Abstract
It has been postulated that fetal vascular abnormalities in aryl hydrocarbon receptor null (ahr−/−) mice may alter cardiovascular homeostasis in adulthood. We tested the hypothesis that blood pressure regulation in adult heterozygous mice (ahr+/−) would be normal, compared to ahr−/− mice, since no vascular abnormalities have been reported in the heterozygote animals. Mean arterial blood pressure (MAP) was measured using radiotelemetry prior to and during treatment with inhibitors of the autonomic nervous system, nitric oxide synthase (NOS), angiotensin converting enzyme (ACE), or endothelin-1 A receptor (ETA). Also, indices of renin-angiotensin system (RAS) activation were measured. ahr+/− and ahr−/− mice were normotensive and hypotensive, respectively, compared to wild-type (ahr+/+) littermates. Responses of all genotypes to autonomic nervous system inhibition were normal. ahr+/− mice responded normally to NOS inhibition, while the responses of ahr−/− mice were significantly blunted. In contrast, ahr+/− mice were significantly more responsive to inhibition of ACE, an ETA antagonist, or both, while ahr−/− mice were significantly less response to ACE inhibition and more response to an ETA antagonist. ahr+/− mice also exhibited significant increases in plasma renin and ACE activity, plasma sodium, and urine osmolality, indicative of RAS activation. Thus, normotension in ahr+/− mice appears to be maintained by increased RAS and ET-1 signaling, while hypotension in ahr−/− mice may result from decreased RAS signaling. In conclusion, despite the lack of overt fetal vascular abnormalities in ahr+/− mice, the loss of a single ahr allele has a significant effect on blood pressure regulation.
1. Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-activated, basic helix-loop-helix/Per-ARNT-Sim transcription factor best known for mediating the toxicity of environmental pollutants, typified by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). More recently, studies of the ahr null mouse (ahr−/−) have established that the AHR has physiological functions in the absence of exogenous ligands and many of these functions relate to the vasculature. ahr−/− mice exhibit a significant reduction in liver weight, which results from the persistence of the fetal ductus venosus after birth [1]. Conditional deletion of ahr in a cell type-dependent manner demonstrates that AHR expression in endothelial/hematopoietic cells, but not hepatocytes, is necessary for normal postnatal closure of this fetal vascular structure [2]. The loss of ahr also has been shown to enhance new blood vessel development following skeletal muscle ischemia [3].
Given the effects of AHR deficiency on angiogenesis and vascular remodeling, we and others have studied blood pressure regulation in adult ahr−/− mice. ahr−/− mice exhibit increased plasma levels of two potent vasoconstrictors, angiotensin II (Ang II) and endothelin-1 (ET-1), and are hypertensive at modest altitude (1600 m), but hypotensive at sea level [4–6]. The hypertension at modest altitude is associated with hypoxemia and is normalized by exposure to sea level oxygen conditions [5]. Interestingly, after maintaining a colony of ahr−/− mice for multiple generations at modest altitude, our current studies reveal that the ahr−/− mice have adapted and now exhibit hypotension similar to that reported for ahr−/− mice at sea level. Nonetheless, since ahr−/− mice exhibit vascular defects during fetal development [1], it has been suggested that these developmental vascular changes might contribute to the disruption of cardiovascular homeostasis in adulthood [7].
Numerous studies have suggested that the intrauterine fetal environment can contribute to programming of blood pressure in adulthood, although most studies demonstrate hypertension in the adult offspring. Nonetheless, fetal programming of hypotension in adult offspring has been reported, including following prenatal dexamethasone exposure, placental insufficiency, and a shift from a protein to carbohydrate diet [8, 9]. To further evaluate the functional role of AHR in blood pressure regulation in adulthood and the potential contribution of abnormal fetal vascular development, we have conducted studies with mice lacking a single ahr allele, i.e. ahr heterozygous mice (ahr+/−). In contrast to ahr−/− mice, the liver size of ahr+/− mice is normal and the ductus venosus closes properly [10–13]. In fact, no developmental phenotypic changes have been ascribed to the loss of a single ahr allele, suggesting that one copy of ahr is sufficient for normal development, including the vasculature.
In these studies we test the hypothesis that blood pressure regulation would be normal in ahr+/− mice in which fetal vascular abnormalities have not been reported, compared to ahr−/− mice in which numerous fetal vascular abnormalities have been described.
2. Methods
2.1 ET-1 receptor A (ETA) antagonist, PD155080
The orally active ETA antagonist, PD155080, was synthesized as described previously [14]. The sodium salt of PD155080 was dissolved in water, added to transgenic dough (Bio-Serv, Frenchtown, NJ) along with 0.1% bromophenol blue, and the mixture was folded together until the dye was evenly distributed throughout the dough. Pregelatinized corn starch was added to absorb excess water and reduce stickiness. The dough was then formed into 100 mg tablets using a pill mold (Gallipot, St. Paul, MN) coated with 5% magnesium stearate. Pills were stored at 4°C and analyzed for PD155080 concentration at 1, 7, 14, and 28 d after preparation. Individual pills were crushed, extracted with 60% ammonium acetate, 40% acetonitrile, and analyzed by high-pressure liquid chromatography, using 60% ammonium acetate, 40% acetonitrile mobile phase [14]. Control pills were made in the same manner without addition of PD155080. All chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO).
2.2 Mouse model
ahr−/− mice were obtained from Dr. Frank Gonzales (National Cancer Institute, MD) [11] and have been maintained in our laboratory backcrossed to C57B1/6N for 11 generations since 2002. Sibling ahr+/− mice were bred to generate male ahr+/+, ahr+/−, and ahr−/− littermates of the same genetic background (C57Bl/6; 12–16 weeks old). All animal protocols were approved by the University of New Mexico Animal Care and Use Committee and the investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.3 Assessment of functional AHR levels
Male mice from all three genotypes were treated with control or 180 ng/kg TCDD orally (n=3/genotype/treatment) for two consecutive days and cardiac expression of cytochrome P4501A1 (CYP1A1) mRNA was analyzed by real time PCR 72 hr later. Total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with the supplied random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500 nM CYP1A1 sense (5’ CAAAGAGCACTACAGGACA 3’) and antisense primers (5’ TTGGCATTCTCGTCCAGC 3’) (Sigma-Genosys) and 250 pg cDNA/µl. Cycle threshold data for both the target gene and reference gene, RNA polymerase II (POL2; sense primer: 5’ TGACTCACAAACTGGCTGACATT 3’; antisense: 5’ TACATCTTCTGCTATGACATGG 3), were used to calculate mean normalized expression as previously described [15, 16].
