Abstract
A distinct feature of malignant gliomas is the intrinsic ability of single tumor cells to disperse throughout the brain, contributing to the failure of existing therapies to alter the progression and recurrence of these deadly brain tumors. Regrettably, the mechanisms underlying the inherent invasiveness of glioma cells are poorly understood. Here, we report for the first time that engulfment and cell motility 1 (ELMO1) and dedicator of cytokinesis 1 (Dock180), a bipartite Rac1 guanine nucleotide exchange factor (GEF), are evidently linked to the invasive phenotype of glioma cells. Immunohistochemical analysis of primary human glioma specimens showed high expression levels of ELMO1 and Dock180 in actively invading tumor cells in the invasive areas, but not in the central regions of these tumors. Elevated expression of ELMO1 and Dock180 was also found in various human glioma cell lines compared with normal human astrocytes. Inhibition of endogenous ELMO1 and Dock180 expression significantly impeded glioma cell invasion in vitro and in brain tissue slices with a concomitant reduction in Rac1 activation. Conversely, exogenous expression of ELMO1 and Dock180 in glioma cells with low level endogenous expression increased their migratory and invasive capacity in vitro and in brain tissue. These data suggest that the bipartite GEF, ELMO1 and Dock180, play an important role in promoting cancer cell invasion and could be potential therapeutic targets for the treatment of diffuse malignant gliomas.
Introduction
The inherent invasive nature of malignant gliomas contributes to the high frequency of tumor recurrence and disease progression in patients afflicted with these deadly cancers. In spite of the use of multimodal therapies including surgery, radiation, and chemotherapy, the mean survival time in patients with high-grade gliomas is less than 1 year (1). It is established that the mechanisms regulating cell migration are fundamental to the invasive phenotype of gliomas (2). Although studies show that various stimuli promote glioma cell invasion, the mechanisms underlying dysregulation of cell motility during invasion of these tumor cells remain largely unknown.
Cell migration is highly regulated by spatial and temporal changes of the actin cytoskeleton essential for many physiologic and pathologic processes including cancer cell invasion. Rac1, a member of the Rho GTPase family, is a key regulator of actin cytoskeletal dynamics and relays signals from various stimuli such as growth factors, cytokines, and adhesion molecules to downstream effectors modulating cell migration and invasion (3). Importantly, Rac1 has been shown to promote glioma cell migration (4–10). The activation of Rac1 is through a GDP/GTP exchange mechanism catalyzed by the guanine nucleotide exchange factors (GEF) resulting in an active, GTP-bound state (11). The Rho GTPase GEFs are a large family of proteins that contain either a Dbl homology domain involved in nucleotide exchange (12) or a newly characterized Docker domain that facilitates GEF function (13), of which Dock180 (dedicator of cytokinesis 180) is the prototypical mammalian member.
Dock180 was first identified as a CrkII-binding protein that regulates NIH 3T3 cell morphology (14). Studies in C. elegans and Drosophila reveal that Dock180 homologues modulate various functions such as phagocytosis, cell migration, myoblast fusion, dorsal closure, and cytoskeletal organization through the activation of Rac1 (15–18). Furthermore, Dock180 stimulates phagocytosis and filopodia formation downstream of integrin receptor signaling in mammalian cells (19, 20). Importantly, Dock180 facilitates nucleotide exchange on Rac1 through its unconventional Docker GEF domain (21–23) but requires binding to engulfment and cell motility 1 (ELMO1) in achieving GDP/GTP exchange on Rac (21). In mammalian cells and in C. elegans, ELMO1 and its homologue, CED-12, enhance phagocytosis and cell migration by forming a complex with Dock180 (24). This bipartite GEF complex synergistically functions upstream of Rac1, promoting Rac-dependent cell migration (25). Although ELMO1 and Dock180 stimulate cell migration in normal mammalian cells, whether these molecules play a critical role in cancer cell migration and invasion has not been investigated.
In this study, we show for the first time that ELMO1 and Dock180 stimulate glioma cell migration and invasion. We detected high-level expression of ELMO1 and Dock180 in actively infiltrating glioma cells within the invasive regions along blood vessels, neuronal structures, and the corpus callosum as compared with the central tumor areas of primary human glioma specimens representing WHO grades 2 to 4. Furthermore, we found that ELMO1 and Dock180 expression is increased in human glioma cell lines compared with normal human astrocytes. Inhibition of endogenous ELMO1 and Dock180 impeded glioma cell migration and invasion whereas forced expression of ELMO1 and Dock180 in glioma cell lines with low endogenous expression enhanced tumor cell migration and invasion in vitro and ex vivo. These data show a novel function for the bipartite GEF, ELMO1 and Dock180, in stimulating cancer cell migration and invasion.
Materials and Methods
Cell lines, antibodies, and reagents
Human LN18, LN229, U118, and U87MG glioma cells were obtained from American Type Culture Collection; U251MG and U373MG glioma cells were from our collection and their culture was previously described (26). D54MG glioma cells were from Dr. D. Bigner (Duke University, Durham, NC). SNB19 glioma cells were from Dr. Y-H. Zhou (University of California, Irvine, Irvine, CA). Immortalized normal human astrocytes and genetically modified normal human astrocytes (27) were from Dr. R. Pieper (University of California, San Francisco, San Francisco, CA). The following reagents were used in our studies: goat anti-Dock180 antibody (H-4), goat anti-Dock180 antibody (N-19), and goat anti–β-actin (I-19) antibodies (Santa Cruz Biotechnology), goat anti-ELMO1 (Ab2239, Abcam), rabbit anti-ELMO1 antibody (21), Rac1 activation assay kit (Upstate Technology), and a mouse anti-Rac1 antibody (BD PharMingen). The secondary antibodies were from Vector Laboratories or Jackson ImmunoResearch Laboratories. A 3,3′-diaminobenzidine elite kit was from DAKO; AquaBlock was from East Coast Biologics, Inc. Cell culture media and other reagents were from Hyclone, Invitrogen BRL, Sigma Chemicals, and Fisher Scientific.
