Abstract
Exendin-4 (Ex-4), an agonist of the glucagon-like peptide-1 receptor (GLP-1R), shares many of the actions of GLP-1 on pancreatic islets, the central nervous system (CNS), and the gastrointestinal tract that mediates glucose homeostasis and food intake. Because Ex-4 has a much longer plasma half-life than GLP-1, it is an effective drug for reducing blood glucose levels in patients with type 2 diabetes mellitus (T2DM). Here, we report that acute administration of Ex-4, in relatively high doses, into either the peripheral circulation or the CNS, paradoxically increased blood glucose levels in rats. This effect was independent of the insulinotropic and hypothalamic-pituitary-adrenal activating actions of Ex-4 and could be blocked by a GLP-1R antagonist. Comparable doses of GLP-1 did not induce hyperglycemia, even when protected from rapid metabolism by a dipeptidyl peptidase IV inhibitor. Acute hyperglycemia induced by Ex-4 was blocked by hexamethonium, guanethidine, and adrenal medullectomy, indicating that this effect was mediated by sympathetic nervous system (SNS) activation. The potency of Ex-4 to elevate blood glucose waned with chronic administration such that after 6 days the familiar actions of Ex-4 to improve glucose tolerance were evident. These findings indicate that, in rats, high doses of Ex-4 activate a SNS response that can overcome the expected benefits of this peptide on glucose metabolism and actually raise blood glucose. These results have important implications for the design and interpretation of studies using Ex-4 in rats. Moreover, since there are many similarities in the response of the GLP-1R system across mammalian species, it is important to consider whether there is acute activation of the SNS by Ex-4 in humans.
Keywords: glucagon-like peptide-1, glycemia
the actions of glucagon-like peptide-1 (GLP-1) are essential for the normal control of blood glucose. GLP-1 augments insulin secretion after meals in a glucose-dependent manner and is thought to be a key component of the “incretin effect” (7). Because the endogenous GLP-1 signaling system promotes glucose homeostasis through a broad array of effects in addition to stimulation of insulin secretion (6a), there has been great interest in applying it to the treatment of diabetes. However, since GLP-1 has a very short half-life in the circulation due to rapid cleavage by the protease dipeptidyl peptidase IV (DPP IV) (19, 28), alternatives to the naturally occurring peptide have been sought. One of the primary strategies for using GLP-1 signaling in therapeutics has been the development of long-acting GLP-1 receptor (GLP-1R) agonists. Among these, exendin-4 (Ex-4), a reptilian peptide that has nearly 50% amino acid sequence homology with GLP-1 (10), has been developed into an effective drug to treat diabetic patients (8). Ex-4 lacks the discrete peptide sequence required for cleavage by DPP IV but otherwise shares virtually all the effects of GLP-1, including stimulation of insulin secretion, inhibition of glucagon release, reduction of food intake, and inhibition of gastrointestinal motility (16, 24, 29, 30, 36). Each of these actions contribute to lower glycemia, and numerous studies in diabetic rodent models demonstrate that chronic administration of Ex-4 increases insulin secretion, reduces body weight, and improves glucose tolerance and chronic hyperglycemia (17, 35, 39, 42). Although some of these actions are likely due to direct effects on end organs such as the pancreatic islet, several recent studies support neurally mediated effects of GLP-1 signaling to mediate blood glucose levels (23, 37). Interestingly, GLP-1R signaling in the central nervous system (CNS) is known to increase arterial blood pressure and heart rate through autonomic responses (5), activate the hypothalamic-pituitary-adrenal axis (20), and promote visceral illness responses through midbrain and hindbrain pathways (21, 34).
Because some of the CNS responses to Ex-4 activate glucose counterregulation, it is possible that this peptide's effects on glycemia are more complex than previously recognized. We report here a series of experiments demonstrating that acute administration of Ex-4 increases blood glucose levels in lean, nondiabetic rats. This novel effect is abolished by pretreatment with the GLP-1R antagonist exendin-3-(9–39) (Ex-9), appears to be mediated by increased sympathetic activity, which temporarily overrides the insulinotropism of Ex-4, and wanes with repeated administration of Ex-4 over several days. These findings suggest that pharmacological activation of neural GLP-1R can override the typical actions of GLP-1 signaling to reduce blood glucose, at least in rats.
MATERIALS AND METHODS
The procedures described here were approved by the Institutional Animal Care and Use Committees at the University of Vigo and the University of Cincinnati. All experimental procedures were carried out in accordance with the European Union regulations regarding the protection of animals used for experimental purposes (Council Directive CEE 86/609) or in accordance with the National Institutes of Health (NIH) Guidelines for the Care and Use of Laboratory Animals.
Drugs and Peptides
GLP-1-(7–36)-NH2, hexamethonium (HXM), guanethidine (GU), d-glucose, and pentobarbital sodium were all provided by Sigma-Aldrich (Alcobendas, Spain). Ex-9 was obtained from Bachem (Bubendorf, Switzerland). Ex-4 was obtained from Sigma-Aldrich and Bachem. Vildagliptin was kindly provided by Dr. Bryan Burkey (Novartis Institutes for Biomedical Research). Sterile 0.9% NaCl solution was used as vehicle.
Experimental Protocols
Acute effects of peripheral Ex-4 on blood glucose.
ACUTE EFFECTS OF EX-4 AND GLP-1 ON INTRAPERITONEAL GLUCOSE TOLERANCE IN ANESTHETIZED RATS.