2.4 Plasma electrolytes and PD155080 concentration
To determine plasma electrolyte concentrations from all genotypes, heparinized whole blood was collected by cardiac puncture and plasma electrolytes analyzed with an i-STAT (Abbott Point of Care Inc, Princeton, NJ). In addition, the plasma concentration of PD155080 was measured from ahr+/+ mice were given 25 mg/kg PD155080 in pills at 12 hr intervals. Mice were euthanized at 6, 54, or 102 hrs after the first dose, representing 6 hrs after the last dose, and heparinized plasma was collected and stored at −20°C. Plasma was extracted with 1% trifluoracetic acid and applied to a reverse phase C-18 sep column (Phoenix Pharmaceuticals, Burlingame, CA). The column eluate was dried, reconstituted in mobile phase, and analyzed for PD155080 by HPLC. Extraction efficiency was determined by spiking plasma from untreated mice with a known quantity of drug. A mean plasma concentration of 9.6±0.8 µg/ml was achieved that did vary among the three time points of analysis and is within the therapeutic effective range reported previously [17].
2.5 In vivo analysis of blood pressure and heart rate
Arterial blood pressure and heart rate were measured using radiotelemetry (Data Sciences International, St. Paul, MN) as described [5], using PA-C10 telemeters. Mice were allowed to recover for 7 days prior to data collection. Basal blood pressure, including systolic, diastolic, mean and pulse arterial blood pressure, and heart rate were collected for 7 days before drug treatments began. Blood pressure and heart rate were recorded for 10 s every 15 min during baseline measurements and chronic drug treatment, or for 10 s every 1 min for 30 min after prazosin or hexamethonium injection.
2.6 Drug treatments
Prazosin (1 µg/g, ip) or hexamethonium (30 µg/g, ip) was injected into conscious animals to assess acute responses in blood pressure and heart rate [18], while Nω-nitro-L-arginine (LNNA) was administered in the drinking water (250 or 500 mg/L) to assess chronic changes in blood pressure [19]. To determine the effects of Ang II and ET-1 on blood pressure, mice were treated with 4 mg/kg captopril (angiotensin converting enzyme inhibitor, ACEi) in the drinking water for 3 d [15], followed by captopril in the drinking water plus 25 mg/kg PD155080 (ETA antagonist) twice per day in dough pills for 3 d [17], followed by PD155080 alone for 3 d, and finally 3 d of no treatment. In all experiments blood pressure and heart rate were monitored prior to, during and after drug therapy. All drugs, except for PD155080, were purchased from Sigma-Aldrich.
2.7 Urine collection and analysis
Male ahr+/+ and ahr−/− mice were placed into metabolic mouse cages, one animal per cage, with access to food and water. Mice were acclimated to the cage for 24 hrs and urine generated during this period was discarded. Then, 24-hr urine samples were collected twice in the subsequent 48 hrs and pooled. Cages were cleaned and dried between two collections, and urine samples were stored at −80 °C until analyzed. Urine samples were analyzed for NE by an enzyme immunoassay (Alpco Diagnostics) and for osmolality using a Vapro™ Vapor Pressure Osmometer, model 5520 (Wescor, Inc Biomedical Division, Logan, UT).
2.8 Analysis of plasma renin and ACE activity
Plasma renin activity (PRA) was determined using a commercial kit (GammaCoat® Plasma Renin Activity 125I Kit; DiaSorin, Stillwater, MN) [20]. The PRA assay is a two-step process, where first angiotensin I is generated and second angiotensin I is detected by a radioimmunoassay. PRA is expressed as ng/ml/hr of generated angiotensin I. Plasma ACE activity was determined using a commercial kit (Alpco Diagnostics, Salem, NH). Plasma samples were incubated with a synthetic ACE substrate, 3H-hippuryl-glycyl-glycine, and the product, 3H- hippuric acid, was extracted and measured in a beta counter. ACE activity was expressed as Units/Liter. One unit of ACE activity was defined as the amount of enzyme required to release 1 µmol of hippuric acid per minute per liter of plasma at 37° C.
2.9 Ex vivo analysis of aortic reactivity
The thoracic aorta was removed, placed in ice-cold physiological saline (PSS) containing 130 nM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15 mM NaHCO3, 5.5 mM glucose, 26 µM CaNa2EDTA, 1.8 mM CaCl2, pH 7.4, and cleaned of connective tissue and adventitial fat. The vessel was cut into 3 mm segments and individual rings were suspended in an organ bath containing PSS at 37°C bubbled with 21% O2, 6% CO2, balanced N2. The rings were attached to a force transducer (Grass Technologies, West Warwick, RI) with steel hangers and resting tension was increased step wise to 1.5 g over 1 hr. After initial contraction with 10 µM phenylephrine (PE) followed by relaxation with 10 µM acetylcholine (ACh), dose-response curves to KCl (2.5–80 mM), PE (0.001–10 µM), or PE following a 30 min preincubation with 100 µM LNNA were performed. All chemicals were purchased from Sigma-Aldrich.
2.10 Aortic eNOS protein analysis
Thoracic aortas were homogenized in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA), the homogenate frozen at −80 °C, thawed, sonicated and centrifuged at 15,000 × g 4 °C for 10 min. Protein concentration in the supernatant was measured using Bio-Rad protein assay (Bio-Rad Laboratories). A 30 µg aliquot of protein was analyzed on a 7.5% Tris-HCL polyacrylamide gel for total eNOS, using mouse anti-eNOS antibody (BD Biosciences, San Jose, CA), and β-actin (Santa Cruz Biotechnology) as a normalization control.
2.11 Statistical analyses
Differences among genotypes were analyzed by one-way analysis of variance with post hoc Holm-Sidak comparisons. The dose-dependent changes in vasocontraction and treatment-related changes in blood pressure among genotypes were analyzed by repeated measures, two-way analysis of variance with post hoc Holm-Sidak comparisons. A p < 0.05 was considered statistically significant in all cases.
