Abstract
Context:
The 5-HTTLPR polymorphism in the promoter region of the serotonin transporter gene (SLC6A4) has been found to moderate several categories of emotional response after stressful life events. Previous studies generally focused on its effect on depressive symptoms; little is known about its moderation of the development of post-traumatic stress disorder (PTSD).
Objective:
To examine the effects of childhood adversity, adult traumatic events, 5-HTTLPR genotypes, and gene×environment interactions on the etiology of PTSD.
Design:
A cross-sectional study in which participants in several studies investigating the genetics of substance dependence were also screened for lifetime PTSD. The triallelic system of 5-HTTLPR was genotyped. Logistic regression modeling was used in the analyses.
Setting:
General community.
Participants:
Five hundred eighty-two European American and 670 African American individuals who reported experiences of childhood adversity, adult traumatic events, or both.
Main Outcome Measure:
Diagnosis of PTSD, defined by DSM-IV diagnostic criteria and assessed through the Semi-Structured Assessment for Drug Dependence and Alcoholism interview.
Results:
Childhood adversity and adult traumatic events both predicted PTSD. Although the 5-HTTLPR genotype alone did not predict the onset of PTSD, it interacted with adult traumatic events and childhood adversity to increase the risk for PTSD, especially for those with high rates of both types of trauma exposure (European American: odds ratio [OR], 2.86; 95% confidence interval [CI], 1.50-5.45; P=.002; African American: OR, 1.88; 95% CI, 1.04-3.40; P=.04; pooled: OR, 2.31; 95% CI, 1.50-3.56; P<.001).
Conclusions:
Participants who had both childhood adversity and adult traumatic events were more likely to develop lifetime PTSD compared with those who experienced either type of adverse event. The risk was increased in individuals with 1 or 2 copies of the S′ (S) allele compared with the L′ (L) homozygotes. Our study provides additional direct evidence that PTSD is influenced by the interactive effect of environmental and genetic factors.
Posttraumatic stress disorder (PTSD) is a complex and multifactorial anxiety disorder. According to the DSMIV, it requires the presence of symptoms from each of 3 clusters, reexperiencing, avoidance, and increased arousal, that occur following exposure to a life-threatening traumatic event. While 40% to 70% of the US population have experienced high-level traumatic events,1,2 the lifetime prevalence for PTSD is 8% in adult Americans based on community epidemiological studies (DSM-IV). Several risk factors, including those that characterize the traumatic exposure as well as those that characterize traits of the individual, have been identified for PTSD. Among the former, traumatic events that involve the infliction of harm by others (eg, rape, physical assault, military combat) carry higher risks for PTSD than other traumas (eg, natural disasters).3 Female sex, preexisting psychiatric disorders, and premorbid personality characteristics such as neuroticism have also been shown to increase risk for PTSD.4,5
Childhood adversity, including sexual and physical abuse, neglect, and other traumas, has received considerable attention from researchers in the studies of the etiology of PTSD. Neurobiological consequences of early life stress can be severe in that they cause enduring changes in brain structure and function.6 These physical changes could influence the response to adult traumas, triggering a cascade of biological events that may ultimately lead to the occurrence of mental disorders. Meanwhile, childhood adversity is associated with an increased risk for behavior problems,7 which, in turn, expose the person more to traumatic events. Numerous studies have shown that childhood adversity is associated with a range of mental disorders, such as major depression,8 antisocial disorder, substance use disorder,9 and PTSD.10 Although most studies of early life stress and mental disorders have focused on childhood abuse or neglect, other traumatic events, such as involvement in a serious accident or witnessing events causing death or serious injury, also constitute an important part of a broad view of early life stress.
In addition to the obvious effect of environmental factors, PTSD has a heritable component. A twin study based on the Vietnam War veteran sample indicated that genetic factors account for approximately 30% of the variance in PTSD symptoms.11 A more recent study outside the context of military trauma showed similar findings.12 Although the idea that PTSD is affected by both environmental and genetic factors has been widely accepted, there have been relatively few molecular genetic studies of PTSD. Association studies have identified a small number of possible susceptibility genes, but the results are inconsistent.13 The difficulty in identifying PTSD-related genes may be due to their low to moderate effect sizes, with consequent insufficient statistical power of many studies, and other methodological shortcomings.14
Study of gene×environment effects on mental disorders has been of great interest recently. This approach is especially promising for investigating the etiology of PTSD, which requires exposure to a traumatic event and has a genetic component. The serotonin transporter gene (SLC6A4) has been shown to moderate several categories of emotional response. In the brain, the serotonin transporter is involved in the rapid reuptake of serotonin following neuronal stimulation; it is thus a key regulator of serotonergic neurotransmission. A common polymorphism (5-HTTLPR) in the promoter region of SLC6A4 was found to regulate gene expression. In vitro studies of SLC6A4 expression in human lymphoblastoid cells and placental choriocarcinoma cell lines consistently showed that the long variant of the polymorphism (L) (16 repeats) produced 2- to 3-fold higher levels of messenger RNA than that of the short variant (14 repeats), resulting in reduced serotonin reuptake.15,16 In vivo studies of messenger RNA level in human postmortem tissue sections, however, yielded inconsistent results.17,18 One possible explanation for this inconsistency between in vitro and in vivo studies is that under normal physiological conditions, the expression level of SLC6A4 may be regulated by many other factors. However, when stressful life events occur, which increases the serotonin release at targeted brain regions,19 the S allele may be less efficient than the L allele at up-regulating the expression level of the serotonin transporter and keeping the extracellular serotonin at a normal level. Studies of humans and rhesus macaques showed that the presence of the S allele interacts with environmental factors to produce effects on behavior and measures of central nervous system function.20-22 Although the most common alleles at 5-HTTLPR in most populations have either 14 or 16 repeated elements, other alleles, generally with more repeated elements (18 or 20 repeats), occasionally occur.23 However, transcriptional efficiencies of these rare 5-HTTLPR alleles are unknown. More recently, an A/G single-nucleotide polymorphism (SNP) (rs25531) in the repeats region was found to be functional; rs25531 is mostly found in the L variant, further dividing it into LA and LG.24 LG and S alleles have equivalent expression levels, which are about half of that of the LA allele; LG and S are thus reclassified as S′ and L, as L′.25 A Although PTSD, like depression, can be considered to reflect a maladaptive response to stressful events, few studies have examined the possible role of 5-HTTLPR in moderating the influence of traumatic events on PTSD risk.
