Abstract
Prolonged endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) have been linked to apoptosis via several mechanisms, including increased expression of C/EBP homologous protein (Chop). Increased long-chain fatty acids, in particular saturated fatty acids, induce ER stress, Chop expression, and apoptosis in liver cells. The first aim of the present study was to determine the role of Chop in lipid-induced hepatocyte cell death and liver injury induced by a methionine-choline-deficient diet. Albumin-bound palmitate increased Chop gene and protein expression in a dose-dependent fashion in H4IIE liver cells. siRNA-mediated silencing of Chop in H4IIE liver cells reduced thapsigargin-mediated cell death by ∼40% and delayed palmitate-mediated cell death, but only at high concentrations of palmitate (400–500 μM). Similar results were observed in primary hepatocytes isolated from Chop-knockout mice. Indices of liver injury were also not reduced in Chop-knockout mice provided a methionine-choline-deficient diet. To ascertain whether ER stress was linked to palmitate-induced cell death, primary hepatocytes were incubated in the absence or presence of the chemical chaperones taurine-conjugated ursodeoxycholic acid or 4-phenylbutyric acid. The presence of either of these chemical chaperones protected liver cells from palmitate-mediated ER stress and cell death, in part, via inhibition of JNK activation. These data suggest that ER stress is linked to palmitate-mediated cell death via mechanisms that include JNK activation.
Keywords: lipoapoptosis, unfolded protein response, nonalcoholic fatty liver disease
nonalcoholic fatty liver disease (NAFLD) is a chronic disease syndrome initially characterized by a pure fatty liver (steatosis) with progression, in some, to nonalcoholic steatohepatitis and end-stage liver disease (1, 2, 14). Hepatocyte apoptosis is increased in patients with nonalcoholic steatohepatitis and correlates with disease severity (13, 60). Therefore, it has been proposed that a progression involving hepatic apoptosis, inflammation, and fibrosis contributes to the pathogenesis of NAFLD (8). A large portion of the elevated hepatic triglyceride stores in NAFLD appears to arise from reesterification of circulating free fatty acids (12). Elevated circulating free fatty acids are a characteristic feature of NAFLD and are positively correlated with liver disease severity (33). Chronic exposure of β-cells to elevated levels of fatty acids in vitro leads to impairments in insulin secretion and induces apoptosis, the latter termed “lipoapoptosis” (7, 47). In fact, exposure to elevated fatty acids, in particular long-chain saturated fatty acids, leads to cellular dysfunction and death in many cell types, including hepatocytes (11, 17, 27, 29, 59). Therefore, lipoapoptosis may be an important stimulus to disease progression in NAFLD.
Although recent evidence suggests that fatty acid-induced apoptosis involves activation of c-Jun NH2-terminal kinase (JNK) and FoxO3a (3, 29), the intracellular pathways regulating lipoapoptosis in hepatocytes remain unclear. Increased fatty acids, in particular long-chain saturated fatty acids, induce endoplasmic reticulum (ER) stress and activate the unfolded protein response (UPR) in a number of cell types, including hepatocytes (6, 41, 59). Prolonged, severe ER stress can target the cell for apoptosis via a number of putative mechanisms, including activation of caspases 7 and 12, interaction of Bcl-2 family proteins with the ER, extrusion of luminal calcium, and induction of CCAAT/enhancer-binding protein homologous protein (Chop)/growth arrest and DNA damage-inducible protein 153 (6, 18–21, 32). Overexpression of Chop can lead to cell cycle arrest and/or apoptosis (37). Recent evidence has demonstrated that Chop deficiency improved β-cell ultrastructure and promoted cell survival in genetic and diet-induced models of insulin resistance and reduced apoptosis and plaque necrosis in atherosclerotic lesions of Apoe−/− and Ldlr−/− mice (49, 53). The aims of the present study were to examine the role of Chop in palmitate-mediated cell death in liver cells and liver injury in response to a methionine-choline-deficient (MCD) diet and to determine whether ER stress was linked to palmitate-mediated cell death.
MATERIALS AND METHODS
Materials and reagents.
Fatty acids (Sigma Chemical, St. Louis, MO) were complexed to bovine serum albumin at a 2:1 molar ratio (27, 58). Thapsigargin (450 nM; Sigma), a tumor-promoting sesquiterpene lactone that induces ER stress via inhibition of the ER-associated calcium ATPase (52), was used as a positive control. Taurine-conjugated ursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid (PBA) were purchased from Sigma. SP600125, an anthrapyrazolone inhibitor of JNK, was purchased from Calbiochem (San Diego, CA).
Cell culture.
H4IIE liver cells (American Type Culture Collection, Manassas, VA), a rat hepatoma cell line, were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin sulfate (58, 59). Each experiment was performed in triplicate.
Animals.
