Abstract
It has been reported that C-nitration of proteins occurs under nitrative/oxidative stress; however, its role in pathophysiological situations is not fully understood. In this study, we determined that nitration of Tyr345 and Tyr368 in the β-subunit of the mitochondrial FoF1-ATPase is a major target for nitrative stress in rat liver under in vivo conditions. The chemical characteristics of these Tyr make them suitable for a facilitated nitration (solvent accessibility, consensus sequence, and pKa). Moreover, β-subunit nitration increased significantly with the age of the rats (from 4 to 80 weeks old) and correlated with decreased ATP hydrolysis and synthesis rates. Although its affinity for ATP binding was unchanged, maximal ATPase activity decreased between young and old rats by a factor of two. These changes directly impacted the available ATP concentration in vivo, and it was expected that they would affect multiple cellular ATP-dependent processes. For instance, at least 50% of available [ATP] in the liver of older rats would have to be committed to sustain maximal Na+-K+-ATPase activity, whereas only 30% would be required for young rats. If this requirement was not fulfilled, the osmoregulation and Na+-nutrient cotransport in liver of older rats would be compromised. On the basis of our studies, we propose that targeted nitration of the β-subunit is an early marker for nitrative stress and aging.
Keywords: adenosine 5′-triphosphate, adenosine 5′-triphosphatase, mitochondria, bioenergetics, biomarker
it has been shown that mitochondria produce nitric oxide and that this molecule modulates the oxygen consumption of the organelle by competitive and noncompetitive inhibition of cytochrome c oxidase (18, 30). Inhibition of oxygen consumption is transient as long as small quantities of nitric oxide are generated. However, at high or sustained levels of nitric oxide, the inhibition of the respiratory chain leads to an increased rate of formation of oxygen-free radicals that may damage mitochondria and/or initiate apoptosis (30). Other studies have demonstrated novel roles for nitric oxide through S-nitrosation of proteins (Ref. 43 and references therein). The finding that protein modification by nitrosation may be dynamically regulated and, in certain cases, coupled to cell surface signals has potential implications for other signaling pathways and cellular control mechanisms.
However, in addition to its transient effects mediated through interaction with cytochrome c oxidase and/or S-nitrosation of proteins, nitric oxide may exhibit relatively long-term effects through the nitration of proteins (32, 38). It has been proposed that C-nitration of proteins constitutes a novel chemical modification that occurs as a consequence of the “normal” oxidative background (39). Nitration of tyrosine residues is a posttranslational modification associated with more than 50 diseases, including shock, cancer, and stroke (6, 36, 38). Common nitrating agents are created from the reaction of nitric oxide produced by nitric oxide synthase1 and superoxide anion to form peroxynitrite or through enzymatic oxidation of nitrite to form·NO2 (6). Only a few nitrated proteins have been identified, thus restricting our understanding of the role of nitration in pathophysiological situations. Most of the studies did not demonstrate a direct correlation between the observed nitration and altered protein activity or document protein nitration that occurs as a consequence of “normal” oxidative/nitrative metabolism (1).
Previous work from our laboratory showed that purified, intact mitochondria from rat liver exhibited a higher specific Tyr nitration in particulate fractions [outer membrane (OM), inner membrane (IM), and contact site (CS), 11 ± 2 nmol nitrotyrosine/g protein] than in soluble ones [matrix (M) and intermembrane space (IMS), 2.5 ± 0.3 nmol nitrotyrosine/g protein] (25). Although we identified several mitochondrial proteins that were significantly nitrated in rat liver, we found no correlation between higher specific Tyr nitration and protein half-lives (assuming that longer-lived proteins may exhibit more extensive nitration), abundance (the more abundant the protein, the higher the extent of nitration), isoelectric point (pI), or size (the more Tyr residues, the more nitration) (25). Thus, colocalization of the target protein (i.e., target Tyr), the putative nitrating agent, and the presence of a consensus sequence for nitration (25) seemed to be the main factors that determined the likelihood of protein nitration. However, when mitochondria were actively producing nitric oxide (supplemented with l-arginine for 30 min), protein nitration significantly shortened the half-life of glutamate dehydrogenase, carbamoyl phosphate synthetase I, and the β-subunit of FoF1-ATPase (25). These results suggested that nitrated or otherwise modified proteins have a higher turnover rate, leading to this critical question: Is protein nitration quantitatively relevant to affect a specific, important mitochondrial function? To answer this question, we focused on the β-subunit of FoF1-ATPase, a protein that we recently found to be specifically nitrated at Tyr345 and Tyr368 in vitro (25). The purpose of this study was to further investigate whether these residues of the β-subunit were indeed the most susceptible to nitration under physiological conditions of endogenous nitric oxide release in rat liver mitochondria and whether such nitration correlated with decreased ATP synthase activity. Furthermore, to evaluate the possible effects of age on cell function, we performed all activity measurements with liver mitochondria isolated from rats ranging from 4 to 80 wk old.
EXPERIMENTAL PROCEDURES
Chemicals and biochemicals.
Mannitol, sucrose, EGTA, HEPES, fatty acid-free BSA, cytochrome c, DEAE cellulose, ADP, ATP, NAD+, NADP+, NADPH, NADH, lactic dehydrogenase, and normal goat serum were purchased from Sigma Chemical (St. Louis, MO). Goat anti-mouse IgG and mouse anti-nitrotyrosine were purchased from Upstate Biotechnology (Lake Placid, NY). Goat anti-rabbit IgG was purchased from Transduction Laboratories (Lexington, KY). Rabbit polyclonal anti-β-F1-ATPase was a kind gift from Dr. J. Cuezva of the Universidad Autónoma de Madrid (Madrid, Spain). Hexokinase, glucose-6-phosphate dehydrogenase, and alcohol dehydrogenase were purchased from Worthington (Lakewood, NJ). All other reagents were of analytical grade.
Animal studies.
The experiments described below were approved by the University of California Davis Animal Care and Use Committee. Male Wistar rats (of various ages as indicated) were purchased from a commercial supplier (Charles River Laboratories, Wilmington, MA). All cages were kept in a vivarium maintained at 22 ± 1°C, with a 12:12-h light-dark cycle, following National Institutes of Health guidelines for animal care and use. The lights were set to go out at 1900. Cage maintenance was conducted during the light cycle. Briefly, after arrival in our laboratory, rats were fed a commercially available diet and allowed a few days to relieve any stress that they may have incurred during the travel and get acquainted with the new location. Over the testing period, the rats had free access to their food 24 h/day, except when cage maintenance was conducted. On the 3rd or 4th day, the rats were removed from their cages and taken to the surgery room, where they were euthanized with CO2. Rats were euthanized between 9 and 10 AM. The livers were quickly removed from the rats, their wet weights were recorded, and they were rapidly homogenized for mitochondria isolation.
Isolation and subfractionation of rat liver mitochondria.
Percoll-purified mitochondria and their fractions were isolated from male Wistar rats, as described before (31).
Preparation of phosphorylating submitochondrial particles.