In the present study, gene×environment interaction was examined to explore its effect on the development of PTSD. We recruited a large number of patients in the course of studies investigating the genetics of drug and alcohol dependence, disorders that are associated (perhaps causally) with high rates of PTSD,26,27 and administered the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), a comprehensive polydiagnostic instrument that includes assessment of PTSD.28,29 The SSADDA covers a wide range of traumatic events and contains additional items related to childhood adversity. Using these environmental data and the triallelic system (LA/LG/S) for 5-HTTLPR, individual and interactive effects of genetic and environmental factors were investigated.
METHODS
SAMPLE COLLECTION AND DEMOGRAPHIC INFORMATION
Samples in this study were a subgroup of 1793 European American and African American individuals who participated in linkage studies focused on the genetics of substance dependence (and were recruited as members of families, each of which included at minimum an affected sibling pair for cocaine or opioid dependence),30,31 as well as more recently recruited subjects, substance dependence cases and unaffected controls, related and unrelated, all of whom were recruited and ascertained with similar methods. The participants were recruited at 4 sites: Yale University School of Medicine, University of Connecticut Health Center, Medical University of South Carolina, and McLean Hospital of Harvard Medical School. Written informed consent was obtained from all participants. The institutional review board at each of the participating sites approved the study protocol. All subjects were interviewed using the SSADDA, which provides diagnostic criteria for a variety of psychiatric and substance use disorders, as well as information on childhood environment.32,33 Since the purpose of the present study was to explore the factors affecting the risk for PTSD when individuals face traumatic events, 510 people who reported never having experienced childhood adverse events or adult traumatic events were excluded. In addition, 31 individuals with rare 5-HTTLPR alleles were excluded from the study, leaving 1252 informative subjects.
The mean (SD) age of the 1252 participants (52% male) was 38.9 (11.0) years, ranging from 17 to 79 years. To derive ancestry information from each individual, a panel of 41 ancestry-informative markers was genotyped, including FY and 36 short tandem repeats markers described in our previous studies,34,35 and augmented by 4 highly ancestry-informative SNPs, rs1540771 (6p23.5), rs1805007 (MC1R), rs12896399 (SLC24A4), and rs1426654 (SLC24A5), which are all associated with hair, eye, and/or skin pigmentation.36,37 These ancestry-informative markers were analyzed by a Bayesian cluster model using STRUCTURE software.38-40 We used admixture and allele frequencies–correlated models and 500 000 Markov chain Monte Carlo repeats after 500 000 burn-in repetitions.
DIAGNOSIS OF PTSD AND ADULT TRAUMATIC EVENTS INDEX
Sample collection and diagnostic interviews were performed by trained interviewers. Interviewing methods and the diagnostic reliability of the SSADDA, including the PTSD section (which showed interrater reliability and test-retest reliability [κ] of 0.59 and 0.76, respectively), have been described previously.32,33 In the SSADDA PTSD section, 12 separate types of traumatic events are assessed. These events are experienced direct combat in a war; seriously physically attacked or assaulted; physically abused as a child; seriously neglected as a child; raped; sexually molested or assaulted; threatened with a weapon; held captive or kidnapped; witnessed someone being badly injured or killed; involved in a flood, fire, or other natural disaster; involved in a life-threatening accident; suffered a great shock because one of these events happened to someone close to you; and other. Participants were asked to list up to 3 traumatic events and their age when these events happened. Those reporting traumatic experiences were then interviewed for potential PTSD symptoms. After all of the data were scored, a PTSD diagnosis was generated based on DSM-IV criteria requiring symptoms from each of 3 clusters, reexperiencing, avoidance, and increased arousal, that occur following the events.
CHILDHOOD ADVERSITY INDEX
Although childhood physical abuse and neglect are ascertained in the PTSD section of the SSADDA, we used the Environment section as the main instrument to assess participants' childhood experiences. All of the participants were asked whether they had witnessed or experienced a violent crime or been sexually abused, physically abused, or neglected by age 13 years. Since neglect is more subjective than the other childhood adversities, participants were asked 3 questions to assess it: “whether the person you were closest to was usually available to you when you needed him or her,” “whether you felt you could confide in this person when necessary,” and “whether this person was aware of who your friends were.” Respondents who answered “no” to any of the 3 questions were considered to have been neglected.
