Abstract
The orphan nuclear receptor small heterodimer partner (SHP) regulates metabolic pathways involved in hepatic bile acid production and both lipid and glucose homeostasis via the transcriptional repression of other nuclear receptors. In the present study, we generated fat-specific SHP-overexpressed transgenic (TG) mice and determined the potential role of SHP activation, specifically in adipocytes, in the regulation of adipose tissue function in response to stressors. We determined in 2 mo-old SHP TG mice body weight, fat mass index, adipose tissues morphology, thermogenic and metabolic gene expression, metabolic rates at baseline and in response to β adrenergic receptor agonists, and brown fat ultrastructural changes in response to cold exposure (6–48 h). Mice were fed a 10-wk high-fat diet (HFD; 42% fat). Weight gain, fat mass index, adipose tissues morphology, glucose tolerance, and metabolic rates were determined at the end of the feeding. Young TG mice had increased body weight and adiposity; however, their energy metabolism was increased and brown fat function was enhanced in response to cold exposure through the activation of thermogenic genes and mitochondrial biogenesis. SHP overexpression exacerbated the diet-induced obesity phenotype as evidence by marked weight gain over time, increased adiposity, and severe glucose intolerance compared with wild-type mice fed a HFD. In addition, SHP-TG mice fed HFD had decreased diet-induced adaptive thermogenesis, increased food intake, and decreased physical activity. In conclusion, SHP activation in adipocytes strongly affects weight gain and diet-induced obesity. Developing a synthetic compound to antagonize the effect of SHP may prove to be useful in treating obesity.
Keywords: small heterodimer partner, metabolism, obesity, energy expenditure, nuclear receptor
obesity is the result of an imbalance between energy intake and expenditure (17). Whereas energy intake is solely dependent on food ingestion, energy expenditure depends on several factors, such as exercise and heat production, or so-called adaptive thermogenesis. Despite extensive research focused on the understanding of obesity, the molecular mechanisms underlying increased adiposity are not fully understood.
Brown adipose tissue (BAT) is the major site for adrenergic mediated adaptive thermogenesis involving the uncoupling protein-1 (UCP1), whereas white adipose tissue (WAT) is mostly implicated in the regulation of lipid storage and catabolism (8, 13, 15, 26). Adaptive thermogenesis allows rodents to slow the development of obesity when overfed and maintain body temperature when exposed to a cold environment. Excessive caloric intake can be sensed by the sympathetic nervous system through β-adrenergic receptors (βARs), i.e., β1AR, β2AR, and β3AR, and the increased cyclic AMP (cAMP) leads to an activation of UCP1 and the induction of the uncoupling process in mitochondria that results in heat production (2, 6). cAMP can also activate deiodinase 2 (Dio2) which generates the active form of the thyroid hormone triiodothyronine (T3) via deiodination of thyroxine (T4), which in turn leads to the induction of T3-responsive thermogenic genes (3, 13, 22, 27). Moreover, peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α (PGC1α) is an important regulator of mitochondrial biogenesis and oxidation through the activation of mitochondrial DNA replication and the modulation of the expression of subunits of the respiratory chain via Nrf1 and Nrf2 nuclear factors (16, 20, 34).
The orphan nuclear receptor small heterodimer partner (SHP, also called NR0B2 for nuclear receptor subfamily 0, group B, member 2) regulates several metabolic pathways involved in fatty liver and obesity by acting as a transcriptional repressor of other nuclear receptors (5, 12, 31, 32). It was previously shown in SHP-null mice (SHP−/−) that in the absence of SHP repression basal gene expression of UCP1 and PGC1α increased in BAT (33) and was associated with increased oxygen consumption and heat production, the latter representing whole body energy expenditure, suggesting, therefore, that SHP may be a negative regulator of energy utilization. Besides, even though body weight of SHP−/− mice was not significantly different from that of wild-type (wt) mice on a normal chow diet (NC) at a young age (8–9 wk old), these mice were resistant to diet-induced obesity (DIO) when challenged with a high-fat diet (HFD) for 12 wk. The resistance to DIO was the consequence of increased energy expenditure rather than a decrease in lipid availability (33).
A more recent study showed that SHP deletion in obese leptin-deficient mice (ob/ob), an animal model of severe obesity and insulin resistance, prevented the development of nonalcoholic fatty liver (12). This effect was associated with changes in hepatic expression of lipogenic genes. SHP deletion in ob/ob mice also improved peripheral insulin sensitivity; however, it did not overcome the severe obesity caused by leptin deficiency (12). The lack of significant protective effect from obesity by SHP deficiency is likely associated with the low basal level of SHP expressed in fat.
Since adipocytes play a major role in the regulation of lipid storage and energy expenditure, in the present study we generated transgenic mice overexpressing SHP, specifically in WAT and BAT, and subjected them to cold exposure or HFD to further assess the specific role of SHP activation in adipose tissues, in response to these stressors. Unexpectedly, SHP overexpression in young mice enhanced whole body energy metabolism, increased basal levels of thermogenic gene β1AR expression as well as PGC1α in BAT, and induced a rapid adaptation of this tissue to cold exposure through the induction of mitochondrial biogenesis and the modulation of lipid homoeostasis. Despite this, SHP transgenic mice increased adiposity and weight gain with age and profoundly affected DIO. These surprising findings indicate that SHP activation in fat has a dominant role in obesity and exacerbates the obesity phenotype associated with HFD.
MATERIALS AND METHODS
Animals.
