Abstract
The cellular mechanisms whereby prior exercise enhances insulin-stimulated glucose transport (GT) are not well understood. Previous studies suggested that a prolonged increase in phosphorylation of Akt substrate of 160 kDa (AS160) may be important for the postexercise increase in insulin sensitivity. In the current study, the effects of in vivo exercise and in vitro contraction on subsequent insulin-stimulated GT were studied separately and together. Consistent with results from previous studies, prior exercise resulted in an increase in AS160 642Thr phosphorylation immediately after exercise in rat epitrochlearis muscles, and this increase remained 3 h postexercise concomitant with enhanced insulin-stimulated GT. For experiments with in vitro contraction, isolated rat epitrochlearis muscles were electrically stimulated to contract in the presence or absence of rat serum. As expected, insulin-stimulated GT measured 3 h after electrical stimulation in serum, but not after electrical stimulation without serum, exceeded resting controls. Immediately after electrical stimulation with or without serum, phosphorylation of both AS160 (detected by phospho-Akt substrate, PAS, antibody, or phospho-642Thr antibody) and its paralog TBC1D1 (detected by phospho-237Ser antibody) was increased. However, both AS160 and TBC1D1 phosphorylation had reversed to resting values at 3 h poststimulation with or without serum. Increasing the amount of exercise (from 1 to 2 h) or electrical stimulation (from 5 to 10 tetani) did not further elevate insulin-stimulated GT. In contrast, the combination of prior exercise and electrical stimulation had an additive effect on the subsequent increase in insulin-stimulated GT, suggesting that these exercise and electrical stimulation protocols may amplify insulin-stimulated GT through distinct mechanisms, with a persistent increase in AS160 phosphorylation potentially important for increased insulin sensitivity after exercise, but not after in vitro contraction.
Keywords: insulin sensitivity, Akt substrate of 160 kDa, TBC1D1, TBC1D4, glucose transporter 4
a single bout of exercise leads to a subsequent increase in insulin-dependent glucose transport that can last for hours after exercise (6, 8, 10, 12, 21, 29, 31). The enhanced insulin-stimulated glucose transport postexercise occurs as a result of greater insulin-stimulated cell-surface GLUT4 localization (20), but the cellular mechanisms that lead to this event are not well understood. Many studies have found that prior exercise does not amplify insulin effects on proximal insulin signaling steps [e.g., insulin receptor tyrosine kinase activity, insulin receptor substrate tyrosine phosphorylation, insulin receptor substrate-associated phosphatidylinositol 3-kinase activity, protein kinase B (Akt) serine phosphorylation, and Akt activity] (3, 13, 18, 20, 22, 36, 38, 39). These results suggested that exercise might improve insulin sensitivity by altering an insulin signaling step distal to Akt.
The first substrate of Akt to be linked to the regulation of GLUT4 translocation was Akt substrate of 160 kDa (AS160; also known as TBC1D4) (23, 33). Under basal conditions, AS160's active Rab GTPase-activating protein domain is believed to restrain the exocytosis of intracellular GLUT4 storage vesicles of 3T3-L1 adipocytes (7, 9, 24, 32, 33). Insulin-stimulated phosphorylation of AS160 on specific Akt motifs, with 642Thr being especially important, appears to relieve this restraint and allow GLUT4 to be recruited to the cell surface membranes.
Bruss et al. (4) demonstrated that either insulin or in vitro contractile activity leads to phosphorylation of AS160 in skeletal muscle. Arias et al. (1) found that AS160 phosphorylation is also elevated in rat epitrochlearis muscle immediately after in vivo exercise. Furthermore, the elevated AS160 phosphorylation was still evident at 3–4 h postexercise, which led to the hypothesis that this prolonged effect on AS160 may be important for the enhanced insulin-stimulated glucose transport at this time. Consistent with this idea, Funai et al. (15) found that, when rats were allowed to eat rat chow after exercise, both the enhanced AS160 phosphorylation and increased insulin-stimulated glucose transport were reversed to resting levels, but, when rats remained fasted postexercise, the elevated AS160 phosphorylation persisted concomitant with enhanced insulin-stimulated glucose transport for as long as 27 h after exercise. A persistent elevation in AS160 phosphorylation has also been observed in human skeletal muscle several hours after acute exercise, suggesting it may be important for the improvement in insulin sensitivity in humans after exercise (34, 37).