GENOTYPING
DNA was extracted from whole blood or immortalized cell lines. Polymerase chain reaction amplification was used to differentiate the short allele from the long allele of the 5-HTTLPR polymorphism. Polymerase chain reaction conditions are described in detail elsewhere.23 To genotype the additional SNP (rs25531), the polymerase chain reaction product was digested by MspI and size-fractionated on agarose gel.41
Detailed genotyping methods and characteristics for 37 of the 41 ancestry-informative markers were described previously.34 The remaining 4 SNPs were genotyped by the TaqMan technique using the ABI PRISM 7900 Sequence Detection System (Applied Biosystems Inc, Foster City, California).
STATISTICAL ANALYSIS
Based on the levels of transcriptional efficiency, the triallelic geno-types were reclassified: LA/LA was reclassified as L′L′; LA/S and LA/LG were reclassified as L′S′; and LG/LG, LG/S, and S/S were reclassified as S′S′. We used logistic regression models to examine the association between the PTSD diagnosis and the potential explanatory variables. In the first model, the effects of 5-HTTLPR geno-type (coded as 0 for L′L′, 1 for L′S′, and 2 for S′S′), childhood adversity (coded as 0 for none, 1 for exposure), adult traumatic events (coded as 0 for none, 1 for exposure), gene×childhood adversity, and gene×adult traumatic events were explored. In the second model, we focused on the participants who experienced both childhood and adulthood traumas. Exposure to dual stressful event types (both childhood and adulthood) was coded as 1, and exposure to either type of stressful events was coded as 0. The interactive effect of gene×stressful events was calculated. After that, in the third model, we analyzed the dual stressful events linearly: exposure to adulthood trauma and 1 type of childhood adversity was coded as 1, adulthood trauma and 2 types of childhood adversity was coded as 2, adulthood trauma and 3 or more types of childhood adversity was coded as 3, and exposure to either type of stressful events was coded as 0. In the first 2 models, we first analyzed each population, European American individuals and African American individuals, separately, using sex and age as covariates. Then we combined the data across the 2 populations using sex, age, and ancestry proportion scores as covariates. In the third model, since the group of dual stressful events was already small, dividing it into 3 groups further decreased the sample size. To increase the statistical power, only analyses with combined data (sex, age, and ancestry proportion scores were used as covariates) were performed. In addition, generalized estimating equations (GEE)42 were applied to fit the logistic regression model to take into account the potential dependence of the data from individuals within the same nuclear family. An exchangeable correlation structure was used in this logistic GEE regression model. Since many previous 5-HTTLPR×stressful events studies did not include rs25531, analyses using the diallelic L-S classification were also performed. Comparisons between groups of categorical variables were made using χ2 tests. All analyses were performed by SAS 9.1 (SAS Inc, Cary, North Carolina).
RESULTS
DEMOGRAPHICS
Approximately one-fifth of the 1252 participants (n=229; 18.3%) who experienced childhood adverse events and/or adult traumatic events met the criteria for lifetime PTSD. The mean (SD) age of participants developing PTSD was 39.3 (9.6) years, which was similar to those without a diagnosis of PTSD (mean [SD], 38.9 [11.3] years). Table 1 presents information on the prevalence of PTSD by sex and population. Among women, 22.3% received a lifetime diagnosis of PTSD, which was significantly higher than for male participants (14.6%) (; P<.001). The rate of PTSD among European American individuals (19.6%) and African American individuals (17.1%) was statistically indistinguishable (; P=.26). In addition, many psychiatric disorders were observed to be comorbid with PTSD (eTable, http://www.archgenpsychiatry.com).
Table 1.
Prevalence of PTSD by Sex and Population
| Characteristics | PTSD, No. (%) | P Value | |
|---|---|---|---|
| Sex | 12.3 | <.001 | |
| M (n = 656) | 96 (14.6) | ||
| F (n = 596) | 133 (22.3) | ||
| Population | 1.3 | .26 | |
| EA (n = 582) | 114 (19.6) | ||
| AA (n = 670) | 115 (17.1) |
Abbreviations: AA, African American; EA, European American; PTSD, posttraumatic stress disorder.
EFFECT OF ADULT TRAUMATIC EVENTS ON RISK OF PTSD
Eight hundred eighty of the 1252 participants (70.3%) reported that they had experienced 1 or more traumatic events as adults. Among them, 21.5% (n=189) met criteria for lifetime PTSD. In contrast, only 10.7% of the participants who never experienced adult traumatic events developed PTSD. Using a logistic GEE regression model, after adjusting for age, sex, ancestry proportion score, 5-HTTLPR genotype, and childhood adversity, adult traumatic events were significantly associated with an increased risk for PTSD (OR, 3.57; 95% CI, 2.43-5.25; P<.001) (Table 2).
Table 2.