Male Sprague-Dawley (SD) (275–325 g) rats were anesthetized with pentobarbital sodium (50 mg/kg), and a silastic cannula was placed in the right jugular vein. Intravenous (iv) injections of saline, Ex-4 (5 μg/kg), or GLP-1-(7–36)-NH2 (20 μg/kg) were given immediately before an intraperitoneal (ip) bolus of glucose (1.5 g/kg d-glucose) and blood samples taken at fixed intervals for 120 min. A second group of rats was fasted for 48 h, anesthetized, and given iv saline or Ex-4 (0.5, 1, 5, or 20 μg/kg iv). The blood glucose response was also tested in 48-h-fasted rats given Ex-4 (5 μg/kg) or the GLP-1R antagonist exendin-(9–39) (100 μg/kg ip) together or separately. Finally, groups of rats were given low (15 μg/kg) or high (75 μg/kg) doses of GLP-1 with or without pretreatment with vildagliptin (12 μg/kg ip at time −15 min). We have found in previous studies that a similar pretreatment efficiently inhibits DPP IV activity (Aulinger BA and D'Alessio DA, unpublished data).
ACUTE EFFECTS OF EX-4 IN CONSCIOUS RATS.
A permanent polyethylene catheter was placed in the right jugular vein under pentobarbital anesthesia (50 mg/kg) in SD rats 1 wk before challenge with Ex-4. On the day of the experiment, a heparin bolus (1,000 IU) was administered. Immediately, a blood sample was obtained and Ex-4 (5 μg/kg) or saline administered. Samples (250 μl) were taken under freely moving conditions. To avoid stress due to manipulation, rats were submitted to a sham-sampling procedure at least twice in the previous days before the experiment. The acute response to Ex-4 was tested as well in a cohort of conscious male Long-Evans rats. Ex-4 (15 μg/kg) was injected ip after an overnight fast, 30 min prior to an ip glucose tolerance test (IPGTT).
Effects of CNS administration of Ex-4 on blood glucose.
A permanent polyethylene cannula (PE-50, Intramedic; Becton Dickinson, Sparks, MD) was stereotactically implanted into the lateral ventricle of SD rats anesthetized with pentobarbital sodium (50 mg/kg), using the coordinates established by Paxinos (31). The rats recovered in individual cages for ≥7 days before the experiment. Ex-4 (0.1, 1, or 5 μg) was given intracerebroventricularly (icv) in 5 μl of saline to 24-h-fasted rats. Trunk blood was collected 1 or 2 h later as indicated in prechilled tubes and immediately centrifuged (3,500 rcf for 5 min at 4°C), and the serum was stored at −35°C prior to glucose determination. The correct placement of the cannula was confirmed by the staining of the third ventricle after postmortem injection of 10 μl of Trypan blue.
Mediation of Ex-4 actions by the autonomic nervous system.
HXM or GU (both at 30 mg/kg) was administered ip to male SD rats (275–325 g) 30 min before an ip (5 μg/kg), iv (5 μg/kg), or icv (1 μg) dose of Ex-4. Blood glucose was measured after 1 or 2 h, as indicated. To study specifically the involvement of the parasympathetic branch of the autonomic nervous system (ANS), a cervical vagotomy was performed. Under pentobarbital anesthesia, male SD rats (275–325 g) had both dorsal and ventral branches of the vagus nerve exposed at the cervical level and carefully severed. Control sham-operated animals underwent the same surgical manipulations, except that the nerve branches were not severed. The rats were injected ip with Ex-4 (20 μg/kg) or vehicle 20 min after vagotomy and blood samples were withdrawn from a catheter implanted in the right jugular vein. To study the involvement of the adrenal medulla, we studied the glucose responses to Ex-4 in medullectomized rats. SD rats were anesthetized with pentobarbital, and the adrenal glands were exposed. The cortex was finely cut with microsurgery scissors, and adrenal medulla was removed with fine forceps. After 1 wk of recovery, the animals were fasted overnight and challenged with Ex-4 (5 μg/kg ip) under pentobarbital anaesthesia and blood samples collected at specific time points through a sylastic catheter placed in the right jugular vein. A group of sham-operated rats was used as control. At end of the experiment, the rats were euthanized and adrenal glands collected for histological assessment of the adrenal medullectomy.
Effects of chronic administration of Ex-4 on blood glucose.
To investigate the effect of Ex-4 on blood glucose levels after chronic administration, two different experiments were performed.
REPEATED INJECTION OF EX-4.
Male Wistar rats (750–950 g) fed a high-fat diet (58% kcal from fat plus sucrose, D12331; Research Diets, New Brunswick, NJ) were injected ip with Ex-4 (10 μg/kg) or saline daily for 14 days before the onset of the dark phase. On the 14th day of the experiment, rats received their treatment, but food was withheld. The following morning, baseline blood glucose was measured, and all animals, both chronic Ex-4 and saline treated, were injected with Ex-4 (15 μg ip). Blood glucose levels were monitored, with measurements taken at 0.5, 1, 3, and 6 h after the injection.
CHRONIC SUBCUTANEOUS INFUSION OF EX-4.
Osmotic minipumps (1007D; Durect) delivering Ex-4 or saline were implanted subcutaneously under isoflurane-induced anesthesia into the intrascapular space of male Long-Evans rats (300–350 g) after an overnight fast. Glucose levels were measured before anesthesia and 3 and 6 h after surgical implantation of the minipumps while food was withheld. Both groups had ad libitum access to food during the first dark phase to elucidate the effect of a continuous infusion of Ex-4 and saline on food intake. For the rest of the study, we gave the saline-treated rats the same amount of food that was eaten by the Ex-4-treated rats. After 6 days of treatment with Ex-4 or saline, rats were fasted overnight, and an IPGTT was done while the minipump infusions continued. Body composition was measured before surgery and after the IPGTT using NMR imaging (Whole Body Composition Analyzer; EchoMRI).
