Abstract
Purpose
To estimate the 4-year incidence and progression of lens opacities.
Design
Population-based longitudinal study.
Methods
4,658 adult Latinos from Los Angeles County, were examined at baseline and 4-year follow-up. Examination included assessment of lens opacities using the Lens Opacities Classification System II (LOCS II). Incidences of cortical, nuclear, and posterior subcapsular opacities (with LOCS II scores ≥2) were defined as opacity development in persons without that opacity at baseline. Single and mixed opacities were defined in persons without any opacity at baseline. Incidence of all lens changes included development of at least one opacity or cataract surgery among those without any opacity at baseline. 4-year progressions were defined as increase of ≥2 in LOCS II score.
Results
The 4-year incidence of all lens opacities was 14.2%. 4-year incidence of cataract surgery was 1.48%. The incidences were 4.1% for cortical-only, 5.8% for nuclear-only, 0.5% for PSC-only, and 2.5% for mixed. The incidences for any opacities were 7.5% for cortical, 10.2% for nuclear, and 2.5% for PSC. Incidence increased with age (P<0.0001 for all). The progressions were 8.5% for cortical, 3.7% for nuclear, and 2.9% for PSC opacities.
Conclusions
Our Latino population had a higher incidence of nuclear than cortical opacities, but a greater progression of cortical than nuclear opacities. Incidence and progression of PSC was low. Additional understanding of the natural history and progression of various lens opacities will give us a better understanding of how and when to screen for, monitor, and treat cataracts.
INTRODUCTION
Cataract is one of the main causes of blindness in the world1, and is the leading cause of visual impairment in the United States (U.S.).2 Lens opacification is an eye disease of aging, and it is estimated that cataract accounts for approximately half of low vision cases in adults over age 40.2 This costs Medicare approximately 3 billion dollars per year.3 Understanding the annual burden of cataract in various populations is important to estimate health service needs in the future and to inform interventions that improve delivery of cataract care.
Some population-based epidemiologic data on the incidence and progression of cataract has recently become available for various ethnic groups worldwide. For example, the Melbourne Visual Impairment Project reported population-based data consisting of Anglo-Saxon and other primarily White people in Melbourne, Australia4; the Beaver Dam Eye Study, studied a Wisconsin population, consisting primarily of Whites of northern European descent5; and the Barbados Eye Study evaluated a Caribbean population of primarily African descent6,7. Our study aims to provide such longitudinal cataract data for Latinos, the largest and fastest-growing minority group in the U.S. The U.S. Census Bureau reported that 35.6 million people or 12.5% of the nation's residents were Latino in 2004, but this proportion is expected to increase to 20.1% by the year 2030.8 Additionally, the median age of Latinos is nearly ten years less than the rest of the U.S. population9, so the burden of age-related cataract among Latinos is likely to rise in the future as the Latino population ages. Longitudinal cataract data on U.S. Latinos will improve the planning of future health services for the aging U.S. population.
The Los Angeles Latino Eye Study (LALES) is a population-based study examining ocular disease in Latinos, primarily Mexican American, ages 40 and older living in La Puente, California. Baseline findings have shown cataract to be the leading cause of low vision.10 The baseline prevalence of cortical, nuclear, and posterior subcapsular lens opacities in the LALES population has been previously reported, with cortical opacities being the most prevalent11. The objective of this report is to describe the 4-year incidence and progression of lens opacity in our population-based cohort of Los Angeles Latinos.
METHODS
Study Population
LALES consists of self-identified Latinos 40 years of age and older living in 6 census tracts in the city of La Puente, Los Angeles County, California. The majority of participants were Mexican American. The Los Angeles County, University of Southern California Medical Center Institutional Review Board Ethics Committee approved this study, and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written, informed consent was obtained from all participants. Study design, sampling plan, and baseline data details have been previously reported.12 Baseline examinations were performed between 2000 and 2003, and 4-year follow-up examination were performed between 2004-2008. At baseline, 6357 of 7789 eligible participants (82%) completed an in-home questionnaire and a clinical ophthalmic examination.
Interview and Examination Procedures
All eligible participants who completed the baseline LALES examination were invited to participate in a second home interview and a clinical examination. Similar questionnaire and examination procedures were used for both baseline and follow-up studies. Trained ophthalmologists and technicians performed a comprehensive ocular examination using standardized protocols.