Results of Logistic Regression Analysesa
| EA |
AA |
Pooled |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sex | 0.53 (0.34-0.83) | .005b | 0.63 (0.42-0.96) | .03c | 0.58 (0.43-0.79) | <.001d |
| Age,y | 1.01 (0.99-1.02) | .58 | 1.02 (1.00-1.04) | .08 | 1.01 (1.00-1.02) | .09 |
| Ancestry proportion score | 1.12 (0.81-1.55) | .50 | ||||
| Adult traumatic events | 4.09 (2.31-7.26) | <.001d | 3.22 (1.90-5.45) | <.001d | 3.57 (2.43-5.25) | <.001d |
| Childhood adversity | 5.70 (2.89-11.26) | <.001d | 3.44 (1.98-5.97) | <.001d | 4.30 (2.81-6.60) | <.001d |
| 5-HTTLPR genotype | 1.24 (0.91-1.69) | .17 | 1.22 (0.91-1.63) | .18 | 1.22 (0.99-1.51) | .07 |
| 5-HTTLPR× adult traumatic events | 2.60 (1.19-5.66) | .02c | 2.06 (1.02-4.14) | .04c | 1.93 (1.17-3.17) | .01c |
| 5-HTTLPR× childhood adversity | 3.29 (1.17-9.22) | .02c | 1.71 (0.81-3.61) | .16 | 1.81 (1.01-3.24) | .046c |
| 5-HTTLPR× dual stressful events | 2.86 (1.50-5.45) | .002b | 1.88 (1.04-3.40) | .04c | 2.31 (1.50-3.56) | <.001d |
Abbreviations: AA, African American; CI, confidence interval; EA, European American; OR, odds ratio.
Sex, age, adult traumatic events, and childhood adversity were used as covariates in all the models. In addition, ancestry proportion scores were included in the pooled model.
P<.01.
P<.05.
P<.001.
EFFECT OF CHILDHOOD ADVERSITY ON RISK OF PTSD
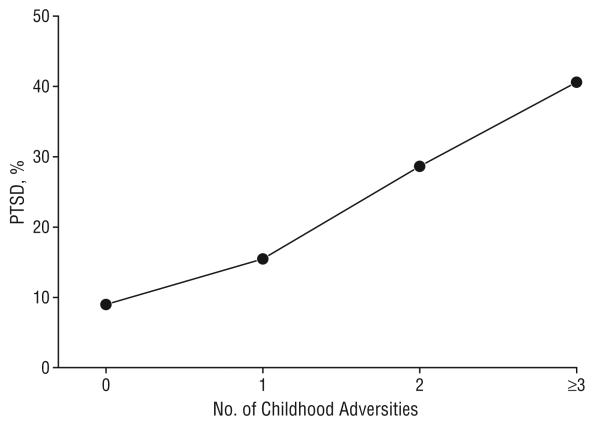
Four types of childhood adversities, namely witnessing or experiencing a violent crime, sexual abuse, physical abuse, or neglect, were assessed in this study. Most of the participants (n=924; 73.8%) experienced at least 1 type of childhood adversity. Among them, 64.4% (n=595) experienced 1 type of adverse event; 23.8% (n=220) experienced 2 types; and 11.8% (n=109) experienced 3 or 4 types of childhood adversities. The percentage of participants with a lifetime diagnosis of PTSD increased from 8.8% in individuals with no childhood adversity to 15.4% in those with 1 type, 28.6% in those with 2 types, and 40.5% in those with 3 or 4 types of childhood adversity (Figure 1). Using the same logistic GEE regression model described previously, a significant effect of childhood adversity on PTSD development was observed (OR, 4.30; 95% CI, 2.81-6.60; P<.001) (Table 2).
Figure 1.
The number of childhood adversity types is associated with the onset of posttraumatic stress disorder (PTSD).
EFFECT OF 5-HTTLPR GENOTYPES ON RISK OF PTSD
In European American individuals, the frequency of the long allele was 56.3% and the short allele, 43.7%. The genotype distribution was 31.1% LL, 50.3% LS, and 18.6% SS. The minor allele frequency of rs25531 was 7.1%. After reclassification, the genotype frequencies were 23.5% L′L′, 51.2% L′S′, and 25.3% S′S′. In African American individuals, the frequencies for the long and short alleles were 77.8% and 22.2%, respectively, and the genotype distribution was 61.0% LL, 33.6% LS, and 5.4% SS. The minor allele frequency of rs25531 was 22.5%. After reclassification, the genotype distribution was 32.2% L′L′, 46.1% L′S′, and 21.6% S′S′. The allele frequencies of the 5-HTTLPR polymorphism were consistent with previous studies,21,23,24 and all were in Hardy-Weinberg equilibrium.
After adjusting for age, sex, childhood adversity, and adult traumatic events, logistic GEE regression analysis showed no significant association between 5-HTTLPR genotype and PTSD diagnosis in either European American or African American individuals (European American: OR, 1.24; 95% CI, 0.91-1.69; P=.17; African American: OR, 1.22; 95% CI, 0.91-1.63; P=.18); the association in the combined sample, in which ancestry proportion score was entered as a covariate, was not statistically significant (OR, 1.22; 95% CI, 0.99-1.51; P=.07). The use of diallelic genotypes, which include only the L and S alleles of the 5-HTTLPR polymorphism, in the logistic regression analysis also showed that 5-HTTLPR genotypes were not associated with PTSD (OR, 0.95; 95% CI, 0.67-1.35; P=.77). In addition, none of the psychiatric disorders that were found to be comorbid with PTSD were associated with 5-HTTLPR genotypes (eTable).