Control C57BL/6J mice and Chop−/− mice (B6.129S-Ddit3tm1Dron/J) were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were housed in a temperature- and humidity-controlled environment with a 12:12-h light-dark cycle. Following a 1-wk acclimatization period, mice were either used for hepatocyte isolation (see below) or provided a purified control high-starch diet (no. D11724; Research Diets, New Brunswick, NJ) or MCD diet (no. 518810; Dyets, Bethlehem, PA) ad libitum for 3 wk. Experiments were performed on 6- to 8-h-fasted mice. All experimental protocols were approved by the Animal Use and Care Committee at Colorado State University.
Hepatocyte isolation and culture.
Hepatocytes were isolated from 6- to 8-h-fasted wild-type and Chop−/− mice immediately following the 1-wk acclimatization period using single-pass collagenase perfusion (4). Cells were first incubated in RPMI (HyClone, Logan, UT) containing 11 mM glucose, 10−7 M dexamethasone, and 10−7 M insulin on Matrigel-coated plates (for RNA) or on collagen-coated plates containing 5% fetal bovine serum (for protein) for 4 h (attachment period). The medium was then changed to one containing RPMI, 8 mM glucose, 10−7 M dexamethasone, and 10−8 M insulin. The following morning, experimental treatments were performed using RPMI that contained 8 mM glucose and 10−7 M dexamethasone. Each independent experiment was performed in triplicate.
RNA isolation and analysis.
Total RNA was extracted with TRIzol reagent using the manufacturer's protocol (Invitrogen, Carlsbad, CA). For analysis of X-box-binding protein-1 splicing, a two-step protocol was used for reverse transcription PCR using Superscript II reverse transcriptase and Taq polymerase (56). Real-time PCR was performed following reverse transcription using 0.5 μg of DNAase-treated RNA, Superscript II RnaseH, and random hexamers. PCR reactions were performed using transcribed cDNA and IQ-SYBR green master mix (Bio-Rad, Hercules, CA). Primer sets can be found in a previous publication (59). PCR efficiency was between 90 and 105% for all primer and probe sets and linear over five orders of magnitude. The specificity of products generated for each set of primers was examined for each fragment using a melting curve and gel electrophoresis. Reactions were run in triplicate and data calculated as the change in cycle threshold (ΔCT) for the target gene relative to the ΔCT for β2-microglobulin and cyclophilin (control genes) according to the procedures of Muller et al. (31). Results were similar regardless of the control gene used, and data in the results section are reported using β2-microglobulin.
siRNA and transfections.
CHOP siRNA (5′→3′: GGAAGAACUAGGAAACGGTT; antisense: UCCGUUUCCUAGUUCUUCCTT) was purchased from Ambion (Austin, TX). CHOP siRNA or control scrambled oligonucleotides (Ambion) were transfected into H4IIE cells using Lipofectamine RNAi Mix (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells were treated with thapsigargin or palmitate 24 h following transfections.
Western blot analysis.
Western blot analysis was performed as described in detail previously (40, 59). Membranes were incubated with antibodies against CHOP (Sigma), glucose-regulated protein 78 (Stressgen, Victoria, BC, Canada), growth arrest and DNA damage-inducible protein 34 (GADD34; Santa Cruz Biotechnology, Santa Cruz, CA), total and phosphorylated eukaryotic initiation factor 2α (Cell Signaling, Beverly, MA), and actin (Sigma). Proteins were detected with horseradish peroxidase-conjugated secondary antibodies (Amersham) and an enhanced chemiluminescence reagent (Pierce, Rockford, IL). Density was quantified using a UVP BioImaging system (UVP, Upland, CA).
Cell viability analysis.
Caspase-3 activity was determined with the colorimetric caspase-3 activation assay, which uses a caspase-specific peptide conjugated to the color reporter p-nitroanaline (R & D Systems, Minneapolis, MN). Caspase activity was normalized to cell lysate protein concentration. DNA fragmentation was evaluated using a modification of the protocol of Bialik et al. (5), as described previously (59). Cell death was also evaluated using the Cell Death Detection ELISA kit (Roche Diagnostics, Penzberg, Germany). This assay is based on the quantitative sandwich enzyme immunoassay principle using mouse monoclonal antibodies directed against DNA and histones. Cell number was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] Cell Proliferation Assay Kit (Biotium, Hayward, CA), which is based on the cleavage of the yellow tetrazolium salt MTT to purple formazan crystal by metabolically active cells.
JNK activity.
JNK activity was determined using the NH2-terminal c-Jun fusion protein bound to glutathione Sepharose beads (Cell Signaling). In this assay the reaction is initiated with 100 μM ATP, and Western blot analysis is used to detect the level of c-Jun phosphorylation using an antibody specific for Ser63 (57).
Liver histology.
Liver samples were fixed in 10% buffered formalin and processed by the paraffin slice technique. Sections were stained with hematoxylin and eosin (Premier Laboratory, Longmont, CO).