The subfractionation of mitochondria was accomplished by subsequent treatments with digitonin and sonication, as described previously (62). Submitochondrial particles (SMP) were prepared by a procedure similar to that described by Pedersen and Hullihen (56) with the following minor modifications. Mitoplasts (mitochondria with the OM removed) were prepared by adding digitonin to the mitochondrial suspension, prepared as described before. The mitoplasts were disrupted by sonication and the SMP collected from a centrifugation step (62). The inverted orientation of the inner mitochondrial membrane in SMP was determined from the rate of oxygen uptake in the presence of 10 mM succinate and stimulated by 100 μM cytochrome c essentially as described by Guerrieri and Papa (34). Under our experimental conditions, the percentage of inversion was found to be >95%. Since rat liver mitochondria are known to contain an ATPase inhibitor peptide [or IF1 (58)], it remained possible that SMP prepared as described above may contain a large population of IF1-inhibited ATPase molecules. To obtain SMP with low IF1, a final step that essentially followed the procedure described by Racker and Horstman (60) was incorporated with the following modifications. The step involving the chromatography through Sephadex G-50 was replaced by concentrating and purifying the SMP in Centricon tubes, with a nominal molecular mass cut off of 30 kDa. The retentate was washed three times with buffer (2 mM EDTA, 75 mM sucrose, 250 mM KCl, and 30 mM HEPES, pH 8.0) at room temperature. By doing this procedure, the ATPase activity increased by three to five times. SMP were characterized for contamination with other submitochondrial compartments by evaluating the activities of monoamine oxidase (OM), adenylate kinase (IMS), and malate dehydrogenase (M), as described before (25); the contamination with mitochondrial components present at compartments other than the IM was 2.3, 3.3, and 7.6%, respectively, of the activities present in the original rat liver mitochondria preparation.
Preparation of F1-deficient SMP and F1-enriched fractions and procedure for reconstituting F1 with F1-deficient SMP.
SMP were treated with urea to remove the F1 portion of the ATP synthase (56). These urea-treated particles were devoid of F1 but still contained the membrane sector subunits and the interface subunits of the ATP synthase. Residual ATPase activity of F1-stripped SMP, evaluated as described in Enzymatic assays, was always <10%, as described by others (56). The uncoupling agent FCCP, at concentrations that markedly stimulated the ATPase activity of intact mitochondria (10 μM), had no significant effect on the ATPase activity of urea particles (55). In addition, concentrating and purifying these urea particles through Centricon (NMWL = 30 kDa) was without a significant effect on the residual ATPase activity. These experiments showed that most of the SMP had no detectable IF1-inhibited ATPase. To monitor that the treatment with urea removed F1 but did not damage or alter the remaining Fo-bound SMP, the rate of oxygen consumption by F1-stripped SMP sustained by succinate (and inhibited by 3 μg/ml oligomycin) was followed as described by Pedersen and Hullihen (55).
The F1 portion was prepared from coupled [with respiratory control ratios of ≥5 (17)] mitochondria, using the procedure originally described by Catterall and Pedersen (15, 16). The F1 particles prepared by using this method were then separated by DEAE chromatography followed by Sephadex G-25. The different fractions were analyzed in parallel dot blots, utilizing antibodies against nitrotyrosine and β-subunit. The fractions that had the highest nitration level per area of β-subunit as indicated by the optical density ratio of nitrated Tyr/total β-subunit were pooled and lyophilized immediately. Reconstitution of F1 with the urea-treated particles was achieved using the procedure described in detail by Pedersen and Hullihen (56).
Immunoprecipitation procedure.
Mitochondrial fractions [T (total mitochondria), IM, and CS], each containing 100 μg of protein, were incubated for 1 h with either 2.5 μg/ml of antibody against ATPase β-subunit (catalog no. 612518; BD Biosciences) or 50 μg/ml of antibody against nitrotyrosine (mouse antibody, catalog no. 05-233; Upstate Biotechnology). Each of these samples (100 μl each) was then mixed with 0.9 ml of immunoprecipitation (IP) buffer [1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.2 mM sodium vanadate, 0.2 mM phenylmethanesulfonyl fluoride (PMSF), 0.5% NP-40] and 0.5% vol/vol of 10% protein A-agarose. The samples were then incubated for 1 h and centrifuged, and the pellet was washed three times with IP buffer. The pellets were resuspended in 50 μl of 2× Laemmli buffer, boiled for 5 min, and centrifuged, with the supernatants subsequently cooled on ice. The immunoprecipitated proteins were separated using 10% SDS-PAGE. Gels were used for Western blots of β-subunit or nitrotyrosine essentially as described below, except that blocking was carried out with either 1% Carnation nonfat dry milk (NFDM) or 1% NFDM supplemented with 250 μg/ml of mouse F(Ab)2 where indicated.
Evaluation of protein nitration under conditions of endogenous nitric oxide production.
Percoll-purified coupled rat liver mitochondria (1 mg protein/sample) were incubated with 0.1 mM l-arginine or NG-monomethyl-l-arginine (NMMA) and a respiratory substrate in an incubation medium containing calcium in amounts shown to be sufficient to elicit sustained production of nitric oxide (65). At each time point (0, 30, 60, and 120 min), mitochondria were pelleted and washed extensively to remove remaining l-arginine or NMMA, and the mitochondrial proteins were analyzed using two-dimensional (2D) gels (25). The gels were stained with SYPRO Ruby (Molecular Probes), and parallel ones were blotted and probed for nitrotyrosine as described for Western blots below. The spot on the 2D gels that was identified as the β-subunit of FoF1-ATPase using the respective antibody was excised, trypsinized in situ, and analyzed by matrix-assisted laser desorption/ionization time of flight [MALDI-TOF; as explained before; (25)] at the Mass Spectrometry Consortium for the Life Sciences, University of Minnesota, Twin Cities. To follow nitric oxide production, we performed two parallel assays, the NMMA-sensitive oxidation of oxymyoglobin in the presence of nitric oxide to yield metmyoglobin and the scintillation proximity assay by using beads labeled with l-arginine. The reaction medium for the former assay contained substrate (either malate-glutamate or succinate) and 0.1 mM oxymyoglobin. Oxymyoglobin was prepared as described previously (29). The reaction was monitored at 581–592 nm at 22°C, as described previously (29). For the latter assay, the activity of mitochondrial nitric oxide synthase was monitored using the scintillation proximity assay. Essentially, nitric oxide production was determined through the conversion of l-[3H]arginine to l-[3H]citrulline in the previous medium with mitochondria, l-arginine, and substrate, to which 0.1 μM l-[3H]arginine was added. Aliquots were taken at various time points, and the reaction was stopped by adding 1 mg of SPA Arg-binding beads (Amersham Biosciences) in 50 mM NaOH. Beads were allowed to settle for 2 h, and then the remaining amount of l-[3H]arginine in the reaction was determined by using the Yttrium Silicate SPA in conjunction with the Beckman 1450 MicroBeta plate counter.
2D gel electrophoresis.