Presenting visual acuity (PVA) and best corrected visual acuity (BCVA) were documented using the Early Treatment of Diabetic Retinopathy Study (ETDRS) protocol. The PVA, which was scored as the total number of lines read correctly, was documented for each eye with participants’ existing refractive correction at 4 meters, and a retroilluminated modified ETDRS distance chart was used. The modified ETDRS near-vision acuity chart was used for near vision measurements, which were based on participants’ present reading prescription.
Lens Opacities Classification System II (LOCS II) Grading
Slit lamp examination following dilation with tropicamide and phenylephrine 2.5% was used for lens assessment. The Lens Opacities Classification System II (LOCS II), which is based on photographic standards, was used to categorize opacities into 5 posterior subcapsular (P0, PI, PII, PIII, PIV), 5 nuclear (N0, NI, NII, NIII, NIV), and 7 cortical (C0, Ctr, CI, CII, CIII, CIV, CV) gradings of increasing severity.13 Phakic status (phakic, pseudophakic, or aphakic) was recorded for each eye. In cases where lens assessment was not possible, reasons for not grading certain regions in one or both eyes were documented.
The reproducibility of LOCS II grading was evaluated between 2 examiners on a regular basis throughout the study. Two examiners performed replicate grading on 50 participants independently every 5 to 6 months. Their LOCS II gradings were compared and measured for agreement using proportionally weighted kappa (ĸ) statistics. Results showed moderate to good inter-grader agreement for all opacity types (cortical opacities, weighted ĸ [95% confidence interval (CI)] = 0.67 [0.56-0.79]; nuclear opacities, weighted ĸ = 0.76 [0.66-0.87]; and PSC opacities, weighted ĸ = 0.66 [0.24-1.0].
Definitions of Lens Opacity
Posterior subcapsular (PSC) lens opacity was defined by LOCS II grading of PII, PIII, or PIV; nuclear lens opacity was defined by LOCS II grading of NII, NIII, or NIV; and cortical lens opacity was defined by LOCS II grading of CII, CIII, CIV, or CV.
Definitions of Incidence and Progression
In our study we present two measurements of 4-year incidence for various lens opacity types.
In the first measurement, we assessed the incidence of the development of any cortical, any nuclear, or any PSC opacity in either or both eyes among participants without that opacity type in either eye at baseline. This measured the risk of each specific opacity type and did not take into account whether other opacity types were present at baseline or follow-up in the same person.
In the second measurement, we assessed the incidence of single opacity types (when only one type developed) and mixed opacities (if more than one type developed) among participants without any type of opacities in either eye at baseline. This gave insight into the new development of certain single-type versus mixed opacities, and consisted of 4 mutually exclusive groups: the development of cortical-only, nuclear-only, PSC-only, or mixed opacities. Incidence of the 4 mutually exclusive outcomes was based on person (not eye). For example, if a person developed one type of opacity in one eye and another opacity in the other eye, the person was considered to have developed “mixed opacities”. Only participants with gradable LOCS II scores for all three types were considered.
The incidence of all lens changes was defined by participants with the development of at least one type of opacity or cataract surgery, among those without opacities at baseline. This allowed us to understand the total risk of all new opacities in our population.
The 4-year progression of each opacity type was defined by an increase of ≥ 2 in LOCS II score in at least one eye among people with that opacity type in the same eye at baseline. Participants that could not have progressed by this criteria (≥C4, ≥N3, or ≥P3 in both eyes) were excluded.
Data and Statistical Analysis
All clinical and grading data were entered into a central database with internal automated quality control checks. The Statistical Analysis System (version 9.1, SAS Institute Inc, Cary, NC) was used for tabulations and statistical analyses, conducted at the 0.05 significance level. Age at baseline was presented and categorized into 4 groups (40-49 years, 50-59 years, 60-69 years, and 70 or more years) for all analyses. Chi-square tests were used to detect differences by opacity types and gender. Tests for trend were used to detect any linear trends in age.