Immunohistochemical analyses of primary human glioma specimens
A total of 53 human malignant glioma specimens that contain an identifiable center and border/invasive area were used and included 6 diffuse astrocytomas (grade 2), 1 oligoastrocytoma (grade 2), 5 oligodendrogliomas (grade 2), 8 anaplastic astrocytomas (grade 3), 5 anaplastic oligodendrogliomas (grade 3), 3 anaplastic oligoastrocytomas (grade 3), and 25 glioblastoma multiforme (grade 4). Additionally, four normal human brain specimens obtained at autopsy from patients without brain lesions were included as controls. Immunohistochemical analyses and scoring were done as previously described (28) with no (−), weakest (±), low (1+), medium (2+), and strong (3+) staining with a polyclonal rabbit anti-ELMO1 (1:150) and a polyclonal goat anti-Dock180 (N-19; 1:1,000) antibody.
Microdissection and protein extraction of paraffin-embedded glioma tissue
Microdissection of paraffin-embedded human glioma tissue was done as previously described (28). Briefly, the paraffin-embedded glioma specimens were sectioned at 5- and 50-μm thicknesses and mounted onto glass slides. To identify the center, border, and invasive regions within the glioma specimen, the 5-μm-thick sections were stained with H&E. Three 50-μm-thick sister sections of each sample were then deparaffinized in xylenes, rehydrated in graded ethanol, immersed in distilled water, and air-dried. To exclusively collect the center or border regions of the tissue, the targeted areas were cut microscopically under an Olympus SZ-STS stereomicroscope with a fine needle using the sister H&E-stained section as a guide. Next, total protein was extracted from the microdissected, formalin-fixed paraffin-embedded glioma tissue as previously described (28).
Immunoblotting
Thirty micrograms of total protein from various whole-cell lysates were separated by NuPAGE 10% Bis-Tris polyacrylamide gel (Invitrogen) electrophoresis under reducing conditions and transferred onto Immobilon-P transfer membranes (Millipore). The membranes were blocked, incubated with the indicated primary antibodies, and subsequently probed with peroxidase-labeled secondary antibodies. The reacted proteins were visualized by enhanced chemiluminescence reaction (Amersham Biosciences).
Inhibition of ELMO, Dock180, and Rac1 expression
Small interfering RNA (siRNA) was synthesized by Invitrogen. The target sequences of ELMO1 were 5′-GGCACUAUCCUUCGAUUAACCACAU-3′ (designated E1) and 5′-CCGAGAGGAUGAACCAGGAAGAUUU-3′ (designated E2; ref. 29). The target sequences of Rac1 were 5′-AAGGAGAUUGGUGCUGUAAAA-3′ (designated R1; ref. 6) and 5′-AACCUUUGUACGCUUUGCUCA-3′ (designated R2; refs. 6, 7). A pool containing three separate Dock180 siRNAs was from Santa Cruz Biotechnology. The glioma cells were plated at 40% to 50% confluency, were allowed to attach to the tissue culture dish for ~3 h, and transfected with 60 to 120 nmol/L of the indicated siRNA for 24 h in the presence of 10% fetal bovine serum (FBS) in DMEM using Lipofectamine 2000 following the manufacturer’s instructions (Invitrogen). Mock transfection was done in parallel using the Stealth RNAi Negative Control Med GC (Invitrogen). After 24 h, the siRNA/lipid complexes were removed and the cells were maintained in complete medium for an additional 48 h or treated with the proteasome inhibitors MG132 (2 μmol/L) or ALLN (4 μmol/L) for 24 h. The inhibition of protein expression was determined by Western blot analysis.
Exogenous expression of ELMO1 and Dock180
A pCG plasmid encoding c-Myc– and His-tagged full-length human ELMO1 (from Dr. J. Skowronski, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY; ref. 30) and/or a pCXN2 plasmid encoding Flag-tagged full-length human Dock180 (from Dr. M. Matsuda, Kyoto University, Kyoto, Japan) was transfected into U87MG and U251MG cells using Effectene following the manufacturer’s instructions (Qiagen). Forty-eight hours posttransfection, the cells were used in in vitro cell migration, invasion, and ex vivo brain slice assays. The expression of exogenous ELMO1 and Dock180 expression was determined by Western blot analysis.
Rac1 activation assay
GTP loading of Rac1 was measured using the Rac1 Activation Assay Kit (Upstate Technology) according to the manufacturer’s instructions. Briefly, cells were lysed in ice-cold magnesium lysis buffer and cleared with glutathione-agarose beads. Cell extracts were then incubated with PAK-1 PBD agarose beads, pelleted, and washed. The beads were resuspended in sample buffer and separated by 10% PAGE. GTP-bound Rac1 was detected using an anti-Rac1 antibody.
In vitro migration and invasion assays
In vitro migration and invasion assays were done as previously described (31). Briefly, 50 μL of transiently transfected (siRNA or plasmid DNA) glioma cells (5 × 105/mL in serum-free DMEM plus 0.05% bovine serum albumin) were separately placed into the top compartment of a Boyden chamber. For migration assays, the cells were allowed to migrate through an 8-μm pore size membrane precoated with fibronectin (10 μg/mL) overnight at 37°C. For invasion assays, the cells were allowed to invade through a growth factor–reduced Matrigel–coated (0.78 mg/mL) membrane overnight at 37°C. Afterwards, the membrane was fixed and stained, nonmigrating and noninvading cells were removed, and the remaining cells were counted.
Ex vivo brain slice invasion assay
The ex vivo brain slice assay was done as previously described with minor modifications (9, 10). Briefly, fresh sections (500-μm thickness) of mouse cerebrum from 8-week-old mice (C57/BL6, The Jackson Laboratory) were placed onto transwell membranes (0.4 μm pore size, Corning) in a six-well dish containing DMEM with 10% FBS, 100 units/mL of penicillin, and 100 μg/mL of streptomycin, allowing the surface of the brain slice to be semidry. Afterwards, green fluorescent protein (GFP)–expressing glioma cells (5 × 104 in 0.5-μL DMEM) are placed onto the putamen of both sides of each brain slice and incubated in a humidified environment at 37°C, 5% CO2 and 95% air. After 48 h, the brain slices were gently rinsed with PBS and fixed in 4% paraformaldehyde overnight at 4°C. Lateral cell migration/invasion was assessed by direct epifluorescent examination of GFP-expressing glioma cells using a stereomicroscope (SZX12, Olympus) at ×10 magnification. Images were captured with a SPOT digital camera (Diagnostic Instrument). Depth of cell invasion into the brain slice was determined by optical sectioning using a Zeiss LSM 510 Confocal Microscope (Carl Zeiss MicroImaging, Inc.).