5-HTTLPR GENOTYPES INTERACTED WITH CHILDHOOD ADVERSITY AND ADULT TRAUMATIC EVENTS TO INCREASE RISK FOR DEVELOPING PTSD
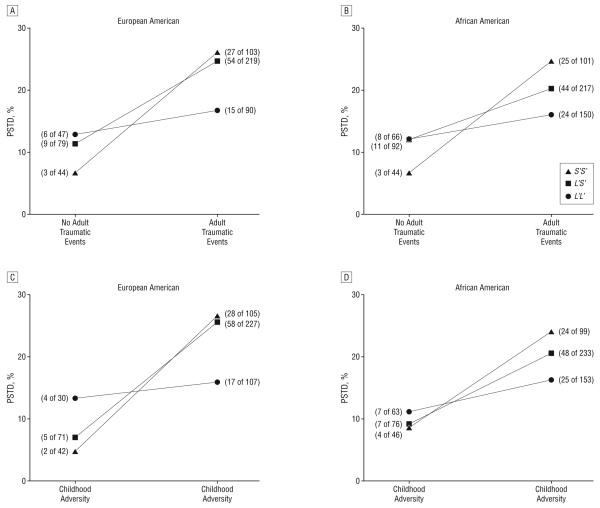
We first tested two 2-way interactions, genotype×adult traumatic events and genotype×childhood adversity, as predictors in the model. After adjusting for all the main effects, including age, sex, genotype, childhood adversity, and adult traumatic events, the genotype×adult traumatic events interaction was significantly associated with risk for PTSD in both European American and African American individuals separately (European American: OR, 2.60; 95% CI, 1.19-5.66; P=.02; African American: OR, 2.06; 95% CI, 1.02-4.14; P=.04) (Figure 2A and B), with the combination of the 2 populations providing stronger evidence of association (OR, 1.93; 95% CI, 1.17-3.17; P=.01). The genotype×childhood adversity interaction was found to be significantly associated with risk for PTSD in European American individuals, but not in African American individuals (European American: OR, 3.29; 95% CI, 1.17-9.22; P=.02; African American: OR, 1.71; 95% CI, 0.81-3.61; P=.16) (Figure 2C and D). When all the participants were combined together, the interactive effect of genotype×childhood adversity was significant (OR, 1.81; 95% CI, 1.01-3.24; P=.046).
Figure 2.
In European American and African American individuals, 5-HTTLPR genotype interacted with adult traumatic events (A and B) to increase risk for posttraumatic stress disorder (PTSD). In European American individuals, the genotype also interacted with childhood adversity (C); in African American individuals, the interactive effect was a trend (D). The numbers of patients with PTSD of the total numbers of participants in the group are shown.
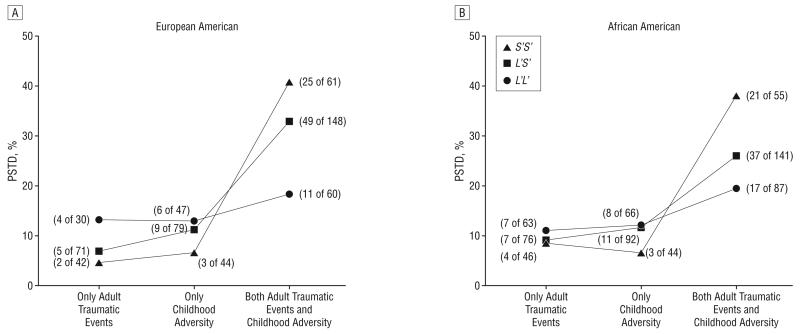
Because in our study the participants reported experiencing at least 1 type of trauma, and many of them experienced both childhood adversity and adult traumatic events, we then investigated how the high rates of both types of trauma exposure interacted with 5-HTTLPR to influence risk for PTSD. Five hundred fifty-two of the 1252 participants (44.1%) experienced both childhood adversity and adult traumatic events. Among this group, 29.0% (n=160) received a lifetime diagnosis of PTSD. In contrast, only 9.9% of participants who experienced either (but not both) childhood adversity or adult traumatic events developed PTSD. Moreover, among European American participants who experienced both stressful events, 41.0% with the S′S′ genotype developed PTSD, compared with 33.1% in the L′S′ genotype group and only 18.3% in the L′L′ genotype group. In African American participants, 38.2% of individuals with the S′S′ genotype, 26.2% with the L′S′ genotype, and 19.5% with the L′L′ genotype were diagnosed with PTSD (Figure 3). If we used the dual stressful events as a predictor, and computed the second logistic regression model described in the “Methods” section, after adjusting for all the main effects, the genotype×dual stressful events interaction was significantly associated with risk for PTSD in both European American and African American individuals (European American: OR, 2.86; 95% CI, 1.50-5.45; P=.002; African American: OR, 1.88; 95% CI, 1.04-3.40; P=.04), with the combination of the 2 populations providing stronger evidence of association (OR, 2.31; 95% CI, 1.50-3.56; P<.001) (Table 2). If the L-S diallelic classification of the 5-HTTLPR genotype was used, the results of the genotype×dual stressful events interaction were comparable with those obtained using the triallelic clasification (European American: OR, 2.37; 95% CI, 1.19-4.72; P=.01; African American: OR, 2.97; 95% CI, 1.43-6.17; P=.003; pooled: OR, 2.64; 95% CI, 1.63-4.27; P<.001). In addition, this interactive effect was observed in both male and female participants (triallelic genotypes: male: OR, 2.38; 95% CI, 1.20-4.72; P=.01; female: OR, 2.31; 95% CI, 1.31-4.09; P=.004; diallelic genotypes: male: OR, 1.99; 95% CI, 1.01-3.92; P=.04; female: OR, 3.38; 95% CI, 1.72-6.64; P<.001).
