Abstract
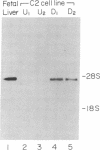
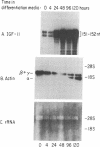
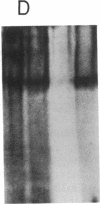
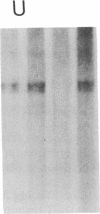
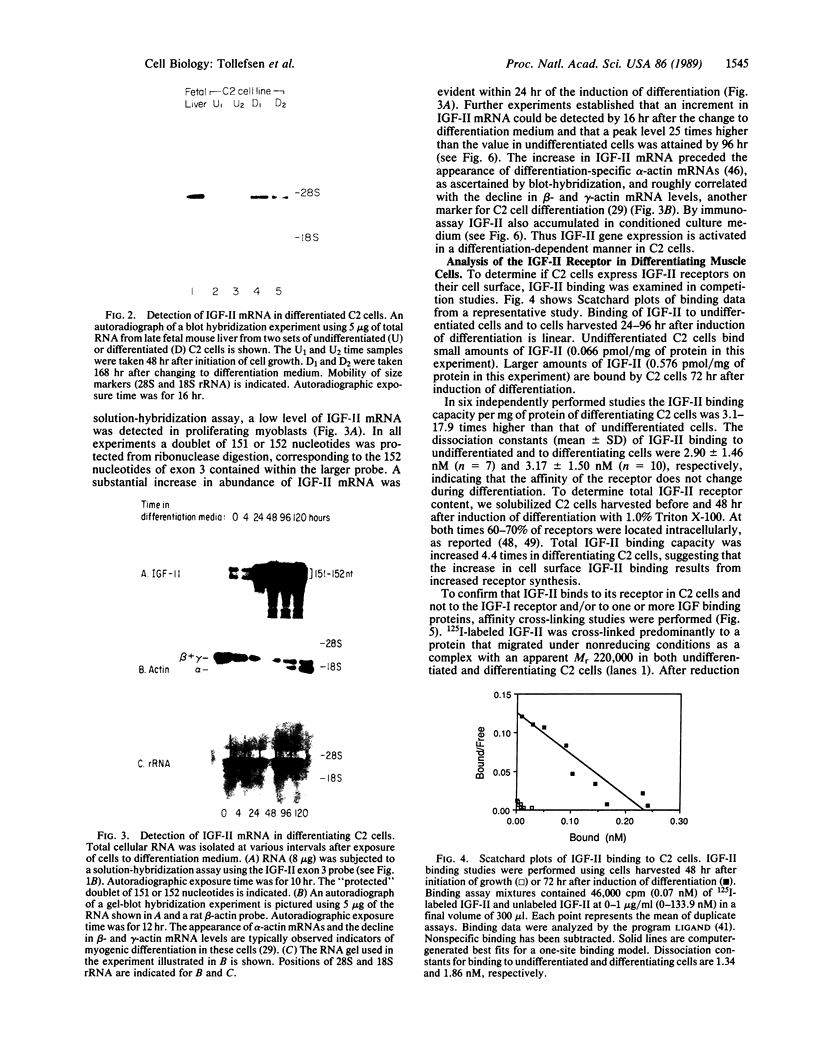
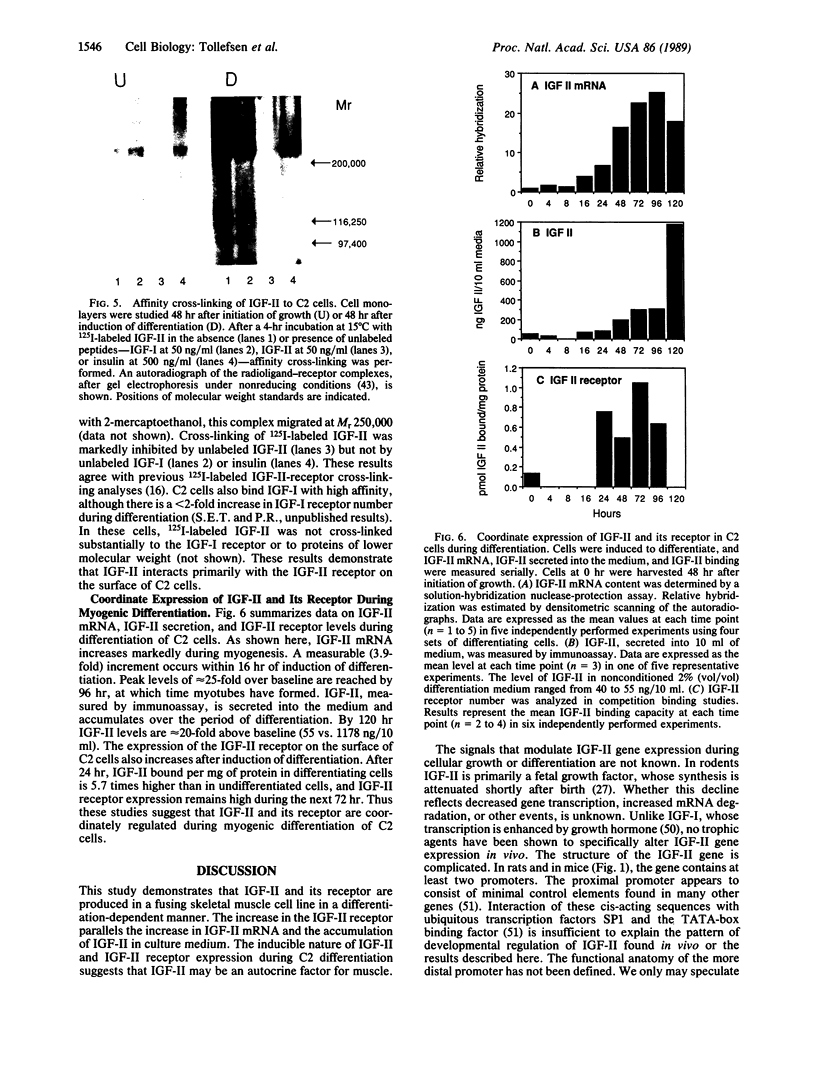
The role of polypeptide growth factors in promoting muscle differentiation is uncharacterized. We have used a fusing skeletal muscle cell line, C2, to examine the endogenous expression of one peptide, insulin-like growth factor II (IGF-II), and its receptor during differentiation. The synthesis of IGF-II is low during proliferation of myoblasts; IGF-II mRNA can be detected only through use of a highly sensitive solution-hybridization assay. Competition binding studies reveal that the IGF-II receptor is similarly nonabundant in myoblasts. During differentiation IGF-II mRNA rises rapidly. A nearly 4-fold increase is seen within 16 hr of onset of the differentiation process, and levels are 25 times higher than those in myoblasts by 96 hr, when myotubes have formed and muscle-specific alpha-actin mRNAs are synthesized. IGF-II accumulates in conditioned culture medium with similar kinetics. The expression of IGF-II receptors on the cell surface increases almost 6-fold 24 hr after the onset of differentiation and remains high. These studies suggest that IGF-II and its receptor are coordinately regulated during myogenic differentiation in C2 cells and that IGF-II may be an autocrine factor for skeletal muscle.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bains W., Ponte P., Blau H., Kedes L. Cardiac actin is the major actin gene product in skeletal muscle cell differentiation in vitro. Mol Cell Biol. 1984 Aug;4(8):1449–1453. doi: 10.1128/mcb.4.8.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., Smith R. J. Distinct biologically active receptors for insulin, insulin-like growth factor I, and insulin-like growth factor II in cultured skeletal muscle cells. J Biol Chem. 1985 Dec 15;260(29):15892–15898. [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Brown A. L., Graham D. E., Nissley S. P., Hill D. J., Strain A. J., Rechler M. M. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986 Oct 5;261(28):13144–13150. [PubMed] [Google Scholar]