Mitochondrial fractions were separated by pI on precast gel strips (IPG strips) with a linear gradient ranging from pH 3 to 10, using the Multiphor II with the Immobiline Strip tray and following the manufacturer's instructions (Amersham Pharmacia Biotech, Piscataway, NJ). The IPG strips were rehydrated overnight in rehydration buffer (6 M urea, 2 M thiourea, 2% NP-40, 2% IPG buffer, pH 3–10, 0.1 M dithiothreitol) prior to use. Twenty micrograms of mitochondrial sample was solubilized in the rehydration buffer for 30 min at room temperature prior to being separated, using the rehydrated strips. The voltage was provided by a Hoefer power supply PS 3501 XL, and the program utilized was as follows: 300 V for 1 min, followed by a linear gradient from 300 to 3,500 V for 1.5 h, then fixed voltage at 3,500 V for 4.5 h. The strips were preequilibrated with 6 M urea, 30% glycerol, 1% SDS, 5 mg/ml dithiothreitol, and 50 mM Tris, pH 6.8, for 10 min at room temperature prior to the samples being separated in the second dimension using SDS-PAGE; the 10% gel was run in a Hoefer SE600 overnight at 50 V. Gels were electroblotted and analyzed using antibodies against nitrotyrosine and the β-subunit of FoF1-ATPase using standard Western blot procedures (see below).
Enzymatic assays.
ATP synthesis was detected spectrophotometrically at 340 nm and 22°C by coupling the production of ATP to the reduction of NADP+ via the hexokinase and glucose-6-phosphate dehydrogenase reactions (57, 59). The assay mixture, in a final volume of 1 ml, contained 1 mM ADP, 10 mM potassium phosphate, pH 7.5, 10 mM succinate, 50 mM HEPES, 1 mM glucose, 1 mM NADP+, 2 mM MgCl2, 2 mg/ml fatty acid-free BSA, 0.2 M sucrose, 20 IU hexokinase, and 7.0 IU glucose-6-phosphate dehydrogenase. The reaction was initiated by addition of mitochondrial protein from 50 to 200 μg of SMP to ensure linear dependency of activity with protein. The rate of NADPH production was followed for 1–3 min, and then 5 μg/ml oligomycin was added and the reaction followed for another 2 min. The rate of NADPH production sensitive to oligomycin was taken as the rate of ATP synthesis.
ATPase activity was measured spectrophotometrically at 340 nm by coupling the production of ADP to the oxidation of NADH via the pyruvate kinase and lactic dehydrogenase reactions, as described previously (51). The reaction mixture, in a volume of 1 ml at pH 7.5 and 22°C, contained 4 mM ATP, 4.8 mM MgCl2, 0.40 mM NADH, 0.6 mM phosphoenolpyruvate, 5 mM potassium cyanide (KCN), 1 IU lactic dehydrogenase, 1 IU pyruvate kinase, 2.5 mM potassium phosphate, 80 mM HEPES, and 10–60 μg of SMP. Initial rates sensitive to oligomycin inhibition were used in all specific activity calculations (57). ATPase activity was also determined using a microplate approach, as described previously (27).
Western blots.
SDS-PAGE was performed using 10% gels. The gels were electroblotted and treated with monoclonal antibodies against nitrotyrosine (Upstate Biotechnology) or with serum raised in rabbits against the rat liver β-ATPase (kind gift from Dr. J. Cuezva, Universidad Autónoma de Madrid, Spain), as described previously (25, 27). To assure specificity for nitrotyrosine, blots were blocked with 10 mM nitrotyrosine or the mitochondrial samples were pretreated with 10 mM sodium persulfate (Na2S2O8) or ascorbic acid to reduce nitrotyrosine to aminotyrosine, a molecule not recognized by the antibody (Supplemental Fig. S1; Supplemental Material for this article can be found at the AJP-Endocrinology and Metabolism web site). The immunocomplexes were developed using an enhanced horseradish peroxidase-luminol chemiluminescence reaction (Amersham) and detected using photographic film (Hyperfilm ECL). The level of nitration was evaluated by processing the spots with the KodakImager 2000MMM and using the software provided by the manufacturer. The original gels were loaded with varying amounts of protein to ensure a linear response between chemiluminescence and protein. The specific nitration (defined as the nmol of nitrotyrosine/g protein) was determined using semiquantitative dot blots and nitrated BSA as a standard. The nitration of peroxynitrite-treated BSA was evaluated by dialyzing the protein after exposure to peroxynitrite, followed by acid hydrolysis, and evaluating the content of nitrotyrosine by using high-performance liquid chromatography (HPLC) with diode array and electrochemical detections. Protein was quantified using the Lowry assay and BSA as a standard (45).
HPLC with fluorescence detection for evaluation of nitrotyrosine in mitochondria.
HPLC samples were prepared by digesting mitochondria in the presence of proteinase K [1:20 (wt/wt), enzyme-protein] in 0.2 M phosphate buffer (pH 7.4) for 2–3 h at 50°C and then treating with a second equal aliquot of the protease and incubating for an additional 12–16 h. Following digestion, samples were divided into three parts. One was left untreated, the second was treated with 60 mM sodium dithionite to reduce nitrotyrosine to aminotyrosine, and the third was also reduced with 60 mM sodium dithionite and spiked with a known amount (10 pmol) of aminotyrosine. The digested samples were analyzed by reversed-phase HPLC using two C18 columns (4.6 × 250 mm) in line. A linear gradient was maintained at a flow rate of 0.8 ml/min as follows: from 100 to 98% mobile phase A (A) in 10 min, 98 to 50% A in 20 min, 50 to 25% A in 25 min, and then from 25 to 0% A in 40 min. Mobile phase A consisted of 50 mM sodium citrate and 50 mM acetic acid (pH 3.1), whereas mobile phase B was 10% methanol, 50 mM sodium citrate, and 50 mM acetic acid (pH 3.1). The eluted fractions were monitored with a diode array and fluorescence detectors (excitation and emission wavelengths 277 and 307 nm, respectively). The aminotyrosine quantification was performed by measuring the peak area and calculating its amount from a standard curve (ensured to be linear). The limit of detection using this method was <3 pmol/mg mitochondrial protein. Nitrated BSA was evaluated using the same method as for mitochondrial proteins.
As a control to ensure efficient proteolysis of mitochondrial proteins, azocasein hydrolysis was measured by following the absorbance of the azo dye at 390 nm. Proteolytic activity was evaluated using 1 mg/ml azocasein (Sigma Chemical) incubated with 1 mg/ml of mitochondrial protein that had been digested with proteinase K, as described above. Upon completion of proteolysis (when no significant changes in absorption at 390 nm were obtained), 10% trichloroacetic acid was added to the hydrolysate and samples were centrifuged for 10 min at 12,000 g. The supernatants were analyzed by following the absorbance at 390 nm. Absorbance measurements were compared with those of azocasein digested alone (100% digestion) and azocasein without proteinase K (0% proteolysis).
Statistical analysis.
The results presented represent the mean ± SD of three replicates from one experiment. Each experiment was carried out a minimum of three times, and the results shown represent all results obtained. The effect of treatment was compared with control values using one-way analysis of variance. Tests were carried out using StatSimple version 2.0.5 from Nidus Technologies (Toronto, ON, Canada).
RESULTS AND DISCUSSION
β-Subunit of the mitochondrial FoF1-ATPase is a preferential target of nitrative stress.