3. Results
3.1 Functional AHR levels as measured by TCDD-inducible CYP1A1 mRNA expression
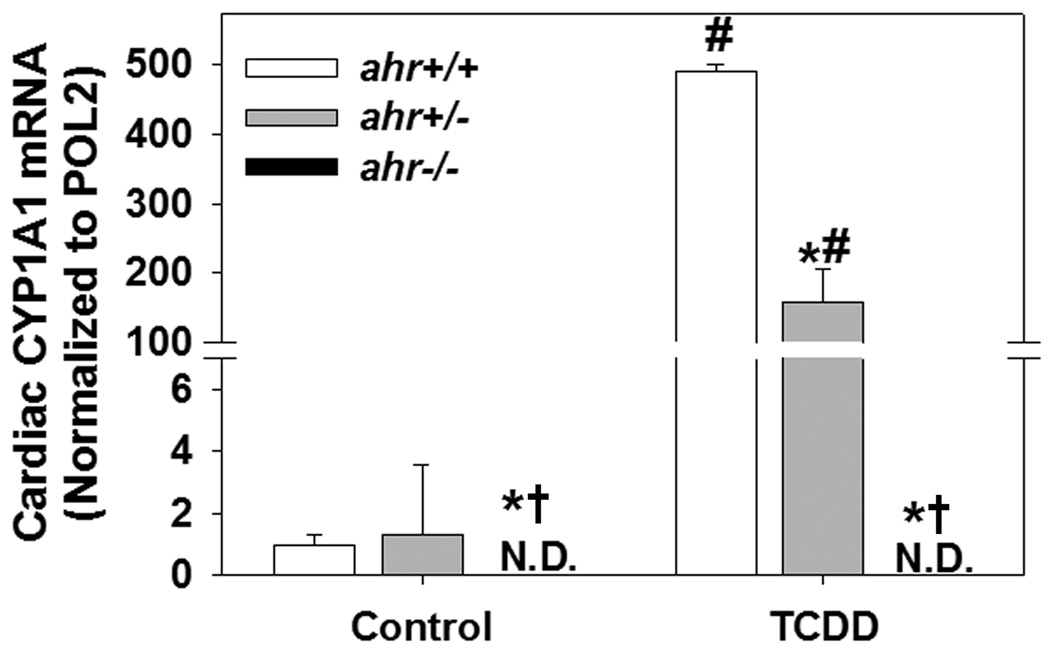
Since inducible expression of CYP1A1 mRNA is controlled by AHR, we used this as a sensitive, quantitative assessment of functional AHR levels. Neither constitutive nor TCDD-inducible cardiac CYP1A1 mRNA expression was detectable in ahr−/− mice (Fig. 1). While the constitutive expression of cardiac CYP1A1 mRNA was very low and not significantly different between ahr+/− and ahr+/+ mice, the TCDD-inducible cardiac CYP1A1 mRNA expression was significantly reduced in ahr+/− mice (3× fold lower), demonstrating that loss of a single ahr allele resulted in reduced levels of functional AHR.
Fig. 1.
Loss of a single ahr allele results in reduced levels of functional AHR as assessed by TCDD induction of CYP1A1 mRNA. Cardiac CYP1A1 mRNA expression was measured by real time PCR from ahr+/+, ahr+/− and ahr−/− male mice 72 hrs after exposure to control or a total of 320 ng TCDD/kg (n=3/genotype). Data represent mean ± SEM and were analyzed by two-way ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to ahr+/+ in the same treatment; †p < 0.05 compared to ahr+/− in the same treatment; #p < 0.05 compared to control-treated mice of the same genotype.
3.2 Basal blood pressure and organ weights
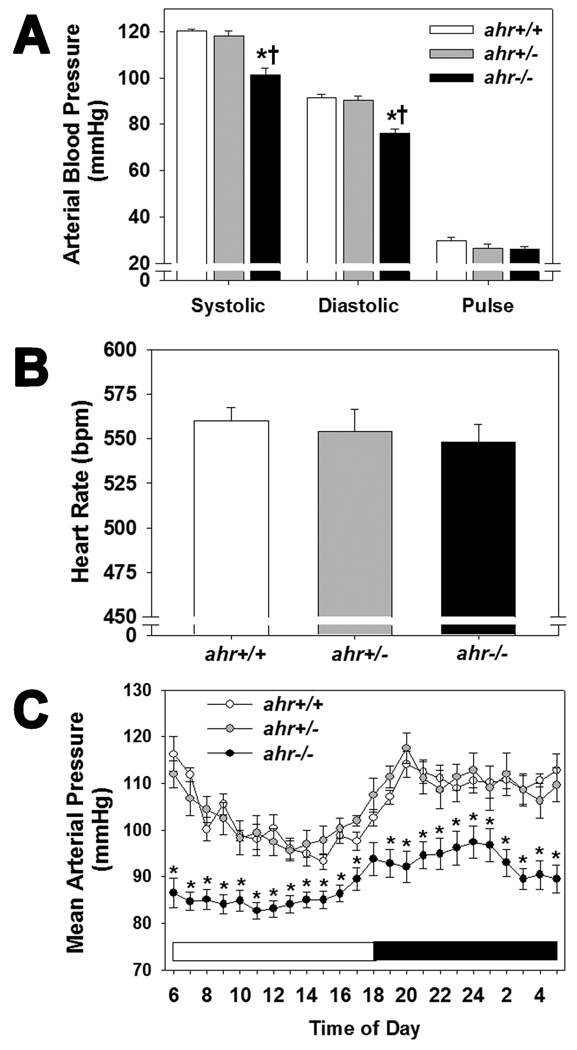
Blood pressure of ahr+/+, ahr+/−, and ahr−/− littermates was measured by radiotelemetry. While ahr+/− mice exhibited normal systolic, diastolic, and pulse pressures, ahr−/− exhibited significantly lower systolic and diastolic blood pressures (Fig. 2A). ahr+/− and ahr−/− mice exhibited a normal heart rate and 24 hour circadian pattern of mean arterial pressure (MAP), although MAP of ahr−/− mice remained significantly lower throughout the 24 hour period (Fig. 2B and C). Lastly, ahr+/− mice exhibited normal heart and kidney weights with a significant increase in body weight, while ahr−/− mice exhibited significant increases in heart and kidney weights as previously reported (Table 1) [15, 21].
Fig. 2.
Loss of a single ahr allele does not affect systolic or diastolic blood pressure or heart rate, while loss of both alleles results in significantly decreased systolic and diastolic blood pressure. (A) Systolic, diastolic and pulse arterial blood pressure, (B) heart rate, and (C) hourly mean arterial pressure over a 24 hr period of male ahr+/+, ahr+/− and ahr−/− mice as measured by radiotelemetry (n=8–10/genotype). Data represent mean ± SEM and were analyzed by one-way ANOVA, *p < 0.05, compared to ahr+/+; †p < 0.05 compared to ahr+/− (A and B), or by repeated measures two-way ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to both ahr+/+ and ahr+/−.
Table 1.
Body and organ weights of 4 month old male ahr+/+, ahr+/− and ahr−/− mice.