Transgenic mice overexpressing SHP specifically in adipose tissues were generated under the control of Ap2 promoter. A 0.8-kb fragment containing the mouse SHP cDNA was ligated downstream of the 7.1-kb Ap2 enhancer/promoter region in the pBluescript SK vector (Stratagene, La Jolla, CA), and the SV40 small tumor antigen splice site and polyadenylylation signal sequences were added to the mSHP cDNA. The DNA used for injections was liberated from the plasmid as an 8.5-kb HindIII/NotI fragment. DNA was injected as previously described (35). Swiss Webster mice were purchased from the National Institutes of Health Frederick Cancer Research Facility (Frederick, MD). Transgenic lines were identified by PCR and Southern blot analysis, and SHP was specifically expressed in WAT and BAT, but not in liver, muscle, and other tissues. Mice were maintained on a hybrid background C57BL6/SJL and on a 12:12-h light-dark cycle at room temperature (21–23°C), and had free access to normal chow (NC) and water. Littermates from intercrossed non-transgene and transgenic mice were genotyped each time, and age- and sex-matched mice for each genotype were used for the experiments. Because it will take about two to three years to backcross the mice into a pure C57 background, which will significantly impact the progress of this research project, we used mixed-background mice for this study. Eight- to nine-wk-old wt and Ap2-SHP (TG) male mice were fed an HFD (42% fat, TD.88137; Harlan Teklad, Madison,WI) for 10 wk. Weight gain was measured twice a wk. Body fat mass was determined at the end of the feeding by a dual-energy X-ray absorptiometry (DEXA) scanner and expressed as percentage of body weight (fat mass index). Age-matched mice on NC were used as controls. Animal care and experimentation were approved by the Institutional Animal Care and Use Committee of the University of Utah and were in accordance with the National Health Institute guidelines.
Glucose tolerance.
Glucose tolerance tests (GTT) were performed after a 6-h fast in mice fed a 10-wk HFD or NC, as previously described (30). The dose of glucose for GTT is 1 mg/g body wt.
Morphological analysis of adipose tissues.
Epididymal WAT and interscapular BAT were removed, fixed in 4% formaldehyde, dehydrated, and embedded in paraffin for sectioning. Histological slides of 6- to 8-μm sections were stained with hematoxylin and eosin (Sigma, St. Louis, MO).
To determine cold exposure ultrastructural changes of BAT, interscapular BAT was removed from mice exposed to 4°C for 6 and 48 h, fixed with 2% glutaraldehyde in PBS overnight at 4°C, postfixed in 1% OsO4 in 0.1 M phosphate buffer (PH 7.4), dehydrated in graded ethanol series, and embedded in Epon-Araldite resin. Ultrathin sections were obtained. Ultramicroscopy image analyses were performed by Nancy Chandler at the Transmission Electron Microscopy (EM) core facility of the University of Utah.
RNA analysis.
For gene expression analysis in basal conditions and in response to cold exposure, WAT and/or BAT was removed from mice, and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Semiquantitative or real-time PCR analysis was performed using primers specific to each gene, which was normalized to β-actin.
Metabolic rates and heat production.
Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2) were measured over a 24-h period with the Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) using a four-chamber open-circuit system. Four mice for each group (wt and TG) were measured simultaneously, and data obtained from each mouse in the same group were averaged. Animals were acclimatized to the chambers overnight with free access to food and water prior to data collection and maintained at 24°C under a 12:12-h light-dark cycle. Mice were housed individually in Plexiglas cages through which 0.6 liters of air were passed per minute. Each chamber was sampled for 1.5 min at 15-min intervals. The O2 and CO2 content of the exhaust air from each chamber was compared with the O2 and CO2 content of the ambient sample air. Food consumption was monitored by electronic scales, water by electronic sipper tubes, and movement by XY/Z laser beam interruption. V̇o2 and V̇co2 measurements were normalized to body weight. The following parameters were calculated as indicated: heat production = caloric value (CV) × V̇o2; CV = 3.815 × respiratory exchange ratio (RER); RER = V̇co2/V̇o2.
To determine the potential effects of adrenergic receptor activation, baseline measurements were collected over a 3-h period. Mice were then injected (ip) with a β1 (Dobutamine), β2 (Clenbuterol), or β3 (CL-316243) agonist (5 mg/kg body wt), and V̇o2 and V̇co2 were measured for 1 h.
Statistics.
Data are expressed as means ± SE. Significance (P < 0.05) was determined by one-way ANOVA followed by a Fisher's protected least significance test, when comparing four groups (wt, TG, wt HFD, TG HFD), and by Student's t-test when only two sets of data were determined in the experiment. Statistical calculations were performed with the Statview 5.0.1 software package (SAS Institute, Cary, NC).
RESULTS
Generating fat-specific SHP tg mice.
SHP KO miceexhibited alteration of metabolic phenotype related to obesity, diabetes, and fatty liver (33); however, it remains to be determined which tissue plays a major role that contributes to this phenotype. To further dissect out the tissue-specific effect of SHP and to direct high-level expression of the SHP to adipocytes, we created a transgene in which the mouse SHP cDNA was under the control of the promoter of the aP2 lipid-binding protein gene, which can direct expression of SHP transgene to WAT and BAT (Fig. 1, A and B). The total level of SHP in the adipose tissues was increased at minimum three to fivefold, at least at the RNA level. Several independent founder animals bearing the aP2-SHP transgene were produced and used to generate pedigrees (Fig. 1C). The total level of SHP in the adipose tissues was increased at minimum three- to fivefold, at least at the RNA level (Fig. 1D). SHP was not detected in wt WAT and BAT due to its low basal expression and low PCR cycles (20x) used to determine the overexpressed SHP. Two mouse lines that showed about three- to fivefold overexpression of SHP in WAT and BAT were chosen. Body weight gains between those two lines for a period of 4 mo were compared and similar results were obtained. Thus, one line was used for further detailed studies.
Fig. 1.