Figure 3.
5-HTTLPR genotype interacted with dual stressful life events to increase the risk for posttraumatic stress disorder (PTSD) in both European American and African American individuals. The numbers of patients with PTSD of the total numbers of participants in the group are shown.
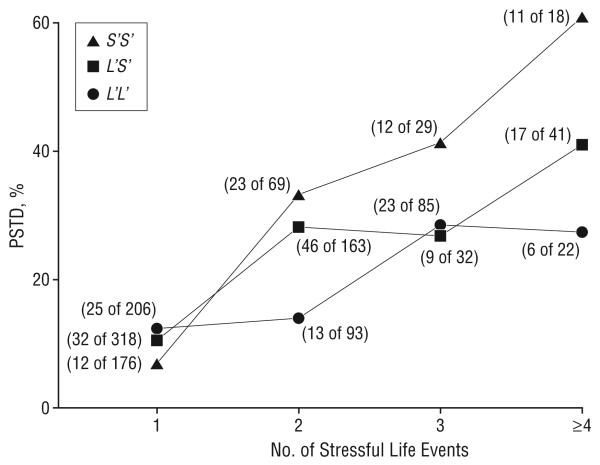
Because the number of childhood adversity types was associated with the risk of PTSD, we then modeled the dual stressful events linearly, as was described in the “Methods” section. The interaction further suggested that the effect of life events on the onset of PTSD was stronger among individuals with an S′ (S) allele than among those without (triallelic genotypes: OR, 1.41; 95% CI, 1.20-1.66; P<.001; diallelic genotypes: OR, 1.60; 95% CI, 1.32-1.94; P<.001) (Figure 4).
Figure 4.
5-HTTLPR genotype interacted with the number of stressful life events to increase the risk for posttraumatic stress disorder (PTSD) (group 1: exposure to either adult trauma or childhood adversity; group 2: exposure to adult trauma and 1 type of childhood adversity; group 3: exposure to adult trauma and 2 types of childhood adversity; and group 4: exposure to adult trauma and more than 2 types of childhood adversity).
DISTRIBUTION OF CHILDHOOD ADVERSITY AND ADULT TRAUMATIC EVENTS BY GENOTYPE GROUPS
As mentioned previously, different traumas influence people differently. Certain life-threatening events may carry higher risk for developing PTSD than others. To exclude the possibility that trauma type was a confounding factor in our study, we examined the distribution of childhood adversity and adult traumatic events across the genotype groups. For childhood adversity, the distribution of events did not differ significantly among the 3 genotype groups (Table 3). Based on the characteristics of the trauma types, and previous studies showing that events involving the element of interpersonal assault are associated with a higher rate of PTSD than events without the element,4,12 we grouped the 10 types of adulthood adverse events into 3 broad groups, namely, sexual trauma (rape or molestation), physical trauma (physical attack, threatened with a weapon, held captive, or kidnapped), and other trauma (witness, natural disaster, accident, shock, or other qualifying trauma). The distribution of this measure also did not differ significantly among the 3 genotype groups (Table 3).
Table 3.
Distribution of Childhood Adversity and Adult Traumatic Events by Genotype Groups
| % |
||||
|---|---|---|---|---|
| L′L′ | L′S′ | S′S′ | P Value | |
| Childhood adversity | ||||
| Neglect | 50.4 | 50.0 | 51.5 | .78 |
| Witnessing or experiencing a violent crime | 41.5 | 42.2 | 40.7 | .91 |
| Sexual abuse | 29.6 | 35.9 | 31.9 | .26 |
| Physical abuse | 28.5 | 24.6 | 28.9 | .36 |
| Adult traumatic events | ||||
| Sexual harassment | 21.7 | 20.0 | 17.6 | .57 |
| Physical attack | 42.9 | 46.1 | 45.6 | .72 |
| Accidents | 70.8 | 69.5 | 70.1 | .94 |
COMMENT
In this study, we examined the effects of childhood adversity, adult traumatic events, the 5-HTTLPR polymorphism, and their interactions on the risk of receiving a lifetime diagnosis of PTSD. We found that both childhood adversity and adult traumatic events predicted PTSD. Although the 5-HTTLPR polymorphism alone did not predict the onset of PTSD, it regulated risk for PTSD in people who experienced childhood adversity and/or adult traumatic events, especially in those who experienced both types of stressful life events, with comparable findings in both European American and African American individuals. We initially based the hypothesis on evidence from prior studies that SLC6A4 was associated with emotional responses, especially the response to a stressful life event. Indeed, our previous work showed that this locus influenced risk for PTSD-related symptoms in a sample of subjects exposed to a hurricane.43
Although the role of serotonergic function in the patho-physiology of PTSD is still unclear, it is believed to regulate a wide array of stress-related processes: anxiety, arousal, depression, aggression, and impulsivity, which are all common symptoms of PTSD. Since the serotonin transporter regulates serotonergic neurotransmission, and is the primary target of efficacious antidepressants such as selective serotonin reuptake inhibitors, SLC6A4 has received particular attention in research on mood and anxiety disorders. Compared with the L′ allele, the S′ allele of the 5-HTTLPR polymorphism was found to reduce SLC6A4 expression and function, resulting in decreased serotonin reuptake. Previous studies have shown that presence of the short allele was associated with anxiety-related personality traits15 and increased amygdala neuronal activity under fearful stimuli44 and may influence selective serotonin reuptake inhibitor response rate among European American patients.41 Studies of gene×environment interactions have also provided evidence for a functional link among serotonin, psychosocial stressors, and risk of depression21,22,45 and an intermediate phenotype of anxiety,25 although some other studies failed to replicate these findings.46-48