| Weight (Organ/body weight) |
ahr+/+ (n=16) | ahr+/− (n=19) | ahr−/− (n=10) |
|---|---|---|---|
| Body (g) | 31.1 ± 0.8 | 35.7 ± 1.2*# | 30.2 ± 0.8 |
| Heart (mg) | 129 ± 3 (0.417 ± 0.010) |
137 ± 4 (0.388 ± 0.012) |
162 ± 6*† (0.535 ± 0.014)*† |
| Kidney (mg) | 379 ± 10 (0.604 ± 0.014) |
409 ± 17 (0.576 ± 0.024) |
433 ± 9* (0.719 ± 0.019)*† |
Values are expressed as mean ± SEM
p < 0.05 versus ahr+/+
p < 0.05 versus ahr+/−
p < 0.05 versus ahr−/−
3.3 Aortic reactivity to phenylephrine
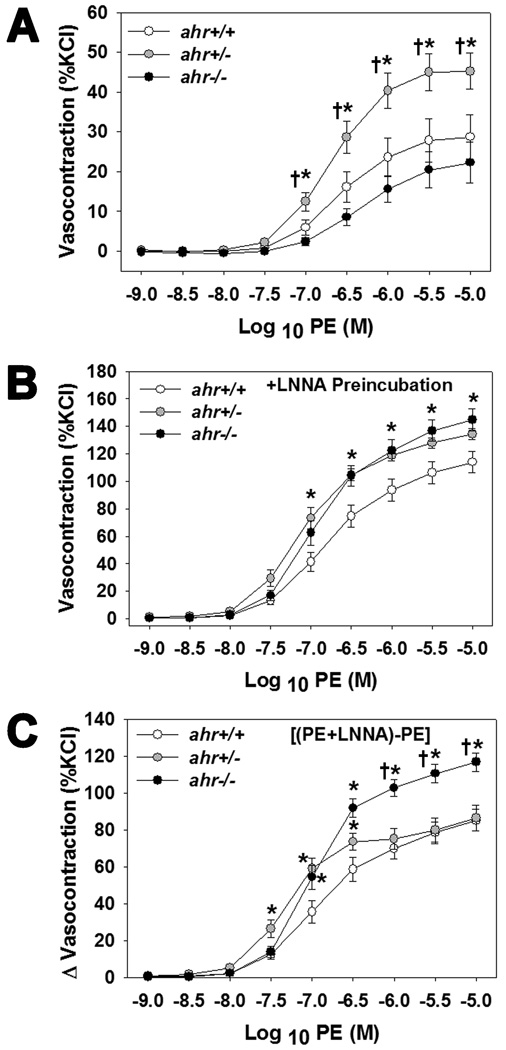
To determine if vascular reactivity to alpha adrenergic receptor agonists in the presence or absence of vascular nitric oxide (NO) was altered among the three genotypes, we assessed aortic vasocontraction responses to PE ± the NOS inhibitor, LNNA. Aortas from ahr+/− mice exhibited an enhanced dose-dependent vasocontraction to PE, compared to both ahr+/+ and ahr−/− mice (Fig. 3A), suggesting an enhanced response to adrenergic stimuli. However, when NO production was blocked by LNNA both ahr+/− and ahr−/− mice exhibited an enhanced dose-dependent vasocontraction to PE (Fig. 3B). Furthermore, the degree of vasocontraction that was blocked by basal NO (vasocontraction of PE+LNNA minus PE alone) was significantly greater in aortas from ahr−/− mice and from ahr+/− mice in the middle of the dose-response curve (Fig. 3C), suggesting an increase in basal NO.
Fig. 3.
Loss of one or both ahr alleles increases the aortic sensitivity to PE-mediated contraction in the presence of the NOS inhibitor, LNNA. (A) PE-induced contraction (% KCl), (B) PE-induced contraction in the presence of LNNA, and (C) difference (Δ) in PE-induced contraction in the presence and absence of LNNA of endothelium-intact thoracic aortic rings from male ahr+/+, ahr+/−, and ahr−/− mice (n=6–9/genotype). Data represent mean ± SEM and were analyzed by two-way repeated measures ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05, compared to ahr+/+ and †p < 0.05 compared to ahr−/− (A) or compared to ahr+/− (C).
3.4 Sympathetic nervous system activity
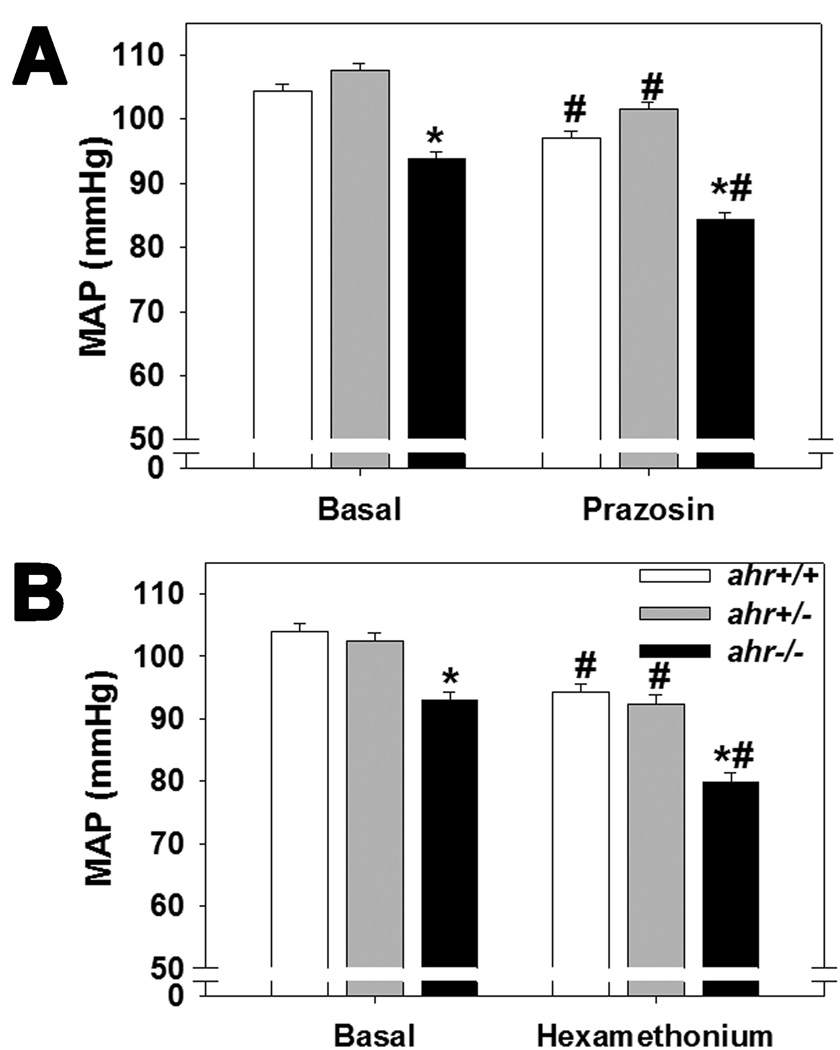
Since the aortic vasoreactivity results suggested that ahr+/− and ahr−/− mice may exhibit an increase in alpha adrenergic agonist sensitivity, we analyzed urinary NE as an indirect index of sympathetic nervous system activity and MAP following acute exposure to prazosin, an alpha1 adrenoceptor antagonist, or to hexamethonium, a ganglionic blocker. We found that urinary NE was not different among genotypes (34.6 ± 8.0 ng/ml/g BW, ahr+/+; 36.3 ± 5.6 ng/ml/g, ahr+/−; 43.7 ± 6.8 ng/ml/g, ahr−/−, n=5–7/genotype; p>0.6) and there were no significant differences in the responsiveness among the genotypes to prazosin- or hexamethonium-induced decreases in MAP (Fig. 4A and B).
Fig. 4.
