Abstract
Most previous studies that determined the effect of estradiol on angiogenesis used endothelial cells from nonpituitary sources. Because pituitary tumor tissue receives its blood supply via portal and arterial circulation, it is important to use pituitary-derived endothelial cells in studying pituitary angiogenesis. We have developed a magnetic separation technique to isolate endothelial cells from pituitary tissues and have characterized these cells in primary cultures. Endothelial cells of the pituitary showed the existence of endothelial cell marker, CD31, and of von Willebrand factor protein. These cells in cultures also showed immunore-activity of estrogen receptors alpha and beta. The angiogenic factors, vascular endothelial growth factor and basic fibroblast growth factor, significantly increased proliferation and migration of the pituitary-derived endothelial cells in primary cultures. These results suggest that a magnetic separation technique can be used for enrichment of pituitary-derived endothelial cells for determination of cellular mechanisms governing the vascularization in the pituitary.
Keywords: Endothelial cells, CD31cell marker, Pituitary, Vascular endothelial growth factor, Basic fibroblast growth factor
Introduction
Angiogenesis is a multistep process which involves interaction with various extracellular matrix components, endothelial cell proliferation, migration, and differentiation into capillaries [1, 2]. Various angiogenic and antiangiogenic molecules control the process of neovascularization by increasing the activity of endothelial cells which carry information regarding the capillary network [3]. During disease conditions, like cancer or tumor formation, an increase in capillary network takes place due to changes in activities of proangiogenic and antiangiogenic factors [4].
Estrogen acts as a mitogen on the lactotropes and promotes the development of pituitary tumors [5]. The steroid also stimulates pituitary tumor vascularization [6-8]. Previous studies have shown that estrogen increases the levels of proangiogenic factors like basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) and its receptors in pituitary tumors [9, 10]. The mechanism by which the angiogenesis-regula tory factors modulate the activity of endothelial cells in the pituitary is not well studied. The pituitary consists of various types of cells, and, therefore, it is difficult to analyze the effects of these factors at the cellular level on these cells in vitro. Until now, there are no reports on the isolation of rat pituitary endothelial cells and their culturing under in vitro conditions. In this study, we isolated endothelial cells from pituitaries by immunomagnetic separation using the anti-CD31 antibody. The pituitary-derived CD31-positive cells were grown in culture and characterized based on morphology, presence of characteristic protein markers, and functionality.
Materials and Methods
Animals
Female Fischer 344 rats with a body weight of 160–200 g were obtained from Simonsen Laboratories (Gilroy, Calif., USA), housed in a controlled environment (temperature 22°C, lights on 05.00–19.00 h), and provided with rodent chow and water ad libitum. The animals were ovariectomized bilaterally and subcutaneously implanted with a 1-cm estradiol-17β (Sigma, St. Louis, Mo., USA) filled Silastic capsule (Dow Corning, Midland, Mich., USA) or an empty Silastic capsule using sodium pentobarbital anesthesia (40–50 mg/kg i.p.). The animals were kept for a period of 4 weeks after surgery. Animal surgery and care were performed in accordance with the institutional guidelines and complied with the NIH policy. The animal protocol was approved by the Rutgers Animal Care and Facilities Committee.
Isolation of Endothelial Cells from Pituitaries Using Magnetic Beads
Endothelial cells were separated from pituitaries of animals with or without estradiol implant by magnetic separation, using a positive selection method and an autoMACS magnetic cell sorter (Miltenyi Biotec, Auburn, Calif., USA). Briefly, 6–8 pituitaries were incubated with collagenase and DNAse, and cells were dissociated as described by us previously [11]. The separated pituitary cell aggregates were resuspended in 500 μl of binding buffer (phosphate-buffered saline, 0.5% bovine serum albumin, 2 mM EDTA, 0.05% sodium azide) containing mouse anti-rat CD31-phycoerythrin (PE) conjugated antibody (1 μg antibody/million cells; Research Diagnostics, Flanders, N.J., USA) for 30 min at 4°C. The cells were washed with buffer (phosphate-buffered saline, 2 mM EDTA, 0.05% sodium azide) once and then incubated with anti-PE antibody labeled with magnetic beads (anti-PE beads; 20 μ1/10 million cells; Miltenyi Biotec) for 15 min at 4°C in binding buffer. The cells were washed and then resuspended in 500 μl of binding buffer and passed through magnetic columns (AutoMacs; Miltenyi Biotec). Positive cells were collected, washed, and resuspended in standardized EGM complete endothelial cell medium (phenol-red-free basal EGM-2 medium containing growth factors; Bullet kit; Cambrex, East Rutherford, N.J., USA). The cells were 95–100% viable, as determined by the trypan blue exclusion method. We performed flow cytometric analysis using anti-CD31 antibody conjugated with PE to estimate the percentage of total pituitary cells that were CD31-positive endothelial cells. We found that animals treated with empty implants had a relatively low number of CD31-positive cells (about 3 × 104 cells/ pituitary) due to a lower mass of the pituitary. However, animals treated with estradiol implants had 5–8% pituitary cells that were CD31 positive (about 1 × 105 cells/pituitary). Hence, we used estradiol-treated animals for isolation of endothelial cells to conduct the cell characterization studies. After isolation, CD31-positive cells were plated at a concentration of 10,000 cells/ml in 25-cm2 flasks, and medium was changed after 24 h and every 2–3 days thereafter. Once cells formed a confluent monolayer, they were trypsinized and split for culture expansion for a maximum of six generations. The experiments were conducted on endothelial cells isolated from various pools as well as from various passages of one pool.