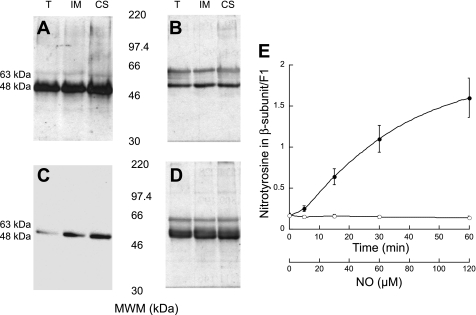
Previous work from our laboratory showed that particulate fractions of rat liver mitochondria exhibited the highest specific nitration (25). These results were confirmed in the present study by using immunoprecipitation assays targeting 3-nitrotyrosine from T, IM, and CS from livers of adult rats. Western blots of the purified, immunoprecipitated fractions were then probed for antibodies specific to 3-nitrotyrosine (Fig. 1A). Although several nitrated proteins had been found by our laboratory in rat liver mitochondria (25), when immunoprecipitation experiments were performed, only one major protein was pulled down. This can be understood by a combination of higher abundance of this nitrated subunit and/or a stronger binding of the antibody for the epitope present in this modified protein. This most intense band on the blot corresponded to an approximate molecular mass of 48 kDa, consistent with previously reported results (25). This protein was identified as the β-subunit of the mitochondrial FoF1-ATPase given its localization (only present in the IM and CS fractions and absent on 2D gels of ureatreated SMP; data not shown), molecular weight, cross-reactivity to antibodies against this subunit (Fig. 1B), and MALDI-TOF analysis of tryptic fragments. To confirm these results, immunoprecipitations of the same mitochondrial fractions (T, IM, and CS) against antibodies directed to the β-subunit were carried out. These immunoprecipitated fractions were analyzed using Western blots probed for 3-nitrotyrosine (Fig. 1C) and the β-subunit (Fig. 1D), and the results indicated a major hybridizing band at the same molecular weight as the bands evident in Fig. 1, A and B. An additional band at 63 kDa corresponded to the heavy chain of immunoglobulins from the immunoprecipitation procedure (Fig. 1, B and D), a result confirmed when the band was abrogated (Fig. 1, A and C) by addition of the Fab fragment [F(Ab)2] of unconjugated polyclonal IgG antibody against mouse to the blocking solution. The blots probed in Fig. 1, B and D, were not blocked using the Fab fragment. These results showed a specific nitration of the β-subunit in vivo.
Fig. 1.
Immunoprecipitation of nitrated proteins from mitochondria. Mitochondrial fractions [total (T), inner membrane (IM), contact sites (CS)] were immunoprecipitated with nitrotyrosine (A and B) or β-subunit antibodies (C and D). Western blots of the immunoprecipitated fractions were then probed for either nitrotyrosine (A and C) or β-subunit (B and D) antibodies. Blots in A and C were blocked with nonfat dry milk (NFDM) with the addition of F(Ab)2, whereas blots in B and D were blocked with NFDM only. This control was done to identify the band appearing at 63 kDa as the heavy chain of immunoglobulins from the immunoprecipitation procedure. E: l-argininne-supplemented rat liver mitochondria isolated from young rats were incubated for 0, 5, 15, 30, and 60 min (●). Samples at each time point were separated by two-dimensional gels, probed for nitrotyrosine, and then stripped and probed for the β-subunit by using Western blots. Control experiments were performed by incubating mitochondria with NG-monomethyl-l-arginine (○), an inhibitor of nitric oxide (NO) synthase. MWM, molecular weight markers.
To evaluate the effects of nitration under conditions of sustained production of nitric oxide, mitochondria were incubated with l-arginine for a period of 0–60 min. Control experiments were performed by treating mitochondria with an inhibitor of nitric oxide synthase, NMMA. After incubation at various times (0, 5, 15, 30, and 60 min), the reactions were stopped and 2D gels were run. These gels were first blotted and quantified for 3-nitrotyrosine and then stripped and reblotted for the β-subunit. The quantitative results are shown in Fig. 1E and indicate that the nitration of the β-subunit increased significantly during the first 30 min of incubation with l-arginine (Fig. 1E, •) and leveled off at 60 min with a value of ∼2 mol nitrotyrosine/mol F1, as assessed from the blots of 2D gels. This plateau suggests that there are only a certain number of reactive Tyr residues available for nitration; Tyr nitration might be limited by protein conformation (e.g., other subunits may affect the exposure of Tyr residues) and/or accessibility of the nitrating agent (e.g., hydrophilic vs. hydrophobic pockets).
Studies were directed at identifying the nitrated residue(s) in the β-subunit from the experiments conducted in adult rat liver shown in Fig. 1. The β-subunit from 2D gels containing control (+NMMA) and treated (+l-arginine) mitochondria was excised and subjected to tryptic digestion. The samples were then analyzed by tandem mass spectrometry (Supplemental Figs. S2 and S3). From the differential peptide masses, Tyr368 was identified as the predominant nitrated residue in samples obtained at time zero (i.e., no l-arginine added), whereas in l-arginine-supplemented mitochondria, both Tyr345 and Tyr368 were the predominant residues nitrated after 60 min. If Tyr nitration proceeds through the phenolate intermediate, then nitration at Tyr368 would proceed faster at pH 7.4 due to the lower pKa of its phenolic group (7.6 vs. 8.2) (11, 27). Previous studies from our laboratory performed in vitro (27) and the results presented here obtained under in vivo conditions support this chemical mechanism.
From these data, it is clear that specific Tyr nitration on the β-subunit is modulated in vivo and by the endogenous production of nitric oxide. However, one question puzzled us. Why is the β-subunit preferentially nitrated, as opposed to the α-subunit? Both subunits are similarly abundant and proximally located in the crystal structure (2), and in fact, the α-subunit has more Tyr residues (16 vs. 12). One possible explanation is the low homology (26%) shared by the mature proteins (vide infra; Fig. 2). The sequence alignment shows that Tyr345 and Tyr368 (indicated by asterisks in Fig. 2) of the β-subunit are substituted with Lys and Arg residues, respectively, in the α-subunit, suggesting that the higher resistance of this subunit to nitration could be due to these substitutions. Another possibility is that nitrating agents such as peroxynitrite are preferentially located to a pocket of the β-subunit containing Tyr345 and Tyr368. To show that the nitration was not simply an accessibility effect, we used the program naccess (http://www.bioinf.manchester.ac.uk/naccess/) to calculate the solvent-accessible surface area from the PDB 1BMF (2). The output file from the calculation (1BMF.rsa) that gives the residue specific accessibility results is given in the Supplemental Material, and it shows that the four most accessible Tyr residues in the β-subunits are 180, 345, 368, and 458. However, if nitration were only an accessibility effect, the α-subunits would also be significantly modified, since several Tyr residues (e.g., 256, 358, 397, 433, and 452) have a similar extent of accessibility. Taken together, these results suggest a possible combined effect that might lead to the specific nitration of Tyr345 and Tyr368 in the β-subunit in vivo: solvent accessibility, sequence homology, and surrounding environment of these Tyr to modify their pKa. Additionally, Tyr345 is involved in nucleotide binding and therefore has different accessibilities in the three β-subunits within F1-ATPase: nucleotide free (β), ADP bound (βDP), and AMP-PNP bound (βTP). Specifically, the apo-β state is highly accessible at Tyr345, the βTP is partially accessible, and βDP is almost completely solvent inaccessible (relative Tyr345 side chain accessibilities of 50.0, 24.1, and 6.5, respectively). For comparison, Tyr368 has relative accessibilities of 29.8, 16.5, and 14.9 in the β-, βDP-, and βTP-subunits, respectively. This suggests that Tyr345 and Tyr368 residues could be more susceptible to nitration in the absence of nucleotide.
Fig. 2.