RESULTS
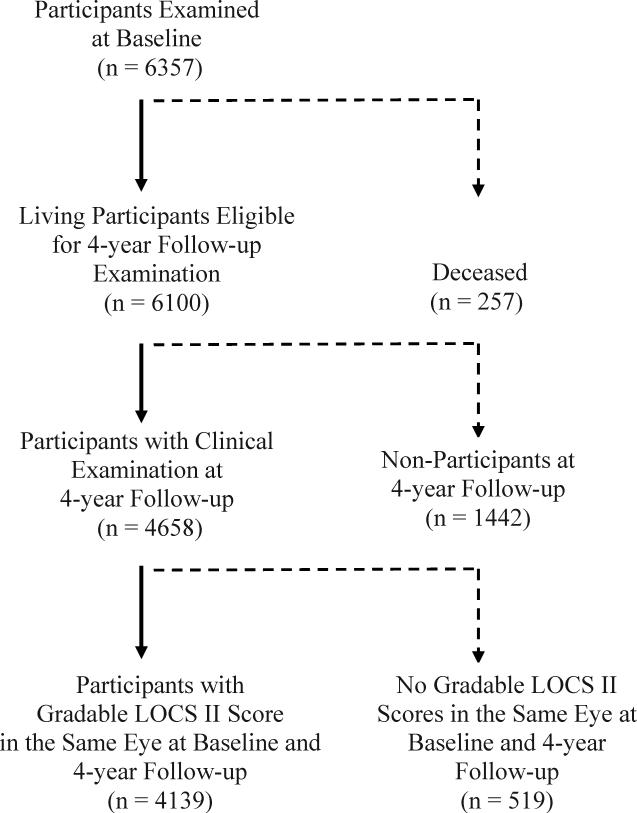
Of the 6357 participants examined at baseline, 6100 living participants were eligible for the 4-year follow-up study; and 4658 (76%) completed a clinical examination at the 4-year follow-up. Mean follow-up period was 4.2 ± 0.5 years. Mean age of participants was 54.7 years, 60% were females, 76% were born outside of the U.S., and 88% had Mexican American ancestry. Nonparticipants were more likely to be: males (44% versus 40%; P=0.003), not married (33% versus 29%; P<0.001), and without health insurance coverage (46% versus 33%; P<0.001) in comparison to participants at follow-up.
Of the 4658 participants who completed the clinical examination at follow-up, 4139 (89%) had gradable LOCS II scores in the same eye(s) at baseline and at 4-year follow-up examination (Figure 1). Nuclear gradings were available for 4139 (89%) participants in at least one eye, and cortical and PSC gradings were available on 3814 (82%) participants in at least one eye. LOCS II grading could not be performed on 519 (11%) participants due to refusal, poor fixation, or poor dilation.
Figure 1.
Participation Flowchart for Assessing 4-Year Incidence and Progression of Lens Opacity in the Los Angeles Latino Eye Study.
The changes in LOCS II scores over 4 years for each lens opacity type are shown in Table 1. There were no changes in LOCS II scores for 63.2% of cortical, 53.3% of nuclear, and 91.8% of PSC gradings. One-step increases were the most frequent changes in score for cortical, nuclear, and PSC lens opacities. One-step decreases in LOCS II scores were much less common, with 2-step decreases being extremely rare.
Table 1.
Four-year Changes in LOCS II* Scores by Type of Opacity in the Los Angeles Latino Eye Study
| Change in LOCS II Scores | Cortical, % (n=3814) | Nuclear, % (n=4139) | PSC†, % (n=3814) |
|---|---|---|---|
| -2 | 1.8 | 0.2 | 0.2 |
| -1 | 9.0 | 16.2 | 1.3 |
| 0 | 63.2 | 53.3 | 91.8 |
| 1 | 15.5 | 27.9 | 4.3 |
| ≥2 | 10.5 | 2.4 | 2.3 |
Lens Opacities Classification System II
PSC=posterior subcapsular.
Cohort for each opacity type included individuals with gradable opacity type at baseline and follow-up.
3578 (77%) participants were free of cataract at baseline and were thus considered at-risk of developing incident lens opacities. Table 2 shows the estimated 4-year incidence of any cortical, any nuclear, and any PSC lens opacity, stratified by baseline age, regardless of new incidence of other lens opacity types. While there was a clear trend of increasing incidence for all lens opacity types with age (P<0.0001 for each), gender was not significantly associated with incidence of any lens opacity type (P=0.31 for any lens opacity; P=0.72 for any cortical; P=0.82 for any nuclear; P=0.80 for any PSC; data not shown in table 2). The total incidences for all participants (ages 40 and over) were 7.5% for any cortical opacities, 10.2% for any nuclear opacities, and 2.5% for any PSC opacities.