- Buonanno A., Merlie J. P. Transcriptional regulation of nicotinic acetylcholine receptor genes during muscle development. J Biol Chem. 1986 Sep 5;261(25):11452–11455. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Linkhart T. A., Olwin B. B., Hauschka S. D. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987 Aug;105(2):949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday W. H. Radioligand assays for insulin-like growth factor II. Methods Enzymol. 1987;146:248–259. doi: 10.1016/s0076-6879(87)46027-8. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Evans T., DeChiara T., Efstratiadis A. A promoter of the rat insulin-like growth factor II gene consists of minimal control elements. J Mol Biol. 1988 Jan 5;199(1):61–81. doi: 10.1016/0022-2836(88)90379-8. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Falen S. L., Florini J. R. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987 Jan;120(1):115–123. doi: 10.1210/endo-120-1-115. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Ewton D. Z., Falen S. L., Van Wyk J. J. Biphasic concentration dependency of stimulation of myoblast differentiation by somatomedins. Am J Physiol. 1986 May;250(5 Pt 1):C771–C778. doi: 10.1152/ajpcell.1986.250.5.C771. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Frunzio R., Chiariotti L., Brown A. L., Graham D. E., Rechler M. M., Bruni C. B. Structure and expression of the rat insulin-like growth factor II (rIGF-II) gene. rIGF-II RNAs are transcribed from two promoters. J Biol Chem. 1986 Dec 25;261(36):17138–17149. [PubMed] [Google Scholar]
- Gossett L. A., Zhang W., Olson E. N. Dexamethasone-dependent inhibition of differentiation of C2 myoblasts bearing steroid-inducible N-ras oncogenes. J Cell Biol. 1988 Jun;106(6):2127–2137. doi: 10.1083/jcb.106.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. J., Crace C. J., Nissley S. P., Morrell D., Holder A. T., Milner R. D. Fetal rat myoblasts release both rat somatomedin-C (SM-C)/insulin-like growth factor I (IGF I) and multiplication-stimulating activity in vitro: partial characterization and biological activity of myoblast-derived SM-C/IGF I. Endocrinology. 1985 Nov;117(5):2061–2072. doi: 10.1210/endo-117-5-2061. [DOI] [PubMed] [Google Scholar]
- Jennische E., Skottner A., Hansson H. A. Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand. 1987 Jan;129(1):9–15. doi: 10.1111/j.1748-1716.1987.tb08034.x. [DOI] [PubMed] [Google Scholar]