Electrically stimulated contraction of isolated skeletal muscle has been widely used as a valuable model for elucidating the mechanisms that regulate the increased glucose transport after in vivo exercise. When isolated rat epitrochlearis muscles are stimulated to contract in the presence of rat serum, there is a substantial increase in the subsequent insulin-stimulated glucose transport measured 3 h postcontraction, reminiscent of the results observed after in vivo exercise (12, 13, 16). However, when isolated rat epitrochlearis muscles are electrically stimulated using an identical protocol in the absence of serum, there is an increase in insulin-independent glucose transport immediately after contraction but no subsequent improvement in insulin-stimulated glucose transport at 3 h postcontraction (8, 12, 13, 16). AS160 phosphorylation is increased immediately after contraction by isolated epitrochlearis muscles (4, 14) but whether this phosphorylation persists for hours after contraction remains to be determined. Thus electrical stimulation of isolated skeletal muscles in the presence of serum (which leads to increased insulin-stimulated glucose transport) or in the absence of serum (which does not) provides an opportunity for probing the idea that a persistent increase in AS160 phosphorylation can lead to enhanced insulin sensitivity.
Accordingly, we hypothesized that contraction induced by electrical stimulation of isolated rat epitrochlearis muscles would lead to increased AS160 phosphorylation immediately postcontraction, whether or not serum was present. We further hypothesized that the presence of serum during contraction would be required for the persistent increase in AS160 phosphorylation and insulin-stimulated glucose transport at 3 h postelectrical stimulation. We also studied isolated muscles that were electrically stimulated to contract after rats performed in vivo exercise. We hypothesized that, at 3 h postelectrical stimulation, the levels of AS160 phosphorylation and insulin-stimulated glucose transport in these muscles would not differ from the levels in muscles studied after in vivo exercise without in vitro electrical stimulation. Data supporting these hypotheses would be consistent with the idea that in vivo exercise and in vitro contraction in serum lead to increased insulin sensitivity by a similar mechanism that is dependent on a persistent increase in AS160 phosphorylation. In addition, we evaluated exercise and in vitro contraction effects on phosphorylation of TBC1D1 (a paralog of AS160, also implicated in glucose transport regulation), GLUT4 abundance, and other potential modulators of insulin sensitivity [muscle glycogen concentration and AMP-activated protein kinase (AMPK) activation].
METHODS
Materials.
Serum from male Wistar rats (120–200 g, fasted for 12 h) was purchased from Gemini Bio-Products (West Sacramento, CA). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for SDS-PAGE and immunoblotting were purchased from Bio-Rad (Hercules, CA). Bicinchoninic acid protein assay reagent (no. 23227), T-PER tissue protein extraction reagent (no. 78510), and West Dura Extended Duration Substrate (no. 34075) were from Pierce Biotechnology (Rockford, IL). Anti-phospho-308ThrAkt (p308ThrAkt, no. 9275), anti-phospho-172ThrAMPK (p172ThrAMPK, no. 2531), anti-phospho-(Ser/Thr) Akt substrate (PAS, no. 9611), and goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate (no. 7074) were from Cell Signaling Technology (Danvers, MA). PAS was designed to recognize Akt phosphorylation motif peptide sequences (RXRXXpT/S). Total TBC1D1 and phospho-237Ser TBC1D1 (p237SerTBC1D1) polyclonal antibody and were provided by Dr. Makoto Kanzaki at Tohoku University (25). Anti-AS160 (no. 07–741), anti-phospho-642Thr AS160 (p642ThrAS160, no. 07–802), and protein G agarose beads (no. 16–266) were from Upstate USA (Charlottesville, VA). 3-O-methyl-[3H]glucose (3-[3H]MG) was from Sigma-Aldrich. [14C]mannitol was from Perkin Elmer (Waltham, MA). Other reagents were from Sigma-Aldrich and Fisher Scientific (Pittsburgh, PA).
Insulin concentration in serum.