Alignment of rat ATP synthase-α and -β chains. The sequences for these subunits of ATP synthase were downloaded from the SWISSPROT database and aligned with CLUSTALW. The alignment exhibited 24% identities (fully conserved residues: 100/401; dark gray) and 42% positive residues (conservation of strong groups: 174/401; light gray). *Tyr345 and Tyr368.
The second question to address was whether additional residues on the F1 α-, β-, or γ-subunits were modified in residues other than Tyr, such as Met and Cys. Since the oxidation of these other residues can be used as early markers for protein oxidation during oxidative and/or nitrative stress (22), this point was pertinent to address because mitochondria were subjected to nitrative stress (+l-arginine). The α-, β-, and γ-subunits of F1 particles from rat liver mitochondria (previously incubated with either l-arginine or NMMA) were therefore separated by 2D gels, identified by Western blots, isolated and trypsinized, and then analyzed by mass spectrometry similarly to the β-subunit for 3-nitrotyrosine. These results, shown in Table 1, indicated that Met residues in the β-subunit had been affected by the sustained production of nitric oxide and that this subunit presented the highest amount of Met sulfoxide. Although all of the Met residues in the subunits could not be detected, oxidation to the β-subunit was preferential over the α- and γ-subunits (Table 1). The implications of these findings will be discussed later in this article.
Table 1.
Met residues in F1 subunits
| Met Residues |
|||
|---|---|---|---|
| Experimental |
|||
| Subunit | Theoretical | Total | Oxidized* |
| α | 11 | 3 | 0.4 |
| β | 12 | 6 | 4.2 |
| γ | 9 | 6 | 0 |
Met residues based on the primary sequence of mature subunits (theoretical); Met (total) and Met sulfoxide (oxidized) residues detected by mass spectrometry (experimental).
Since oxidation of Met residues is quite common from residual persulfate (26, 53, 54) in polyacrylamide gels during and after digestion (67), horse heart myoglobin was used to quantify the % artifactual Met sulfoxide introduced in our system. Our experimental values obtained by mass sprectrometry analyses were corrected by those artifactually introduced (15 ± 2%). The 4 Met residues that were oxidized in the β-subunit are 113, 145, 127, and 272 (residue nos. correspond to the precursor rat subunit).
Impact of nitrative stress on ATPase activity.
The F1-ATPases contain six nucleotide-binding sites in the beef heart enzyme. Three of these sites bind and exchange nucleotides readily during catalysis (19, 21), suggesting a direct role in enzyme activity. In contrast, the three other sites, once filled with adenine nucleotides, exchange very slowly during turnover and are therefore called noncatalytic. Chemical modification studies with 2-azido-ATP have provided evidence that Tyr345 of the F1 β-subunit is located at catalytic sites of the beef enzyme, whereas Tyr368 is located at noncatalytic sites (20). Given the observed nitration of the rat β-subunit in vivo and in vitro, we investigated the effect of nitration on FoF1-ATPase activity by measuring ATP synthesis and hydrolysis rates through the use of coupled enzyme assays. These activities were evaluated in reconstituted samples consisting of either control or nitrated F1 with F1-deficient particles (or urea particles) from rat liver. The nitrated F1 particles were obtained from l-arginine-supplemented rat liver mitochondria incubated for 60 min, separated, and collected by chromatography. Control F1 particles were not subjected to l-arginine but were purified in a similar manner. Reconstitution of F1 with urea particles (also obtained from rat liver) provided the means for evaluating the sole effect of F1 subunits on the ATPase. Our results indicated that there was a reciprocal correlation between Tyr nitration of the β-subunit and the ATP hydrolysis and synthesis rates (Table 2); that is, the more nitration, the lower the rates. Since mass spectrometry does not provide quantitative information on the extent of nitration at each residue, we cannot comment on the relative contribution of each nitrated Tyr residue toward decreasing the synthesis and hydrolysis activities, only that both Tyr345 and Tyr368 were nitrated to some extent.
Table 2.
Effect of β-subunit nitration on ATP synthase activities
| SMP | mol Nitrotyrosine/mol Tyr β-Subunit | ATP Hydrolysis, nmol·min−1·mg protein−1 | ATP Synthesis, nmol·min−1·mg protein−1 |
|---|---|---|---|
| Control | 0.17 | 1,134 | 25.2 |
| Nitrated | 0.90* | 306* | 8.7* |
SMP, submitochondrial particles. Mitoplasts (prepared by digitonin treatment of mitochondria) were sonicated, and SMP were prepared by centrifugation (15). SMP were treated with urea to remove the F1 portion of the ATP synthase (24, 57). The F1 particles were separated by DEAE chromatography followed by Sephadex G-25. The fractions were anayzed by parallel dot blots with antibodies to nitrotyrosine and β-subunit (see experimental procedures). Fractions with the highest nitration per β-subunit were pooled and lyophilized immediately. Reconstitution of F1 with the urea particles was performed as described previously (69). Nitration was evaluated by HPLC. The enzymatic activities were measured as described in experimental procedures. Each value represents the mean of 3 independent determinations for which the SD was always ≤10%.
P < 0.01.
As mentioned in the previous section, in addition to the Tyr nitration, we also detected Met sulfoxide formation from mitochondria subjected to nitrative stress. Chemical modification to Met residues can be a double-edged sword, sometimes inducing conformational changes causing loss of protein activity (8, 9), whereas other modifications can have a crucial effect on protein interaction without large conformational changes or, at other times, protecting the protein by acting as an antioxidant (66). Nevertheless, considering that Met sulfoxide has been implicated in activity loss and the fact that oxidized Met residues detected (see Table 1) are located at conserved regions in the β-subunit, it could be speculated that these residues may have contributed to the activity loss. However, there are two observations that undermine this argument. 1) Substitution of Met with a slightly more polar amino acid, such as its sulfoxide derivative, does not substantially change the hydropathy index,2 and 2), most importantly, a double-point mutation (Y345F/Y368F) of the β-subunit completely preserved ATPase activity under nitrative stress in vitro (27). Similar experiments conducted on wild-type β-subunit documented ∼34% residual ATPase activity (28), a value that agrees well with the residual ATPase activity under nitrative stress reported in this study (∼27%; Table 2). These arguments suggest that the Met sulfoxide formation is not the main cause for decreased ATPase activity during nitrative stress.
Role of nitrated β-subunit in ATPase activity under in vivo conditions.
The experiments depicted above ascertained an effect of nitrated Tyr on ATPase activity but did not provide any information as to whether this effect was relevant to the animal under physiological and pathological conditions. To investigate this possibility, the level of nitration and the ATPase activities of SMP from animals of various ages, namely young (4 wk old), adult (16 wk old), and old rats (80 wk old), were evaluated. As demonstrated previously using mass spectrometry (see above), nitration of Tyr368 was evident in young rats, and nitration was predominantly of Tyr368 in adult rats, albeit with a minor contribution of nitration of Tyr345. In older animals, the nitration of both residues was significant. In experiments carried out under the same conditions, a decline in enzymatic activity, Vmax, was observed to accompany the increase in the nitration of the β-subunit (Table 3). These results corroborate the conclusions obtained previously with an in vitro system (27); nitration of Tyr368 and/or Tyr345 decreases the activity of ATPase, suggesting that maintenance of these residues in a nonnitrated state is critical to the full biological activity of this enzyme.
Table 3.