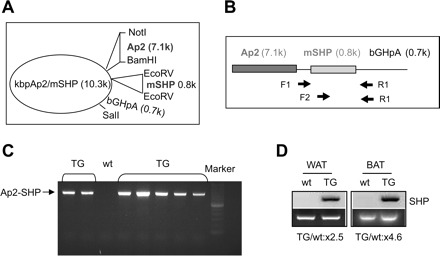
Generating adipocyte-specific nuclear receptor small heterodimer partner (SHP)-overexpressed transgenic mice (Ap2-SHP-TG). A: target gene construct for overexpression of mouse SHP (mSHP) in adipocytes. Mouse Ap2 promoter was ligated into kbpa1b vector between NotI and BamHI sites to obtain kbpAp2 construct. Then, mSHP was ligated into the EcoRV site of the construct. Final construct was confirmed by enzyme digestion and sequencing. Construct was cut by NotI/SalI, and the resulting Ap2-SHP fragment was gel purified and quantified for microinjection. B: schema of mouse Ap2 promoter-mSHP construct. Primers used to amplify the exogenous SHP transgene are indicated: F1 is located 80 bp upstream of SHP start codon ATG; F2 is located 429 bp downstream of SHP start codon ATG; and R1 is located 42 bp downstream of SHP stop codon TGA. The size of the PCR product is 905 bp for F1/R1 and 400 bp for F2/R1 primers. C: identifying mice overexpressing mSHP in adipocytes. Genomic DNA was extracted from the tail of each mouse and was subjected to PCR analysis. One representative genotyping result is shown. Each number represents an individual mouse or negative (neg) control. D: semiquantitative PCR analysis of SHP mRNA in white (WAT) and brown adipose tissue (BAT). SHP was not detected in WAT and BAT of wild-type (wt) mice due to low PCR cycles used. Bottom: fold induction of SHP from real-time qPCR analysis.
SHP overexpression in adipose tissues increases fat mass index, thermogenic gene expression, and energy metabolism under basal conditions.
Two-month-old TG mice showed an ∼8% increase in body weight (Fig. 2A) compared with wt mice (P = 0.05) and an ∼65% increase (P = 0.07 vs. wt) in fat mass index (Fig. 2B) with no marked alterations in WAT morphology (Fig. 2C), as indicated by adipocyte number and size (not shown).
Fig. 2.
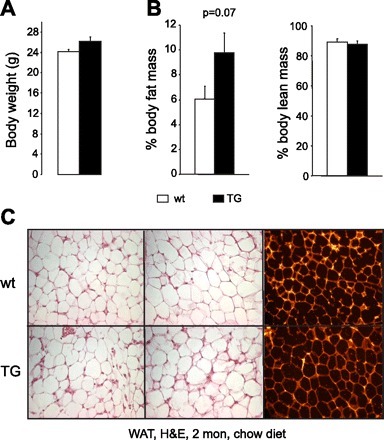
Body weight, fat mass index, and WAT morphology in Ap2-SHP-TG mice. A: total body weight was determined in 2-mo-old nontransgene control wild-type (wt) and Ap2-SHP (TG) mice. B: whole body fat and lean mass were measured by DEXA scanner and expressed as %total body weight (n = 7/group). C: WAT morphology was determined by hematoxylin and eosin (H&E) staining of slides of paraffin-embedded epididymal fat pads (left 4). Cell images (right 2) showing cell boundary were used to analyze white adipocyte cell number and size. Representative slide is shown for each group (n = 3–4/group). Bar graphs are represented as means ± SE (6 fields/slide, 4 slides/mouse).
The expression of lipid metabolism and thermogenic genes was determined in WAT and BAT. In TG mice, WAT (Fig. 3A), the most notable change was a decrease in gene expression of lipolytic enzyme lipoprotein lipase (LPL) and sterol-regulatory element-binding protein-1 (SREBP1). β3AR was not detected under our low PCR cycle conditions, although it was reported to be expressed in WAT. Dio2 and UCP1 are BAT-specific genes and thus were not expected to be expressed in WAT.
Fig. 3.
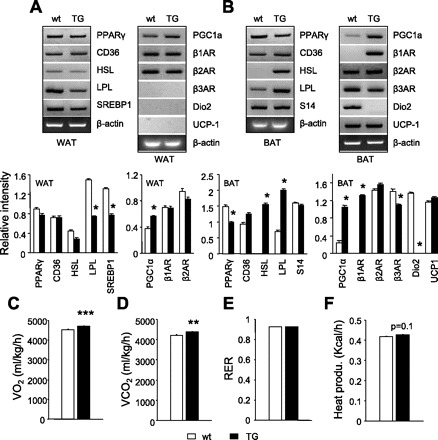
WAT and BAT expression of genes involved in lipid metabolism and thermogenesis and measurement of metabolic rates and heat production in Ap2-SHP-TG mice. Expression of mRNA was determined in epididymal WAT (A) and interscapular BAT (B) by semiquantitative PCR in 2-mo-old wt and TG mice. Each lane represents RNA pooled from 3 mice/group. Blank (β3AR, Dio2, UCP1) in WAT (A, right) indicates undetected gene expression. Relative intensity of bands is presented at the bottom. Oxygen consumption (V̇o2; C), CO2 production (V̇co2; D), respiratory exchange ratio (RER; E), and heat production (F) were determined in 2-mo-old mice in basal conditions over a 24-h period using indirect calorimetric measurements. AR, adrenergic receptor; PPAR, peroxisome proliferator-activated receptor; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; Dio2 deiodinase 2; SREBP, sterol-regulatory element-binding protein; UCP, uncoupling protein. Bar graphs are represented as means ± SE (***P = 0.001, **P = 0.01 vs. wt, n = 4/group).
In TG mice, BAT (Fig. 3B) and mRNA levels of PGC1α, β1AR, LPL, and hormone-sensitive lipase (HSL) increased compared with wt mice. β1AR was not detected in wt BAT because a low PCR cycle was used. Surprisingly, basal gene expressions of UCP1 and β3AR were unchanged or moderately decreased, respectively. Interestingly, Dio2 mRNA was decreased in TG mice, consistent with its increase in SHP−/− mice (33). In contrast to the upregulated genes, the levels of PPARγ mRNA were ∼30% reduced in BAT (Fig. 3B), similar to its downregulation by SHP in 3T3-L1 cells (29). The observation of the upregulation of some of those genes, particularly PGC1α, was unexpected, because SHP generally functions as a transcriptional repressor, and our previous studies using SHP−/− mice identified an increased expression of PGC1α (33). It is postulated that the increased PGC1α is likely due to a secondary effect, probably a result of activation of β1AR. However, it remains to be determined in future studies at the molecular level how β1AR was induced by SHP in BAT.
