Abstract
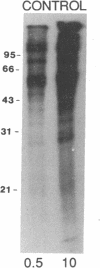
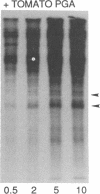
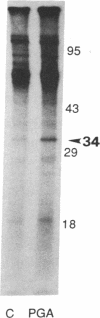
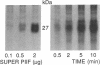
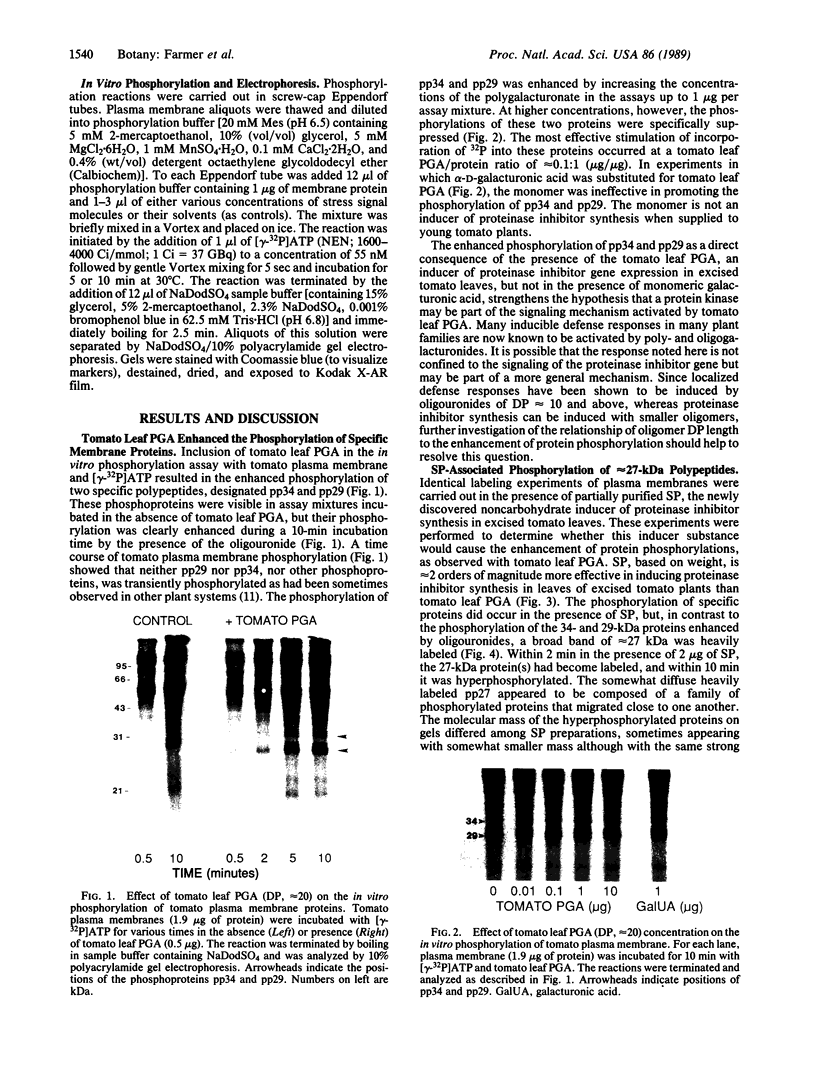
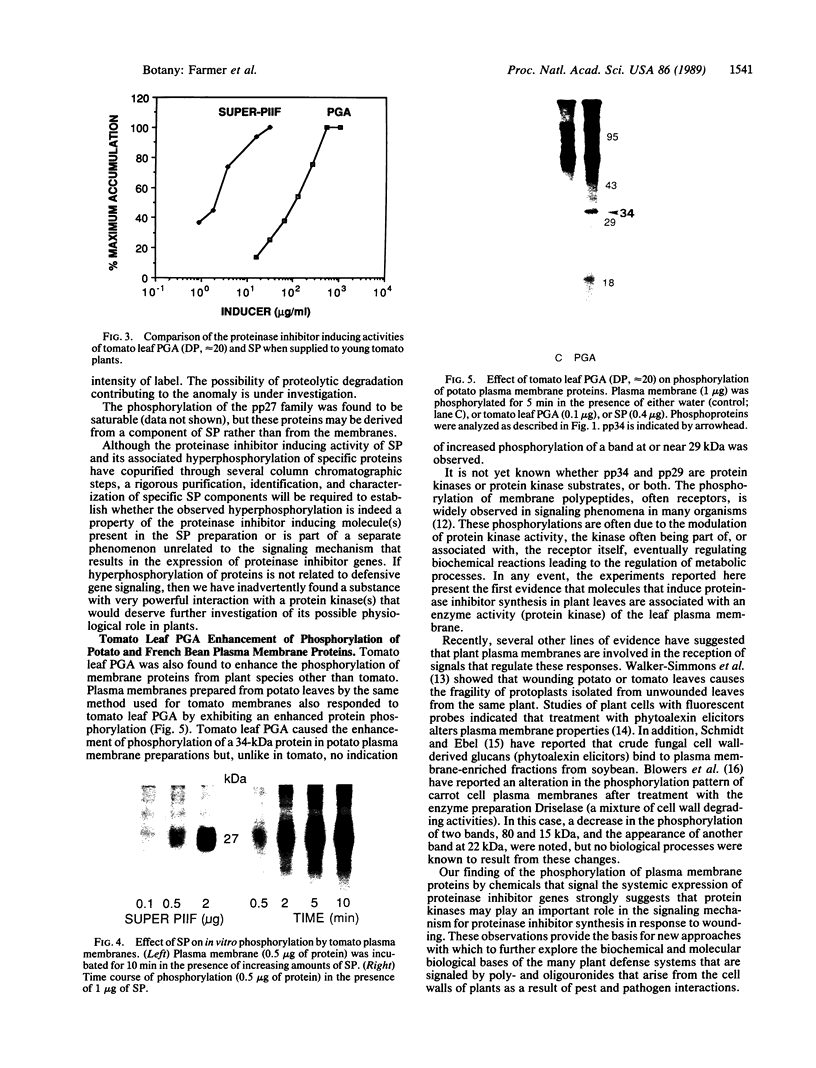
A polygalacturonide purified from a tomato leaf pectic polysaccharide that induces the systemic synthesis of proteinase inhibitors in tomato plants enhances the phosphorylation of specific proteins in plasma membrane fractions isolated from tomato and potato leaves. In tomato plasma membranes, two proteins of 34 and 29 kDa show enhanced phosphorylation in response to the polyuronide. In potato plasma membranes, only a protein of 34 kDa exhibited enhanced phosphorylation due to the polyuronide. A noncarbohydrate class of proteinase inhibitor inducing factor, recently identified by workers in this laboratory, resulted in the in vitro hyperphosphorylation of a family of proteins of ≈27 kDa. The phosphorylation of specific polypeptides in leaves in response to the same factors that induce the expression of proteinase inhibitor genes suggests that protein kinases may play an important role in the mechanism of signal transduction leading to defense gene expression.
Keywords: signal transduction, wound response, protein kinase, induced plant defense
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwai A. P., Takemoto J. Y. Bacterial phytotoxin, syringomycin, induces a protein kinase-mediated phosphorylation of red beet plasma membrane polypeptides. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6755–6759. doi: 10.1073/pnas.84.19.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Blowers D. P., Boss W. F., Trewavas A. J. Rapid Changes in Plasma Membrane Protein Phosphorylation during Initiation of Cell Wall Digestion. Plant Physiol. 1988 Feb;86(2):505–509. doi: 10.1104/pp.86.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Oligosaccharide signalling in plants. Annu Rev Cell Biol. 1987;3:295–317. doi: 10.1146/annurev.cb.03.110187.001455. [DOI] [PubMed] [Google Scholar]
- Ryan C. A. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967 Jun;19(3):434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Ebel J. Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautman R., Cowan K. M., Wagner G. G. Data processing for radial immunodiffusion. Immunochemistry. 1971 Oct;8(10):901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Holländer-Czytko H., Andersen J. K., Ryan C. A. Wound signals in plants: A systemic plant wound signal alters plasma membrane integrity. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3737–3741. doi: 10.1073/pnas.81.12.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]