Biochemical analyses.
Plasma glucose levels were measured on an automated analyzer (Beckman Instruments, Fullerton, CA). Plasma insulin levels were determined by ELISA (Linco, St. Louis, MO). Plasma alanine aminotransferase and aspartate aminotransferase levels were determined using kits from Genzyme Diagnostics (Charlottetown, PEI, Canada).
Data analysis and statistics.
Statistical comparisons were calculated using analysis of variance and post hoc comparisons among means using the Scheffe's or Tukey's test. Statistical significance was set at P < 0.05. All data are reported as means ± SD.
RESULTS
Palmitate increases Chop gene and protein expression in H4IIE liver cells.
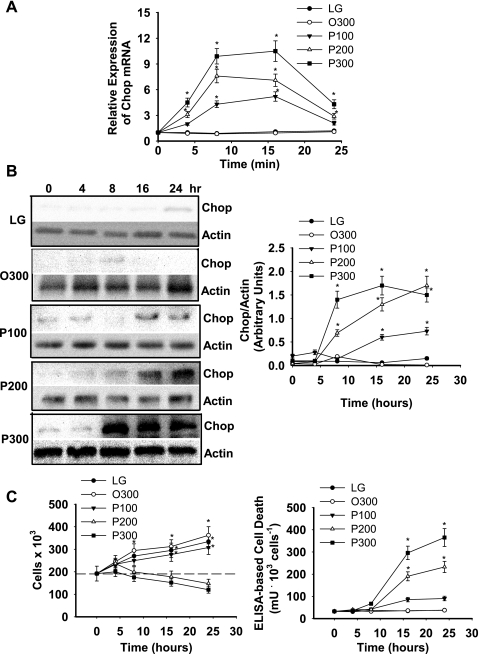
Palmitate increased Chop mRNA and protein in a dose-dependent fashion, reaching maximum levels between 8 and 24 h of incubation (Fig. 1, A and B). In contrast, oleate did not increase Chop mRNA or protein (Fig. 1, A and B). Net cell growth was reduced and cell death increased when liver cells were incubated in the presence of 200 or 300 μM palmitate (Fig. 1C). As demonstrated previously (41, 59), incubation of liver cells with palmitate, but not oleate, increased several markers of ER stress and UPR activation and caspase-3 activity (Supplemental Fig. S1; Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site).
Fig. 1.
Fatty acid-mediated regulation of Chop gene and protein expression. H4IIE liver cells were incubated for varying periods of time in control medium (LG), control medium supplemented with albumin-bound oleate at 300 μM (O300), or palmitate at 100 (P100), 200 (P200), or 300 μM (P300). A: Chop mRNA. B: Chop protein. C: cell number and ELISA-based cell death as a function of palmitate concentration and duration of exposure. Data in graphs are reported as the mean ± SD of triplicate samples from 5 to 7 independent experiments. Representative gels are shown for Chop and actin protein expression. *Significantly (P < 0.05) different from LG and O300.
siRNA-mediated silencing of Chop delays cell death but only at high concentrations of palmitate.
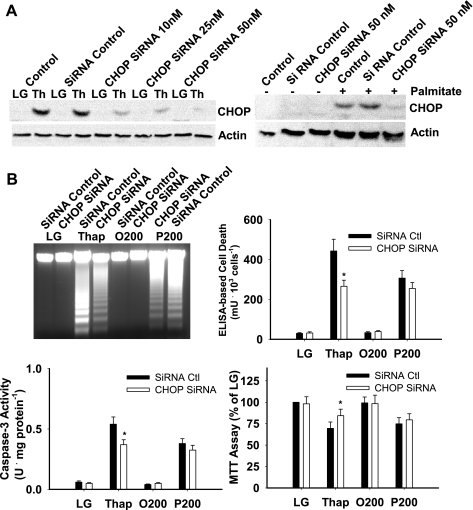
Chop siRNA (50 nM) reduced the induction of Chop protein in response to thapsigargin or palmitate by ∼90% (Fig. 2A). When all measurements of cell death (DNA fragmentation, ELISA, caspase-3 activity) and survival (MTT assay) were considered, siRNA-mediated silencing of Chop reduced cell death significantly in thapsigargin-treated cells but not in cells incubated with 200 μM palmitate over a 16-h period (Fig. 2B). We next examined whether Chop expression influenced palmitate-mediated cell death over a range of fatty acid concentrations and time points. siRNA-mediated silencing of Chop delayed but did not prevent cell death in response to high concentrations (400 and 500 μM) of palmitate (Table 1 and Supplemental Figs. S2 and S3).
Fig. 2.