These observations raise the question of whether SHP overexpression specifically in adipose tissues has a functional impact, and if so, whether it affects whole body metabolism. Thus, we next determined whole body metabolic rates, RER, and heat production over a 24-h period. V̇o2 (Fig. 3C) increased in TG mice compared with wt mice (4,700 ± 14 vs. 4,524 ± 17 ml·kg−1·h−1, P = 0.001), as did V̇co2 (Fig. 3D; 4,370 ± 44 vs. 4,198 ± 48 ml·kg−1·h−1, P = 0.01), resulting in an unchanged RER (Fig. 3E). Heat production (Fig. 3F) was slightly but not significantly increased (P = 0.1). The increased V̇o2 may be associated with the activation of PGC1α and β1AR.
The β1AR, β2AR, and β3AR responses are altered in mice overexpressing SHP in adipose tissues.
In wt mice, V̇o2 and V̇co2 increased in response to β1AR agonist Dobutamine (Fig. 4A +28%, P = 0.001, Fig. 4B +21%, P < 0.01 vs. wt, respectively). RER was slightly decreased (Fig. 4C −5%, P = 0.001), suggesting a moderate increase in whole body lipid utilization, and heat production increased (Fig. 4D +31%, P = 0.001).
Fig. 4.
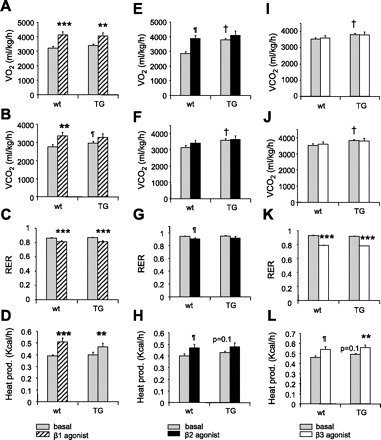
Metabolic rates and heat production in response to β1-, β2-, and β3-adrenergic receptor (AR) agonists in Ap2-SHP-TG mice. V̇o2 (A, E, I), V̇co2 (B, F, J), RER (C, G, K), and heat production (D, H, L) were determined in 2-mo-old mice after injection of Dobutamine, Clenbuterol or CL-316243 (5 mg/kg ip). **P ≤ 0.01, ***P = 0.001 vs. respective basal; ¶P ≤ 0.05, †P ≤ 0.05 vs. wt basal, n = 4/group, 1-h stimulation vs. 3 h at baseline.
In TG mice, basal V̇o2 and V̇co2 were higher then in wt mice, as previously described (24-h period). V̇o2 increased in TG mice in response to Dobutamine (Fig. 4A), whereas no further increase in V̇co2 (Fig. 4B) was observed after treatment with the agonist. Nevertheless, TG mice showed a similar reduction in RER (Fig. 4C) and the thermogenic response as wt mice with Dobutamine (Fig. 4D), indicating that the β1AR response was preserved in these mice.
Clenbuterol, a β2AR agonist, induced in wt mice similar changes in V̇o2 (Fig. 4E +35%, P < 0.05), RER (Fig. 4G −4%, P < 0.05), and heat production (Fig. 4H +17.5%, P < 0.05), as did Dobutamine. In TG mice, although basal rates of V̇o2 (Fig. 4E) and V̇co2 (Fig. 4F) were significantly higher (P < 0.05) than in wt mice, there was no further increase in these rates and no significant changes in RER (Fig. 4G) and heat production (Fig. 4H) in response to the β2AR agonist, indicating that β2AR activity was impaired in TG mice. TG mice showed similar alterations in V̇o2 (Fig. 4I) and V̇co2 (Fig. 4J) with β3AR agonist CL-316243. Nevertheless, these mice had a significant reduction in RER (Fig. 4K −15%, P = 0.001, vs. TG baseline) and increase in heat production (Fig. 4L +14%, P < 0.05 vs. TG baseline) in response to CL-316243, indicating that β3AR activity was partially maintained.
SHP overexpression in BAT induces a rapid adaptation to cold exposure.
TG mice have increased basal thermogenic gene expression such as PGC1α and β1AR in BAT compared with wt mice and increased V̇o2, suggesting that these mice may adapt to cold temperatures through adaptive thermogenesis. Therefore, we investigated the potential role of SHP overexpression in adaptive thermogenesis by determining in BAT the expression of thermogenic genes in response to cold exposure (6–48 h, 4°C).
β1AR gene expression, which was greatly increased in BAT of TG mice at baseline (Fig. 3B) was even higher in response to cold exposure in a time-dependent manner (Fig. 5A). Cold exposure increased β3AR gene expression in BAT of wt mice (Fig. 5A) but not in TG mice, which may be associated with its somewhat lower expression under basal conditions. We used lower PCR cycles in Fig. 5A (22×) than in Fig. 3B (30×) in order to see more marked changes of β3AR expression by cold exposure; thus, the basal level of β3AR in Fig. 5A before the cold exposure appeared to be lower than that presented in Fig. 3B. Even though basal mRNA levels of Dio2 were lower and PGC1α higher in BAT of TG mice compared with wt mice, cold exposure seemed to induce a similar increase in the expression of these genes in both groups of mice. Body temperatures of mice after exposure to cold appeared not significantly different in both groups of mice (not shown), suggesting that activation of β1AR in TG and β3AR in wt contributed to the thermogenic effect to a similar extent.
Fig. 5.