Immunocytochemical Characterization
After two passages, CD31-positive cells were immunocytochemically stained with various endothelial cell marker proteins to characterize the identity of their phenotypes and their purity. Endothelial cells were seeded in eight-well-chambered slides and grown for 2–4 days. After development of monolayer, the cells were fixed and processed for immunocytochemical detection of various cellular proteins, as described by us previously [12]. Briefly, cells in cultures were incubated with various primary antibodies for 24 h at 4°C and then incubated with respective secondary antibodies (Molecular Probes, Eugene, Oreg., USA) for 30 min. After washing in Tris-HCl, cell-containing chambers were dried and were mounted using DAPI-containing mounting medium (H-1200; Vector Laboratories, Burlingame, Calif., USA). The primary antibodies used for immunocytochemistry were mouse anti-CD31 (1:25; Chemicon International, Temecula, Calif., USA), goat anti-vWF (von Willebrand factor; 1:25; Santa Cruz Biotechnology, Santa Cruz, Calif., USA), rabbit antiprolactin antibody (1:5,000; NIDKK, Bethesda, Md., USA), rabbit anti-S100 antibody (1:500; Zymed Laboratories, South San Francisco, Calif., USA), rabbit anti-LH antibody (luteinizing hormone; 1:500; Biomeda, Foster City, Calif., USA), rabbit anti-GH (growth hormone; 1:100,000; NIDDK), and rabbit antibody for β-endorphin (1:1,000; Peninsula Laboratories, San Carlos, Calif., USA). Secondary antibodies used were Alexa Fluor 594 conjugated anti-mouse (1:2,500) or antirabbit or antigoat antibodies (1:2,500; Molecular Probes). The specificities of these antibodies have been previously determined [5, 12, 13]. The CD31 and vWF antibodies are very specific and have been well characterized by the suppliers. We found complete absence of staining, when the cells were processed for immunofluorescence staining in the absence of primary antibodies. Fluorescent images were captured with a Nikon TE 2000 inverted microscope coupled to a CoolSNAP-Pro CCD camera. Images were processed, and double merges were made with Adobe Photoshop 7.0.
Western Blot Analysis
Cell lysates from endothelial cells, PR1 cells (prolactin-producing cell line), folliculostellate (FS) cells, and pituitary tissue homogenate were subjected to SDS-PAGE. Proteins were transferred to immobiloP membranes, and the expression of various proteins was detected by immunoblotting as described by us previously [11]. The antibodies used were mouse anti-CD31 antibody (1:250; Chemicon International), rabbit anti-S100 antibody (1:1,000; Zymed Laboratories), mouse antiprolactin antibody (1:10,000; NIDKK), mouse anti-LH antibody (1:5,000; Biomeda), anti ER-α (1:500; Santa Cruz Biotechnology), and beta antibodies (1:1,000; EMD Biosciences, San Diego, Calif., USA).
Cell Proliferation Assay
For cell proliferation experiments, 10,000 endothelial cells/well in 96-well plates were grown in EGM complete endothelial cell medium for 48 h. The cells were then washed twice with serum-free medium and treated with various doses of VEGF (R & D Systems, Minneapolis, Minn., USA) or of bFGF (R&D Systems) in 2.5% fetal bovine serum containing EGM-2 basal medium for 24 h. The cultures were treated with 0.5 μCi [methyl-3H] thymidine (specific activity 82.2 Ci/mM; Amersham, Arlington Heights, Ill., USA) per well for 8 h prior to harvesting. The cells were harvested using a cell harvester (Packard Bioscience, Meriden, Conn., USA) and counted in a liquid scintillation counter.