Small interfering RNA (siRNA)-mediated knockdown of Chop. H4IIE liver cells were transfected with control siRNA or Chop siRNA, as described in materials and methods, and cells were harvested for Western blot or RNA analysis following a 16-h incubation in LG or control medium supplemented with thapsigargin (Thap; 450 nM), 200 μM albumin-bound oleate (O200), or 200 μM albumin-bound palmitate (P200). A: Western blot analysis of Chop and actin protein in cells that were not transfected (control) and cells that were transfected with either control scrambled oligonucleotides (siRNA control) or Chop siRNA at the indicated concentrations. The gel shown is representative of 4 independent experiments. B: DNA fragmentation, ELISA-based cell death, caspase-3 activity, and cell survival based on MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. Data in graphs are reported as the mean ± SD of triplicate samples from 5 independent experiments. Gel for DNA fragmentation is representative from a total of 5 independent experiments. *Significantly (P < 0.05) different from siRNA control.
Table 1.
Effects of Chop SiRNA on the time course of palmitate-mediated cell death in H4IIE liver cells
| 6 h |
9 h |
12 h |
16 h |
|||||
|---|---|---|---|---|---|---|---|---|
| Palm, μM | Ctl | siRNA | Ctl | siRNA | Ctl | siRNA | Ctl | siRNA |
| 100 | 33 ± 4 | 31 ± 5 | 36 ± 5 | 39 ± 4 | 65 ± 7 | 67 ± 8 | 92 ± 9 | 90 ± 11 |
| 200 | 29 ± 4 | 30 ± 3 | 33 ± 5 | 32 ± 4 | 101 ± 10 | 109 ± 11 | 177 ± 12 | 180 ± 13 |
| 300 | 35 ± 3 | 40 ± 4 | 96 ± 9 | 105 ± 11 | 196 ± 17 | 187 ± 16 | 256 ± 19 | 267 ± 22 |
| 400 | 39 ± 5 | 33 ± 4 | 137 ± 16 | 94 ± 9* | 276 ± 19 | 145 ± 16* | 361 ± 29 | 334 ± 30 |
| 500 | 26 ± 4 | 28 ± 2 | 195 ± 20 | 107 ± 11* | 363 ± 29 | 241 ± 27* | 427 ± 31 | 411 ± 39 |
Values are reported as means ± SD of triplicate samples from 6 independent experiments and are in mU/103 cells. Chop, CCAAT/enhancer-binding protein homologous protein; Palm, palmitate; Ctl, small interfering (si)RNA control; siRNA, Chop siRNA. Cell death was measured by ELISA, as described in materials and methods.
Significantly (P < 0.05) different from Ctl at corresponding time point.
Cell death is delayed in primary hepatocytes from Chop-knockout mice but only at high concentrations of palmitate.
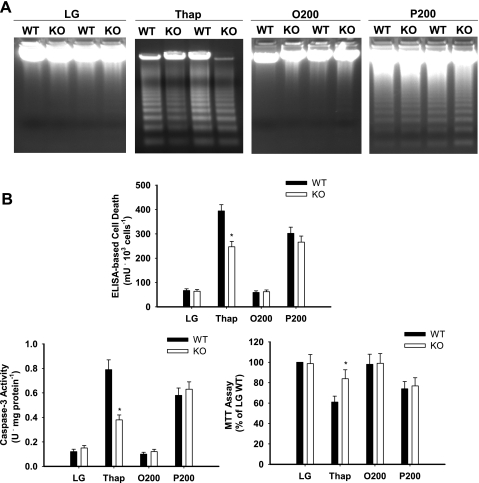
Thapsigargin and palmitate (200 μM) induced cell death and reduced cell survival in primary hepatocytes from wild-type mice (Fig. 3). Although thapsigargin-mediated cell death was reduced significantly and cell survival increased in primary hepatocytes from Chop-knockout mice, no significant reduction in cell death or increase in cell survival was observed in the presence of 200 μM palmitate (Fig. 3). There were no differences in several ER stress markers between wild-type and knockout mouse primary hepatocytes (Supplemental Fig. S4). We next examined whether Chop expression influenced palmitate-mediated cell death over a range of concentrations and time points. The absence of Chop delayed but did not prevent cell death in response to high concentrations (400 and 500 μM) of palmitate (Table 2 and Supplemental Fig. S5).
Fig. 3.
Fatty acid and Thap-mediated cell death in primary hepatocytes from wild-type (WT) and Chop-knockout (KO) mice. Primary hepatocytes were incubated in LG or control medium supplemented with Thap (450 nM), albumin-bound oleate at 200 μM (O200), or palmitate at 200 μM (P200) for 16 h. Measurements of cell integrity include DNA fragmentation (representative gel), ELISA-based cell death, caspase-3 activity, and MTT assay. Data in graphs are reported as the mean ± SD of triplicate samples from a total of 6 independent experiments. *Significantly (P < 0.05) different from WT.
Table 2.