Metabolite and Hormone Measurements
Plasma glucose was measured using a commercial kit based on the glucose oxidase method (Biomerieux). Whole blood glucose samples were measured with standard glucose strips (Free Style; Abbott Laboratories). Insulin, corticosterone, C-peptide, and glucagon levels were measured with commercially available radioimmunoassay kits (DRG Systems, Marburg, Germany, for insulin and corticosterone; and Linco Research, St. Charles, MO, for C-peptide and glucagon) according to the manufacturer's instructions. Metanephrine (MN) and normetanephrine (NMN) were determined using electrochemical detection after reverse-phase isocratic HPLC following cationic exchange chromatography.
Statistical Analysis
The data are presented as means ± SE. Student's t-test for independent samples was used for comparison between two groups. One-way ANOVA followed by Tukey's post hoc test or two-way ANOVA followed by Bonferroni's post hoc test was used for comparisons between multiple independent groups.
RESULTS
Acute Effects of Peripheral Ex-4 on Blood Glucose
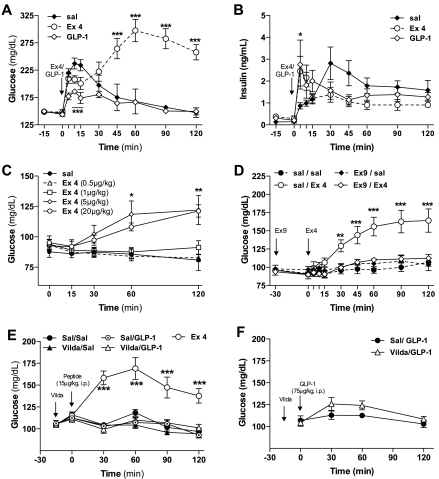
Intravenous administration of GLP-1 or Ex-4 to anesthetized ad libitum-fed rats reduced the immediate glucose excursion after an ip bolus of glucose (P < 0.05 for the 0- to 15-min time points; Fig. 1A). This effect was accompanied by the expected increase of insulin secretion (P < 0.05 for the 5-min time point; Fig. 1B). In saline- and GLP-1-treated rats, glycemia was maximal after 15 min and returned to baseline by 120 min (Fig. 1A). In marked contrast, starting 30 min after the glucose bolus, blood glucose levels of the Ex-4 group increased continuously up to values of 280 mg/dl at 60 min and remained above 250 mg/dl for ≤2 h (Fig. 1A). Despite the persistent hyperglycemia, plasma insulin levels in the rats given Ex-4 were comparable with the other groups.
Fig. 1.
Exendin-4 (Ex-4) increases blood glucose levels acutely in anesthetized rats. Glucose (A) and insulin levels (B) following intravenous (iv) administration of Ex-4 (5 μg/kg) or glucagon-like peptide-1 (GLP-1)-(7–36)-NH2 (20 μg/kg) combined with intraperitoneal (ip) glucose tolerance test (IPGTT; d-glucose, 1 g/kg) in ad libitum-fed anesthetized Sprague-Dawley rats (n = 7/treatment). C: dose-dependent glucose levels following iv administration of Ex-4 (0.5, 1, 5, and 20 μg/kg) in 48-h-fasted anesthetized Sprague-Dawley rats (n = 7). D: glucose levels following iv administration of Ex-4 (5 μg/kg iv) in anesthetized 48-h-fasted Sprague-Dawley rats pretreated with the GLP-1 receptor antagonist exendin-3-(9–39) (Ex-9) (100 μg/kg ip, −30 min) (n = 7). E and F: glucose levels following ip administration of GLP-1-(7–36)-NH2 (15 or 75 μg/kg) in 6-h-fasted Sprague-Dawley rats pretreated with vildagliptin (vilda) (12 mg/kg ip, −15 min) (n = 8). Values are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. saline (sal)-treated control group. Student's t-test or 2-way ANOVA with Bonferroni post hoc test for multiple comparisons.
The hyperglycemic activity of Ex-4 was reproduced in conditions in which the insulinotropic activity of the peptide is minimal, after 48 h of fasting in the absence of exogenous glucose (Fig. 1C). In this setting, Ex-4 caused a dose-dependent increase of plasma glucose 60 and 120 min after injection compared with saline-treated rats, with a threshold dose of 5 μg/kg to induce hyperglycemia (Fig. 1C). The hyperglycemic action of Ex-4 appeared to be mediated through the GLP-1R, since anesthetized rats pretreated with the GLP-1R antagonist Ex-9 before the administration of Ex-4 had glucose values similar to controls (Fig. 1D). In contrast to the acute hyperglycemia induced by Ex-4, GLP-1 did not have this effect, even when given at a very high dose and protected from rapid inactivation by coadministration of the DPP IV inhibitor vildagliptin (Fig. 1, E and F).