Statistical analysis
One-way ANOVA with Newman-Keuls posttest or paired two-way Student’s t test was done using GraphPad Prism version 4.00 for Windows (GraphPad software). A χ2 test was done as previously described to examine the association between immunohistochemical staining for ELMO1 and Dock180 and glioma invasion (28). P < 0.05 was considered statistically significant.
Results
ELMO1 and Dock180 are co-overexpressed in actively invading glioma cells of primary human glioma specimens
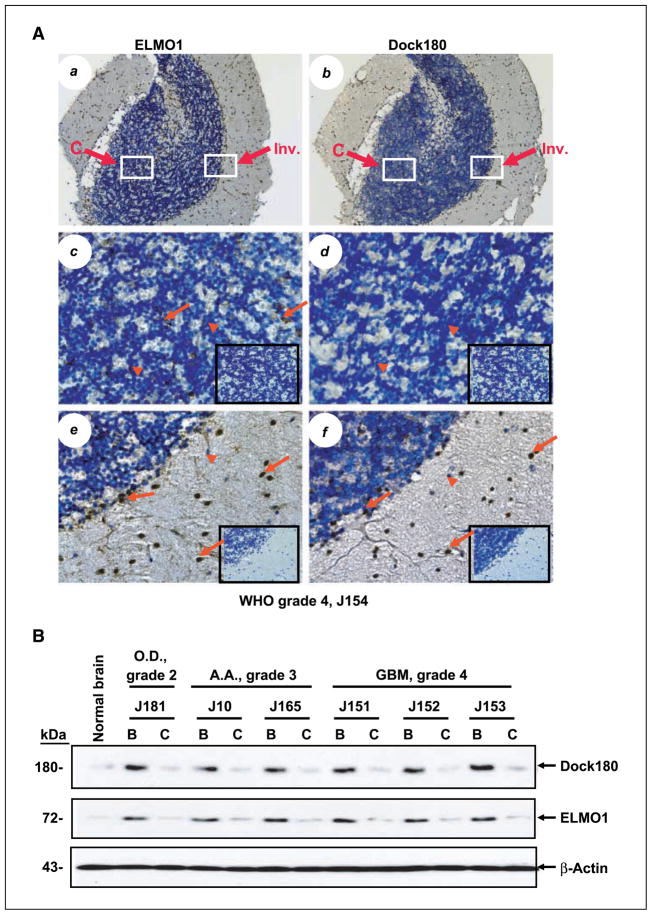
The ELMO1-Dock180 complex has been shown to stimulate cell migration through the activation of Rac1 (25). Recently, we identified the up-regulation of ELMO1 gene expression in an angiopoietin-2–induced astrocytoma invasion model using global gene array followed by real-time PCR analysis,8 signifying the potential importance of ELMO1 in promoting glioma invasion. To determine the role of ELMO1 and its binding partner Dock180 (21) in glioma cell invasion, we began by carrying out immunohistochemical analyses on a collection of primary human glioma specimens to assess whether ELMO1 and Dock180 are associated with the invasive phenotype of gliomas. We examined 53 tumors representing WHO grades 2 to 4 containing an identifiable central region and invasive areas and four normal human brain specimens (J140–J143) obtained at autopsy from patients without brain lesions as controls (28). Little to no immunoreactivity for ELMO1 and Dock180 was detected in the normal brain specimens (Supplementary Table S1; Fig. S1, a and b). Interestingly, the co-overexpression of ELMO1 and Dock180 was found in infiltrating tumor cells within the invasive areas of the glioma specimens independent of tumor grade (Fig. 1A, e and f; Supplementary Fig. S1, e, f, i, and j). Actively invading glioma cells observed at distant sites such as the gray matter of normal brain parenchyma, along blood vessels, neuronal structures, and the corpus callosum showed a high immunoreactivity for ELMO1 and Dock180 (data not shown). For example, in a glioblastoma multiforme (grade 4) specimen, the glioma cells invading into the adjacent brain structure (Fig. 1A, e and f, arrows) exhibited strong immunostaining by the ELMO1 and Dock180 antibodies. In contrast, Dock180 protein was not detected (Fig. 1A, d) and ELMO1 was expressed at low levels in the central core region of this same glioma tissue (Fig. 1A, c). Additionally, we failed to detect the expression of ELMO1 and Dock180 in reactive astrocytes or cells that are morphologically similar to astrocytes in invasive areas or center regions in these primary glioma specimens.
Figure 1.
ELMO1 and Dock180 are co-overexpressed in invading tumor cells of primary human glioma specimens. A, a total of 53 individual primary tumor specimens (WHO grades 2–4) were analyzed, and representative staining of serial sections of glioblastoma multiforme (specimen J154, grade 4) tissue using a polyclonal rabbit anti-ELMO1 antibody (a, c, and e) and a polyclonal goat anti-Dock180 antibody (b, d, and f) is shown. c to f, insets, isotype-matched immunoglobulin G controls of the identical areas shown. c and d, enlarged central regions of the tumor mass shown in a and b (white rectangle). e and f, enlarged invasive areas shown in a and b (red rectangle). Arrows, positive staining for ELMO1 (c and e) and Dock180 (f). Arrowheads, unstained cells. Original magnification, ×100 (a and b); 400× (c–f). Representative results; immunohistochemical (IHC) analyses were done two additional times with similar results. B, immunoblot analysis of the tumor center (C) and invasive border (B) of primary glioma specimens. Total protein extracted from normal brain and microdissected glioma tissue from oligodendroglioma (O.D.; specimen J181, grade 2), anaplastic astrocytoma (A.A.; specimens J10 and J165, grade 3), and glioblastoma multiforme (GBM; specimens J151, J152, and J153, grade 4) was examined by immunoblotting with anti-Dock180 and anti-ELMO1 antibodies, respectively. The membranes were also probed with an anti–β-actin antibody as a loading control. Representative of three independent experiments with similar results.