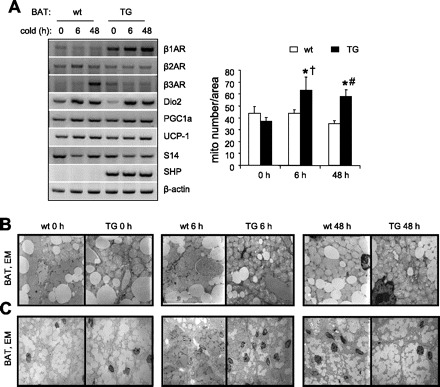
Thermogenic gene expression and ultrastructural changes of BAT in Ap2-SHP-TG mice in response to cold exposure. A: effect of SHP overexpression on thermogenesis was investigated in BAT by determining, in 2-mo-old wt and TG mice, changes in mRNA expression of thermogenic genes in response to cold exposure (4°C, 6 h, 48 h). Each lane represents RNA pooled from 3 mice/group, *P < 0.05 vs. TG basal, †P < 0.05 vs. wt 6 h, #P = 0.02 vs. wt 48 h (n = 3/group). Gene expression was analyzed by semiquantitative PCR. Ultrastructural changes in BAT were determined by electron microscopy (EM). B: ×10,000 magnification shows mitochondria proliferation in response to cold exposure. C: ×2,700 magnification shows changes in size and number of lipid droplets in response to cold exposure (n = 3/group); representative images are shown for each group. A, right: statistical analysis of mitochondria numbers in BAT of wt and TG mice after cold exposure (counted 6 fields/slide, 4 slides/mouse).
We next determined the cold-induced ultrastructural changes in BAT by EM. Interestingly, images at the higher magnification (×10,000; Fig. 5B) indicate a rapid increase in mitochondrial number in TG mice in response to cold exposure (+69% at 6 h, P < 0.01; +56% at 48 h, P < 0.05 vs. basal TG), whereas no such affect was observed in wt mice.
In addition, acute cold exposure (6 h) caused a marked reduction in the size and number of lipid droplets in BAT of TG mice (×2,700 magnification; Fig. 5C). This effect was less pronounced in wt mice, suggesting that TG mice have a more rapid lipolytic response to cold exposure than wt mice.
Interestingly, in BAT of TG mice, by 48 h, the cytoplasm was filled again with bigger lipid inclusions, indicating their rapid reorganization possibly by fusion and/or increased lipogenesis. Although wt mice seem to go through a similar process, at 48 h they had more of the small lipid vacuoles then did TG mice. These data indicate that SHP overexpression in BAT induces a faster lipolytic phase and reorganization of lipid inclusion during cold acclimatization.
SHP overexpression in adipose tissues exacerbates DIO.
The initial characterization of TG mice indicates that they have increased fat mass index and body weight, suggesting that SHP activation in adipose tissues may play an important role in modulating weight gain and adiposity. Therefore, we investigated the potential role of SHP overexpression in DIO.
When fed NC, TG mice gained more weight over time than wt mice (+17.6% vs. +13.1% at day 67, P < 0.05; Fig. 6A). When challenged with HFD, they gained almost twice as much weight by the end of the feeding period as did wt mice on a similar diet (+50% vs. +32% at day 67, P < 0.002). Besides, fat mass index was significantly higher in TG mice both on NC and HFD (Fig. 6B) then in wt mice on a similar diet (NC 15.2% vs. 5.6%, HFD: 66.7% vs. 58% of total body wt, P < 0.05). Furthermore, TG mice fed HFD were severely glucose intolerant compared with their controls (Fig. 6C). These effects occurred without any increase in daily food intake (see below) and are therefore not a response to overfeeding. These data clearly indicate that SHP overexpression in adipose tissues leads to higher susceptibility to DIO and exacerbates the obesity phenotype.
Fig. 6.
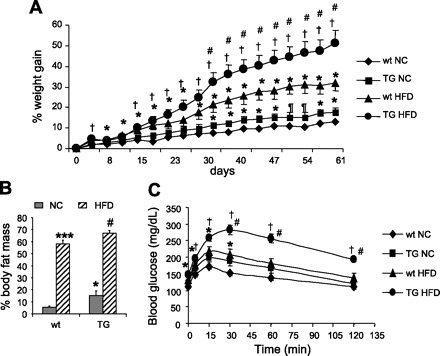
Diet-induced obesity in Ap2-SHP-TG mice fed high-fat diet (HFD). Mice of 2-mo-old (wt and TG) were fed 42% HFD for 10 wk. A: weight gain was determined twice a week and compared with that of their respective controls [normal chow diet (NC)]. Values were expressed as %weight gain from starting body weight. B: whole body fat mass was measured at the end of the experiment by DEXA scanner and expressed as %body weight. C: glucose tolerance tests (GTT) were performed in these mice at the end of high fat feeding. *P < 0.05 vs. TG NC, ¶P = 0.08 vs. wt NC (n = 7–8/group).
WAT (Fig. 7A) and BAT (not shown) morphology, indicated by adipocyte number and size, were not markedly altered in TG mice on NC. Adipocyte size was enlarged in WAT (Fig. 7A) and BAT (Fig. 7B) of wt mice fed HFD as would be expected, and this effect was even more pronounced in adipose tissues of TG mice fed HFD, consistent with increased adiposity.
Fig. 7.
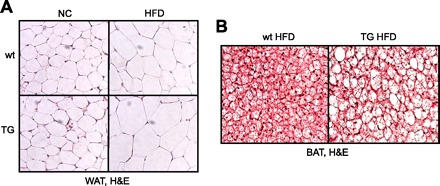
WAT and BAT morphology in Ap2-SHP-TG mice fed HFD. H&E staining of slides of paraffin-embedded epididymal WAT (A) and interscapular BAT (B). Representative slide is shown for each group (×40 magnification, n = 3 mice/group).
SHP overexpression in adipose tissues decreases diet-induced adaptive thermogenesis.
Basal V̇o2 (Fig. 8A) was slightly but significantly lower in older TG mice fed NC than in (3,246 ± 41 vs. 3,382 ± 44 ml·kg−1·h−1, P < 0.05), as was V̇co2 (3,050 ± 38 vs. 3,220 ± 36 ml·kg−1·h−1, P = 0.001; Fig. 8B). RER (Fig. 8C) and heat production (Fig. 8D) were also slightly decreased (0.939 ± 0.003 vs. 0.954 ± 0.003, P < 0.001, 0.46 ± 0.006 vs. 0.48 ± 0.006 kcal/h, P < 0.05, respectively).