Loss of one or both ahr alleles does not alter the blood pressure responses to acute exposure to an alpha adrenergic receptor blocker, prazosin, or a ganglionic blocker, hexamethonium. MAP prior to (basal) and during 30 min immediately after ip injection of (A) 1.0 µg/g prazosin or (B) 30.0 µg/g hexamethonium of male ahr+/+, ahr+/−, and ahr−/− mice (n=6–7/genotype). Data represent mean ± SEM and were analyzed by repeated measures two-way ANOVA, using post hoc Holm-Sidak comparisons, *p < 0.05 compared to ahr+/+ and †p < 0.05 compared to ahr+/− within the same treatment group; #p < 0.05 compared to untreated, basal MAP of the same genotype.
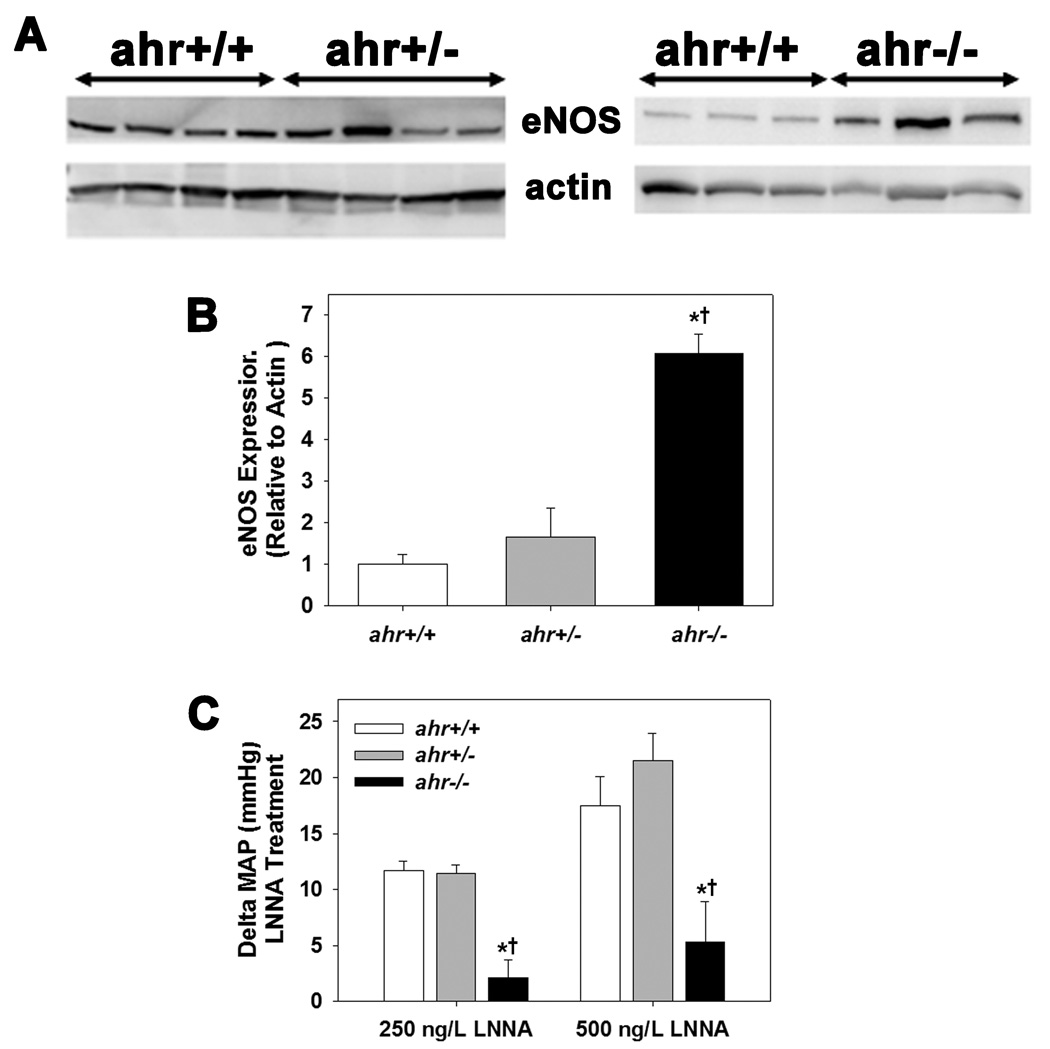
3.5 NOS expression and activity
Since the aortic vasoreactivity studies also suggested that ahr+/− and ahr−/− mice may exhibit an increase in basal aortic NO, we measured aortic eNOS expression and blood pressure responses to chronic LNNA treatment in vivo. Aortic expression of total eNOS was significantly increased in ahr−/− mice but not changed in ahr+/− mice, compared to ahr+/+ mice (Fig. 5A and B). We also found that MAP of ahr+/+ and ahr+/− mice increased to an equivalent degree following treatment with LNNA, while the MAP of ahr−/− mice increased, but to a significantly lower degree than either ahr+/+ or ahr+/− mice (Fig. 5C).
Fig. 5.
Loss of both ahr alleles significantly increases eNOS expression, but blunts the MAP response to NOS inhibition by LNNA in vivo, while loss of a single ahr allele has no effect on these two parameters. (A) Representative western blot of total eNOS protein expression in the aorta, (B) quantification of total aortic eNOS protein expression relative to β-actin, and (C) MAP 5 d after treatment with 250 and 500 mg/L LNNA in the drinking water of male ahr+/+, ahr+/−, and ahr−/− mice (n=5/genotype for all experiments). Data represent mean ± SEM and were analyzed by one-way ANOVA, using post hoc Holm-Sidak comparisons, *p < 0.05, compared to ahr+/+ and †p < 0.05 compared to ahr+/−.