Our study provides additional evidence that genetic and environmental factors interact to play a role in psychiatric disorders. Exposure to trauma earlier in life increases risk for PTSD on exposure to subsequent trauma.49 Our study suggests that this risk is heightened among individuals with 1 or 2 copies of the 5-HTTLPRS′(S) allele. Interestingly, because of the design of our study (people who reported never experiencing traumas were not included), we were unable to ascertain an effect of 5-HTTLPR genotype on risk for PTSD among persons with either (but not both) childhood maltreatment or adult trauma. It was only in the group of subjects who could be characterized as having had the highest rates of trauma exposure (ie, in both childhood and adulthood) that an impact of 5-HTTLPR could be detected. This suggests that there may be many neurobiological (including genetically determined) “buffers” to PTSD; only in instances of extreme and/or repeated trauma exposure (which, it should be pointed out, characterizes those trauma “types” with the highest conditional risk for PTSD, eg, domestic violence and military combat), in which these buffers are overwhelmed, can the impact of specific genes such as 5-HTTLPR be detected. To our knowledge, this is the first study to examine the diagnosis of PTSD as an outcome of a gene×environment interaction. Two previous studies have focused on gene×environment interaction effects on PTSD symptom severity.43,50 Kilpatrick and colleagues43 were the first to provide evidence that the S′ allele of the 5-HTTLPR polymorphism modulated the influence of hurricane exposure and social support on PTSD symptoms. A limitation of that epidemiologic study is that the number of affected subjects was small (n=19). Further, it focused on 1 specific (and acute) trauma. This decreased the heterogeneity of the trauma experience between patients, enhancing the internal validity of the study but limiting its generalizability. The study by Binder and colleagues50 showed that 4 SNPs in the FKBP5 gene interacted with childhood abuse to increase the level of PTSD symptoms. Two of the 4 SNPs in FKBP5 were previously found to be associated with peritraumatic dissociation, a well-established risk factor for PTSD, in medically injured children.51 Binder and colleagues used a PTSD symptom scale score as an outcome measure based on the assumption that it could be used to make inferences about the disorder. Although the score correlates with a diagnosis of PTSD, it remains uncertain as to the relevance of the measured symptoms to the disorder.
In comparison with these previous studies, the current study has several strengths that deserve mention. Instead of using PTSD symptoms as an outcome, participants were directly interviewed by trained interviewers, resulting in a reliable PTSD diagnosis. Consistent with the necessity that PTSD follows exposure to traumatic event(s), we studied only people who reported having had 1 or more extreme adverse experiences in their lives, which increased the statistical power. In addition, we examined a range of childhood adversity and adult traumatic events. These events covered most (although certainly not all) trauma types known to cause PTSD. For the 5-HTTLPR genotypes, we performed analyses using genotypes based on the triallelic reclassification to compare the results with those using the genotypes from the L-S diallelic classification. They were nearly identical. Therefore, this study provided a systematic and clear view of the gene×environment interaction as it contributes to the risk of a PTSD diagnosis.
Different allele frequencies of 5-HTTLPR in European American and African American individuals have been previously reported23 and were observed in our study. A series of methods to exclude population stratification as a potential confounder were used in this study. First, a Bayesian clustering method was applied to analyze the genotype data of 41 ancestry-informative markers, dividing the participants into 2 subgroups. Second, statistical analyses were performed separately within the 2 population subgroups. The interaction of 5-HTTLPR by dual stressful life events was observed to be significantly associated with the PTSD diagnosis in both populations individually. Third, when analyzing the whole sample, ancestry proportion score was used as a covariate in the logistic regression model, although no significant association was observed between PTSD and population. Therefore, the different 5-HTTLPR allele frequencies between the 2 populations were not likely confounding our analyses.
However, interpretation of this study should consider the following limitation. Retrospective recall of early adverse experiences might be inaccurate or biased, including the potential for there to be bias introduced by a subsequent experience of PTSD. The interval, significance of events, and personal characteristics could cause recall bias. Especially since PTSD rarely occurs alone, an abnormal mental state related to other disorders, such as substance use disorder or major depression, may also cause recall bias. Future gene×environment studies based on a cohort longitudinal design would address this potential bias in recall of life events.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health grants R01 DA12690, R01 DA12849, K24 DA15105, K24 AA013736, 24 DA022288, and R01 AA11330.
Footnotes
Financial Disclosure: Dr Stein receives or has in the past 3 years received research support from Eli Lilly and Co and GlaxoSmithKline and is currently or has in the past 3 years been a consultant for AstraZeneca, Avers Pharmaceuticals, BrainCells Inc, Bristol-Myers Squibb, Eli Lilly and Co, EPI-Q, Forest Laboratories, Hoffmann-La Roche Pharmaceuticals, Integral Health Decisions Inc, Jazz Pharmaceuticals, Johnson & Johnson, Mindsite, Sanofi-Aventis, Transcept Pharmaceuticals Inc, and Virtual Reality Medical Center.