Dose-response and time course of palmitate-mediated cell death in primary hepatocytes from WT and Chop-KO mice
| 6 h |
9 h |
12 h |
16 h |
|||||
|---|---|---|---|---|---|---|---|---|
| Palm, μM | WT | KO | WT | KO | WT | KO | WT | KO |
| 100 | 55 ± 6 | 61 ± 4 | 67 ± 7 | 68 ± 6 | 111 ± 12 | 120 ± 13 | 148 ± 13 | 144 ± 15 |
| 200 | 64 ± 7 | 61 ± 6 | 84 ± 9 | 81 ± 7 | 175 ± 20 | 188 ± 14 | 269 ± 24 | 255 ± 22 |
| 300 | 65 ± 5 | 71 ± 6 | 90 ± 10 | 82 ± 9 | 244 ± 21 | 227 ± 26 | 345 ± 26 | 337 ± 28 |
| 400 | 71 ± 6 | 68 ± 6 | 168 ± 13 | 104 ± 11* | 313 ± 25 | 128 ± 14* | 392 ± 30 | 351 ± 33 |
| 500 | 61 ± 5 | 59 ± 6 | 202 ± 17 | 111 ± 13* | 340 ± 27 | 186 ± 19* | 468 ± 34 | 439 ± 30 |
Values are reported as means ± SD of triplicate samples from 6 independent experiments and are in mU/103 cells. WT, wild type; KO, knockout. Cell death was measured by ELISA, as described in materials and methods.
Significantly (P < 0.05) different from Ctl at corresponding time point.
Indices of liver injury are not reduced in Chop-knockout mice fed a MCD diet.
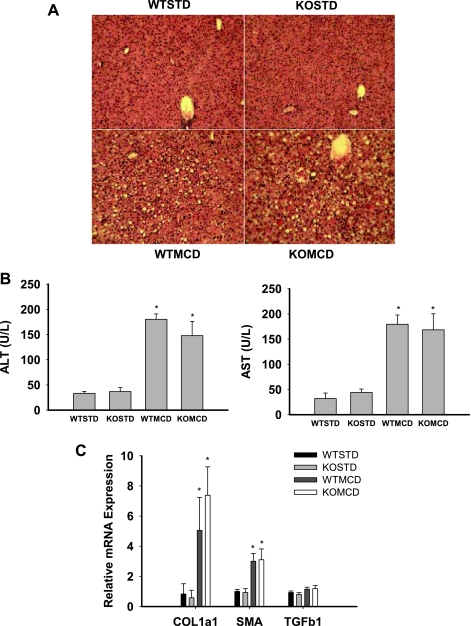
Diets deficient in methionine and choline induce hepatic steatosis, inflammation, and liver injury and are a widely used dietary model of nonalcoholic steatohepatitis (22, 24, 43, 46). Recent data have demonstrated that saturated fatty acids mediate hepatic toxicity in this model (26, 43). Therefore, we next examined the effects of Chop on liver injury induced by a MCD diet. Body weight, food intake, and plasma levels of glucose and insulin were not significantly different in wild-type and Chop-knockout mice fed the MCD diet (Supplemental Fig. S6). Hematoxylin and eosin staining revealed minimal steatosis and inflammation in wild-type and Chop-knockout mice on the control starch diet (Fig. 4A). In contrast, wild-type and Chop-knockout mice on the MCD diet developed histological evidence of gross hepatic steatosis and mixed inflammatory infiltrate (Fig. 4A). The MCD diet increased liver enzymes and the expression of collagen-α1 and α-smooth muscle actin mRNA in both wild-type and Chop-knockout mice (Fig. 4B). The expression of gene and protein markers of ER stress were not significantly differently between wild-type and Chop-knockout mice, with the exception of Chop and GADD34 (Supplemental Fig. S7), a gene that is regulated by Chop (30).
Fig. 4.
Liver injury in WT and Chop-KO mice. WT and Chop-KO mice were provided a purified high-starch diet (STD) or a methionine-choline-deficient (MCD) diet for 3 wk. A: hematoxylin and eosin staining. B: liver enzymes, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). C: collagen-α1 (COL1a1), α-smooth muscle actin (SMA), and transforming growth factor-β1 (TGFb1) mRNA from liver samples. Data in graphs are reported as the mean ± SD; n = 6/group. *Significantly (P < 0.05) different from STD.
Chemical chaperones reduce palmitate-mediated ER stress and cell death.