Table 2.
Four-year Incidence of Any Lens Opacity Stratified by Age at Baseline in the Los Angeles Latino Eye Study
| Four-year Incidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at baseline (years) |
Any Cortical Opacities |
Any Nuclear Opacities |
Any PSC* Opacities |
||||||
| N | n | % (CI) | N | n | % (CI) | N | N | % (CI) | |
| 40-49 | 1454 | 35 | 2.4 (1.6, 3.2) | 1626 | 16 | 1.0 (0.5, 1.5) | 1480 | 13 | 0.9 (0.4, 1.4) |
| 50-59 | 1145 | 75 | 6.6 (5.1, 8.0) | 1321 | 86 | 6.5 (5.2, 7.9) | 1232 | 17 | 1.4 (0.7, 2.0) |
| 60-69 | 553 | 95 | 17.2 (14.0, 20.3) | 701 | 186 | 26.5 (23.2, 29.8) | 711 | 35 | 4.9 (3.3, 6.5) |
| 70+ | 170 | 44 | 25.9 (19.3, 32.5) | 194 | 104 | 53.6 (46.6, 60.6) | 271 | 28 | 10.3 (6.7, 14.0) |
| P < 0.001 | P < 0.001 | P < 0.001 | |||||||
| Overall | 3322 | 249 | 7.5 (6.6, 8.4) | 3842 | 392 | 10.2 (9.2, 11.2) | 3694 | 93 | 2.5 (2.0, 3.0) |
N=number at risk at baseline, n=number of incident cases, CI=95% confidence interval, P=test of trend, PSC=posterior subcapsular.
No incident cases.
Note: Incidences presented were for each opacity type. Participants with more than one type of opacity may be included in more than one category.
Table 3 shows the estimated 4-year incidence of single type lens opacities (cortical only, nuclear only, PSC only), mixed type lens opacities, and all lens opacities, stratified by baseline age. Age was significantly associated with the incidence of cortical-only opacities (P<0.001), nuclear-only opacities (P<0.001), mixed type opacities (P<0.001), and all lens opacities (P<0.001), but not with PSC-only opacities (P=0.25). Gender was significantly associated with PSC-only opacities (P=0.005), with females having a higher incidence than males (0.73% vs. 0.07%). However, gender was not associated with the other opacity types (P=0.90 for cortical-only, P=0.90 for nuclear-only, P=0.95 for mixed-type opacities; gender data not shown in table 3). The total incidences of each single type opacity were: 4.1% for cortical-only opacities,5.8% for nuclear-only opacities, and 0.5% for PSC-only opacities. The total incidence of mixed type opacities was 2.5%. For the four-year incidence of all lens opacities, persons with a history of cataract surgery were included and considered in the group having lens opacities. The 4-year incidence of all lens opacities was 14.2% overall, and increased from 3.4% among ages 40-49 and to 63.5% for ages 70 and over.
Table 3.
Four-year Incidence of Single and Mixed Type Lens Opacities and Incidence of All Lens Opacities Stratified by age at baseline in the Los Angeles Latino Eye Study
| Four-year Incidence |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at baseline (years) | Cortical Only Opacities |
Nuclear Only Opacities |
PSC* Only Opacities |
Mixed Type Opacities |
All Lens Changes† |
|||||||
| |
N‡ |
n¥ |
% (CIo) |
n |
% (CI) |
n |
% (CI) |
n |
% (CI) |
N |
n |
% (CI) |
| 40-49 | 1595 | 29 | 1.8 (1.2, 2.5) | 9 | 0.6 (0.2, 0.9) | 6 | 0.4 (0.1, 0.7) | 7 | 0.4 (0.1, 0.8) | 1626 | 55 | 3.4 (2.5,4.3) |
| 50-59 | 1219 | 55 | 4.5 (3.4, 5.7) | 52 | 4.3 (3.1, 5.4) | 5 | 0.4 (0.1,0.8) | 18 | 1.5 (0.8, 2.2) | 1286 | 153 | 11.9 (10.1,13.7) |
| 60-69 | 540 | 45 | 8.3 (6.0, 10.7) | 100 | 18.5 (15.2, 21.8) | 4 | 0.7 (0.0, 1.5) | 43 | 8.0 (5.7, 10.3) | 608 | 219 | 36.0 (32.2,39.8) |
| 70+ | 117 | 12 | 10.3 (4.8, 15.8) | 39 | 33.3 (24.8, 41.9) | 1 | 0.9 (0.0, 2.5) | 20 | 17.1 (10.3, 23.9) | 148 | 94 | 63.5 (55.8,71.2) |
| P<0.001 | P<0.001 | P=0.25 | P<0.001 | P<0.001 | ||||||||
| Overall | 3471 | 141 | 4.1 (3.4, 4.7) | 200 | 5.8 (5.0, 6.5) | 16 | 0.5 (0.2, 0.7) | 88 | 2.5 (2.0, 3.1) | 3668 | 521 | 14.2 (13.1,15.3) |
PSC=Posterior Subcapsular
Incidence of all lens changes was defined by those with cortical only, nuclear only, PSC only, mixed -type, or cataract surgery (n=76) among those with no lens opacity in either eye at baseline. Mixed refers to cases where more than one type of opacity was present.