Kinetic parameters of ATPase and β-subunit nitration in rat liver mitochondria with aging
| Age, wk | Km, μM | Vmax, nmol·min−1·mg protein−1 | β-Subunit Nitration, pmol/mg protein |
|---|---|---|---|
| 4 | 300 ± 25 | 903 ± 10 | 191 ± 5 |
| 16 | 320 ± 37 | 800 ± 13* | 310 ± 7* |
| 80 | 340 ± 37 | 534 ± 12* | 381 ± 6* |
Values are means ± SE. The kinetic parameters shown in this table were obtained from Fig. 3.
P < 0.05 when compared with 4 wk old.
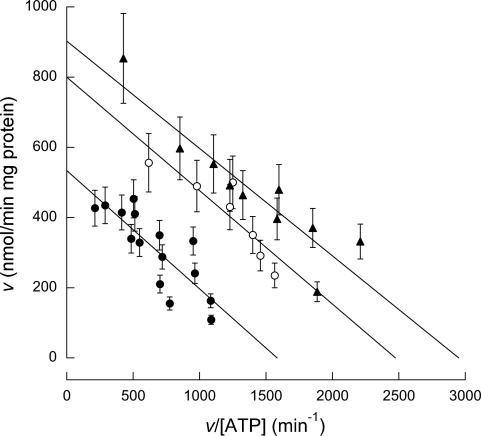
To provide a more complete understanding of how nitration of these Tyr residues leads to decreased ATPase activity, we performed kinetic studies on the ATPase from rats of different ages. To this end, the initial rates for ATP hydrolysis for each group were tested in the presence of 10–2,000 μM ATP (Fig. 3). Woolf-Augustinsson-Hofstee plots of the kinetic data resulted in parallel lines, indicating that the nitration of Tyr residues resulted in an effect similar to that observed with an irreversible, noncompetitive inhibitor. This suggests that, although nitration had no effect on substrate affinity (similar Km values; Table 3), nitration at either Tyr site created a substantial alteration to the ATPase structure that resulted in decreasing Vmax by a factor of two (Fig. 3 and Table 3). In agreement with this possibility, we have shown significant changes in the structure of ATPase as a result of nitration by modeling X-ray crystallography structures of F1 (27).
Fig. 3.
Activity of liver ATPase in young, adult, and old animals. The activity of ATPase was evaluated (following the procedure described in experimental procedures) in rat liver mitochondria from young (▲), adult (○), and old rats (●).
Lower ATPase activity impacts cellular functions in vivo.
It could be hypothesized that increased oxidative/nitrative stress that occurs as a result of aging (13, 33, 49) targets mitochondrial ATPase, resulting in the decrease of its activity. However, it could be argued that ATP-dependent biological activities can still be sustained with a lower supply of ATP without the cellular functions being compromised. To address whether lower levels of ATP would limit cellular functions, we tested the threshold of ATP required to sustain the maximal activity of Na+-K+-ATPase, a critical ATP-dependent activity in liver.
To this end, the oxygen consumption of liver slices from young and old rats was determined in the presence of 10 mM glucose. Most of the oxygen consumed by liver slices from young and old rats was attributed to oxidative phosphorylation because addition of KCN, an inhibitor of cytochrome c oxidase, caused a 70% inhibition in oxygen consumption in both samples. The remaining O2 consumption (KCN resistant) could be the result of uncoupling proteins, production of reactive oxygen species, microsomal enzymes, or other effects. In the presence of 1 mM KCN, the concentration of ATP in liver slices declined by 80–90%, regardless of the age of the animal, in agreement with the inhibition of oxygen consumption observed. When 1 mM ouabain, an inhibitor of Na+-K+-ATPase, was added to the liver slices from young and old rats, it resulted in a (average) 60% inhibition of the oxygen consumption, indicating that ion transport is coupled to energy utilization and ATP production in young and old rats and that the Na+-K+-ATPase activity is one of the main processes sustained by oxidative phosphorylation.
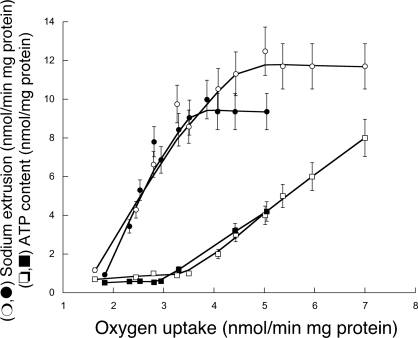
To determine the threshold of ATP concentrations that would be sufficient to support maximal Na+/K+ exchange in liver from young and old rats, liver slices from both groups were titrated with KCN, and oxygen consumption, ATP content, and Na+ extrusion were evaluated (Fig. 4). Our results indicated that to sustain maximal Na+-K+-ATPase activity in young rats (∼11 nmol·min−1·mg−1 protein; Fig. 4, ○), ∼2.4 nmol ATP/mg protein would be required (Fig. 4, ☐), a value in agreement with previously reported values (4, 28, 71). The Na+-K+-ATPase activity in older rats was 20% lower than that in young rats (n = 10, P < 0.05; Fig. 4). The lower Na+-K+-ATPase activity in liver is in agreement with the reported 20–70% decline of this enzyme with age in various brain regions (3, 41). The threshold of ATP to sustain maximal Na+-K+-ATPase activity in older rats was not significantly different from that observed for young rats (Fig. 4). The concentrations of ATP in the liver slices of young and old rats supplemented with glucose were 8.0 ± 0.5 and 4.2 ± 0.3 nmol ATP/mg protein, respectively (Fig. 4). Using the ATPase inhibitor oligomycin, we found that the ATPase activity of older rats was 60% that of control young rats, consistent with the ATP concentrations observed in liver and the data shown in Table 3. Considering the threshold of ATP for sustaining maximal Na+-K+-ATPase activity and the ATP concentration found in young rat liver (and assuming no significant changes in ATP production from substrate level phosphorylation), it can be expected that maximal Na+-K+-ATPase activity can be sustained even when 30% of the ATP would be available for this process. In contrast, 50% or more of the ATP concentration should be committed to sustain full Na+-K+-ATPase activity in older rats. Any additional changes, such as increased nitration of the FoF1-ATPase or increases in the demand for ATP of other processes, without the simultaneous increase in ATP via oxidative phosphorylation would directly affect the osmoregulation, the electrochemical Na+ gradient required for Na+-nutrient cotransport, and the action potential of excitable cells.
Fig. 4.
ATP concentrations, Na+-K+-ATPase activity, and oxygen uptake of rat liver slices incubated in the presence of different concentrations of potassium cyanide (KCN). Liver slices (0.4 μm) from young (○ and ☐) and old (● and ■) rats were incubated in Krebs-Henseleit buffer for 60 min at 30°C. After the slices in this medium were incubated (and after the medium was changed several times), the buffer was supplemented with various amounts of [KCN]. Oxygen consumption was evaluated with a Clark-type electrode. Tissue slice incubations, [ATP] (☐ and ■), and Na+ extrusion used to evaluate Na+-K+-ATPase activity (○ and ●) were performed as described previously by others (4, 71).