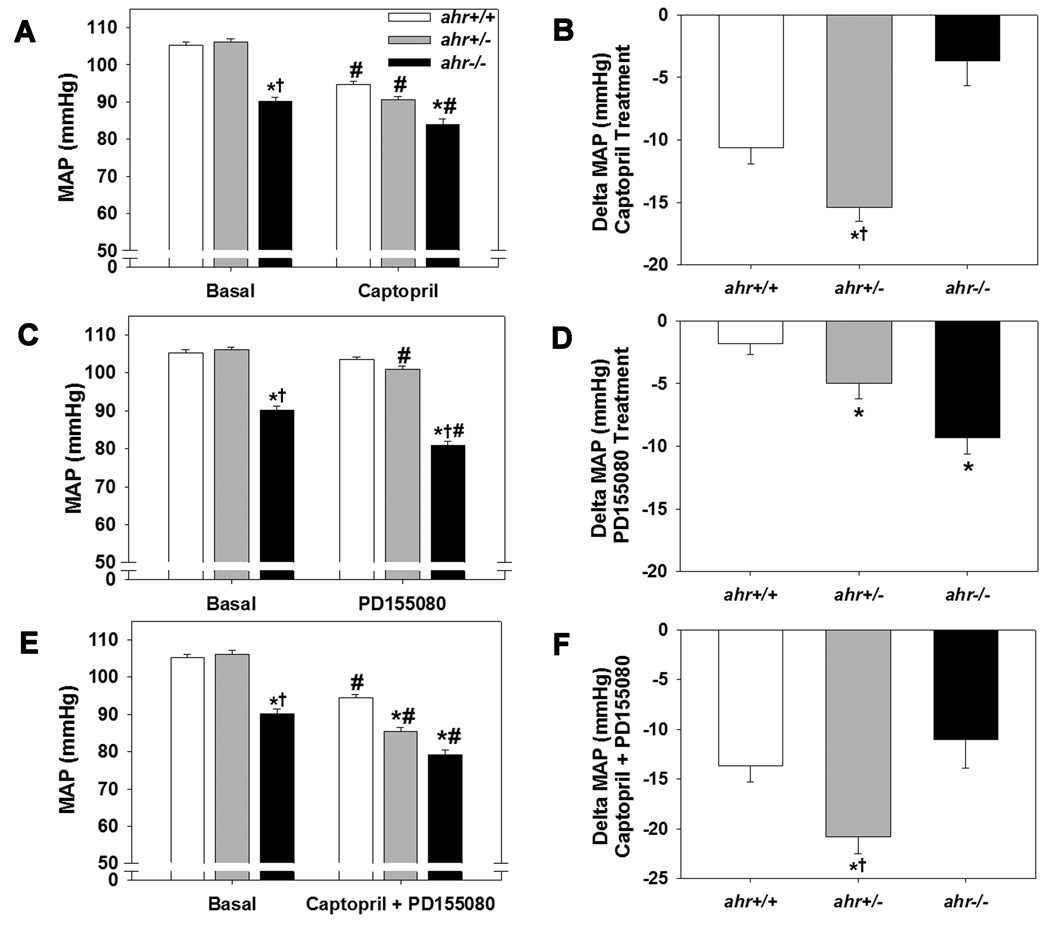
3.6 Inhibition of RAS and ET-1 on blood pressure
Since RAS and ET-1 are key components in blood pressure regulation, we next investigated the responsiveness of all three genotypes to inhibitors of the RAS and ET-1 signaling. We found that the ACEi, captopril, significantly decreased MAP in mice of all three genotypes, but the decrease was significantly greater in ahr+/− mice and significantly less in ahr−/− mice (Fig. 6A and B). In contrast, the ETA antagonist, PD155080, significantly decreased MAP in ahr+/− and ahr−/− mice, but not ahr+/+ mice (Fig. 6C and D), while the combination drug therapy showed the greatest decrease in ahr+/− mice (Fig. 6E and F).
Fig. 6.
Loss of a single ahr allele increases the hypotension response following exposure to an ACEi (captopril), ETA receptor antagonist (PD155080), or the two in combination, while loss of both ahr alleles attenuates the response to an ACEi but increases the response to an ETA receptor antagonist. (A) MAP and (B) Change in MAP following treatment with 4 mg/ml ACEi, captopril, in the drinking water for 3 d. (C) MAP and (D) Change in MAP following treatment with 25 mg/kg PD155080 twice per day for 3 d. (E) MAP and (F) Change in MAP following treatment with captopril in the drinking water plus oral PD155080 for 3 d (n=8/genotype). Data represent mean ± SEM and in panels (A, C, and E) were analyzed by repeated measures two-way ANOVA (A, C, E); *p < 0.05 compared to ahr+/+ and †p < 0.05 compared to ahr+/− within the same treatment group; #p < 0.05 compared to untreated, basal MAP of the same genotype. Data in panels (B, D, and F) were analyzed by one-way ANOVA, using post hoc Holm-Sidak comparisons; *p < 0.05 compared to ahr+/+ and †p < 0.05 compared to ahr−/−.
3.7 Indices of RAS activity
The increased responsiveness to in vivo inhibition of ACE in ahr+/− mice, but not in ahr−/− mice, suggested that the RAS may be activated only in ahr+/− animals. We found that four indices of RAS activation were significantly increased only in ahr+/− mice. Both the rate limiting step, plasma renin activity (PRA), and the final step, ACE activity, in Ang II formation were significantly elevated in ahr+/− mice (Table 2). In addition, downstream responses that would be consistent with an activated RAS, including sodium retention and an increased concentration of electrolytes in the urine, were evident in ahr+/− mice. ahr+/− mice showed a small but significant increase in plasma sodium and a significant increase in urine osmolality (Table 2).
Table 2.
Indices of RAS activation, plasma electrolytes, and urine osmolality in ahr+/+, ahr+/−, and ahr−/− mice.
| Parameter | ahr+/+ (n) | ahr+/− (n) | ahr−/− (n) |
|---|---|---|---|
| PRA (ng Ang I/ml/hr) | 2.1 ± 0.4 (6) | 3.3 ± 0.3 (6)*† | 2.1 ± 0.6 (5) |
| Plasma ACE (units/L) | 148 ± 12 (5) | 176 ± 4 (6)*† | 142 ± 11 (5) |
| Plasma Na (mM) | 144.9 ± 1.13 (11) | 148.3 ± 0.4 (6)*† | 145.4 ± 0.6 (7) |
| Plasma K (mM) | 7.5 ± 0.4 (11) | 7.3 ± 0.3 (6) | 8.0 ± 0.4 (7) |
| Plasma Cl (mM) | 118 ± 1 (11) | 118 ± 1 (6) | 123 ± 2 (7) |
| Urine Osmolality | 2069 ± 245 (7) | 2733 ± 223 (6)*† | 1695 ± 104 (5) |
Values are expressed as mean ± SEM
p < 0.05 versus ahr+/+
p < 0.05 versus ahr−/−
4. Discussion
Although ahr+/− mice do not exhibit overt vascular abnormalities during development [12, 13] and are normotensive in adulthood, our studies show that blood pressure regulation in ahr+/− mice is different from both ahr−/− and ahr+/+ mice. Notably, ahr+/− mice exhibit an activated RAS and increased ETA signaling, which are responsible for maintaining their blood pressure at normotensive levels. In contrast, ahr−/− mice do not have an activated RAS and are significantly less responsive to ACE inhibition, suggesting that hypotension may result, in part, from reduced contribution of RAS signaling. While we cannot rule out the possibility that ahr+/− mice are devoid of vascular abnormalities, the results of our studies clearly show that loss of a single ahr allele has a significant impact on blood pressure regulation in adulthood.
The role of the RAS in contributing to basal blood pressure regulation is well known and is demonstrated by a number of studies where ACE inhibition decreases blood pressure in experimental animals that are hypertensive, normotensive, and hypotensive [22]. Further evidence that the RAS is essential to regulating basal blood pressure comes from gene ablation studies. Mice harboring a genetic deletion of angiotensinogen, ACE, angiotensin 1A (AT1A) receptor or AT1B receptor are all hypotensive [23–26]. Thus, our observation that ACE inhibition reduces blood pressure is not novel. What is novel about our data, however, is that the set point for basal blood pressure is reached by a greater degree of RAS and ET-1 signaling in ahr+/− mice. In contrast, this set point is not reached in ahr−/− mice, at least in part, because RAS is not activated and ahr−/− mice appear to exhibit reduced responsiveness to Ang II, the downstream product of ACE and one prohypertensive effector of the RAS.