Rat serum purchased from Gemini Bio-Products was submitted to the Chemistry Laboratory Core of the Michigan Diabetes Research and Training Center for the measurement of insulin concentration. The insulin concentration of serum (21 μU/ml) was determined with a double-antibody radioimmunoassay using an 125I-Human insulin tracer (Linco Research), a rat insulin standard (Novo), a guinea pig anti-rat insulin first antibody (Linco Research), and a sheep anti-guinea pig gamma globulin-polyethylene glycol second antibody (MDRTC).
Animal treatment.
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (120–200 g; Harlan, Indianapolis, IN) were provided with rodent chow (Lab Diet; PMI Nutritional International, Brentwood, MO) and water ad libitum until 1700 the night before the experiment when their food was removed. On the following day between 0800 and 1000, rats were randomly assigned to 1) resting (REST), 2) postelectrical stimulation (in vitro electrical stimulation of epitrochlearis muscles: PES), 3) postexercise (in vivo exercise: PEX), or 4) postexercise and electrical stimulation (in vivo exercise followed by in vitro electrical stimulation: PEX + PES) groups. For all experiments, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg wt). While rats were under deep anesthesia, both epitrochlearis muscles were rapidly removed by dissection.
In vitro electrical stimulation.
Paired isolated epitrochlearis muscles were incubated in flasks containing either Krebs-Henseleit buffer (KHB) + 0.1% BSA + 8 mM glucose + 32 mM mannitol (solution 1) or in serum for 30 min in a shaking water bath at 35°C. For all incubation steps, flasks were continuously gassed from above with 95% O2-5% CO2. After 30 min, one of the paired muscles was transferred to a vial containing identical media (REST). The contralateral muscle was attached to a glass rod and a force transducer (Radnoti, Litchfield, CT). Mounted muscles were incubated in media identical to the first incubation step and were stimulated to contract (PES) as previously described (0.1 ms twitch, 100 Hz train for 10 s, 10 trains, 10 min; Grass S48 Stimulator; Grass Instruments, Quincy, MA) (12). For the experiments immediately postelectrical stimulation (0 h REST and 0 h PES; Fig. 1A), muscles were either freeze-clamped or transferred to vials containing KHB + 0.1% BSA + 2 mM pyruvate + 36 mM mannitol (solution 2; 30°C, 10 min) before incubation with 3-MG. For the experiments 3 h postelectrical stimulation [3 h REST and 3 h PES; Fig. 2A and Supplemental Fig. S2, A (Supplemental data for this article can be found on the American Journal of Physiology: Endocrinology and Metabolism website)], muscles were incubated according to the protocol previously used (12, 16). Immediately after electrical stimulation, all muscles (regardless of whether the previous incubation was with or without serum) were transferred to vials containing solution 1 for a 5-min wash step at 35°C. Muscles were then transferred to other vials containing solution 1 for 3 h at 35°C. After 3 h, muscles were transferred to flasks containing solution 2 for 30 min at 30°C. During this step, for some muscle pairs, solution 2 contained 50 μU/ml of insulin; for other muscle pairs, solution 2 contained no insulin. After 30 min, all muscles were incubated with 3-MG (see 3-MG incubation, muscle homogenization, and 3-MG transport determination).
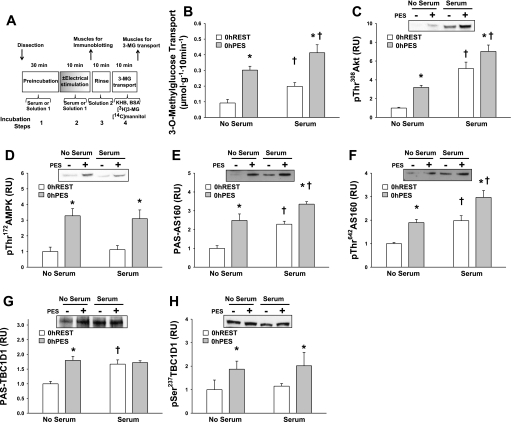
Fig. 1.