N=number at risk at baseline
n=number of incident cases
CI=95% confidence interval
Table 4 shows the estimated 4-year progression of any lens opacities, stratified by baseline age. The total progression of any cortical opacities was 8.5% (38/447), with age not being significantly associated (P=0.33). The total progression of any nuclear opacities was 3.7% (9/242), with age also not being significantly associated (P=0.95). The total progression of any PSC opacities was 2.9% (2/69), which occurred only in the 60-69 age group. Gender was not significantly associated with progression of any opacity types (data not shown).
Table 4.
Four-Year Progression of Any type of Lens Opacity Stratified by age at baseline in the Los Angeles Latino Eye Study.
| Four-year Progression | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at baseline (years) |
Any Cortical Opacities |
Any Nuclear Opacities |
Any PSC* Opacities |
||||||
| N† | n‡ | % (CI¥) | N | n | % (CI) | N | n | % (CI) | |
| 40-49 | 32 | 3 | 9.4 (0.0, 19.5) | 8 | * | * | 3 | * | * |
| 50-59 | 100 | 13 | 13.0 (6.4, 19.6) | 21 | 1 | 4.8 (0.0, 13.9) | 10 | * | * |
| 60-69 | 185 | 11 | 5.9 (2.5, 9.4) | 92 | 4 | 4.4 (0.2, 8.5) | 29 | 2 | 6.9 (0.0, 16.1) |
| 70+ | 130 | 11 | 8.5 (3.7, 13.3) | 121 | 4 | 3.3 (0.1, 6.5) | 27 | * | * |
| P-value (trend test) | 0.33 | 0.95 | 0.78 | ||||||
| Overall | 447 | 38 | 8.5 (5.9, 11.1) | 242 | 9 | 3.7 (1.3, 6.1) | 69 | 2 | 2.9 (0.0, 6.9) |
PSC=posterior subcapsular
N=number at risk at baseline
n=number of progression cases
CI=confidence interval
No progression cases.
Note: Progression defined as ≥ 2-step increase in score in at least one eye for a specific type of lens opacity, and exclude persons with scores ≥ C4 (for cortical opacity), ≥ N3 (for nuclear opacity), or ≥ P3 (for PSC opacity) at baseline.
Table 5 suggests that visual acuity loss between baseline and follow-up occurred more frequently in incident cases than cataract-fee participants. VA loss was greatest for any PSC opacities, where 26% of incident cases had visual acuity worse than 20/40 at 4-year follow-up; 11% of persons with any-nuclear incident cases, and 7% with any-cortical incident cases had visual acuity 20/40 or worse at follow-up. Additionally, this table reveals that visual acuity worse than 20/40 occurred most frequently for those with any PSC opacities, compared to those with any cortical and any nuclear opacities.
Table 5.
Proportion of cases with a specific type of lens opacity and a visual acuity that is worse than 20/40 at 4-year follow-up in Participants of the Los Angeles Latino Eye Study
| Follow-up VA worse than 20/40 |
|||
|---|---|---|---|
| Opacity Type | Incident cases** (%) | Cataract Free participants | p-value† |
| Any cortical | 6.7 | 1.4 | 0.003 |
| Any nuclear | 10.9 | 1.0 | <0.001 |
| Any PSC* | 26.0 | 1.9 | <0.001 |
Note: Only included subjects with VA 20/40 or better at baseline
Posterior subcapsular
age,adjusted
Incident Cases: persons who developed new opacity type at the 4-year follow-up.