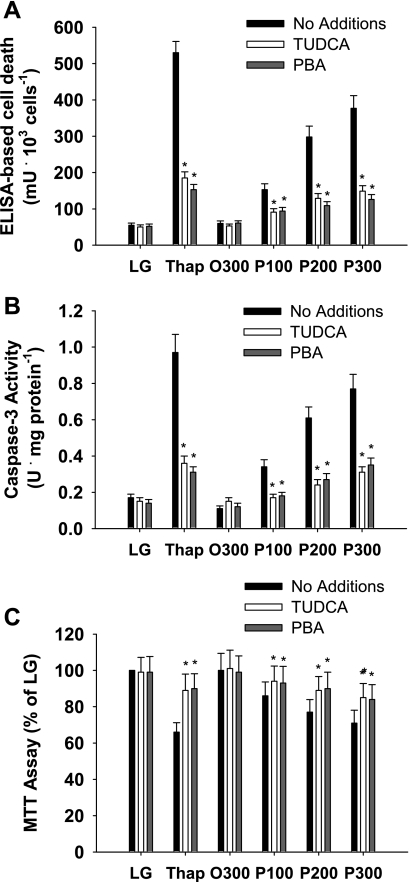
Chemical chaperones, such as glycerol, trimethylamine-N-amide, methyl-β-cyclodextrin, and PBA, represent a group of low-molecular-weight compounds that can stabilize protein conformation, improve ER folding capacity, and facilitate the appropriate trafficking of mutant proteins (23, 39). Endogenous bile acids and bile acid derivatives, such as TUDCA, can also modify ER function (16, 61). Previous studies have demonstrated that chemical chaperones reduce markers of ER stress in murine models of obesity and cell systems exposed to chemical inducers of ER stress or increased free fatty acid delivery (35, 39, 61). Given the limited role of Chop in the present study, we sought to establish a link between ER stress and palmitate-mediated cell death using chemical chaperones. TUDCA and PBA reduced markers of ER stress, cell death, and caspase-3 activation in response to thapsigargin and palmitate in primary hepatocytes from wild-type mice (Fig. 5 and Supplemental Fig. S8).
Fig. 5.
Cell integrity in primary hepatocytes isolated from WT mice. Primary hepatocytes were incubated in LG or control medium supplemented with Thap (450 nM), albumin-bound oleate at 300 μM (O300) or palmitate at 100 (P100), 200 (P200), or 300 μM (P300) in the absence or presence of chemical chaperones, taurine-conjugated ursodeoxycholic acid (TUDCA; 200 μM), or 4-phenylbutyric acid (PBA; 500 μM) for 16 h. Measurements of cell integrity include ELISA-based cell death (A), caspase-3 activity (B), and MTT assay (C). Data in graphs are reported as the mean ± SD of triplicate samples from a total of 6 independent experiments. *Significantly (P < 0.05) different from no addition.
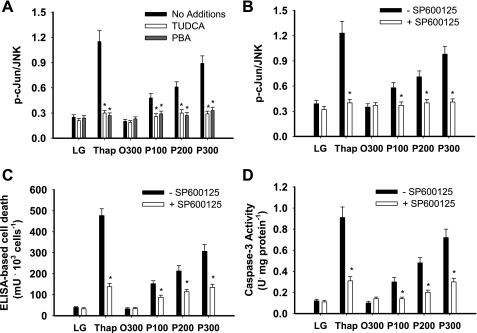
Previous studies have suggested that JNK activation is linked to fatty acid-mediated cell death, particularly at physiological fatty acid concentrations (29, 41). TUDCA and PBA reduced thapsigargin- and palmitate-mediated activation of JNK, and selective inhibition of JNK, using SP600125, also reduced thapsigargin- and palmitate-mediated cell death (Fig. 6).
Fig. 6.
JNK activity and cell integrity in primary hepatocytes. Primary hepatocytes were incubated in LG or control medium supplemented with Thap (450 nM), albumin-bound oleate at 300 μM (O300), or palmitate at 100 (P100), 200 (P200), or 300 μM (P300) in the absence or presence of chemical chaperones, TUDCA (200 μM), PBA (500 μM), or SP600125 (10 μM) for 16 h. A and B: ratio of phosphorylation of c-Jun to total JNK. C: ELISA-based cell death. D: caspase-3 activity. Data in graphs are reported as the mean ± SD of triplicate samples from a total of 6 independent experiments. *Significantly (P < 0.05) different from no addition or SP600125. Representative Western blots are provided in Supplemental Fig. S9.
DISCUSSION
Much of what we presently know about the UPR and its role in cell death has been derived from studies that employ pharmacological agents such as thapsigargin and tunicamycin, which induce severe, and typically unrecoverable, ER stress (20, 45). However, there have been an increasing number of studies that have observed ER stress and UPR activation in the context of normal physiological regulation and disease states characterized by prolonged pathogenic time courses (15, 25, 38, 45, 50, 55). The pathogenesis of NAFLD appears to include both ER stress and apoptosis (13, 44, 60). Increased fatty acids, a characteristic feature of obesity and NAFLD (33), in particular long-chain saturated fatty acids, induce ER stress, activate the UPR, and lead to cell death in hepatocytes (29, 59). The present study sought to determine whether the proapoptotic protein Chop contributed to cell death in response to palmitate and in MCD diet-induced steatohepatitis and to establish a link between ER stress and cell death in hepatocytes. Results demonstrate that Chop deficiency delays but does not prevent cell death in response to high palmitate concentrations and that ER stress appears to be linked to cell death, in part, via JNK activation.