Fig. 8.
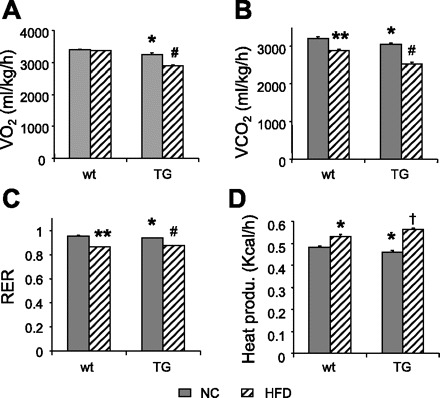
Metabolic rates and heat production in Ap2-SHP-TG mice fed HFD. V̇o2 (A), V̇co2 (B), RER (C), and heat production (D) were determined in wt and TG mice fed HFD or NC, over a 24 h period using the metabolic cage. *P < 0.05 vs. wt NC, **P < 0.0001 vs. wt NC, #P ≤ 0.0001 vs. wt HFD and TG NC, †P = 0.006 vs. wt HFD (n = 4–6/group).
HFD did not affect V̇o2 in wt mice, whereas V̇co2 was markedly reduced (2,883 ± 43 vs. 3,220 ± 36 ml·kg−1·h−1, P < 0.0001), as was RER (0.859 ± 0.001 vs. 0.954 ± 0.003, P < 0.0001). Heat production increased in response to HFD (0.53 ± 0.009 vs. 0.48 ± 0.006 kcal/h, P = 0.0001).
The decrease in basal V̇o2 observed in TG mice (Fig. 8A) was more dramatic with HFD (2,906 ± 9 vs. 3,246 ± 41 ml·kg−1·h−1, P < 0.0001). HFD also induced a marked decrease in V̇co2 in TG mice (Fig. 8B) compared with wt mice (2,542 ± 30 vs. 2,883 ± 43, P < 0.0001). RER was slightly higher (Fig. 8C), and interestingly, HFD induced a more significant increase in heat production (Fig. 8D) in TG mice then in wt mice (P = 0.006 vs. wt HFD), an effect that was even more pronounced when rates were compared with those of respective controls (TG HFD + 22% vs. TG NC, wt HFD +10% vs. wt NC, P ≤ 0.0001). The overall decreased V̇o2 and V̇co2 in both older and HFD-fed TG mice compared with the rather increased V̇o2 and V̇co2 in the less obese young TG mice suggest that the severe obesity developed with age or by HFD in TG mice plays a more dominant role in controlling the whole body energy metabolism. The increased heat production in TG mice with HFD is likely due to a compensatory response.
SHP-overexpressing mice fed HFD show decreased locomotor activity.
Food intake was considered markedly different between wt and TG mice on HFD (P = 0.05; Fig. 9A), suggesting that it may contribute to the increased body weight. In addition, the overall locomotor activity, as measured by a series of laser lights along the x, y, and z (up) axes in two ways, was decreased in TG mice fed a HFD compared with the wt mice (Fig. 9, B–F). Although some of the differences in activity did not reach statistical significance due to large error bars introduced from individual mouse variations, the overall reduced physical activity in TG mice may likely play a role in their obese phenotype.
Fig. 9.
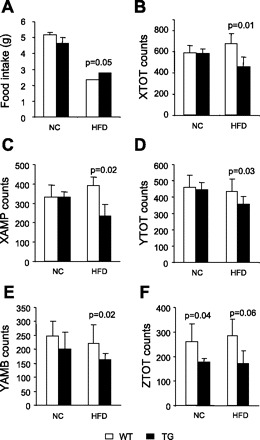
Food intake and locomotor activity in Ap2-SHP-TG mice fed HFD. Food intake (A) and locomotor activity (B–F) were determined in wt and TG mice fed HFD or NC over a 24-h period using a Comprehensive Laboratory Animal Monitoring System (n = 5–7/group). Locomotor activity is measured by a series of laser lights along x, y, and z (up) axes in two ways. If at least one laser beam is broken, it is registered in the columns x, y, or z TOT. If at least two beams are broken, indicating locomotion, beams are totaled in column x, y, or z AMB (ambulation).
SHP overexpression is increased in obese mice.
Thus far, we have shown that exogenous SHP overexpression in fat promoted the obese phenotype. To further determine the physiological relevance of SHP in the development of obesity, SHP expression was analyzed in leptin receptor-deficient db/db mice, a well-studied obesity mouse model. SHP mRNA expression was increased in the WAT of db/db mice compared with the wt lean mice (Fig. 10), confirming its important role in the development of obesity.
Fig. 10.
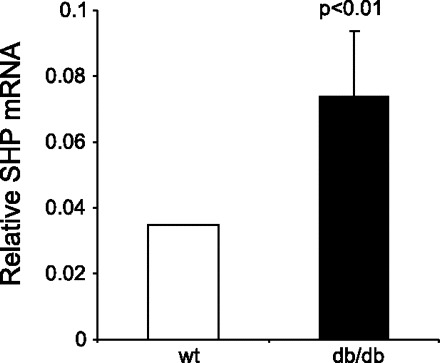
Real-time PCR analysis of SHP mRNA expression in WAT of C57/BL6 db/db mice. C57/BL6 wt mice were used as controls. Total RNA was isolated from WAT of an individual mouse (n = 3/genotype) and used for qPCR analysis. qPCR was assayed in triplicate, and results were normalized to β-actin. Relative SHP mRNA from 3 mice per genotype was presented.
DISCUSSION
Obesity is a serious public health problem in the United States (9, 10, 21, 28) that generally results from an imbalance between energy intake and expenditure. It is conceivable that a better understanding of the molecular mechanisms involved in adiposity and the modulation of energy expenditure will facilitate the development of novel therapeutics for the treatment and prevention of this metabolic disorder.