The role of ET-1 in basal blood pressure regulation is less clearly defined. While nonselective ETA/ETB receptor blockade fails to decrease blood pressure in normotensive rats [27], selective ETA receptor blockade reduces MAP in normotensive nonhuman primates, rats, and mice [17, 28, 29], supporting a role of ET-1 and ETA receptors in basal blood pressure regulation. While administration of the selective ETA receptor antagonist (PD155080) in this study did not significantly decrease MAP in ahr+/+ mice, this drug significantly decreased MAP in both ahr+/− and ahr−/− mice. Thus, the contribution of ET-1 and the ETA receptor to basal blood pressure is greater in ahr+/− and ahr−/− mice than in ahr+/+ mice, although it is not sufficient to normalize blood pressure in hypotensive ahr−/− mice.
It is not clear if loss of a single ahr allele results in direct activation of the RAS and ET-1 or if these are compensatory responses to changes in other blood pressure regulatory pathways. Our current studies fail to reveal changes in other regulatory blood pressure pathways that might lead to RAS and ET-1 activation, including the sympathetic nervous system and NO signaling. A reduction in sympathetic nervous system activity, such as genetic deletion of alpha1 adrenoceptors, can induce hypotension [30]. Treatment of ahr+/− with the alpha1 adrenoceptor blocker, prazosin, or with the ganglionic blocker, hexamethonium, reduced blood pressure to an equal degree as in ahr+/+ mice, suggesting that sympathetic nervous system regulation of blood pressure is not altered by AHR. Alternatively, increased eNOS expression and NO release can also induce hypotension [31, 32]. Although ahr+/− mice exhibit increased aortic basal NO activity, the degree to which blood pressure increases when NOS is chronically inhibited does not differ between ahr+/+ and ahr+/− mice, suggesting that NO-dependent regulation of blood pressure also is not altered in ahr+/− mice. Thus, the mechanism that leads to activation of RAS and ET-1 in ahr+/− mice remains to be elucidated.
The blood pressure and vascular reactivity phenotypes of the ahr+/− mice share some similarities with ahr−/− mice. Both genotypes exhibit increased aortic basal NO activity and increased vasocontraction to PE when NOS is inhibited. Despite these changes, the regulation of blood pressure was not significantly affected by inhibition of alpha adrenergic signaling or NO production. This discrepancy could result from the fact that changes in vasoreactivity of a conduit vessel, like aorta, are not always predictive of changes occurring in resistance vessels that are the vascular regulators of blood pressure. Nonetheless, these changes in vessel reactivity could potentially contribute to alterations in other vascular functions, such as angiogenesis, that have been reported by others in ahr−/− mice [3, 33, 34].
Despite the similarities between ahr+/− and ahr−/− mice, there also are some significant differences. First, and most importantly, ahr+/− mice are normotensive, while ahr−/− mice are hypotensive. Second, ahr+/− mice have an activated RAS, while ahr−/− mice do not. Since the activated RAS maintains normal blood pressure in ahr+/− mice, it is possible that the failure of the RAS to up-regulate under normoxic conditions in ahr−/− mice may be one contributor to their hypotension. Third, ahr+/− and ahr−/− mice exhibit significantly different blood pressure responses to NOS inhibition. NOS inhibition results in a sustained increase in blood pressure in ahr+/− mice, which is identical to that observed in ahr+/+ mice. In contrast, ahr−/− mice are almost completely refractory to increases in blood pressure following NOS inhibition. It has been reported that sustained hypertension induced by NOS inhibition is mediated, in part, by RAS activation [35]. Thus, the differences in blood pressure responses to NOS inhibition between ahr+/− and ahr−/− mice may result from their respective abilities to up-regulate the RAS. Future studies will investigate the regulation of the RAS components and their ability to increase in ahr+/− and ahr−/− mice following typical physiological stimuli, such as a low salt diet.
Finally, there are some significant differences in organ and body weights between ahr+/− and ahr−/− mice. Body weight is significantly increased in ahr+/− animals, but not in ahr−/−, when compared to wild-type mice. The reason for this difference is not known, but it is possible that ahr+/− mice retain more water as a result of RAS activation. Both kidney and heart weights are significantly increased in ahr−/− mice, while these organ weights are normal in the ahr+/− animals, when compared to wild-type mice. The increased heart weight in ahr−/− mice could represent a compensatory physiological hypertrophy to increase cardiac output in an attempt to increase blood pressure. Consistent with this idea, hearts from ahr−/− mice exhibit hypertrophic remodeling without induction of fetal hypertrophy marker genes, which would suggest a physiological, rather than pathological, hypertrophy [36]. Since ahr+/− mice are normotensive, there would be no stimulus for a compensatory physiological hypertrophy. Lastly, altered vascular branching has been described in the kidneys of ahr−/− mice [1]. Since no vascular structural changes have been reported in the ahr+/− mice, the increase in kidney weight in ahr−/− mice, but not in the ahr+/− mice, could be related to disruption of renal vascularization during fetal development.
The specific mechanisms by which AH R contributes to blood pressure regulation remain to be fully elucidated. It is well established that vascular endothelium plays an important role in the maintenance of vascular tone and blood pressure. A number of studies have shown that physiological shear stress of endothelial cells is a potent stimulus for AHR activation [37–39]. Further, changes in gene expression associated with shear of endothelium can be protective or deleterious, depending on the level shear and the vascular site, and some of these changes are likely controlled by AHR. Thus, ahr+/− mice could be a useful tool for investigating changes in gene expression that contribute to altered vasoreactivity and blood pressure regulation under conditions of increased shear, such as during exercise or exposure to vasoconstricting agents. In addition, we are developing a new mouse model in which ahr is deleted only from endothelial cells in adult animals to further investigate its role in the vascular control of blood pressure.