Chop is among the best characterized of the UPR-regulated proapoptotic proteins (37). Chop expression is regulated by activating transcription factor (ATF)4 and perhaps ATF6, and the deletion of Chop provides some protection from ER stress-induced cell death in both cells and animals (20, 28, 34, 37, 45, 49). siRNA-mediated silencing of Chop suppressed celecoxib-induced apoptosis; thus it has been proposed that Chop plays a role in celecoxib-induced cell death in cervical cancers (21). In addition, targeted disruption of the Chop gene delayed the development of ER stress-mediated diabetes in Akita mice (36). Finally, Chop deficiency attenuated cholestasis-induced liver fibrosis (51). Thus, Chop not only plays a role in ER-stress mediated apoptosis but also may contribute to the development or progression of chronic diseases. In the present study, palmitate increased Chop mRNA and protein in H4IIE liver cells. siRNA-mediated silencing of Chop reduced thapsigargin-mediated DNA laddering, ELISA-based cell death, and caspase-3 activity and improved cell viability (MTT assay). siRNA-mediated silencing of Chop did not have a significant effect on any of these parameters in response to physiological concentrations of palmitate (100–300 μM). At high, and potentially unphysiological, concentrations of palmitate (400–500 μM), siRNA-mediated silencing of Chop delayed but did not prevent cell death.
We next examined palmitate-mediated cell death in primary hepatocytes isolated from wild-type and Chop-knockout mice. Although thapsigargin-mediated DNA laddering, ELISA-based cell death, and caspase-3 activity were all reduced significantly in hepatocytes isolated from Chop-knockout mice, palmitate-mediated cell death was not significantly different between wild-type and Chop-knockout mice hepatocytes at palmitate concentrations between 100 and 300 μM. Similarly to H4IIE liver cells, Chop deletion delayed but did not prevent cell death in response to high concentrations of palmitate (400–500 μM). Finally, we examined the role of Chop on liver injury in vivo in C57BL/6J wild-type and Chop-deficient mice provided a methionine-choline-deficient diet. This diet causes weight loss accompanied by steatosis, inflammation, and fibrosis in the liver (22, 24), and recent data suggest that saturated fatty acids mediate hepatic toxicity in this dietary model (26, 43). Chop deletion did not reduce steatosis, inflammation, gene markers of fibrosis, or gene markers of ER stress when studied after a 3-wk dietary period. In total, these data suggest that Chop plays a limited role in lipid-mediated cell death in hepatocytes. This role appears to be restricted to influencing the kinetics of cell death at high concentrations of palmitate.
Chop-mediated regulation of cellular integrity appears to be critical to pancreatic function, and a recent study demonstrated that Chop knockdown delayed palmitate-mediated cell death in INS-1E cells, FACS-purified primary β-cells, and human islets (9, 49). However, in the latter study, experiments were performed with palmitate concentrations of 500 μM; therefore, it is presently unclear whether Chop would also mediate cell death at lower, more physiological fatty acid concentrations in β-cells. It is also important to mention that at least one other study has demonstrated that although sustained induction of Chop is correlated with cell death in mature β-cells, Chop is not required for this process (48). It may be that palmitate-mediated upregulation of Chop reflects the presence of ER stress and UPR activation and/or may serve to regulate downstream targets that do not necessarily lead to cell death, such as DOCs (downstream of Chop), and/or influence the cellular redox state (37). Recent evidence has suggested that the relative instability of Chop may ensure that its expression reflects the actual stress level experienced by the cell (45). In this context, the ability of Chop to promote cell death in the face of UPR activation may be directly related to the magnitude of the stress imposed (45).
It has been postulated that chemical chaperones, such as TUDCA and PBA, have the ability to stabilize proteins in their native conformation and enhance ER functional capacity (10, 39, 42). Therefore, we used chemical chaperones to determine whether ER stress was linked to liver cell death in response to palmitate. The presence of TUDCA or PBA reduced palmitate-mediated ER stress markers, JNK activation, and cell death. In addition, chemical inhibition of JNK also reduced palmitate-mediated cell death. Taken together, these data support the notion that palmitate-mediated ER stress is linked to cell death via activation of JNK. Thus, our data are consistent with previous studies that have demonstrated the important role of JNK in fatty acid-mediated cell death in hepatocytes and JNK1 in the development of steatohepatitis in the methionine-choline-deficient diet model (29, 41, 46). Previous studies have demonstrated that IRE1α not only physically interacts with JNK but that IRE1α-mediated activation of JNK is an important determinant of obesity-induced insulin resistance in liver and adipose tissue (38, 54). The role of IRE1α in palmitate-mediated activation of JNK and cell death is currently under investigation.