- Kiess W., Haskell J. F., Lee L., Greenstein L. A., Miller B. E., Aarons A. L., Rechler M. M., Nissley S. P. An antibody that blocks insulin-like growth factor (IGF) binding to the type II IGF receptor is neither an agonist nor an inhibitor of IGF-stimulated biologic responses in L6 myoblasts. J Biol Chem. 1987 Sep 15;262(26):12745–12751. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobel P., Dahms N. M., Kornfeld S. Cloning and sequence analysis of the cation-independent mannose 6-phosphate receptor. J Biol Chem. 1988 Feb 15;263(5):2563–2570. [PubMed] [Google Scholar]
- MacDonald R. G., Pfeffer S. R., Coussens L., Tepper M. A., Brocklebank C. M., Mole J. E., Anderson J. K., Chen E., Czech M. P., Ullrich A. A single receptor binds both insulin-like growth factor II and mannose-6-phosphate. Science. 1988 Mar 4;239(4844):1134–1137. doi: 10.1126/science.2964083. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews L. S., Norstedt G., Palmiter R. D. Regulation of insulin-like growth factor I gene expression by growth hormone. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9343–9347. doi: 10.1073/pnas.83.24.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko A. S., Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987 Oct 9;51(1):51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Edman J. C., Standring D. N., Fried V. A., Smith M. C., Roth R. A., Rutter W. J. Insulin-like growth factor II receptor as a multifunctional binding protein. Nature. 1987 Sep 24;329(6137):301–307. doi: 10.1038/329301a0. [DOI] [PubMed] [Google Scholar]
- Mottola C., Czech M. P. The type II insulin-like growth factor receptor does not mediate increased DNA synthesis in H-35 hepatoma cells. J Biol Chem. 1984 Oct 25;259(20):12705–12713. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Oka Y., Czech M. P. The type II insulin-like growth factor receptor is internalized and recycles in the absence of ligand. J Biol Chem. 1986 Jul 15;261(20):9090–9093. [PubMed] [Google Scholar]
- Oka Y., Mottola C., Oppenheimer C. L., Czech M. P. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Roth R. A. Structure of the receptor for insulin-like growth factor II: the puzzle amplified. Science. 1988 Mar 11;239(4845):1269–1271. doi: 10.1126/science.2964085. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Webster C., Morgan D. O., Blau H. M., Roth R. A. Insulin and insulinlike growth factor receptors and responses in cultured human muscle cells. Am J Physiol. 1986 Nov;251(5 Pt 1):E611–E615. doi: 10.1152/ajpendo.1986.251.5.E611. [DOI] [PubMed] [Google Scholar]