Cataract Free participants: persons with no opacities
The estimated annual incidence and progression of lens opacities stratified by type and baseline age for LALES participants, as compared to BISED participants, are presented in Tables 6 and 7, respectively. The age-specific annual incidence and progression rates of any cortical opacity are lower in LALES participants as compared to Afro-Caribbeans (BISED participants). The age-specific annual incidence of any nuclear opacity is slightly higher in Latinos compared to Afro-Caribbeans, while the age-specific annual progression of any nuclear opacity is comparable in Latinos and Afro-Caribbeans. For any PSC opacity, age-specific annual incidence is similar in Latinos and Afro-Caribbeans. However, few cases with evidence of progression of PSC in our study precluded a robust comparison of these rates between Latinos and Afro-Caribbeans.
DISCUSSION
The Los Angeles Latino Eye Study is a large population-based survey of eye disease, and it provides comprehensive data on the natural history of lens opacities in a U.S. Latino population. This study demonstrates the significant incidence and progression of lens opacities in our study population, and explores the natural history of various lens opacities in Latinos.
At baseline assessment, 19.5% of LALES participants had presence of 1 or more lens opacities, with cortical opacity being the most prevalent (13.5%) followed by nuclear opacity (9.0%) and PSC opacity (3.2%).11 At follow-up assessment, 26.6% of LALES participants had presence of 1 or more lens opacities, with 18.1% having any cortical opacity, 16.3% having any nuclear lens opacity , and 4.6% having any PSC lens opacity.
In the 4-year study period, the trend for greater risk of cortical and nuclear opacities remained: 1 in 13 developed a new cortical opacity, 1 in 10 developed a new nuclear opacity, while only 1 in 40 developed a new PSC opacity. Furthermore, the effect of age on incidence of any cortical, nuclear, or PSC opacity was strong. For example, in the 70 and over age group, 1 in 4 developed a new cortical opacity, 1 in 2 developed a new nuclear opacity, and 1 in 10 developed a new PSC opacity.
While the lowest risk appeared to exist for developing a PSC opacity compared to the other two opacity types, a drop in visual acuity most often occurred in those who had developed any PSC opacities, compared to cortical or nuclear opacities (table 5). Visual acuity dropped to worse than 20/40 in 26% of persons who developed any PSC opacities in the study period, as compared to 7% of incident cases for any cortical and 11% of incident cases for any nuclear. Thus, while PSC opacities occur less frequently, their presence may more often be visually significant than a cortical or nuclear opacity. In analyzing the incidence of single versus mixed-type lens opacities (table 4), it was observed that more new cases of lens opacities arose as single types (mostly cortical and nuclear) as opposed to mixed-type opacities. Thus, the natural history of lens opacification is such that a lens opacity will more often arise as a single-type. In the cases of both cortical-only and nuclear-only opacities, the frequency of new cases rose as age increased. However, a higher proportion of new cortical-only opacity cases were in the younger age groups, as compared to nuclear-only opacity cases. The observations that a lens opacity arise most often as a single-type, and that new cortical-only cases occur more often in the younger age groups (compared to nuclear-only) have also been made in the Barbados Eye Study.6
In comparing the age-specific incidences of any cortical opacities (table 2) and cortical-only opacities (table 3), incidence is similar in ages 40-49 (2.4% vs. 1.8%, respectively) and in ages 50-59 (6.6% vs. 4.5%, respectively), but is much higher in any cortical opacities than cortical-only opacities in ages 60-69 (17.2% vs. 8.3%, respectively) and in ages 70 and older (25.9% vs. 10.3%, respectively). This suggests that in younger age groups, lens opacity changes are predominated by cortical changes of the lens, but in older age groups, there are more often coexisting lens changes taking place. A similar but less profound trend is evident when comparing age-specific incidence of any nuclear opacities and nuclear-only opacities. This data suggests that these younger individuals have a lower risk for mixed type opacities, as compared to cortical-only and nuclear-only, but this risk increases with age.