CONCLUSIONS
Mitochondria serve dual roles in cellular metabolism, that is, as the focal point of cellular bioenergetics as well as the centers of programmed cell death (63). The mitochondrial theory of aging postulates that intracellular reactive oxygen and nitrogen species produced during normal cellular metabolism cumulatively contributes to the progressive damage of macromolecules and, hence, organ dysfunction that collectively define aging in organisms (5, 14, 46, 72). Earlier studies in isolated mitochondria from young/aged animals were unable to provide a realistic picture since they ignored physiologically relevant regulatory cross-talks between mitochondria and cytosol/nucleus, and no clear molecular markers whose loss of function is correlated with a physiological dysfunction found in aging had been identified. Although the decline of ATP synthesis with age has been reported by several laboratories, the molecular mechanism for such decline is still poorly understood (35, 44, 47, 49, 61, 70). This study provides a molecular mechanism for the occurrence of this decline, which may result from the nitration of the β-subunit of the FoF1-ATP synthase. In 4-wk-old rats, nitration of the β-subunit was detected only at Tyr368, but the concentration of nitro-Tyr368 as well as that of Tyr345 increased with the animal's age. Importantly, this increase in nitrated Tyr residues had a detrimental impact on the activity of the FoF1-ATP synthase. This suggests that protein nitration, a stable posttranslational modification, is an important pathway by which the activity of an enzyme can be modulated. In addition, nitration of complex V may have a direct impact on cellular bioenergetics and maintenance of ATP levels, as documented in this study. Due to the detection limitations of mass spectrometry, a potential role for other nitrated residues in modulating β-subunit activity cannot be entirely excluded. However, in vitro studies performed with single and double mutants, the mutations of which involve these two particular Tyr residues, suggest that nearly all the activity loss observed in this study can be accounted for from nitration at only Tyr345 and Tyr368.
With regard to pathology, the amount of Tyr nitration needed to elicit a detrimental biological response is important. Studies performed with 5′-p-fluorosulfonylbenzoylinosine indicated that modification of Tyr345 in a single copy of the β-subunit led to inactivation of the ATPase, whereas all three copies must be modified at Tyr368 to completely inactivate the ATPase (10, 11, 27). These results suggested that the modification at Tyr345 might be a prevailing force in driving enzyme inactivation. Due to its proximity to a catalytic ATP binding site, nitrated Tyr345 might be expected to elicit a higher impact on activity loss than modification to Tyr368. However, the results reported here and those by us utilizing single/double mutations at these Tyr sites (27) suggest a more central role for Tyr368. Although Tyr345 might be closer to the catalytic binding site, it is also well known that long-range allosteric communication plays a crucial role in catalysis of this enzyme (12, 48).
Although the mechanisms by which β-subunit nitration increases with age may involve increased nitrative stress (13, 33, 49), a less efficient proteolytic system (7, 23, 50), or a deficiency in the antioxidant/repair system (37, 40, 64), it is clear from our studies that it leads to a significant decrease in ATP synthase activity. We hypothesize that most of the diverse and pathological changes that occur during aging could ultimately stem from inadequate ATP production, since that would lead to a variety of cellular dysfunctions, especially those that require a substantial amount of energy for their completion, such as Na+-K+-ATPase activity and biomolecular syntheses. The targeted nitration of the β-subunit documented here can certainly be used as an early marker for nitrative stress. Further studies of the regulation or prevention of β-subunit nitration should also reveal additional aspects of the aging process itself.
GRANTS
This study was supported by National Institute of Environmental Health Sciences Grants 012691 and 005707.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Theresa Sarkela for technical expertise during the immunoprecipitation studies.
Footnotes
REFERENCES
- 1.Abello N, Kerstjens HA, Postma DS, Bischoff R. Protein tyrosine nitration: selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J Proteome Res 8: 3222–3238, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Arivazhagan P, Panneerselvam C. Alpha-lipoic acid increases Na+K+ATPase activity and reduces lipofuscin accumulation in discrete brain regions of aged rats. Ann NY Acad Sci 1019: 350–354, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baines A, Ho P. Glucose stimulates O2 consumption, NOS, and Na/H exchange in diabetic rat proximal tubules. Am J Physiol Renal Physiol 283: F286–F293, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 120: 483–495, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion 1: 33–49, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Brot N, Weissbach H. Biochemistry and physiological role of methionine sulfoxide residues in proteins. Arch Biochem Biophys 223: 271–281, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Brot N, Weissbach H. Biochemistry of methionine sulfoxide residues in proteins. Biofactors 3: 91–96, 1991 [PubMed] [Google Scholar]
- 10.Bullough DA, Allison WS. Inactivation of the bovine heart mitochondrial F1-ATPase by 5′-p-fluorosulfonylbenzoyl[3H]inosine is accompanied by modification of tyrosine 345 in a single beta subunit. J Biol Chem 261: 14171–14177, 1986 [PubMed] [Google Scholar]
- 11.Bullough DA, Allison WS. Three copies of the beta subunit must be modified to achieve complete inactivation of the bovine mitochondrial F1-ATPase by 5′-p-fluorosulfonylbenzoyladenosine. J Biol Chem 261: 5722–5730, 1986 [PubMed] [Google Scholar]
- 12.Bullough DA, Brown EL, Saario JD, Allison WS. On the location and function of the noncatalytic sites on the bovine heart mitochondrial F1-ATPase. J Biol Chem 263: 14053–14060, 1988 [PubMed] [Google Scholar]
- 13.Byrne CD, Olufadi R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci (Lond) 116: 539–564, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med 25: 17–26, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Catterall WA, Pedersen PL. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem 246: 4987–4994, 1971 [PubMed] [Google Scholar]
- 16.Catterall WA, Pedersen PL, Lambeth DO, Lardy HA. Purification of F1 ATPase from rat liver mitochondria. Methods Enzymol 55: 320–328, 1979 [DOI] [PubMed] [Google Scholar]
- 17.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17: 65–134, 1956 [DOI] [PubMed] [Google Scholar]
- 18.Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: molecular mechanism and tissue physiology. Am J Physiol Cell Physiol 292: C1993–C2003, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Cross RL. The number of functional catalytic sites on F1-ATPases and the effects of quaternary structural asymmetry on their properties. J Bioenerg Biomembr 20: 395–405, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Cross RL, Cunningham D, Miller CG, Xue ZX, Zhou JM, Boyer PD. Adenine nucleotide binding sites on beef heart F1 ATPase: photoaffinity labeling of beta-subunit Tyr-368 at a noncatalytic site and beta Tyr-345 at a catalytic site. Proc Natl Acad Sci USA 84: 5715–5719, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross RL, Nalin CM. Adenine nucleotide binding sites on beef heart F1-ATPase. Evidence for three exchangeable sites that are distinct from three noncatalytic sites. J Biol Chem 257: 2874–2881, 1982 [PubMed] [Google Scholar]
- 22.Davies KJ, Delsignore ME, Lin SW. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem 262: 9902–9907, 1987 [PubMed] [Google Scholar]
- 23.Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology 66: S93–S96, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Drahota Z, Houstĕk J. A simple procedure for isolating adenosine triphosphatase from mitochondria. Biochim Biophys Acta 460: 541–548, 1977 [DOI] [PubMed] [Google Scholar]