To determine whether there is a distinct link between ELMO1 and Dock180 expression and human glioma invasiveness, we did a χ2 test for trend to examine the association between the positive staining of ELMO1 and Dock180 and each area of the glioma specimen (center, border, and invasive areas). As shown in Table 1, a significant correlation was found between the positive immunoactivities for ELMO1 (P < 0.01 for border versus center and P < 0.0001 for invasive versus center, respectively) and Dock180 (P < 0.0001 for both comparisons) and the invasiveness displayed by these gliomas. Of the 53 glioma samples analyzed, 67% (8 of 12) and 92% (11 of 12) WHO grade 2 specimens, 69% (11 of 16) and 75% (12 of 16) grade 3 specimens, and 84% (21 of 25) and 96% (24 of 25) grade 4 specimens showed higher expression of ELMO1 and Dock180 in the border/invasive areas versus the center region of the tumors, respectively (Supplementary Table S1).
Table 1.
ELMO1 and Dock180 expression correlates with glioma invasion
| Immunohistochemical score | ELMO1 |
Dock180 |
||||
|---|---|---|---|---|---|---|
| Center | Border | Invasive | Center | Border | Invasive | |
| 3+ | 0 | 3 | 9 | 0 | 3 | 10 |
| 2+ | 1 | 12 | 19 | 1 | 15 | 22 |
| 1+ | 9 | 14 | 7 | 3 | 15 | 15 |
| ± | 29 | 15 | 10 | 18 | 16 | 5 |
| − | 14 | 9 | 8 | 31 | 4 | 1 |
|
P value of correlation between positivity and tumor area* | ||||||
| Border vs center | <0.01 | <0.0001 | ||||
| Invasive vs center | <0.0001 | <0.0001 | ||||
| Border vs invasive | NS | <0.05 | ||||
NOTE: The immunohistochemical staining intensity for each antibody and specimen was defined as no (−), weakest (±), low (1+), medium (2+), and strong (3+) staining as previously described (28) and is shown in Supplementary Table S1. NS, not significant (P > 0.05).
Analyzed by χ2 test for trend based on the distribution of the scores from each area.
Next, to corroborate our observation of ELMO1 and Dock180 up-regulation in invading glioma cells of the primary glioma specimens, we did immunoblotting on total protein extracted from the border/invasive regions and core area of microdissected primary glioma tissues and four normal brain specimens (28) that were used in the immunohistochemical analyses. An increase in ELMO1 and Dock180 expression was found in the border region of all six primary glioma specimens examined when compared with the center tumor area of the identical sample (Fig. 1B). Little to no expression of ELMO1 and Dock180 was detected in the normal brain specimens (Fig. 1B; Supplementary Fig. S2). These findings support our immunohistochemical data showing that ELMO1 and Dock180 are co-upregulated in the areas of active invasion of primary glioma specimens. Taken together, these data suggest that the expression of ELMO1 and Dock180 is consistent with the intrinsically invasive phenotype of gliomas and independent of tumor grade.
ELMO1 and Dock180 are coexpressed in human glioma cell lines
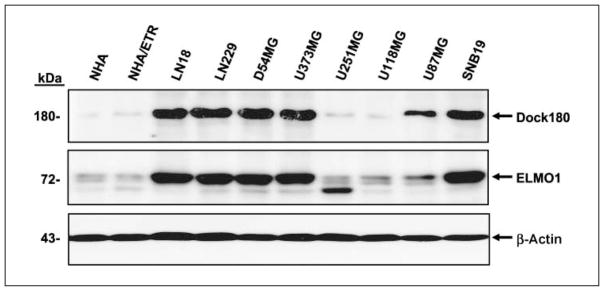
Next, we sought to determine whether ELMO1 and Dock180 play a role in glioma cell migration and invasion. We first examined the expression of ELMO1 and Dock180 in various human glioma cell lines. As shown in Fig. 2, LN18, LN229, D54MG, U373MG, and SNB19 glioma cell lines endogenously express ELMO1 and Dock180 at high levels whereas lower-level expression was found in normal human astrocytes, genetically modified normal human astrocytes (27), U251MG, U118, and U87MG glioma cell lines. In addition, the level of expression of ELMO1 correlated with the expression level of Dock180.
Figure 2.
Endogenous expression of Dock180 and ELMO1 in various human glioma cell lines. Immunoblot analysis of normal human astrocytes (NHA), genetically modified normal human astrocytes (NHA/ETR; see Materials and Methods), and human glioma cell lysates with anti-Dock180 and anti-ELMO1 antibodies. The membranes were also probed with an anti–β-actin antibody as a loading control. Representative of three independent experiments with similar results.