Additional Information: The eTable is available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48(3):216–222. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 2.Norris FH. Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60(3):409–418. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Bromet E, Sonnega A, Kessler RC. Risk factors for DSM-III-R posttraumatic stress disorder: findings from the National Comorbidity Survey. Am J Epidemiol. 1998;147(4):353–361. doi: 10.1093/oxfordjournals.aje.a009457. [DOI] [PubMed] [Google Scholar]
- 5.Bramsen I, Dirkzwager AJ, van der Ploeg HM. Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: a prospective study of former peacekeepers. Am J Psychiatry. 2000;157(7):1115–1119. doi: 10.1176/appi.ajp.157.7.1115. [DOI] [PubMed] [Google Scholar]
- 6.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Levendosky AA, Okun A, Parker JG. Depression and maltreatment as predictors of social competence and social problem-solving skills in school-age children. Child Abuse Negl. 1995;19(10):1183–1195. doi: 10.1016/0145-2134(95)00086-n. [DOI] [PubMed] [Google Scholar]
- 8.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 10.Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry. 2007;64(5):577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- 11.True WR, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 12.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 13.Broekman BF, Olff M, Boer F. The genetic background to PTSD. Neurosci Biobehav Rev. 2007;31(3):348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. J Trauma Stress. 2007;20(5):737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- 15.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 16.Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 17.Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, Del-Proposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry. 1998;155(2):207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- 18.Lim JE, Papp A, Pinsonneault J, Sadee W, Saffen D. Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTLPR. Mol Psychiatry. 2006;11(7):649–662. doi: 10.1038/sj.mp.4001797. [DOI] [PubMed] [Google Scholar]
- 19.Kaehler ST, Singewald N, Sinner C, Thurnher C, Philippu A. Conditioned fear and inescapable shock modify the release of serotonin in the locus coeruleus. Brain Res. 2000;859(2):249–254. doi: 10.1016/s0006-8993(00)01967-3. [DOI] [PubMed] [Google Scholar]
- 20.Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55(7):733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- 24.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33(2):312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- 26.Reed PL, Anthony JC, Breslau N. Incidence of drug problems in young adults exposed to trauma and posttraumatic stress disorder: do early life experiences and predispositions matter? Arch Gen Psychiatry. 2007;64(12):1435–1442. doi: 10.1001/archpsyc.64.12.1435. [DOI] [PubMed] [Google Scholar]
- 27.Schiff M, Zweig HH, Benbenishty R, Hasin DS. Exposure to terrorism and Israeli youths' cigarette, alcohol, and cannabis use. Am J Public Health. 2007;97(10):1852–1858. doi: 10.2105/AJPH.2006.090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelernter J, Kranzler HR, Panhuysen C, Weiss RD, Brady K, Poling J, Farrer L. Dense genomewide linkage scan for alcohol dependence in African Americans: significant linkage on chromosome 10. Biol Psychiatry. 2009;65(2):111–115. doi: 10.1016/j.biopsych.2008.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang BZ, Kranzler HR, Farrer L. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15(24):3498–3507. doi: 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- 30.Gelernter J, Panhuysen C, Wilcox M, Hesselbrock V, Rounsaville B, Poling J, Weiss R, Sonne S, Zhao H, Farrer L, Kranzler HR. Genomewide linkage scan for opioid dependence and related traits. Am J Hum Genet. 2006;78(5):759–769. doi: 10.1086/503631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelernter J, Panhuysen C, Weiss R, Brady K, Hesselbrock V, Rounsaville B, Poling J, Wilcox M, Farrer L, Kranzler HR. Genomewide linkage scan for cocaine dependence and related traits: significant linkages for a cocaine-related trait and cocaine-induced paranoia. Am J Med Genet B Neuropsychiatr Genet. 2005;136B(1):45–52. doi: 10.1002/ajmg.b.30189. [DOI] [PubMed] [Google Scholar]
- 32.Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80(3):303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Pierucci-Lagha A, Gelernter J, Chan G, Arias A, Cubells JF, Farrer L, Kranzler HR. Reliability of DSM-IV diagnostic criteria using the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2007;91(1):85–90. doi: 10.1016/j.drugalcdep.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang BZ, Zhao H, Kranzler HR, Gelernter J. Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol. 2005;28(4):302–312. doi: 10.1002/gepi.20070. [DOI] [PubMed] [Google Scholar]
- 35.Stein MB, Schork NJ, Gelernter J. A polymorphism of the beta1-adrenergic receptor is associated with low extraversion. Biol Psychiatry. 2004;56(4):217–224. doi: 10.1016/j.biopsych.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Dimisianos G, Stefanaki I, Nicolaou V, Sypsa V, Antoniou C, Poulou M, Papadopoulos O, Gogas H, Kanavakis E, Nicolaidou E, Katsambas AD, Stratigos AJ. A study of a single variant allele (rs1426654) of the pigmentation-related gene SLC24A5 in Greek subjects. Exp Dermatol. 2009;18(2):175–177. doi: 10.1111/j.1600-0625.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 37.Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Pálsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39(12):1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65(1):220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology (Berl) 2006;187(1):68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- 42.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 43.Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 44.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 46.Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59(3):224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35(1):101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 48.Chipman P, Jorm AF, Prior M, Sanson A, Smart D, Tan X, Easteal S. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: results from two community surveys. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 49.Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of Trauma. Am J Psychiatry. 1999;156(6):902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- 50.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005;10(12):1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.