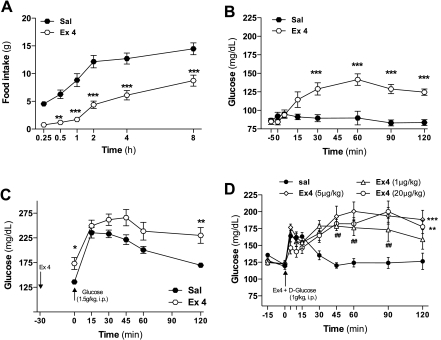
Peripheral administration of Ex-4 also induced a sustained increase in blood glucose levels in conscious rats at a dose that effectively reduces food intake. Ex-4 (5 μg/kg ip) significantly suppressed food intake 30 min after its administration in 24-h-fasted rats (Fig. 2A). The same dose of Ex-4 increased baseline glucose levels in freely moving overnight-fasted SD rats (Fig. 2B) and caused glucose intolerance following an ip bolus of glucose (Fig. 2C). These findings demonstrate that the acute effects of Ex-4 to cause hyperglycemia occur in conscious as well as anesthetized animals and are present in two commonly used strains of rats.
Fig. 2.
Peripheral administration of Ex-4 increases blood glucose levels in conscious rats. A: effect on food intake of Ex-4 (5 μg/kg ip) in 24-h-fasted Sprague-Dawley rats (saline, n = 10; Ex-4, n = 6). B: glucose levels following administration of Ex-4 (5 μg/kg ip) in overnight-fasted, conscious, freely moving Sprague-Dawley rats C: glucose levels following administration of Ex-4 (15 μg/kg ip) 30 min prior to an IPGTT (d-glucose, 1.5 g/kg) in overnight-fasted, conscious Long-Evans rats (sal, n = 7; Ex-4, n = 9). D: glucose levels following iv administration of several doses of Ex-4 (1, 5, and 20 μg/kg) combined with IPGTT (d-glucose, 1 g/kg) in ad libitum-fed anesthetized Sprague-Dawley rats (n = 7). Values are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. saline-treated control group. Two-way ANOVA for multiple comparisons.
To determine whether there is a dose-dependent dissociation of Ex-4 action to improve glucose tolerance and induce hyperglycemia later in the GTT, a range of doses of Ex-4 were combined with an ip bolus of glucose (Fig. 2D). None of the doses (1, 5, and 20 μg/kg) of Ex-4 had a significant effect to improve ip glucose tolerance, and all caused hyperglycemia in the later phase of the test. This result indicates that, in the rat, acute hyperglycemia is the predominant response across the dose range tested.
Central Effects of Ex-4 on Blood Glucose
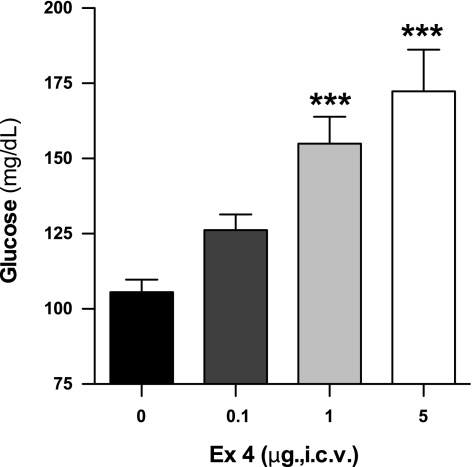
To determine the effects of CNS Ex-4 on blood glucose, Ex-4 was given icv to conscious rats in doses from 0.1 to 5 μg/rat. Intracerebroventricular Ex-4 increased fasting glucose levels in a dose-dependent fashion during 2 h of observation compared with saline-treated control rats (Fig. 3). These data indicate that both central and peripheral administration of Ex-4 can cause hyperglycemia.
Fig. 3.
Central administration of Ex-4 increases blood glucose levels in conscious rats. Effect of intracerebroventricular (icv) administration of Ex-4 (0.1, 1, and 5 μg) after 2 h on blood glucose levels of conscious, 24-h-fasted Sprague-Dawley rats (n = 9–12). Values are expressed as means ± SE. ***P < 0.001 vs. saline-infused control group, 1-way ANOVA.
The Hyperglycemic Effect of Ex-4 is Mediated Through the Sympathetic Nervous System
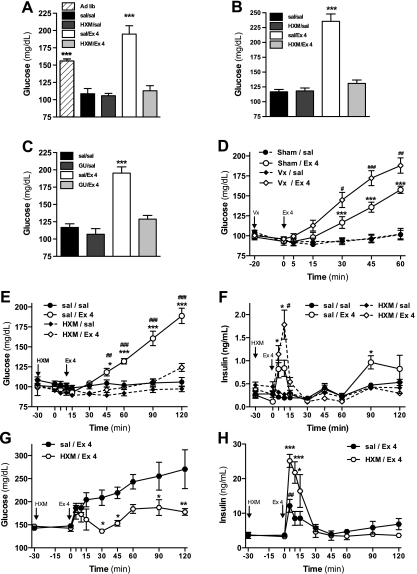
Because previous studies demonstrated effects of GLP-1 to activate the ANS, we hypothesized that hyperglycemia induced by Ex-4 is mediated though the ANS. To test this hypothesis, specific branches of the ANS were selectively blocked during peripheral and central Ex-4 administration. Fasted rats were pretreated with the preganglionic blocker HXM or saline, and Ex-4 or vehicle was given either ip (5 μg/kg; Fig. 4A) or icv (1 μg; Fig. 4B). HXM pretreatment alone had no effect on blood glucose levels in rats given saline ip or icv. Similarly to the previous experiments, Ex-4 caused an increase in blood glucose compared with control 1 h after its administration whether given ip or icv. This effect was completely blocked by HXM in both cases. These findings support a critical role for ANS signaling to mediate the acute effect of Ex-4 to raise blood glucose.
Fig. 4.