The present study sought to examine the role of Chop in palmitate-mediated cell death in liver cells and liver injury in response to a methionine-choline-deficient diet. The results suggest that Chop has a limited role in lipid-mediated cell death and liver injury. Results also demonstrate that palmitate-mediated ER stress is linked to cell death, in part, via activation of JNK.
GRANTS
This work was supported by Grants DK-47416 and DK-072017 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Lillian Smith Foundation Endowment. C. L. Gentile was supported by NIDDK Institutional Training Grant T32-DK-007658-17.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
Present address of K. T. Pfaffenbach: USC Norris Cancer Center, 1441 E. Lake Ave., Los Angeles, CA 90033.
REFERENCES
- 1.Abdelmalek MF, Diehl A. Mechanisms underlying nonalcoholic steatohepatitis. Drug Discov Today Dis Mech 3: 479–488, 2006 [Google Scholar]
- 2.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ 172: 899–905, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem 282: 27141–27154, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Berry M, Friend D. High-yield preparation of isolated rat liver parenchymal cells. A biochemical and fine structural study. J Cell Biol 43: 506–519, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, Lord EM, Koch CJ, Kitsis RN. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest 100: 1363–1372, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 47: 2726–2737, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic B-cells from lipoapoptosis. Diabetes 54: 2917–2924, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Canbay A, Kip SN, Gieseler RK, Nayci A, Gerken G. Apoptosis and fibrosis in non-alcoholic fatty liver disease. Turk J Gastroenterol 16: 1–6, 2005 [PubMed] [Google Scholar]
- 9.Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Overbergh L, Mathieu C, Lupi R, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci 121: 2308–2318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem 282: 27905–27912, 2007 [DOI] [PubMed] [Google Scholar]
- 11.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res 38: 1384–1394, 1997 [PubMed] [Google Scholar]
- 12.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 125: 437–443, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev 5: 27–42, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Gass JN, Gunn KE, Sriburi R, Brewer JW. Stressed out B cells? Plasma-cell differentiation and the unfolded protein response. Trends Immunol 25: 17–24, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gentile CL, Pagliassotti MJ. The endoplasmic reticulum as a potential therapeutic target in nonalcoholic fatty liver disease. Curr Opin Investig Drugs 9: 1084–1088, 2008 [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res 60: 6353–6358, 2000 [PubMed] [Google Scholar]
- 18.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312: 572–576, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ikeyama S, Wang XT, Li J, Podlutsky A, Martindale JL, Kokkonen G, van Huizen R, Gorospe M, Holbrook NJ. Expression of the pro-apoptotic gene gadd153/chop is elevated in liver with aging and sensitizes cells to oxidant injury. J Biol Chem 278: 16726–16731, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Hwang CI, Park WY, Lee JH, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis 27: 1961–1969, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall Pde L. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 18: 1272–1282, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Kolter T, Wendeler M. Chemical chaperones—a new concept in drug research. Chembiochem 4: 260–264, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis 21: 89–104, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem 284: 5637–5644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem 276: 14890–14895, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318: 1351–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques 32: 1372–1379, 2002 [PubMed] [Google Scholar]
- 32.Nakagawa T. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403: 98–103, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci 46: 2347–2352, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366: 585–594, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118: 316–322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum-stress mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun CZ, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagliassotti MJ, Kang J, Thresher JS, Sung CK, Bizeau ME. Elevated basal PI 3-kinase activity and reduced insulin signaling in sucrose-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab 282: E170–E176, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Pagliassotti MJ, Wei Y, Wang D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-Jun NH2 terminal kinase activity. Endocrinology 148: 3338–3345, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res 52: 832–836, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Pickens MK, Yan JS, Ng RK, Ogata H, Grenert JP, Beysen C, Turner SM, Maher JJ. Dietary sucrose is essential to the development of liver injury in the MCD model of steatohepatitis. J Lipid Res In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134: 568–576, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4: 2024–2041, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology 43: 163–172, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95: 2498–2502, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skalet AH, Isler JA, King LB, Harding HP, Ron D, Monroe JG. Rapid B cell receptor-induced unfolded protein response in nonsecretory B cells correlates with pro- versus antiapoptotic cell fate. J Biol Chem 280: 39762–39771, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167: 35–41, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol 294: G498–G505, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular calcium stores by specific inhibition of the endoplasmic reticulum calcium ATPase. Proc Natl Acad Sci USA 87: 2466–2470, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab 9: 474–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 147: 943–951, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology 147: 350–358, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Wei Y, Pagliassotti MJ. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: implications for hepatic insulin resistance. Am J Physiol Endocrinol Metab 287: E926–E933, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem 303: 105–113, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291: E275–E281, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 44: 27–33, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 36: 592–601, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.