Cell Migration Assay
Cell migration was determined using a cell migration assay kit (Chemicon International) as per manufacturer’s instructions. Briefly, endothelial cells were grown in 75-cm2 flasks. The cells were incubated with basal EGM-2 medium for 24 h before plating on assay plates. The cells were trypsinized the next day and resuspended in EGM-2 basal medium; 1.5 × 104 cells were plated in each well of the 96-well plate of the migration chamber. This migration chamber was inserted into a 96-well feeder tray which contained VEGF (50 ng/ml) or bFGF (25 ng/ml). Treatment was done in quadruplicate. Migratory cells on the bottom of the insert membrane were detached using cell detachment buffer in another 96-well plate provided with the kit. The migrated cells were subsequently lysed and detected by CyQuant GR dye. This green fluorescent dye exhibits strong fluorescence enhancement when bound to cellular nucleic acids. The data are presented in relative fluorescent units (RFU).
Results
Isolation of Pituitary Endothelial Cells
We used CD31, a characteristic marker, for isolation of endothelial cells using an autoMACS magnetic cell sorter. Microscopic examination showed that almost 100% of the cells were positive for CD31 (fig. 1A–C). Flow cytometry analysis also detected approximately 94% CD31-positive cells (fig. 1D). Isotype-specific IgG did not show any different fluorescence compared with unstained cells.
Fig. 1.
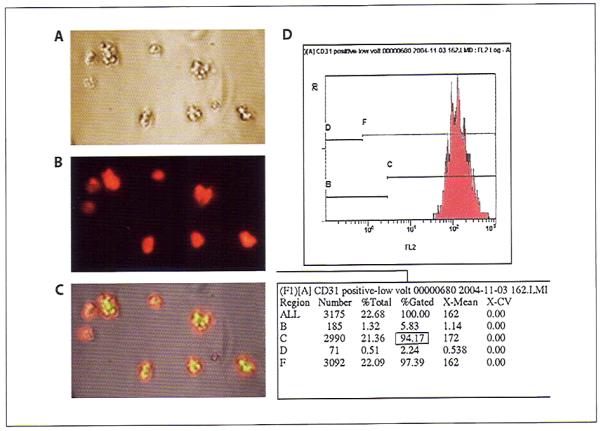
Microscopic images and flow cytometric analysis of pituitary-derived endothelial cells immediately after purification. A Representative phase-contrast image of the isolated cells. B Fluorescent image of the same cells. C Superimposed image, showing that almost all cells were CD31 positive. D An example of flow cytometric analysis of purified CD31-positive cells.
Morphology of Pituitary Endothelial Cells
Pituitary-derived endothelial cells showed similar morphological characteristics to endothelial cells derived from other tissues [14, 15]. Like the other tissue-derived endothelial cells, pituitary-derived endothelial cells showed a long tube-like structure formation during the early period in culture (fig. 2A, B). After 2–3 days in culture, these cells changed to cobblestone morphology (fig. 2C, D). Pituitary endothelial cells started showing adhered growth and proliferation after 3–5 days and reached a monolayer after 7–9 days in culture (fig. 2E). When the cell density was high, the cells were more elongated and tightly closed. After the cells were 80–90% confluent, the cell monolayer was trypsinized and used for specific experiments or for expansion of culture. We maintained these cells in culture for five to six passages only.
Fig. 2.
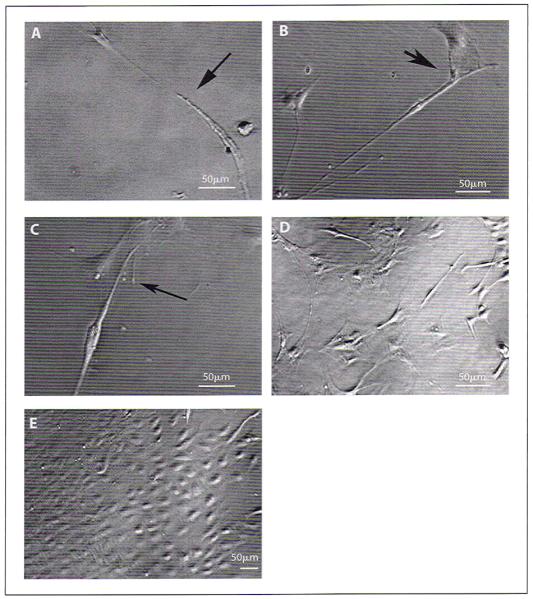
Phase-contrast microscopy images of pituitary-derived endothelial cells at various stages of differentiation in cultures. A-C Endothelial cells after 1–2 days of culture, showing tube-like structures and processes changing to a traditional two-dimensional cobblestone structure (arrows). D, E Semiconfluent monolayer (D) and confluent monolayer (E) of endothelial cells after 7–9 days of culture. Bars indicate 50 μm.