Previous studies showed that total deletion of SHP increased energy expenditure, prevented the development of DIO, and improved insulin sensitivity (33). However, SHP deficiency did not overcome the severe obesity associated with leptin deficiency in ob/ob mice (12), possibility because SHP was minimally expressed in fat thus the effect of loss of SHP was also minimal and not sufficient to counteract the effect of loss of leptin.
In the present study, we determined the effect of SHP overexpression specifically in BAT and WAT, two major sites for the modulation of energy expenditure and lipid metabolism, respectively, and whether it would modulate adaptive thermogenesis in response to both cold exposure (cold-induced adaptive thermogenesis) and 10 wk of HFD (diet-induced adaptive thermogenesis). SHP overexpression in adipose tissues of 2-mo-old mice resulted in a moderate increase in body weight and 65% increase in fat mass index. Despite increased adiposity, whole body energy metabolism was somewhat enhanced, as evidenced by increased V̇o2 and V̇co2, which may be associated with the upregulation of gene expression of β1AR and PGC1α in BAT, two major activators of mitochondrial thermogenesis and biogenesis (2, 6, 16, 34). HSL, a downstream target of βAR signaling, was increased, suggesting a possible enhancement of lipolysis. Moreover, the expression of PPARγ decreased, and mRNA levels of LPL, known to supply BAT with fatty acids for thermogenesis, was greatly increased. It is noteworthy that similar changes in expression of these two genes were observed in BAT of rodents during prolonged (>24 h) cold acclimatization (11, 14). Taken together, these observations suggest that BAT function may be enhanced by SHP ovexepression in the context of cold-induced adaptive thermogenesis.
We further investigated this hypothesis first by determining the expression of thermogenic genes in response to 6 and 48 h of cold exposure and showed that, in TG mice, although both β2- and β3-adrenergic responses to their agonists and to cold exposure were altered, β1AR gene expression, which was already elevated in BAT of TG mice in basal conditions, was even higher in response to cold exposure, suggesting a compensatory effect through β1AR signaling.
Second, the study of cold-induced ultrastructural changes in BAT by EM showed a hyperresponse (6 h) of BAT to cold exposure in TG mice via a rapid increase in lipolysis, which may have been the consequence of increased HSL expression and β1AR signaling. Interestingly, by 48 h, lipid inclusion size had almost returned to that observed at room temperature, indicating a rapid restoration of BAT lipid reserves in TG mice. This response to cold exposure was also associated with a rapid increase in the number of mitochondria, the main thermogenic site (24).
Even though basal levels of PGC1α mRNA were higher in BAT of TG mice then in wt mice, the activation of mitochondrial biogenesis in TG mice occurred independently of any concomitant changes in PGC1α mRNA levels, suggesting that it was not mediated by the activation of PGC1α gene expression. For instance, in BAT of wt mice, the increase in PGC1α mRNA levels after 6 and 48 h of cold exposure was not accompanied by the induction of mitochondria proliferation. In contrast, β1AR showed a marked and constant induction by cold exposure in TG mice and thus is likely the gene contributing to the alterations of mitochondrial biogenesis.
These are unexpected and thus interesting results indicating that on the one hand SHP overexpression in WAT increases body weight and adiposity, whereas on the other hand, surprisingly, SHP overexpression in BAT activates whole body energy metabolism in basal conditions and enhances BAT function in response to cold exposure by activating β1AR gene expression and mitochondrial biogenesis.
Previous studies using SHP-null mice showed that deletion of SHP increased V̇o2, PGC1α gene expression, and heat production and that it seemed to occur independently of β1AR activation (33). It is difficult to compare the present study, where SHP is overexpressed only in adipose tissues, which play a major role in the development of obesity and the modulation of energy expenditure, to previously reported ones that investigated the effects of whole body SHP deficiency in mice and where SHP deletion may have affected other organs that are important in the regulation of whole body homeostasis and metabolism, including liver (5, 12, 31–33). Furthermore, the present study suggests that SHP overexpression may modulate BAT function through β1AR signaling, a different molecular mechanism than those involved in the functional alterations associated with SHP deletion (33). Currently unknown is the molecular basis by which β1AR was highly induced by SHP in TG mice, which would be elucidated in future studies.
When TG mice were challenged with HFD, they gained almost twice as much weight as wt mice on a similar diet, had a higher fat mass index, adipocyte hypertrophy in WAT and BAT, and were severely glucose intolerant. Thus, SHP overexepression in adipose tissues profoundly affects the obesity phenotype. These observations are consistent with a previous report showing that SHP deletion induces resistance to DIO (33).
In the present study, older TG mice, unlike younger ones, had slightly lower basal rates of V̇o2 and V̇co2 than their wt controls, indicating that their oxidative metabolism decreased as they grew older, possibly because of increased weight gain with age.
The increase in weight gain in wt mice induced by HFD was accompanied by a decrease in V̇co2, no change in V̇o2, and therefore a significant decrease in RER, suggesting that these mice may have increased fat oxidation. This effect was associated with an increase in heat production, or so-called diet-induced adaptive thermogenesis, a process that was previously described by others (19, 23). It is well documented that HFD increases UCP expression in adipose tissues, i.e., UCP1 and UCP3 mRNA in BAT and UCP2 mRNA in WAT, of wt mice (18, 25). In pathophysiological states where fatty acid availability is high, excess lipid oxidation or storage leads ultimately to an overproduction of reactive oxygen species, associated with increased mitochondrial uncoupling and energy dissipation (1, 4, 7). However, increased UCP expression and heat production are not always sufficient to prevent weight gain and body fat accumulation (18, 23).