- 25.Elfering SL, Haynes VL, Traaseth NJ, Ettl A, Giulivi C. Aspects, mechanism, and biological relevance of mitochondrial protein nitration sustained by mitochondrial nitric oxide synthase. Am J Physiol Heart Circ Physiol 286: H22–H29, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Fantes KH, Furminger IG. Polyacrylamide gel electrophoresis of highly purified chick interferon. Nature 216: 71–72, 1967 [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa Y, Kato K, Giulivi C. Nitration of tyrosine residues 368 and 345 in the beta-subunit elicits FoF1-ATPase activity loss. Biochem J 423: 219–231, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Galeotti T, van Rossum GD, Russo MA, Palombini G. Interaction of Na+ and K+ transport with aerobic energy metabolism in slices of Morris hepatoma 3924A. Cancer Res 36: 4175–4184, 1976 [PubMed] [Google Scholar]
- 29.Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J 332: 673–679, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giulivi C, Kato K, Cooper CE. Nitric oxide regulation of mitochondrial oxygen consumption I: cellular physiology. Am J Physiol Cell Physiol 291: C1225–C1231, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Giulivi C, Poderoso JJ, Boveris A. Production of nitric oxide by mitochondria. J Biol Chem 273: 11038–11043, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Gole MD, Souza JM, Choi I, Hertkorn C, Malcolm S, Foust RF, 3rd, Finkel B, Lanken PN, Ischiropoulos H. Plasma proteins modified by tyrosine nitration in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 278: L961–L967, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Goto S, Nakamura A, Radak Z, Nakamoto H, Takahashi R, Yasuda K, Sakurai Y, Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev 107: 245–253, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Guerrieri F, Papa S. Effect of chemical modifiers of amino acid residues on proton conduction by the H+-ATPase of mitochondria. J Bioenerg Biomembr 13: 393–409, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, Ames BN. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA 94: 3064–3069, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halliwell B, Zhao K, Whiteman M. Nitric oxide and peroxynitrite. The ugly, the uglier and the not so good: a personal view of recent controversies. Free Radic Res 31: 651–669, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci USA 100: 5634–5639, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys 356: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 298: 431–437, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Kang M, Akbarali HI. Denitration of L-type calcium channel. FEBS Lett 582: 3033–3036, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur J, Sharma D, Singh R. Acetyl-l-carnitine enhances Na(+), K(+)-ATPase glutathione-S-transferase and multiple unit activity and reduces lipid peroxidation and lipofuscin concentration in aged rat brain regions. Neurosci Lett 301: 1–4, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105–132, 1982 [DOI] [PubMed] [Google Scholar]
- 43.López-Sánchez LM, Muntané J, de la Mata M, Rodríguez-Ariza A. Unraveling the S-nitrosoproteome: tools and strategies. Proteomics 9: 808–818, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Lossa S, Lionetti L, Mollica MP, Crescenzo R, Botta M, Liverini G. Mitochondrial respiration and triiodothyronine concentration in liver from postpubertal and adult rats. Horm Metab Res 33: 343–347, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 46.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol 3: 214–220, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Nair KS. Age-related changes in muscle. Mayo Clin Proc 75, Suppl: S14–S18, 2000 [PubMed] [Google Scholar]
- 48.Nalin CM, Cross RL. Adenine nucleotide binding sites on beef heart F1-ATPase. Specificity of cooperative interactions between catalytic sites. J Biol Chem 257: 8055–8060, 1982 [PubMed] [Google Scholar]
- 49.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol 292: C670–C686, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Ngo JK, Davies KJ. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann NY Acad Sci 1119: 78–87, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Okamoto H, Sone N, Hirata H, Yoshida M, Kagawa Y. Purified proton conductor in proton translocating adenosine triphosphatase of a thermophilic bacterium. J Biol Chem 252: 6125–6131, 1977 [PubMed] [Google Scholar]
- 52.Panzenböck U, Stocker R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim Biophys Acta 1703: 171–181, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Patterson SD. Matrix-assisted laser-desorption/ionization mass spectrometric approaches for the identification of gel-separated proteins in the 5–50 pmol range. Electrophoresis 16: 1104–1114, 1995 [DOI] [PubMed] [Google Scholar]
- 54.Patterson SD, Aebersold R. Mass spectrometric approaches for the identification of gel-separated proteins. Electrophoresis 16: 1791–1814, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Pedersen PL, Hullihen J. Adenosine triphosphatase of rat liver mitochondria. Capacity of the homogeneous F1 component of the enzyme to restore ATP synthesis in urea-treated membranes. J Biol Chem 253: 2176–2183, 1978 [PubMed] [Google Scholar]
- 56.Pedersen PL, Hullihen J. Resolution and reconstitution of ATP synthesis and ATP-dependent functions of liver mitochondria. Methods Enzymol 55: 736–741, 1979 [DOI] [PubMed] [Google Scholar]
- 57.Pedersen PL, Hullihen J, Wehrle JP. Proton adenosine triphosphatase complex of rat liver. The effect of trypsin on the F1 and F0 moieties of the enzyme. J Biol Chem 256: 1362–1369, 1981 [PubMed] [Google Scholar]
- 58.Pullman ME, Monroy GC. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. J Biol Chem 238: 3762–3769, 1963 [PubMed] [Google Scholar]
- 59.Pullman ME, Racker E. Spectrophotometric studies of oxidative phosphorylation. Science 123: 1105–1107, 1956 [DOI] [PubMed] [Google Scholar]
- 60.Racker E, Horstman LL. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem 242: 2547–2551, 1967 [PubMed] [Google Scholar]
- 61.Sandhu SK, Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology 4: 19–29, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Schnaitman C, Greenawalt JW. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol 38: 158–175, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skulachev VP. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 11: 473–485, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Smallwood HS, Lourette NM, Boschek CB, Bigelow DJ, Smith RD, Pasa-Tolic L, Squier TC. Identification of a denitrase activity against calmodulin in activated macrophages using high-field liquid chromatography-FTICR mass spectrometry. Biochemistry 46: 10498–10505, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Solien J, Haynes V, Giulivi C. Differential requirements of calcium for oxoglutarate dehydrogenase and mitochondrial nitric-oxide synthase under hypoxia: impact on the regulation of mitochondrial oxygen consumption. Comp Biochem Physiol A Mol Integr Physiol 142: 111–117, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Stadtman ER, Moskovitz J, Berlett BS, Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem 234–235: 3–9, 2002 [PubMed] [Google Scholar]
- 67.Steen H, Mann M. Similarity between condensed phase and gas phase chemistry: fragmentation of peptides containing oxidized cysteine residues and its implications for proteomics. J Am Soc Mass Spectrom 12: 228–232, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Sun H, Gao J, Ferrington DA, Biesiada H, Williams TD, Squier TC. Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase. Biochemistry 38: 105–112, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Thayer WS, Rubin E. Effects of chronic ethanol intoxication on oxidative phosphorylation in rat liver submitochondrial particles. J Biol Chem 254: 7717–7723, 1979 [PubMed] [Google Scholar]
- 70.Tummino PJ, Gafni A. A comparative study of succinate-supported respiration and ATP/ADP translocation in liver mitochondria from adult and old rats. Mech Ageing Dev 59: 177–188, 1991 [DOI] [PubMed] [Google Scholar]
- 71.Van Rossum GD. The effects of oligomycin on energy metabolism and cation transport in slices of rat liver. Inhibition of oxidative phosphorylation as the primary action. Biochim Biophys Acta 423: 111–121, 1976 [DOI] [PubMed] [Google Scholar]
- 72.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.