Immunocytochemical and Western Blot Analyses of Various Pituitary Proteins in Pituitary Endothelial Cells
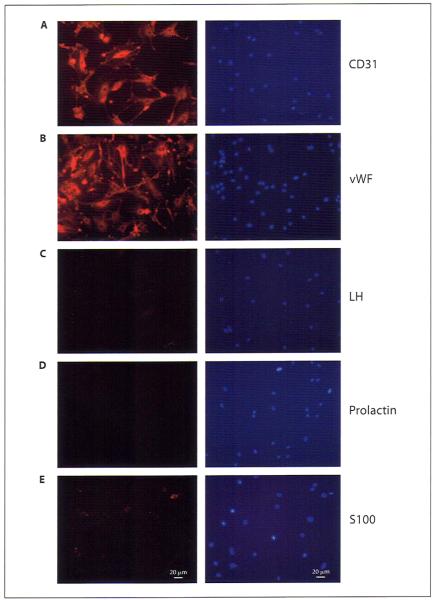
To further confirm the purity of pituitary-derived endothelial cell cultures, after two passages, these cells were examined for the presence of various endothelial cell specific and other pituitary cell specific markers by immunocytochemical analysis. As shown in figure 3, isolated pituitary-derived cells exhibited presence of the endothelial cell markers CD31 and vWF. However, the pituitary-derived endothelial cells did not stain for prolactin (protein produced from lactotropes), S100 (protein of FS cells), and LH (protein produced from gonadotropes). These cells also did not stain for GH (protein produced from somatotropes; data not shown) or β-endorphin (protein produced from corticotropes; data not shown). In order to further confirm the purity of the isolated endothelial cells, we also performed Western blot analysis of various proteins in lysates of these cells as well as in PR1 cells, FS cells, and a pituitary tissue. Figure 4 confirms the data of immunocytochemical studies and demonstrates the cell- and tissue-specific production of pituitary proteins. Additionally, the data presented in figure 4 show that, like many other pituitary cells, endothelial cells of pituitary origin contain both estrogen receptors ER-α and ER-β.
Fig. 3.
Immunocytochemical analysis of various pituitary proteins in pituitary-derived endothelial cells. Endothelial cells were grown in eight-chambered slides and immunostained for various proteins, as indicated. Representative fluorescent images showing immunostaining with CD31 (A), vWF (B), LH (C), prolactin (D), and S100 (E). The left panels show antigen-specific staining and the right panels DAPI staining for nucleus. Bars indicate 20 μm.
Fig. 4.
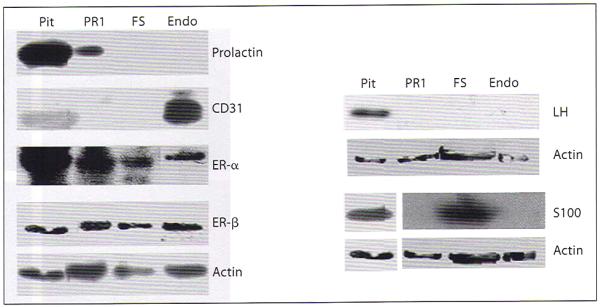
Western blot analysis of various pituitary proteins in pituitary-derived endothelial cells. Cell lysates from endothelial cells (Endo), prolactin-secreting PR1 cells (PR1), S100-positive FS cells (FS), and tissue homogenate from a pituitary (Pit) were used for detection of various endothelial cell or pituitary cell specific proteins by Western blot analysis. Actin was used as loading control.
Effect of VEGF and bFGF on Proliferation and Migration of Endothelial Cells
Angiogenic factors like VEGF and bFGF are known to increase proliferation and migration of endothelial cells [16]. We also determined whether the pituitary-derived endothelial cells show similar responses to these angiogenic factors. Dose-response studies revealed that both VEGF and bFGF increased the proliferation of these cells in a concentration-dependent manner (fig. 5A, B). As shown in figure 5C, both VEGF and bFGF also increased the migration of endothelial cells.