Ex-4 increases blood glucose levels through the sympathetic branch of the autonomous nervous system. A: glucose levels 1 h following ip administration of Ex-4 (5 μg/kg) in 72-h-fasted conscious Sprague-Dawley rats pretreated with the ganglionic nicotinic blocker hexamethonium (HXM) (30 mg/kg at −30 min; n = 6). B: glucose levels 1 h following icv administration of Ex-4 (1 μg) in 72-h-fasted conscious Sprague-Dawley rats pretreated with HXM (30 mg/kg at −30 min; n = 6). C: glucose levels 1 h following ip administration of Ex-4 (5 μg/kg) in 48-h-fasted conscious Sprague-Dawley rats pretreated with the inhibitor of the norepinephrine release guanethidine (GU) (30 mg/kg at −30 min; n = 6). D: glucose levels following ip administration of Ex-4 (20 μg/kg) in 72-h-fasted anesthetized Sprague-Dawley rats, previously vagotomy (Vx) at cervical level (−20 min; n = 8). Glucose (E) and insulin levels (F) following intravenous administration of Ex-4 (5 μg/kg) in 48-h-fasted anesthetized Sprague-Dawley rats pretreated with HXM (30 mg/kg, −30 min; n = 8). Glucose (G) and insulin levels (H) following iv administration of Ex-4 (5 μg/kg) combined with d-glucose administration (1.5 g/kg ip) in ad libitum-fed Sprague-Dawley rats pretreated with HXM (30 mg/kg, −30 min; n = 7–8). Values are expressed as means ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. saline-infused control group. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. nonpretreated (E and F), sham-operated Ex-4-injected group (D), or time 0 min (H). One- (A–C) or two-way ANOVA (D–F), Bonferroni post hoc test.
To determine the role of the sympathetic limb of the ANS in the hyperglycemic effect of acute Ex-4, fasted rats were pretreated with GU, which inhibits the release of norepinephrine from peripheral autonomic nerves, before ip administration of Ex-4 or saline (Fig. 4C). Similarly to HXM, GU had no independent effect on blood glucose under these experimental conditions. However, like HXM, GU abolished the hyperglycemic effect observed 1 h after ip Ex-4. These findings suggest an important role of sympathetic nervous system (SNS) signaling to mediate the acute hyperglycemic effects of Ex-4 in rats.
To investigate the involvement of the parasympathetic limb of the ANS in the hyperglycemic actions of Ex-4, both branches of the vagus nerve were surgically disrupted at the cervical level in fasted, anesthetized rats before the ip administration of Ex-4 (20 μg/kg) or saline. Surgical vagotomy did not affect basal glucose levels compared with the sham-operated controls. Ex-4 significantly increased the blood glucose levels after 30 min (Fig. 4D). Interestingly, the increase in blood glucose levels induced by Ex-4 was enhanced significantly in vagotomized rats. These data demonstrate that hyperglycemia induced by Ex-4 is not caused directly by vagal signals, although there seems to be some influence of the parasympathetic nervous system (PNS) to mitigate this response.
Pretreatment with HXM alone to block the ANS did not affect either basal glucose or insulin concentrations (Fig. 4 E–H) but attenuated Ex-4-induced hyperglycemia in rats with or without a glucose challenge (Fig. 4, E and G). Notably, Ex-4 induced a discrete but significant early increase in insulin secretion in the absence of any hyperglycemia (Fig. 4F). This increase of insulin secretion was not blocked but rather enhanced significantly by the pretreatment with HXM, even in conditions of GTT (Fig. 4, F and H). These results confirm our previous observation that a fully functional ANS is essential to induce the acute increase of blood glucose after the peripheral administration of Ex-4. However, there is an insulinotropic effect of Ex-4 that is apparent soon after administration, and this effect is independent of hyperglycemia and enhanced by the ANS blockade.
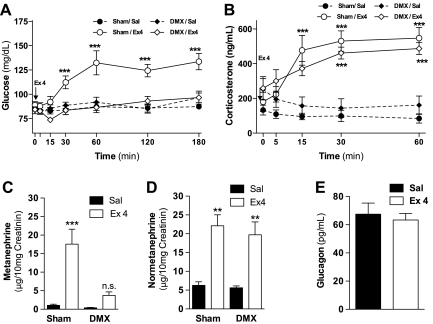
To study whether the catecholamines secreted by the adrenal medulla after the activation of the SNS could be responsible for the acute hyperglycemia, we administered Ex-4 in overnight-fasted rats that had previously undergone adrenal medullectomy. Ex-4 injection failed to induce hyperglycemia in medullectomized rats (Fig. 5A). The integrity of the adrenal cortex in these rats was demonstrated by postmortem histological analysis of the adrenal glands (data not shown) and by the increase in corticosterone induced by Ex-4 (Fig. 5B) as a result of the activation of the hypothalamic-pituitary-adrenal axis. Ex-4 increased urinary content of MN and NMN in sham-operated rats. Adrenal medullectomy prevented the increase of urinary MN, but not NMN, induced by Ex 4 (Fig. 5, C and D). Plasma glucagon levels 30 min after ip administration of Ex-4 or saline did not differ (Fig. 5E). These findings indicate that Ex-4-induced hyperglycemia is independent of glucocorticoid secretion or the direct effects of norepinephrine secreted by SNS efferents but highly dependent on epinephrine released from the adrenal medulla.
Fig. 5.