The severe DIO phenotype observed in TG mice fed HFD was associated with a marked decrease in whole body energy metabolism, an increased food intake, and a moderate reduction in physical activity, most likely because of the greater increase in weight gain. The results suggest that, although the basal energy metabolism was somewhat increased in young TG mice as a result of SHP induction of BAT β1AR signaling, it was not sufficient to overcome the dominant effect of SHP on WAT function to promote obesity in aged and HFD-fed mice. Thus, SHP seems to play distinct roles in BAT and WAT with regard to energy metabolism and the development of obesity. Further investigations focused on the molecular mechanisms by which SHP overexpression specifically in white or brown adipocytes may affect metabolic and thermogenic gene expression would bring more insight into the understanding of the role of SHP activation in modulating obesity.
In conclusion, this study presents strong evidence that SHP overexpression in adipose tissues predominantly increases adiposity and exacerbates the DIO phenotype. Although expressed at low basal levels in fat, SHP is induced under obese conditions. Developing an SHP antagonist may provide a potential new approach in targeting SHP to treat obesity.
GRANTS
This work was supported, in part, by the Junior Faculty Award (7-06-JF-67) from the American Diabetes Association and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-080440 to L. Wang.
DISCLOSURES
No conflicts of interest are reported by the author(s).
ACKNOWLEDGMENTS
We thank Deborah Jones for assistance with indirect calorimetric measurements and E. Dale Abel for kindly giving us access to the DEXA scanner.
REFERENCES
- 1. Argiles JM, Busquets S, Lopez-Soriano FJ. The role of uncoupling proteins in pathophysiological states. Biochem Biophys Res Commun 293: 1145–1152, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116: 2571–2579, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112: 2686–2695, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J 24: 2624–2633, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol 18: 2123–2131, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cortez-Pinto H, Zhi Lin H, Qi Yang S, Odwin Da Costa S, Diehl AM. Lipids up-regulate uncoupling protein 2 expression in rat hepatocytes. Gastroenterology 116: 1184–1193, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Daikoku T, Shinohara Y, Shima A, Yamazaki N, Terada H. Specific elevation of transcript levels of particular protein subtypes induced in brown adipose tissue by cold exposure. Biochim Biophys Acta 1457: 263–272, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell 116: 337–350, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Guardiola-Diaz HM, Rehnmark S, Usuda N, Albrektsen T, Feltkamp D, Gustafsson JA, Alexson SE. Rat peroxisome proliferator-activated receptors and brown adipose tissue function during cold acclimatization. J Biol Chem 274: 23368–23377, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Iqbal J, Saha PK, Liu J, Chan L, Hussain MM, Moore DD, Wang L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 46: 147–157, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol 88: 141–148, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Klingenspor M, Ebbinghaus C, Hulshorst G, Stohr S, Spiegelhalter F, Haas K, Heldmaier G. Multiple regulatory steps are involved in the control of lipoprotein lipase activity in brown adipose tissue. J Lipid Res 37: 1685–1695, 1996 [PubMed] [Google Scholar]
- 15. Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab 30: 294–309, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Lehman JJ, Boudina S, Banke NH, Sambandam N, Han X, Young DM, Leone TC, Gross RW, Lewandowski ED, Abel ED, Kelly DP. The transcriptional coactivator PGC-1α is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol 295: H185–H196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 404: 652–660, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Margareto J, Marti A, Martinez JA. Changes in UCP mRNA expression levels in brown adipose tissue and skeletal muscle after feeding a high-energy diet and relationships with leptin, glucose and PPARgamma. J Nutr Biochem 12: 130–137, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Mercer SW, Trayhurn P. Effect of high fat diets on energy balance and thermogenesis in brown adipose tissue of lean and genetically obese ob/ob mice. J Nutr 117: 2147–2153, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Nisoli E, Clementi E, Moncada S, Carruba MO. Mitochondrial biogenesis as a cellular signaling framework. Biochem Pharmacol 67: 1–15, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Pavelka S, Hermanska J, Baudysova M, Houstek J. Adrenergic control of induction of type II iodothyronine 5′-deiodinase activity in cultured mouse brown adipocytes. Biochem J 292: 303–308, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richard D, Boily P, Dufresne MC, Lecompte M. Energy balance and facultative diet-induced thermogenesis in mice fed a high-fat diet. Can J Physiol Pharmacol 66: 1297–1302, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol 529: 3–10, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rippe C, Berger K, Boiers C, Ricquier D, Erlanson-Albertsson C. Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. Am J Physiol Endocrinol Metab 279: E293–E300, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Samra JS. Sir David Cuthbertson Medal Lecture. Regulation of lipid metabolism in adipose tissue. Proc Nutr Soc 59: 441–446, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Silvestri E, Schiavo L, Lombardi A, Goglia F. Thyroid hormones as molecular determinants of thermogenesis. Acta Physiol Scand 184: 265–283, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Skelton JA, Cook SR, Auinger P, Klein JD, Barlow SE. Prevalence and trends of severe obesity among US children and adolescents. Acad Pediatr 9: 322–329, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song G, Park K, Wang L. Gene expression profiling reveals a diverse array of pathways inhibited by nuclear receptor shp during adipogenesis. Int J Clin Exper Pathol 2: 275–285, 2009 [PMC free article] [PubMed] [Google Scholar]
- 30. Tabbi-Anneni I, Buchanan J, Cooksey RC, Abel ED. Captopril normalizes insulin signaling and insulin-regulated substrate metabolism in obese (ob/ob) mouse hearts. Endocrinology 149: 4043–4050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Han Y, Kim CS, Lee YK, Moore DD. Resistance of SHP-null mice to bile acid-induced liver damage. J Biol Chem 278: 44475–44481, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Lee YK, Bundman D, Han Y, Thevananther S, Kim CS, Chua SS, Wei P, Heyman RA, Karin M, Moore DD. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell 2: 721–731, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Wang L, Liu J, Saha P, Huang J, Chan L, Spiegelman B, Moore DD. The orphan nuclear receptor SHP regulates PGC-1alpha expression and energy production in brown adipocytes. Cell Metab 2: 227–238, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Xu P, Park K, Choi Y, Moore DD, Wang L. Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology (Baltimore, Md) 48: 289–298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