Fig. 5.
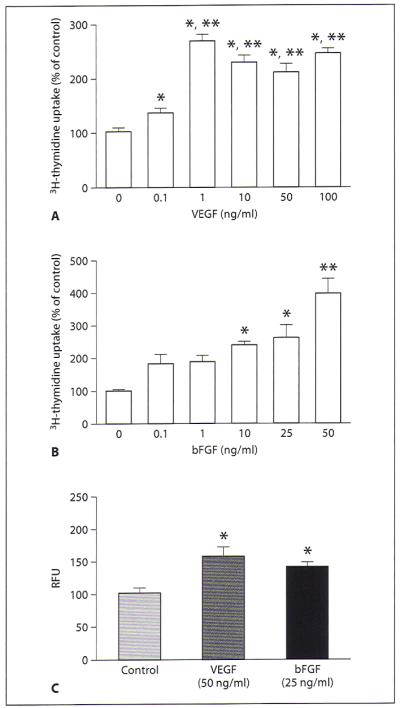
Effect of VEGF and bFGF on proliferation and migration of pituitary-derived endothelial cells. A, B Pituitary-derived endothelial cells were grown in cultures and then treated with various doses of VEGF (0.1–100 ng/ml) (A) or bFGF (0.1–50 ng/ml) (B) for 24 h. The cultures were treated with 0.5 μCi [3H] thymidine per well for 8 h prior to harvesting for determination of thymidine incorporation as a measure of cell proliferation. Data are presented as percent of control. Each column represents the mean ± SEM of n = 5–6. *p < 0.01 compared with the control group; **p < 0.05 compared with the group treated with either 0.1 or 1 ng/ml of growth factor. C Equal numbers of serum-starved cells were plated on the migration tray with filter (8 μm pore size) and incubated with VEGF (50 ng/ml) and bFGF (25 ng/ml) for 24 h. The cell migration to membranes was detected by using CyQuant GR dye. The data are presented as relative fluorescent units (RFU). Each column represents the mean ± SEM of n = 4. * p < 0.01 compared with the control group.
Discussion
The purpose of this study was to isolate endothelial cells from the pituitary and to maintain these cells in culture for determination of the angiogenic-factor-regulated growth and migration of these cells. To our knowledge, this is the first report on isolation of rat pituitary endothelial cells and on maintaining these cells in culture. The methods we described herein provide pure and viable endothelial cells from pituitary tissues. Furthermore, pituitary-derived endothelial cells show morphological characteristics, protein products, and growth and migration responses to angiogenic factors similar to those of endothelial cells of other tissue origins.
We used the CD31 antibody to purify the pituitary endothelial cells by immunomagnetic separation. CD31 is the most widely used endothelial cell marker for studying angiogenesis; it is also known as platelet endothelial cell adhesion molecule, PECAM-1. It is a type I integral membrane glycoprotein and a member of the immunoglobulin superfamily of cell surface receptors. It is strongly expressed by all endothelial cells and weakly by several types of leukocytes [17]. According to several studies, CD31 can be used to measure angiogenesis in several types of malignancies, such as lung cancer [18], breast cancer [19], and colorectal cancer [20]. We used another endothelial cell marker, vWF, to confirm the purity of the isolated endothelial cells. vWF is a multimeric glycoprotein found in endothelial cells; it causes adhesion of platelets to injured vessel walls and functions as carrier and stabilizer for coagulation of factor VIII [21]. The presence of vWF is the most widely used criterion for identification of cells of endothelial origin [22]. Hence, the cells we isolated from the pituitary tissue using magnetic separation techniques are primarily of endothelial origin.
Morphologically, pituitary-derived endothelial cells showed the characteristics of endothelial cells: adhered growth and long tube structure followed by cobblestone morphology [14, 15]. We also found that angiogenic factors like bFGF and VEGF can increase proliferation and migration of pituitary-derived endothelial cells. We performed this isolation at least five times, with similar results. We found that pituitary-derived endothelial cells contain estrogen receptors. Estrogen exposure is known to increase various angiogenic factors, like VEGF and bFGF, in the pituitary [9, 10]. In this study, we found that these factors increased proliferation and migration of endothelial cells derived from pituitaries. Hence, this newly developed pituitary-derived endothelial cell primary culture model can serve as a tool for determining cellular and molecular mechanisms of action of estrogen or other agents that control the pituitary neovascularization.