The adrenal medulla is required for increase in blood glucose levels induced by Ex-4. Glucose (A) and corticosterone levels (B) following ip administration of Ex-4 (5 μg/kg) in overnight-fasted anesthetized Sprague-Dawley rats, which had previously undergone adrenal medullectomy. Metanephrine (C) and normetanephrine (D) levels in urine accumulated in the 4 h following the administration of Ex-4 (5 μg/kg ip) in demedullectomized (DMX) rats. E: plasma glucagon levels 30 min following the administration of Ex-4 (5 μg/kg ip) Values are expressed as means ± SE (n = 7–8). **P < 0.01, ***P < 0.001 vs. saline-infused control group. Two-way ANOVA, Bonferroni post hoc test.
Waning of the Hyperglycemic Effect of Ex-4 After Chronic Administration
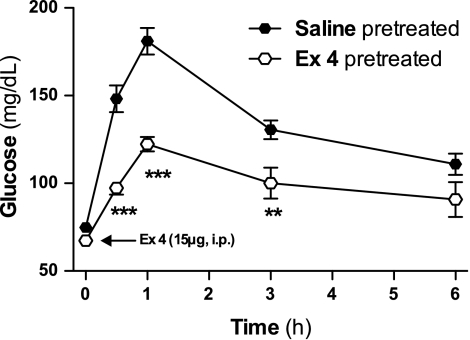
To determine whether the hyperglycemic effect of Ex-4 persists over time, glucose levels were measured after an acute challenge with ip Ex-4 (15 μg) in Wistar rats that had been pretreated with either Ex-4 (10 μg/kg ip) or saline daily for 14 days. Ex-4 induced a significant increase in glucose levels for ≤6 h in animals chronically exposed to saline or Ex-4 administrations (Fig. 6). However, this increase was significantly lower in rats that were previously pretreated with Ex-4 compared with saline-pretreated rats, suggesting that Ex-4-induced hyperglycemia is a phenomenon that is susceptible to adaptation.
Fig. 6.
Waning of the hyperglycemic effect of Ex-4 in Ex-4-pretreated rats. Glucose levels in 24-h-fasted diet-induced obese male Wistar rats after the injection of Ex-4 (15 μg ip). The rats had been injected daily with Ex-4 (10 μg ip) or saline for 10 days. Values are expressed as means ± SE (n = 10). **P < 0.01, ***P < 0.001 vs. saline-pretreated control group. Blood glucose was significantly higher (P < 0.001) at any time point vs. “0 h” for both saline- and Ex-4-pretreated groups. Two-way ANOVA, Bonferroni post hoc test.
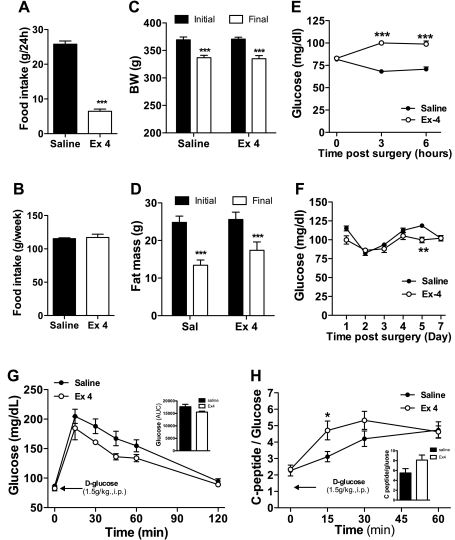
To confirm the apparent adaptation to the hyperglycemic effect of Ex-4, we infused Ex-4 (15 μg/day) or saline to Long-Evans rats for 7 days using osmotic minipumps. Rats infused with Ex-4 showed a significant decrease of food intake during the first dark phase (Fig. 7A). Thereafter, the saline-treated rats were pair-fed the same amount of food that was eaten by the Ex-4-treated animals (Fig. 7B) so that body weight and fat mass were comparable in both groups (Fig. 7, C and D). Consistent with our other observations, Ex-4 induced a significant acute increase in blood glucose levels seen 3 and 6 h after the start of treatment (Fig. 7E). However, after 1 day of treatment both the Ex-4- and saline-treated rats showed similar levels of glycemia (Fig. 7F). These results indicate that although the increase in blood glucose induced by Ex-4 is robust, it becomes attenuated with continuous exposure to the peptide. After 7 days of infusion, an IPGTT was performed in overnight-fasted Ex-4-treated and control rats. Glucose levels showed a strong trend to be lower in the Ex-4-treated rats (Fig. 7G). Although C-peptide levels did not differ statistically from the control group (data not shown), when corrected for differences in glycemia an insulinotropic effect of Ex-4 in treated rats was apparent (Fig. 7H).
Fig. 7.
Continuous chronic exposure eliminates the hyperglycemic effect of Ex-4. A: effect on food intake after the first dark phase following subcutaneous implantation of osmotic minipump containing Ex-4 (15 μg·rat−1·day−1, 7 days) or saline. B: total food intake of Ex-4-treated rats and the pair-fed control group at the end of the study. Body weight (C) and fat mass values (D) of the Ex-4- and saline-treated rats. Glucose levels over 6 h (E) and 7 days (F) following subcutaneous implantation of osmotic minipump containing Ex-4 or saline. Daily values were obtained before the onset of the dark phase, after fasting during the light phase. Glucose (G) and C-peptide/glucose ratio (H) following IPGTT (d-glucose, 1.5 g/kg) in overnight-fasted rats chronically infused for 7 days with Ex-4 or saline. Values are expressed as means ± SE. (A–F: n = 8; G–H: saline = 7, Ex-4 = 6) **P < 0.01 and ***P < 0.001 vs. saline-infused control group. Student's t-test or 2-way ANOVA, Bonferroni post hoc test.