It was interesting that the overall incidences of nuclear opacities were higher than that for cortical opacities (tables 2-3), respectively, yet, as reported above, the overall prevalence of cortical opacities was higher in our LALES population. These findings suggest that, in our population, nuclear opacities may arise more rapidly and perhaps at a later age, whereas cortical opacities may initially arise more subtly at an earlier age. It is also noted that when comparing the progressions of nuclear to cortical opacities (table 4), progression occurs at a faster rate for the cortical opacities. This suggests that while the initial phase of cortical opacity development may be slow and insidious, its later stages of development may occur at a greater rate than nuclear opacities. It is important to note that these observations are dependent on our system of grading, the LOCS II and assume that each grade of cortical classification is congruent to each grade of nuclear classification in LOCS II.
While our data shows that lens opacities are more likely to arise initially as a single-type, especially as cortical type opacity, our data also suggest that the development of various lens opacity types becomes more likely when a person has other existing opacity types. It has been previously reported that the presence of any opacity type can increase the incidence of other opacity types6,14,15,16, so our findings are in agreement with previous studies. This inter-relationship could be attributed to common health and lifestyle risk factors (diabetes, hypertension, smoking), common genetic predispositions, or other unknown lens physiology characteristics caused by the concomitant presence of other lens opacities. However, it is important to note that our analyses, which look at opacities in the whole person, cannot directly comment on the effect of coexisting lens opacities on new opacity development within the same eye.
In order to understand the magnitude of incidence and progression of lens opacities in Latinos, it is useful to compare the estimated annual incidence of various lens opacities among LALES participants to other available population-based data. Our LALES (supplemental table 6) data is most easily compared with the Barbados Incidence Study of Eye Diseases (BISED), a population-based survey of Afro-Caribbeans in Barbados6, because both studies grade lens opacities using the LOCS II grading system. Direct comparisons between our LALES study and BISED revealed a higher age-specific annual incidence of nuclear but lower age-specific annual incidence and progression of cortical opacities in Latinos compared to Afro-Caribbeans (supplemental table 7). When comparing our LALES findings to the Beaver Dam Eye Study and the Melbourne Visual Impairment Project, both of which comprise primarily White populations, it is noted that age-specific annual incidence is 2-3 times higher for cortical opacities in LALES compared to the other two studies.4,5 For nuclear and posterior subcapsular opacities, the incidence for LALES is similar to Melbourne but higher than the Beaver Dam study.4,5 Comparisons to these two studies must be made with caution because they used different methods of lens opacity gradings from LALES. One reason for inter-study differences between incidence rates may be related to differing risk factor burdens between the populations. For example, myopia is well linked to nuclear opacities and may be more prevalent in our LALES population compared to BISED and Beaver Dam participants. Other reasons for differing rates could include differing genetic predispositions of those with Latino versus African or other Caucasian descents, or differing rates of cataract surgery in the various populations.
Strengths and Limitations
One of the greatest strengths of the Los Angeles Latino Eye Study is that it consists of a large, population-based cohort in the real world. This study is particularly strong because it follows study participants over time, and is thus able to contribute longitudinal analyses to our understanding of lens opacity development. Additional strengths include: high participation rate, use of a well-established lens opacity grading system (Lens Opacity Classification System II), and demonstration of high inter-grader reproducibility using this grading system.
One limitation of the current study is that our observations about the longitudinal development of lens opacities are dependent on our system of grading. LOCS II uses photographic definitions for each grade of opacity, and each increasing grade for one opacity type may not be congruent to an increasing grade for another opacity type. A second limitation is that there were a small number of participants who developed posterior subcapsular lens opacities; thus, conclusions drawn about PSC opacities should be taken with caution. A third limitation is a possible bias created by the slight tendency for participants to be female, married, and insured.
Overall, this study revealed that: (1) Our population had a higher incidence of nuclear than cortical opacities, but a greater prevalence and progression of cortical than nuclear opacities; (2) Cortical opacities were more likely than other opacity types to occur in the younger age groups; and (3) While the incidence and progression of PSC opacities was relatively low, the greatest visual acuity loss occurred in those who had developed this type of opacity. Additional efforts to elucidate the natural history and progression of various lens opacities, as well as their inter-relationships, will give us a better understanding of how and when to screen for, monitor, and treat cataract.
