbalance between α- and β-adrenergic signaling is a key condition for susceptibility to obesity (Valet et al., 2000).
The observation that Ucp1−/− mice are more obese than wild-type mice at 30°C led Nedergaard and colleagues to conclude that at thermoneutrality, the capacity for UCP1-dependent diet-induced thermogenesis in wild-type mice limits the development of obesity (Feldmann et al., 2009). On the other hand, the work done by Yamashita and colleagues and corroborated by studies on adrenergic signaling and obesity indicates that enhanced obesity in Ucp1−/− mice at thermoneutral temperatures may be explained by downregulation of adrenergic signaling and lipolysis, without evoking the notion of diet-induced thermogenesis by UCP1 (Kontani et al., 2005). To establish definitively this secondary and indirect role for UCP1 in diet-induced thermogenesis, it will be necessary to determine the effects of variation in adrenergic signaling in UCP1-deficient mice on phenotypes of energy balance.
Summary and Conclusions
Would it matter to our concepts of energy balance if there was no diet-induced thermogenesis? Probably not, since as I have argued from the phenotypes of several genetic models of thermogenesis, no compelling case can be made for diet-driven thermogenesis. Expenditure of energy to balance food intake would come principally from physical activity and maintenance of body temperature in individuals in harmony with their environment; this would exclude most modern humans. The realization this past year that significant levels of BAT continue to exist in adult humans has opened the door to research that will aim to determine how this remarkable thermogenic system may be associated with the obesity epidemic and to discover new ways to utilize the potential to expand and activate BAT thermogenesis to prevent or reduce obesity in individuals. Similar excitement about the potential contribution of variation in BAT to slow metabolism and increased susceptibility to obesity occurred 30 years ago. The effort that flowed from this excitement and energy faded because we failed to understand that the function of BAT in mammals is to maintain body temperature in the face of a cold environment and not to maintain a normal body weight free of insulin resistance in the face of an obesogenic environment. To understand this limitation does not diminish our ability to use this basic thermogenic mechanism of brown adipocytes to maintain body temperature by burning off excess calories. Today, we have the choice of utilizing this system to reduce obesity simply by reducing the ambient temperature, making it analogous to going for a jog. Tomorrow, the promise will also be reached with the development of adrenergic receptor agonists and other drugs that mimic the normal cold response by the CNS to induce brown adipocyte numbers in adipose tissue. These can be administered to obese individuals at abnormally elevated room temperatures. Both approaches have been shown to be very effective in experimental mammals from mice to dogs. On the other hand, searching for genes that cause variation in diet-induced obesity by brown fat must be designed with care so as not to repeat the past when efforts to find individuals with a slow metabolism akin to ob/ob mice failed.
Acknowledgments
I thank Andrew Butler and Barbara Gawronska-Kozak for critical reviews of the manuscript. This research was supported by funding from NIH grant HD008431. I apologize to my colleagues for not directly citing their papers that have contributed to the field due to space restrictions.
References
- Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. J Biol Chem. 2008;283:27688–27697. doi: 10.1074/jbc.M804268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteilla L, Champigny O, Bouillaud F, Robelin J, Ricquier D. Biochem J. 1989;257:665–671. doi: 10.1042/bj2570665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Foster DO, Frydman ML. Can J Physiol Pharmacol. 1978;56:110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A, Flier JS, Lowell BB. Endocrinology. 1996;137:21–29. doi: 10.1210/endo.137.1.8536614. [DOI] [PubMed] [Google Scholar]
- Hayes M, Chustek M, Heshka S, Wang Z, Pietrobelli A, Heymsfield SB. Int J Obes (Lond) 2005;29:151–156. doi: 10.1038/sj.ijo.0802842. [DOI] [PubMed] [Google Scholar]
- Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Bachman ES. J Biol Chem. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- Ma SW, Foster DO, Nadeau BE, Triandafillou J. Can J Physiol Pharmacol. 1988;66:1347–1354. doi: 10.1139/y88-221. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Locke RM. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP. FASEB J. 2008;22:3925–3937. doi: 10.1096/fj.08-108266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Jebb S. Nutr Rev. 2004;62:S98–S104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Robidoux J, Martin TL, Collins S. Annu Rev Pharmacol Toxicol. 2004;44:297–323. doi: 10.1146/annurev.pharmtox.44.101802.121659. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Smith RE, Horwitz BA. Physiol Rev. 1969;49:330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Luquet S, Curtis W, Palmiter RD. Obes Res. 2005;13:1518–1522. doi: 10.1038/oby.2005.185. [DOI] [PubMed] [Google Scholar]
- Stefl B, Janovská A, Hodný Z, Rossmeisl M, Horáková M, Syrový I, Bémová J, Bendlová B, Kopecký J. Am J Physiol. 1998;274:E527–E533. doi: 10.1152/ajpendo.1998.274.3.E527. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thurlby PL, James WPT. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Endocrinology. 2006;147:2468–2480. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- Valet P, Grujic D, Wade J, Ito M, Zingaretti MC, Soloveva V, Ross SR, Graves RA, Cinti S, Lafontan M, Lowell BB. J Biol Chem. 2000;275:34797–34802. doi: 10.1074/jbc.M005210200. [DOI] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]