Acknowledgment
This work was supported by National Institutes of Health Grants AA11591 and CA775500.
References
- 1.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan H, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis. In: Mendelsohn J, Liotta LA, Howley PM, Israel MA, editors. The Molecular Basis of Cancer. Saunders; Philadelpia: 1996. pp. 206–232. [Google Scholar]
- 4.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee SK, De A, Sarkar DK. Colocalization of prolactin and proliferating cell nuclear antigen in the anterior pituitary during estrogen-induced pituitary tumors. Cancer Lett. 1994;87:139–144. doi: 10.1016/0304-3835(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd RV. Estrogen-induced hyperplasia and neoplasia in the rat anterior pituitary gland: an immunohistochemical study. Am J Pathol. 1983;113:198–206. [PMC free article] [PubMed] [Google Scholar]
- 7.Elias KA, Weiner RI. Direct arterial vascularization of estrogen-induced prolactin-secreting anterior pituitary tumors. Proc Natl Acad Sci U S A. 1984;81:4549–4553. doi: 10.1073/pnas.81.14.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schechter J, Ahmad N, Elias K, Weiner R. Estrogen-induced tumors: changes in the vasculature in two strains of rat. Am J Anat. 1987;179:315–323. doi: 10.1002/aja.1001790402. [DOI] [PubMed] [Google Scholar]
- 9.Hentges ST, Sarkar DK. Transforming growth factor-beta regulation of estradiol induced prolactinomas. Front Neuroendocrinol. 2001;22:340–363. doi: 10.1006/frne.2001.0220. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar DK, Hentges ST, De A, Reddy RH. Hormonal control of pituitary prolactin-secreting tumors. Front Biosci. 1998;3:d934–d943. doi: 10.2741/a334. [DOI] [PubMed] [Google Scholar]
- 11.Chaturvedi K, Sarkar DK. Mediation of basic fibroblast growth factor-induced lactotropic cell proliferation by Src-Ras-mitogen-activated protein kinase p44/42 signaling. Endocrinology. 2005;146:1948–1955. doi: 10.1210/en.2004-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CP, Kuhn P, Chaturvedi K, Boyadjieva NI, Sarkar DK. Ethanol induces apoptotic death of developing β-endorphin neurons via suppression of cyclic adenosine monophosphate production and activation of transforming growth factor-β1-linked apoptotic signaling. Mol Pharmacol. 2006;69:706–717. doi: 10.1124/mol.105.017004. [DOI] [PubMed] [Google Scholar]
- 13.Oomizu S, Chaturvedi K, Sarkar DK. Folliculostellate cells determine the susceptibility of lactotropes to estradiol’s mitogenic action. Endocrinology. 2004;145:1473–1480. doi: 10.1210/en.2003-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye CA, Patrick CW., Jr Isolation and culture of rat microvascular endothelial cells. In Vitro Cell Dev Biol Anim. 2002;38:208–212. doi: 10.1290/1071-2690(2002)038<0208:IACORM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Stachon A, Schluter T, Koller M, Weisser H, Krieg M. Primary culture of microvascular endothelial cells from human benign prostatic hyperplasia. Prostate. 2001;48:156–164. doi: 10.1002/pros.1094. [DOI] [PubMed] [Google Scholar]
- 16.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giatromanolaki A, Koukourakis M, O’Byrne K, Fox S, Whitehouse R, Talbot DC, Harris AL, Gatter KC. Prognostic value of angiogenesis in operable non-small cell lung cancer. J Pathol. 1996;179:80–88. doi: 10.1002/(SICI)1096-9896(199605)179:1<80::AID-PATH547>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Horak ER, Leek R, Klenk N, Lejeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992;340:1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- 20.Takebayashi Y, Akiyama S, Yamada K, Akiba S, Aikou T. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996;78:226–231. doi: 10.1002/(SICI)1097-0142(19960715)78:2<226::AID-CNCR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 21.Mazurier C. In vitro evaluation of the haemostatic value of the LFB-von Willebrand factor concentrate. Haemophilia. 1998;4(Suppl 3):40–43. doi: 10.1046/j.1365-2516.1998.0040s3040.x. [DOI] [PubMed] [Google Scholar]
- 22.Hewett PW, Murray JC. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell Dev Biol Anim. 1993;29A:823–830. doi: 10.1007/BF02631356. [DOI] [PubMed] [Google Scholar]