DISCUSSION
Ex-4 combines robust GLP-1R agonism with resistance to degradation by DPP IV, making it a potent antidiabetic agent. The effects of Ex-4 to reduce blood glucose have been demonstrated in animal models of diabetes (38, 39, 42) and in diabetic patients. However, in contrast to these chronic effects of Ex-4 to promote glucose metabolism, the present set of experiments demonstrate that in rats Ex-4 induces an acute effect to increase blood glucose. Ex-4-induced hyperglyemia was dose dependent, developed 15–30 min after peptide administration independent of insulin secretion, and mediated by the GLP-1R. The doses of Ex-4 that cause this response are certainly pharmacological but include doses in the range that has been used to demonstrate chronic benefits on glucose tolerance in both rats and mice (15, 25). The acute effect of Ex-4 to raise blood glucose was observed with either peripheral or CNS administration of peptide and was abolished with sympathetic, but not parasympathetic, blockade and by adrenal medullectomy. These findings indicate that acute administration of Ex-4 activates the sympathetic nervous system and that this response is sufficient to cause hyperglycemia, even in the presence of augmented early insulin secretion. This novel set of observations indicates that the neural activity of Ex-4 in rats is complex and not uniformly protective of glucose homeostasis.
It is now well established that the GLP-1R is expressed in the peripheral and central nervous systems (20, 21, 23, 29, 33, 34, 37, 41) and can mediate a range of behavioral and metabolic effects. Recent findings suggest that some neural activation through GLP-1R signaling promotes glucose metabolism (23, 33, 37) by suppressing hepatic glucose production, increasing hepatic glucose uptake, and enhancing insulin secretion. However, the role of brain GLP-1R signaling on glucose metabolism is complex since chronic central administration of a GLP-1R antagonist seems to have beneficial effects in mice fed a high-fat diet (22). Ex-4 activates the SNS when given peripherally or centrally to mice (40, 41) and causes pressor and tachycardic responses in rats also through SNS activation (4, 5, 13, 14). Our results are consistent with these earlier findings in that an increase in SNS activity of sufficient magnitude to raise blood pressure could also increase blood glucose likely through enhanced hepatic glucose production. The sympathetic activation triggered by Ex-4 causes hyperglycemia independent of steady-state fuel availability, since the glucose increase occurs in rats fed ad libitum or given ip glucose as well as in rats after overnight and prolonged fasting. This effect of Ex-4 presents an important confounder of studies involving acute administration of the peptide in rat models.
Although acute administration of Ex-4 has been shown to stimulate insulin secretion and ameliorate hyperglycemia in animals, most of these demonstrations have been in mice (17, 42). In rats, the acute glycemic effects of Ex-4 are less clear. Parkes et al. (30) reported that iv Ex-4 caused a dose-dependent increase of insulin in anesthetized Lewis rats, without a significant effect on iv glucose tolerance, although both the treated and control animals in this experiment were relatively hyperglycemic with fasting values >10 mM. Similarly, Frangioudakis et al. (12) demonstrated that iv Ex-4 enhanced glucose-stimulated insulin secretion in anesthetized and conscious Wistar rats without significant differences in blood glucose compared with controls. These studies used Ex-4 doses of ≤10 μg/kg, comparable with those used in our experiments. Although they did not note an acute hyperglycemic effect of Ex-4, it is worth noting that their studies were relatively short, and they did not assess glucose across the time periods where we saw the most pronounced effects. However, several other groups have observed that acute administration of Ex-4 causes hyperglycemia. Chronic administration of Ex-4 subcutaneously, in doses similar to what were used in the present studies, increased blood glucose, ACTH, corticosterone, and catecholamines in diabetic rats (27). In addition, Aziz and colleagues (2, 3) gave Ex-4 ip in doses of 1.5 μg/kg to fasted Wistar rats and noted hyperglycemia compared with saline-treated controls, an effect that was potentiated by protein ingestion. Thus, although there is a paucity of reports on acute effects of Ex-4 on glucose metabolism in rats, there exist data that are compatible with the findings reported here.
Although hyperglycemia was prevented by the blockade of both branches of the ANS with HXM, this treatment actually enhanced insulin release stimulated by peripheral Ex-4. This likely reflects a reduction of catecholaminergic signaling in the pancreatic islets, since the catecholamines inhibit insulin secretion (32). The parasympathetic branch of the ANS (PNS) does not seem to contribute to the acute hyperglycemic effect of Ex-4. Rather, cervical vagotomy significantly enhanced Ex-4-induced hyperglycemia, suggesting that parasympathetic signaling provides some counterbalance against Ex-4-induced activation of the SNS. The effects of both the SNS and PNS are blocked by HXM, but specific pharmacological blockade of the sympathetic branch of the ANS by GU and adrenal medullectomy ameliorated Ex-4-mediated hyperglycemia. GU inhibits the release of norepinephrine, present in the presynaptic terminals of the SNS, including those innervating the adrenal medulla, which seems to be the critical mediator of Ex-4-induced hyperglycemia.