Inhibition of endogenously expressed ELMO1 and Dock180 suppresses Rac1 activation in glioma cells
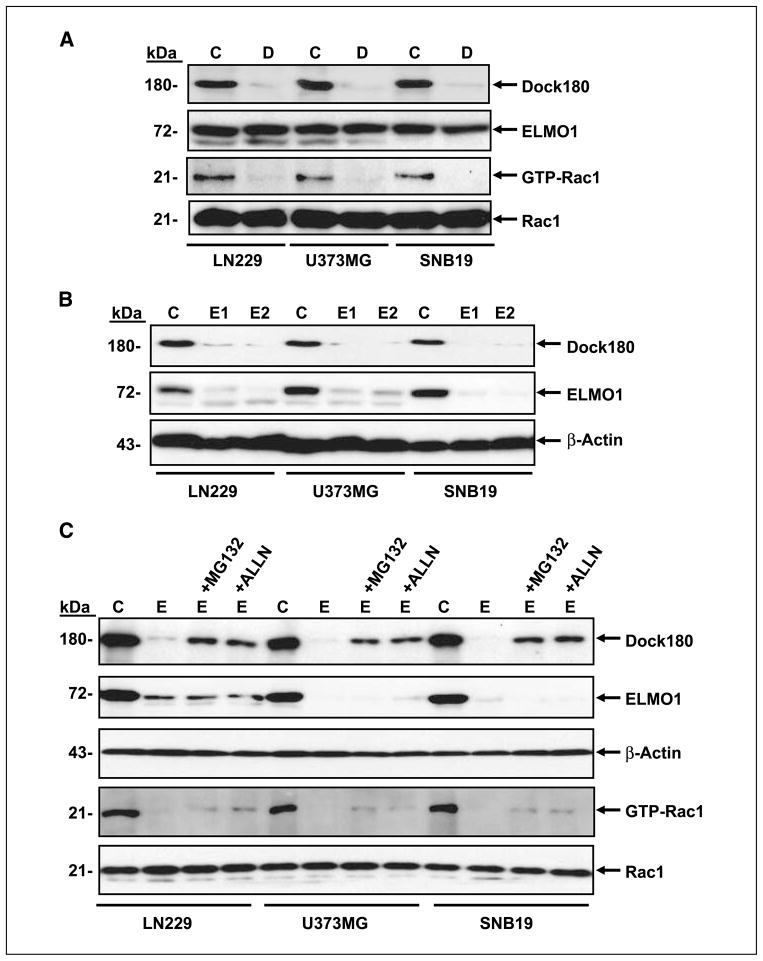
ELMO1 and Dock180 have previously been shown to form a complex and act as a bipartite GEF thereby activating Rac1 (21). Therefore, we evaluated the significance of endogenous ELMO1 and Dock180 expression in glioma cells and determined whether inhibition of their expression by siRNA attenuates Rac1 activation. LN229, U373MG, and SNB19 glioma cells were separately transfected with ELMO1 and Dock180 siRNA. After 48 h, the glioma cells that were transiently transfected with a siRNA pool containing three target-specific sequences for Dock180 completely suppressed Dock180 expression and significantly attenuated Rac1 activation while having no effect on ELMO1 and Rac1 protein levels (Fig. 3A). Similarly, two different siRNAs for ELMO1 (designated E1 and E2) inhibited ELMO1 expression (Fig. 3B), resulting in a decrease in GTP loading of Rac1 without alteration of Rac1 protein expression (Fig. 3C). Interestingly, siRNA knockdown of ELMO1 also reduced the expression of Dock180 in the glioma cells tested (Fig. 3B and C). This effect was partially blocked by the proteasome inhibitors MG132 and ALLN (Fig. 3C), corroborating a previous report showing that ELMO1 protects Dock180 from ubiquitylation-mediated degradation (29). These data suggest that ELMO1 and Dock180 function upstream of Rac1 and play an essential role in its activation in glioma cells.
Figure 3.
Suppression of endogenous ELMO1 and Dock180 inhibits GTP loading of Rac1 in glioma cells. A and B, LN229, U373MG, and SNB19 cells were transiently transfected with ELMO1 siRNA (E1 and E2), Dock180 siRNA (D), or a control siRNA (C). Total cell lysates were analyzed by immunoblotting with anti-ELMO1, anti-Dock180, and anti-Rac1 antibodies and GTP loading of Rac1 using a Rac1 activation assay kit. C, LN229, U373MG, and SNB19 cells were transiently transfected with ELMO1 siRNA and treated with the proteasome inhibitors MG132 (2 μmol/L) or ALLN (4 μmol/L) followed by immunoblotting for ELMO1, Dock180, and Rac1 expression and GTP loading of Rac1. The membranes were also probed with an anti–β-actin antibody as a loading control. Representative of three independent experiments with similar results.
Suppression of endogenously expressed ELMO1 and Dock180 expression inhibits glioma cell migration and invasion
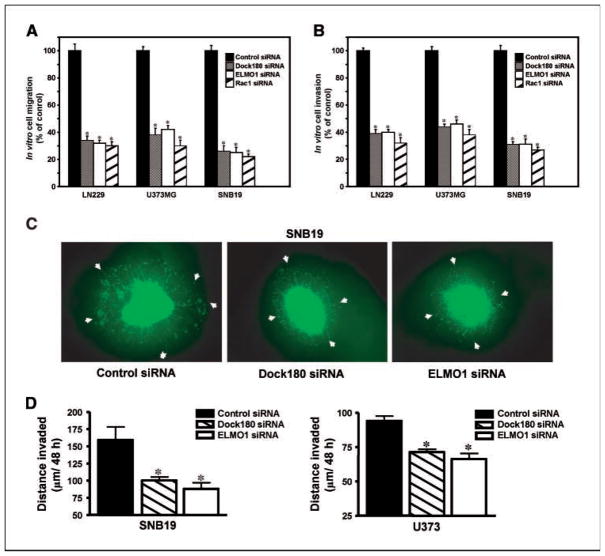
Rac1, a Rho family GTPase member, induces lamellipodia formation, cell migration, and invasion in glioma cells (6). Therefore, we hypothesize that ELMO1 and Dock180 promote glioma cell migration and invasion through their effects on Rac1 activation. To test this hypothesis, we transiently transfected LN229, U373MG, and SNB19 glioma cells with ELMO1, Dock180, and Rac1 siRNA. As shown in Fig. 4A and Supplementary Fig. S3, suppression of ELMO1 and Dock180 expression inhibited in vitro glioma cell migration by 3- to 4-fold, comparable to Rac1 knockdown by siRNA (Supplementary Fig. S3). Consistent with these results, knockdown of endogenous ELMO1 and Dock180 inhibited the ability of LN229, U373MG, and SNB19 cells to invade through a growth factor–reduced Matrigel–coated membrane. Again, in vitro glioma cell invasion was attenuated to the same degree using ELMO1 or Dock180 siRNA as Rac1 suppression (Fig. 4B). These results suggest that ELMO1 and Dock180 have an essential role in promoting glioma cell migration and invasion similar to Rac1.
Figure 4.
Suppression of endogenous ELMO1 and Dock180 inhibits glioma cell migration and invasion. A, in vitro cell migration assay. LN229, U373MG, and SNB19 cells were transiently transfected with the indicated siRNAs followed by cell migration assay. B, in vitro cell invasion assay. LN229, U373MG, and SNB19 cells were transiently transfected with the indicated siRNAs followed by an invasion assay. The migrating or invading cells were counted in 10 random high-powered fields (total magnification, −200). Mean number of migrating or invading control cells: for migration, LN229 cells, 94.2 ± 4.6/field; U373MG cells, 73.1 ± 2.8/field; SNB19, 113.3 ± 3.8/field; and for invasion, LN229 cells, 46.7 ± 1.4/field; U373MG cells, 56.2 ± 1.9/field; SNB19, 37.2 ± 2.4/field. Columns, percent of control siRNA cells; bars, SD. *, P < 0.05, one-way ANOVA followed by Newman-Keuls post hoc. Three independent experiments were done in triplicate with similar results. C, GFP-expressing SNB19 and U373MG cells (data not shown) were transiently transfected with indicated siRNAs followed by an ex vivo brain slice invasion assay. Representative epifluorescent images of the GFP-expressing SNB19 cells were captured using a digital camera attached to a stereomicroscope at ×40 magnification. D, depth of SNB19 and U373MG cell invasion into a murine brain slice. Columns, mean distance (μm) invaded in 48 h from six independent experiments done in five to seven replicates per pair (control siRNA–transfected cells versus specific siRNA–transfected cells); bars, SE. *, P < 0.05, one-way ANOVA followed by Newman-Keuls post hoc. No-transfection controls for both SNB19 and U373MG cell lines were also done showing no observable effects on cell viability or the invasive ability when comparing the control siRNA–transfected and nontransfected cells (data not shown).