Supplementary Material
ACKNOWLEDGEMENTS
A. Support: National Institutes of Health Grants: NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com.
D. Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
E. Other Acknowledgements: The Los Angeles Latino Eye Study Group, University of Southern California, Los Angeles, CA.-Rohit Varma, MD, MPH; Sylvia H. Paz, MS; Fernando Pena, MD; Stanley P. Azen, PhD; Jaime Barrera; Lupe Cisneros, COA; Elizabeth Corona; Carolina Cuestas, OD; Jeanne Dzekov, BS; Ana Evans, OD; Denise R. Globe, PhD; Carlos Lastra, MD; Mei-Ying Lai, MS; George Martinez; Ronald E. Smith, MD; LaVina Tetrow, Mina Torres, MS; Natalia Uribe, OD; Joanne Wu, MPH; Myrna Zuniga.
Battelle Survey Research Center, St. Louis, MO, Lisa John, PhD; Candace Kwong MS; Karen Tucker, MA; Natasha L. Walker; Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI Ronald Klein, MD, MPH.
B. Financial Disclosure: The authors have no proprietary or commercial interests.
REFERENCES
- 1.Pascolini D, Mariotti SP, Pokharel GP, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11:67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Salm M, Belsky D, Sloan FA. Trends in cost of major eye diseases to Medicare, 1991 to 2000. Am J Ophthalmol. 2006;142:976–82. doi: 10.1016/j.ajo.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 4.McCarty CA, Mukesh BN, Dimitrov PN, Taylor HR. Incidence and progression of cataract in the Melbourne Visual Impairment Project. Am J Ophthalmol. 2003;136:10–7. doi: 10.1016/s0002-9394(02)01844-5. [DOI] [PubMed] [Google Scholar]
- 5.Klein BE, Klein R, Lee KE. Incidence of age-related cataract: the Beaver Dam Eye Study. Arch Ophthalmol. 1998;116:219–25. doi: 10.1001/archopht.116.2.219. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC, Wu SY, Nemesure B, Li X, Hennis A, Connell AM. Incidence and progression of lens opacities in the Barbados Eye Studies. Ophthalmology. 2000;107:1267–73. doi: 10.1016/s0161-6420(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Wu SY, Nemesure B, Yang L, Hennis A. Nine-year incidence of lens opacities in the Barbados Eye Studies. Ophthalmology. 2004;111:483–90. doi: 10.1016/j.ophtha.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin. U.S. Census Bureau, Population Division, Population Projections Branch. 2004.
- 9. [June 11, 2009];U.S. Census Bureau News: Hispanic and Asian Americans Increasing Faster than Overall Population. Available at: http://www.census.gov/ Press-Release/www/releases/archives/race /001839.html.
- 10.Cotter SA, Varma R, Ying-Lai M, Azen SP, Klein R, Los Angeles Latino Eye Study Group Causes of low vision and blindness in adult Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2006;11:1574–82. doi: 10.1016/j.ophtha.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Varma R, Torres M, Los Angeles Latino Eye Study Group Prevalence of lens opacities in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1449–56. doi: 10.1016/j.ophtha.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Varma R, Paz SH, Azen SP, et al. Los Angeles Latino Eye Study Group The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–31. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Chylack LT, Leske MC, McCarthy D, et al. Lens Opacities Classification System II (LOCS II). Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 14.Incidence and progression of cortical, nuclear, and posterior subcapsular cataracts. The Italian-American Cataract Study Group. Am J Ophthalmol. 1994;118:623–31. doi: 10.1016/s0002-9394(14)76577-8. [DOI] [PubMed] [Google Scholar]
- 15.Leske MC, Chylack LT, He Q, Wu SY, Schoenfeld E, Friend J, Wolf J. Incidence and progression of cortical and posterior subcapsular opacities: The Longitudinal Study of Cataract. Ophthalmology. 1997;104:1987–93. doi: 10.1016/s0161-6420(97)30043-8. [DOI] [PubMed] [Google Scholar]
- 16.Leske MC, Chylack LT, Wu SY, Schoenfeld E, He Q, Friend J, Wolfe J. Incidence and progression of nuclear opacities in the Longitudinal Study of Cataract. Ophthalmology. 1996;103:705–12. doi: 10.1016/s0161-6420(96)30625-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.