The administration of Ex-4 and GLP-1 in our studies was insulinotropic and lowered the glycemic response to iv glucose, at least in the early phases of the glucose tolerance tests. In contrast to Ex-4, GLP-1 did not trigger any later rise in blood glucose relative to control animals. However, since the hyperglycemia induced by Ex-4 was completely abolished by pretreatment with Ex-9, it seems likely that both peptides were acting through the GLP-1R. The differential effect of GLP-1 and Ex-4 cannot be explained by DPP IV metabolism of the former, since the addition of vildagliptin did not change the response to GLP-1. Although we cannot explain the disparate effects of these two GLP-1R agonists to cause acute activation of the SNS, we have recently reported differential effects of Ex-4 and GLP-1 to cause anorexia that are also metabolism independent (6).
Our data indicate that SNS stimulation by Ex-4 must wane with repeated exposure to the peptide because the hyperglycemic effect diminishes over time. It is notable that chronic exposure to Ex-4 tended to improve glucose tolerance, consistent with the many studies showing beneficial effects on glycemic control in chronic studies in rats (15, 26, 42). Disappearance of the hyperglycemic effect of Ex-4 with chronic administration suggests that the sympathetic mechanisms activated by Ex-4 are susceptible to the development of adaptation or desensitization with protracted exposure to the compound.
Previous reports in animal models show that GLP-1R signaling in the CNS mediates visceral illness (34) and the response to stress (20). Consistent with this, nausea and vomiting are the most common adverse effects of Ex-4 therapy in diabetic patients (1). There is little evidence for acute SNS activation by synthetic Ex-4 in humans, but this has not been a specific focus of research in this area. However, iv administration of Ex-4 to healthy volunteers did not affect heart rate or blood pressure (9). Nonetheless, SNS activation can inhibit gastrointestinal motility acutely and could contribute to the well-known side effects of Ex-4. Interestingly, these side effects reported in humans given Ex-4 disappear after the initial days of treatment (11, 18). Our findings taken in the context of these clinical observations raise the possibility of short-term SNS activation with pharmacological doses of Ex-4.
In summary, we have demonstrated a novel aspect of neural activation by Ex-4, namely hyperglycemia, an effect that was robust and demonstrable across a range of experimental settings in three commonly used strains of rats. Our results demonstrate a response to GLP-1R activation that appears to be specific for Ex-4, is mediated by the SNS, and raises blood glucose even in the face of potentiated insulin secretion. Since the effects we have demonstrated are the result of pharmacological stimulation of the GLP-1R, it seems unlikely that activation of the SNS is a usual component of regulation by endogenous GLP-1. Our findings raise important considerations for investigators using Ex-4 in studies of rats and support direct testing of whether effects derived from the activation of the SNS are also seen in other species and whether those effects could be related to adverse effects of drugs with GLP-1R activity.
GRANTS
This work was carried out with the financial support of the Xunta de Galicia, Spain (PGIDIT05PXIB31001PR to F. Mallo), and NIH (NIH-NIDDK-56863 to M. H. Tschöp and DK-57900-05 to D. A. D'Alessio).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Ahren B, Schmitz O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm Metab Res 36: 867–876, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aziz A, Anderson GH. Exendin-4, a GLP-1 receptor agonist, modulates the effect of macronutrients on food intake by rats. J Nutr 132: 990–995, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Aziz A, Anderson GH, Giacca A, Cho F. Hyperglycemia after protein ingestion concurrent with injection of a GLP-1 receptor agonist in rats: a possible role for dietary peptides. Am J Physiol Regul Integr Comp Physiol 289: R688–R694, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol Endocrinol Metab 277: E784–E791, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept 67: 63–68, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Barrera JG, D'Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58: 2820–2827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.D'Alessio DA, Vahl TP.Glucagon-like peptide 1: evolution of an incretin into a treatment for diabetes. Am J Physiol Endocrinol Metab 286: E882–E890, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281: E155–E161, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 267: 7402–7405, 1992 [PubMed] [Google Scholar]
- 11.Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD. Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev 20: 411–417, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Frangioudakis G, Gyte AC, Loxham SJ, Poucher SM. The intravenous glucose tolerance test in cannulated Wistar rats: a robust method for the in vivo assessment of glucose-stimulated insulin secretion. J Pharmacol Toxicol Methods 57: 106–113, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gardiner SM, March JE, Kemp PA, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol 154: 60–71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther 316: 852–859, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 146: 2069–2076, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268: 19650–19655, 1993 [PubMed] [Google Scholar]
- 17.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 42: 45–50, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 143: 559–569, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136: 3585–3596, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci 23: 6163–6170, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149: 4768–4777, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115: 3554–3563, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88: 3082–3089, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lamont BJ, Drucker DJ. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes 57: 190–198, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Li L, Yang G, Li Q, Tan X, Liu H, Tang Y, Boden G. Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab 10: 921–930, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Malendowicz LK, Neri G, Nussdorfer GG, Nowak KW, Zyterska A, Ziolkowska A. Prolonged exendin-4 administration stimulates pituitary-adrenocortical axis of normal and streptozotocin-induced diabetic rats. Int J Mol Med 12: 593–596, 2003 [PubMed] [Google Scholar]
- 28.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829–835, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Navarro M, Rodriquez de Fonseca F, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blázquez E. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 67: 1982–1991, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Parkes DG, Pittner R, Jodka C, Smith P, Young A. Insulinotropic actions of exendin-4 and glucagon-like peptide-1 in vivo and in vitro. Metabolism 50: 583–589, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Paxinos GW. The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic, 1998 [Google Scholar]
- 32.Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest 46: 86–94, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20: 1616–1621, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141: 1936–1941, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45: 1263–1273, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci 23: 2939–2946, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 110: 43–52, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 48: 1026–1034, 1999 [DOI] [PubMed] [Google Scholar]