To test our hypothesis in a pathophysiologically relevant model, we examined whether inhibition of ELMO1 and Dock180 modulates the invasion of SNB19 and U373MG cells in a murine brain slice model (9, 10). We separately transfected GFP-expressing SNB19 and U373MG cells with control, ELMO1, or Dock180 siRNA. After 48 h, the glioma cells that were transfected with specific or control siRNAs were placed bilaterally onto the putamen of a murine brain slice and allowed to invade into the brain tissue for an additional 48 h. Afterwards, lateral migration/invasion and depth of invasion were evaluated. Inhibition of ELMO1 and Dock180 expression in both cell lines displayed less lateral migration/invasion on the brain slice compared with control siRNA–transfected or nontransfected cells (Fig. 4C and data not shown). Analysis by confocal laser scanning microscopy revealed that ELMO1 and Dock180 suppression significantly blocked the intrinsic invasiveness of the GFP-expressing SNB19 (mean ± SE, 88 ± 8.9 and 100 ± 5.2 μm/48 h, respectively) into the brain slice as compared with control siRNA–transfected or nontransfected SNB19 cells (mean ± SE, 159 ± 19 μm/48 h; Fig. 4D and data not shown). Similarly, glioma cell invasiveness was inhibited in the ELMO1 siRNA–treated (mean ± SE, 66 ± 4.1 μm/48 h) and Dock180 siRNA–treated (mean ± SE, 71 ± 2.2 μm/48 h) U373 cells versus the cells transfected with a control siRNA or nontransfected cells (mean ± SE, 94 ± 3.5 μm/48 h; Fig. 4D and data not shown). Thus, these results indicate that ELMO1 and Dock180 significantly contribute to the inherent invasive phenotype of these glioma cells in vitro and ex vivo.
Exogenous expression of ELMO1 and Dock180 stimulates glioma cell migration and invasion
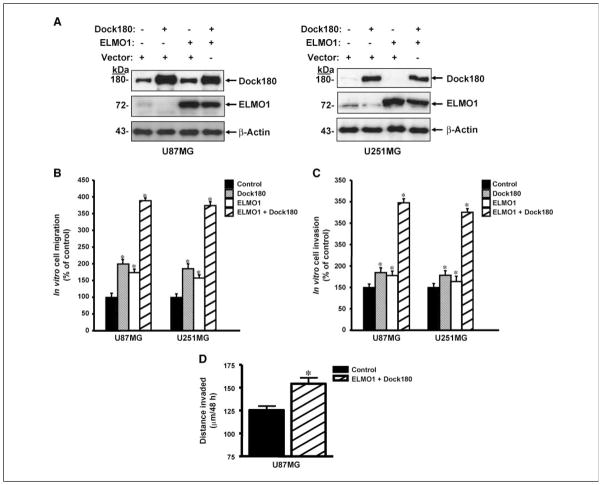
In reciprocal experiments, we examined whether exogenous expression of ELMO1 and Dock180 in glioma cells that have low level endogenous expression increases glioma cell migration and invasion. First, U87MG and U251 glioma cells were transiently transfected with empty vectors, the ELMO1 and/or the Dock 180 plasmids leading to enhanced expression of these two proteins (Fig. 5A) and an increase in activated Rac1 (data not shown). Exogenous expression of ELMO1 and/or Dock180 in U87MG and U251MG cells increased cell migration and invasion in vitro with dual expression having the greatest effect of 3- to 4-fold (Fig. 5B and C).
Figure 5.
Exogenous expression of Dock180 and ELMO1 promotes glioma cell migration and invasion. A, U87MG and U251MG cells were transiently transfected with the indicated plasmids. Total cell lysates were analyzed by immunoblotting for ELMO1 and Dock180 expression. B and C, U87MG and U251MG cells were transiently transfected with the indicated plasmids followed by cell migration assay (B) or cell invasion assay (C). D, GFP-expressing U87MG cells were transiently transfected with the indicated plasmids and analyzed by an ex vivo brain slice invasion assay. Columns, mean distance (μm) invaded in 48 h from four independent experiments done in five to seven replicates per pair (control transfected–cells versus ELMO1- and Dock180-transfected cells); bars, SE. *, P < 0.05, paired Student’s t test.
Next, we tested whether exogenous overexpression of ELMO1 and Dock180 stimulates U87MG glioma cell invasion in the murine brain tissue. U87MG control (vector-transfected) and ELMO1/Dock180–expressing cells were separately seeded onto the murine brain slice. As shown in Fig. 5D, the depth of invasion into the brain slice was significantly increased in the ELMO1/Dock180–expressing U87MG glioma cells (mean ± SE, 154 ± 6.5 μm/48 h) compared with the control transfected cells (mean ± SE, 125 ± 4.5 μm/48 h). Interestingly, the ELMO1/Dock180–expressing U87MG cells showed a similar invasion distance (mean ± SE, 154 ± 6.5 μm/48 h) as the parental SNB19 cells (mean ± SE, 159 ± 19 μm/48 h) that have high endogenous expression of ELMO1 and Dock180. Taken together, these results further confirm that ELMO1 and Dock180 are able to enhance the migration/invasion of glioma cells.