Acknowledgements
This study was supported by a grant from the National Institutes of Health [R01 HL078914 to M.K.W.] and a Pfizer summer internship program. The authors wish to thank Drs. Changjian Feng and Laura Gonzalez Bosc for their technical assistance, and critical analysis and interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci USA. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci USA. 2005;102:17858–17563. doi: 10.1073/pnas.0504757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, et al. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1297–1304. doi: 10.1161/ATVBAHA.106.138701. [DOI] [PubMed] [Google Scholar]
- 4.Villalobos-Molina R, Vazquez-Cuevas FG, Lopez-Guerrero JJ, Figueroa-Garcia MC, Gallardo-Ortiz IA, Ibarra M, et al. Vascular alpha-1D-adrenoceptors are overexpressed in aorta of the aryl hydrocarbon receptor null mouse: role of increased angiotensin II. AutonAutacoidPharmacol. 2008;28:61–67. doi: 10.1111/j.1474-8673.2008.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lund AK, Agbor LN, Zhang N, Baker A, Zhao H, Fink GD, et al. Loss of the aryl hydrocarbon receptor induces hypoxemia, endothelin-1, and systemic hypertension at modest altitude. Hypertension. 2008;51:803–809. doi: 10.1161/HYPERTENSIONAHA.107.100586. [DOI] [PubMed] [Google Scholar]
- 6.Vasquez A, Atallah-Yunes N, Smith FC, You X, Chase SE, Silverstone AE, et al. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by AhR knockout mice. CardiovascToxicol. 2003;3:153–163. doi: 10.1385/ct:3:2:153. [DOI] [PubMed] [Google Scholar]
- 7.McMillan BJ, Bradfield CA. The Aryl Hydrocarbon Receptor sans Xenobiotics: Endogenous Function in Genetic Model Systems. Mol Pharmacol. 2007;27:487–498. doi: 10.1124/mol.107.037259. [DOI] [PubMed] [Google Scholar]
- 8.Louey S, Cock ML, Harding R. Postnatal development of arterial pressure: influence of the intrauterine environment. Arch Physiol Biochem. 2003;111:53–60. doi: 10.1076/apab.111.1.53.15137. [DOI] [PubMed] [Google Scholar]
- 9.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone 'programmes' hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunger MK, Moran SM, Glover E, Thomae TL, Lahvis GP, Lin BC, et al. Resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and abnormal liver development in mice carrying a mutation in the nuclear localization sequence of the aryl hydrocarbon receptor. J Biol Chem. 2003;278:17767–17774. doi: 10.1074/jbc.M209594200. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 12.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279:16326–16331. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty AM, Patt WC, Edmunds JJ, Berryman KA, Reisdorph BR, Plummer MS, et al. Discovery of a novel series of orally active non-peptide endothelin-A (ETA) receptor-selective antagonists. J Med Chem. 1995;38:1259–1263. doi: 10.1021/jm00008a002. [DOI] [PubMed] [Google Scholar]
- 15.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice is correlated with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Toxicol Appl Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 17.Potter GS, Johnson RJ, Fink GD. Role of endothelin in hypertension of experimental chronic renal failure. Hypertension. 1997;30:1578–1584. doi: 10.1161/01.hyp.30.6.1578. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Joaquim LF, Farah VM, Wichi RB, Fazan R, Jr, Salgado HC, et al. Cardiovascular autonomic control in mice lacking angiotensin AT1a receptors. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1071–R1077. doi: 10.1152/ajpregu.00231.2004. [DOI] [PubMed] [Google Scholar]
- 19.Duling LC, Cherng TW, Griego JR, Perrine MF, Kanagy NL. Loss of alpha2B-adrenoceptors increases magnitude of hypertension following nitric oxide synthase inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H2403–H2408. doi: 10.1152/ajpheart.01066.2005. [DOI] [PubMed] [Google Scholar]
- 20.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund AK, Goens MB, Nunez B, Walker MK. Characterizing the role of endothelin-1 in the progression of cardiac hypertrophy in aryl hydrocarbon receptor (AHR) null mice. Toxicology and Applied Pharmacology. 2006;212:127–135. doi: 10.1016/j.taap.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Leckie BJ. The action of salt and captopril on blood pressure in mice with genetic hypertension. J Hypertens. 2001;19:1607–1613. doi: 10.1097/00004872-200109000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, et al. Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature. 1995;375:146–148. doi: 10.1038/375146a0. [DOI] [PubMed] [Google Scholar]
- 25.Esther CR, Jr, Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE. Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Lab Invest. 1996;74:953–965. [PubMed] [Google Scholar]
- 26.Tsuchida S, Matsusaka T, Chen X, Okubo S, Niimura F, Nishimura H, et al. Murine double nullizygotes of the angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of angiotensinogen nullizygotes. J Clin Invest. 1998;101:755–760. doi: 10.1172/JCI1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JS, Schiffrin EL. Effect of chronic treatment of adult spontaneously hypertensive rats with an endothelin receptor antagonist. Hypertension. 1995;25:495–500. doi: 10.1161/01.hyp.25.4.495. [DOI] [PubMed] [Google Scholar]
- 28.Reinhart GA, Preusser LC, Opgenorth TJ, Wegner CD, Cox BF. Endothelin and ET(A) receptors in long-term arterial pressure homeostasis in conscious nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1701–R1706. doi: 10.1152/ajpregu.2000.279.5.R1701. [DOI] [PubMed] [Google Scholar]
- 29.Fryer RM, Rakestraw PA, Banfor PN, Cox BF, Opgenorth TJ, Reinhart GA. Blood pressure regulation by ETA and ETB receptors in conscious, telemetry-instrumented mice and role of ETA in hypertension produced by selective ETB blockade. Am J Physiol Heart Circ Physiol. 2006;290:H2554–H2559. doi: 10.1152/ajpheart.01221.2005. [DOI] [PubMed] [Google Scholar]
- 30.Sanbe A, Tanaka Y, Fujiwara Y, Tsumura H, Yamauchi J, Cotecchia S, et al. Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol. 2007;152:332–340. doi: 10.1038/sj.bjp.0707366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, et al. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Haperen R, de Waard M, van Deel E, Mees B, Kutryk M, van Aken T, et al. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277:48803–48807. doi: 10.1074/jbc.M209477200. [DOI] [PubMed] [Google Scholar]
- 33.Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM, Fernandez-Salguero PM. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem. 2009;284:25135–25148. doi: 10.1074/jbc.M109.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fritz WA, Lin TM, Cardiff RD, Peterson RE. The aryl hydrocarbon receptor inhibits prostate carcinogenesis in TRAMP mice. Carcinogenesis. 2007;28:497–505. doi: 10.1093/carcin/bgl179. [DOI] [PubMed] [Google Scholar]
- 35.Zanchi A, Schaad NC, Osterheld MC, Grouzmann E, Nussberger J, Brunner HR, et al. Effects of chronic NO synthase inhibition in rats on renin-angiotensin system and sympathetic nervous system. Am J Physiol. 1995;268:H2267–H2273. doi: 10.1152/ajpheart.1995.268.6.H2267. [DOI] [PubMed] [Google Scholar]
- 36.Vasquez A, Atallah-Yunes N, Smith FC, You X, Chase SE, Silverstone AE, et al. A role for the aryl hydrocarbon receptor in cardiac physiology and function as demonstrated by AhR knockout mice. Cardiovasc Toxicol. 2003;3:153–163. doi: 10.1385/ct:3:2:153. [DOI] [PubMed] [Google Scholar]
- 37.Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, et al. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovascular Research. 2008;77:809–818. doi: 10.1093/cvr/cvm095. [DOI] [PubMed] [Google Scholar]
- 38.Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: upregulation by shear stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- 39.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, et al. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–677. doi: 10.1093/cvr/cvn360. [DOI] [PMC free article] [PubMed] [Google Scholar]