- Silberstein L., Webster S. G., Travis M., Blau H. M. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986 Sep 26;46(7):1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Spizz G., Roman D., Strauss A., Olson E. N. Serum and fibroblast growth factor inhibit myogenic differentiation through a mechanism dependent on protein synthesis and independent of cell proliferation. J Biol Chem. 1986 Jul 15;261(20):9483–9488. [PubMed] [Google Scholar]
- Stempien M. M., Fong N. M., Rall L. B., Bell G. I. Sequence of a placental cDNA encoding the mouse insulin-like growth factor II precursor. DNA. 1986 Oct;5(5):357–361. doi: 10.1089/dna.1986.5.357. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Weinman S. A., Bradshaw R. A. Dissociation kinetics of 125I-nerve growth factor from cell surface receptors. Acceleration by unlabeled ligand and its relationship to negative cooperativity. J Biol Chem. 1981 Nov 10;256(21):11086–11092. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen S. E., Thompson K., Petersen D. J. Separation of the high affinity insulin-like growth factor I receptor from low affinity binding sites by affinity chromatography. J Biol Chem. 1987 Dec 5;262(34):16461–16469. [PubMed] [Google Scholar]
- Tong P. Y., Tollefsen S. E., Kornfeld S. The cation-independent mannose 6-phosphate receptor binds insulin-like growth factor II. J Biol Chem. 1988 Feb 25;263(6):2585–2588. [PubMed] [Google Scholar]
- Turner J. D., Rotwein P., Novakofski J., Bechtel P. J. Induction of mRNA for IGF-I and -II during growth hormone-stimulated muscle hypertrophy. Am J Physiol. 1988 Oct;255(4 Pt 1):E513–E517. doi: 10.1152/ajpendo.1988.255.4.E513. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardzala L. J., Simpson I. A., Rechler M. M., Cushman S. W. Potential mechanism of the stimulatory action of insulin on insulin-like growth factor II binding to the isolated rat adipose cell. Apparent redistribution of receptors cycling between a large intracellular pool and the plasma membrane. J Biol Chem. 1984 Jul 10;259(13):8378–8383. [PubMed] [Google Scholar]
- Webster K. A., Muscat G. E., Kedes L. Adenovirus E1A products suppress myogenic differentiation and inhibit transcription from muscle-specific promoters. Nature. 1988 Apr 7;332(6164):553–557. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. The type I insulin-like growth factor receptor mediates the rapid effects of multiplication-stimulating activity on membrane transport systems in rat soleus muscle. J Biol Chem. 1984 Mar 10;259(5):3090–3095. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]