Discussion
In the present study, we provide direct functional evidence showing that ELMO1 and Dock180 play a critical role in glioma invasion. Our data suggest that preferential expression of ELMO1 and Dock180 in invading glioma cells is associated with the intrinsically invasive behavior of human gliomas. Recent studies show that localized alterations in gene and protein expression in the actively invading glioma cell population may be responsible for their more migratory and invasive phenotype compared with the glioma cells of the tumor core (5, 9, 28, 32). Our results identify a significant correlation between ELMO1 and Dock180 expression and the invasive phenotype of the gliomas, strongly corroborating this theory. Immunohistochemical analysis revealed a co-overexpression of ELMO1 and Dock180 in the infiltrating glioma cells within the border and invasive regions compared with the central core of the identical glioma specimens, independent of WHO tumor grade. We witnessed glioma cells that were strongly positive for ELMO1 and Dock180 infiltrating along blood vessels, neuronal structures, and the corpus callosum (data not shown). This display of dissemination is consistent with the unique pattern of invasion of diverse anatomic structures observed in glioma biology (2). Importantly, because all malignant gliomas have the propensity to diffusely invade normal brain areas regardless of WHO grade (33), the increased expression of ELMO1 and Dock180 in the infiltrating tumor cells may reveal an important pathologic occurrence during active glioma cell invasion.
Dock180 and ELMO1 are evolutionarily conserved proteins that are essential in multiple biological processes involving cell migration (18, 24). For example, myoblast city, the Dock180 homologue in Drosophila, is necessary for dorsal closure and cytoskeletal organization in the migrating epidermis (16). CED-5 and CED-12, the homologues of Dock180 and ELMO1, are required for phagocytosis and cell migration in C. elegans (18, 24). In normal mammalian cells, ELMO1 and Dock180 form a complex, function as an unconventional Rac1-GEF (21), and stimulate cell migration through Rac1 activation (25). Notably, these latter studies use forced overexpression of ELMO1 and Dock180 in human embryonic kidney 293T and murine fibroblast LR73 cells to examine the effects of both proteins. In this study, we extended these observations for the first time to human glioma models revealing a novel function of this unconventional Rac1-GEF in promoting cancer cell migration and invasion. We found that inhibition of endogenously expressed ELMO1 and Dock180 attenuates the invasive behavior of glioma cell lines concomitant with a reduction in activated Rac1. Conversely, exogenous expression of ELMO1 and Dock180 in low-level expressing glioma cells increased their capacity to migrate and invade in vitro and in the brain, emphasizing the importance of these molecules in the invasive process of these malignant cells.
Tumor cell invasion requires both intrinsic cellular alterations and extrinsic stimuli to trigger cell motility (34). Our study examined how endogenously or exogenously expressed ELMO1 and Dock180 affected the intrinsic aspect of glioma cell migration and invasion independent of exogenous stimuli. The modest increase in invasion of ELMO1- and Dock180-overexpressing U87MG cells into the murine brain slice suggests the need for both intrinsic and extrinsic cues for efficient glioma invasion to occur. Indeed, the lack of exogenous stimuli and the diverse genetic background of glioma cell lines that impart distinct innate characteristics may influence the varying effects of ELMO1 and Dock180 on the invasive behavior of glioma cells. Because various growth factors such as epidermal growth factor (EGF), hepatocyte growth factor/scatter factor (HGF/SF), and platelet-derived growth factor (PDGF) have been implicated in promoting glioma cell invasion (35, 36), we investigated whether these growth factors regulate ELMO1 and Dock180. EGF, HGF/SF, and PDGF did not modulate the protein expression and phosphorylation of ELMO1 and Dock180 in glioma cells. Recent studies have shown that an EGF receptor (EGFR) mutant that lacks exons 3 to 7 of its extracellular domain (EGFRvIII) and is frequently found in high grade gliomas (1) promotes glioma progression and invasion in the brain (37, 38). We have found by immunohistochemical analysis that EGFRvIII is coexpressed with ELMO1 and Dock180 in invading tumor cells within the border regions but not in the center areas of primary glioma specimens. We are currently investigating the mechanisms by which ELMO1 and Dock180 mediate EGFRvIII-promoted glioma cell invasion using in vitro and in vivo models.
Rac1 regulates spatial and temporal changes of the actin cytoskeleton by relaying signals from various stimuli such as growth factors, cytokines, and adhesion molecules to downstream effectors (3) promoting glioma cell migration (4–10). Several reports indicate that activation of Rac1 by ephrin-B3 (9), fibroblast growth factor-inducible 14 (5), neurotensin (4), and P311 (39) stimulates glioma cell motility and invasion through actin cytoskeleton reorganization. It is plausible that ELMO1/Dock180 or other Rac1-GEFs (40) are responsible for mediating these upstream signals to activate Rac1 in these glioma cell lines, and thus further investigation is warranted.
It is well established that the tumor microenvironment composed of extracellular matrix contributes to the invasive behavior of gliomas (41–43). Cell adhesion to the extracellular matrix accomplished by cell-surface receptors such as integrins is a critical first step during glioma invasion (41). Dock180 has been shown to function downstream of the αvβ5 and β1 integrins in GD25 fibroblasts and 293T cells, respectively (19, 20). Furthermore, a ternary complex consisting of RhoG-ELMO1-Dock180 mediates integrin-induced cell spreading of HeLa cells (44). Thus, integrin signaling may constitute another important pathway in facilitating ELMO1- and Dock180-mediated glioma invasion. Within the tumor microenvironment, the stromal cells serve as an ideal source of exogenous stimuli for glioma cells by producing numerous cytokines, proteases, and other extrinsic factors that affect cancer cell motility (34). Given that ELMO1 and Dock180 are co-overexpressed in actively infiltrating glioma cells, it is also possible that these extrinsic factors within the tumor milieu modulate ELMO1 and Dock180 expression through paracrine mechanisms.
In summary, this study identifies the novel function of the unconventional GEF, ELMO1 and Dock180, in promoting glioma cell migration and invasion. Co-overexpression of ELMO1 and Dock180 in actively infiltrating glioma cells illustrates a significant association between these Rac1 regulatory proteins and the invasive phenotype of diffuse gliomas. Because aberrant activation of cell motility pathways may underlie cancer cell invasion, understanding the mechanisms by which ELMO1 and Dock180 mediate glioma cell invasion could establish these proteins as potential targets for effective therapies in the treatment of these deadly tumors.
Supplementary Material
Acknowledgments
Grant support: NIH grant CA102011, American Cancer Society grant RSG CSM-107144 (S.Y. Cheng), and the Hillman Fellows Program (S-Y. Cheng and B. Hu).
We thank G. Wang for assistance in the use of laser confocal microscope, M. Nakada for help with the ex vivo brain slice invasion assay, J. Skowronski for the human ELMO1 plasmid and M. Matsuda for the human Dock180 plasmid, D. Bigner for the D54MG cells, Y-H. Zhou for the SNB19 cells, and R. Pieper for the immortalized normal human astrocytes and genetically modified normal human astrocytes.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Manuscript in preparation.
References
- 1.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–33. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–36. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 4.Servotte S, Camby I, Debeir O, et al. The in vitro influences of neurotensin on the motility characteristics of human U373 glioblastoma cells. Neuropathol Appl Neurobiol. 2006;32:575–84. doi: 10.1111/j.1365-2990.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 5.Tran NL, McDonough WS, Savitch BA, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-κB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–42. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 6.Chan AY, Coniglio SJ, Chuang YY, et al. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–9. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- 7.Salhia B, Rutten F, Nakada M, et al. Inhibition of Rhokinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005;65:8792–800. doi: 10.1158/0008-5472.CAN-05-0160. [DOI] [PubMed] [Google Scholar]
- 8.Murai T, Miyazaki Y, Nishinakamura H, et al. Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J Biol Chem. 2004;279:4541–50. doi: 10.1074/jbc.M307356200. [DOI] [PubMed] [Google Scholar]
- 9.Nakada M, Drake KL, Nakada S, Niska JA, Berens ME. Ephrin-B3 ligand promotes glioma invasion through activation of Rac1. Cancer Res. 2006;66:8492–500. doi: 10.1158/0008-5472.CAN-05-4211. [DOI] [PubMed] [Google Scholar]
- 10.Valster A, Tran NL, Nakada M, et al. Cell migration and invasion assays. Methods. 2005;37:208–15. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 13.Meller N, Merlot S, Guda C. CZH proteins: a new family of Rho-GEFs. J Cell Sci. 2005;118:4937–46. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H, Kiyokawa E, Tanaka S, et al. DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol Cell Biol. 1996;16:1770–6. doi: 10.1128/mcb.16.4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyokawa E, Hashimoto Y, Kobayashi S, et al. Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev. 1998;12:3331–6. doi: 10.1101/gad.12.21.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson MR, Galletta BJ, Abmayr SM. Drosophila myoblast city encodes a conserved protein that is essential for myoblast fusion, dorsal closure, and cytoskeletal organization. J Cell Biol. 1997;138:589–603. doi: 10.1083/jcb.138.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan KM, Barrett K, Lu Y, et al. Myoblast city, the Drosophila homolog of DOCK180/CED-5, is required in a Rac signaling pathway utilized for multiple developmental processes. Genes Dev. 1998;12:3337–42. doi: 10.1101/gad.12.21.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–4. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 19.Albert ML, Kim JI, Birge RB. αvβ5 integrin recruits the CrkII-Dock180-1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 20.Gustavsson A, Yuan M, Fallman M. Temporal dissection of β1-integrin signaling indicates a role for p130Cas-Crk in filopodia formation. J Biol Chem. 2004;279:22893–901. doi: 10.1074/jbc.M309693200. [DOI] [PubMed] [Google Scholar]
- 21.Brugnera E, Haney L, Grimsley C, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–82. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 22.Cote JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J Cell Sci. 2002;115:4901–13. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- 23.Cote JF, Vuori K. In vitro guanine nucleotide exchange activity of DHR-2/DOCKER/CZH2 domains. Methods Enzymol. 2006;406:41–57. doi: 10.1016/S0076-6879(06)06004-6. [DOI] [PubMed] [Google Scholar]
- 24.Gumienny TL, Brugnera E, Tosello-Trampont AC, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 25.Grimsley CM, Kinchen JM, Tosello-Trampont AC, et al. Dock180 and ELMO1 proteins cooperate to promote evolutionarily conserved Rac-dependent cell migration. J Biol Chem. 2004;279:6087–97. doi: 10.1074/jbc.M307087200. [DOI] [PubMed] [Google Scholar]
- 26.Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci U S A. 1997;94:12479–84. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda Y, Ozawa T, Hirose Y, et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–60. [PubMed] [Google Scholar]
- 28.Guo P, Imanishi Y, Cackowski FC, et al. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5γ2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–90. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino Y, Tsuda M, Ichihara S, et al. Elmo1 inhibits ubiquitylation of Dock180. J Cell Sci. 2006;119:923–32. doi: 10.1242/jcs.02797. [DOI] [PubMed] [Google Scholar]
- 30.Janardhan A, Swigut T, Hill B, Myers MP, Skowronski J. HIV-1 Nef binds the DOCK2-1 complex to activate rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2004;2:E6. doi: 10.1371/journal.pbio.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu B, Guo P, Fang Q, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci U S A. 2003;100:8904–9. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–28. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 33.Kleihues P, Cavenee WK International Agency for Research on Cancer. Pathology and genetics of tumours of the nervous system. Lyon: IARC Press; 2000. p. 314. [Google Scholar]
- 34.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Christofori G. New signals from the invasive front. Nature. 2006;441:444–50. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 36.Hamel W, Westphal M. Growth factors in gliomas revisited. Acta Neurochir (Wien) 2000;142:113–37. doi: 10.1007/s007010050015. discussion 37–8. [DOI] [PubMed] [Google Scholar]
- 37.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–77. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q, Clarke L, Scheidenhelm DK, et al. High-grade glioma formation results from postnatal Pten loss or mutant epidermal growth factor receptor expression in a transgenic mouse glioma model. Cancer Res. 2006;66:7429–37. doi: 10.1158/0008-5472.CAN-06-0712. [DOI] [PubMed] [Google Scholar]
- 39.McDonough WS, Tran NL, Berens ME. Regulation of glioma cell migration by serine-phosphorylated P311. Neoplasia. 2005;7:862–72. doi: 10.1593/neo.05190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 41.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–69. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Giese A, Rief M, Loo M, Berens M. Determinants of human astrocytoma migration. Cancer Res. 1994;54:3897–904. [PubMed] [Google Scholar]
- 43.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 44.Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–4. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.