Postelectrical stimulation (in vitro electrical stimulation of epitrochlearis muscles) (PES) for 0 h vs. 0 h in the resting state (REST). Rat epitrochlearis muscles were incubated in either buffer or serum before and during in vitro electrical stimulation or resting control. A: experimental design. KHB, Krebs-Henseleit buffer. B: rate of 3-O-methylglucose (3-MG) transport. C: phosphorylated (p) 308Thr protein kinase B (Akt). D: p172Thr AMP-activated protein kinase (AMPK). E: anti-phospho-(Ser/Thr) Akt substrate (PAS)-Akt substrate of 160 kDa (AS160). F: p642ThrAS160. G: PAS-TBC1D1. H: p237SerTBC1D1. Data were analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (electrical stimulation effect; post hoc test); †P < 0.05 (serum effect; post hoc test). Data are means ± SE, n = 4–6 rats/group. Open bars, resting (0 h REST); filled bars, immediately after electrical stimulation (0 h PES). RU, relative units.
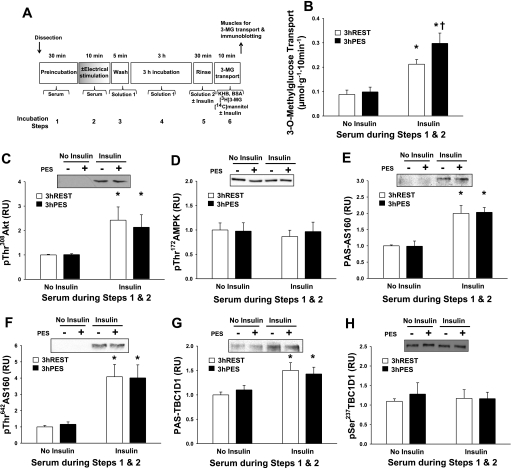
Fig. 2.
3 h PES vs. 3 h REST. Rat epitrochlearis muscles were incubated in serum before and during in vitro electrical stimulation or resting control and were subsequently incubated in buffer solution for 3 h. A: experimental design. B: rate of 3-MG transport. C: p308ThrAkt. D: p172ThrAMPK. E: PAS-AS160. F: p642ThrAS160. G: PAS-TBC1D1. H: p237SerTBC1D1. Data were analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (insulin effect; post hoc test); †P < 0.05 (postelectrical stimulation effect; post hoc test). Data are means ± SE, n = 6–12/group. Open bars, resting in serum followed by 3 h incubation in buffer (3 h REST); filled bars, in vitro electrical stimulation in serum followed by 3 h incubation in buffer (3 h PES).
Serum vs. insulin experiment.
One experiment compared the effect of incubation of muscles in serum with incubation of muscles in 21 μU/ml insulin (which equaled the insulin concentration in serum; Supplemental Fig. S1, A). Immediately after dissection, isolated epitrochlearis muscles were placed in solution 1 with no insulin, solution 1 with 21 μU/ml of insulin, or serum for 30 min at 35°C. Muscles were then transferred to a second flask including the identical media as in step 1 for 10 min before being freeze-clamped.
Increasing the amount of exercise or electrical stimulation.
To determine if exercise and electrical stimulation protocols were maximally effective, the effects of increasing the amount of exercise or electrical stimulation on subsequent insulin-stimulated glucose transport were studied. For in vivo exercise (Fig. 3A), rats performed 1 or 2 h swim exercise in a barrel filled with water (35°C) to a depth of ∼60 cm (6–8 rats/barrel) for 2 or 4 × 30 min bouts, with a 5-min rest period between each bout. Immediately after exercise (1 or 2 h) or rest, both epitrochlearis muscles were rapidly removed and were incubated in serum for 30 min, serum for 10 min, solution 1 for 5 min wash, solution 1 for 3 h rest, solution 2 (±insulin) for 30 min, and then 10 min in a solution containing 3-MG as described below. For in vitro electrical stimulation (Fig. 4A), both epitrochlearis muscles were dissected from unexercised rats and were incubated in serum before and during rest or electrical stimulation (5 or 10 tetani), followed by incubation in solution 1 for 5 min wash, solution 1 for 3 h rest, solution 2 (±insulin) for 30 min, and then 10 min in a solution containing 3-MG as described below.
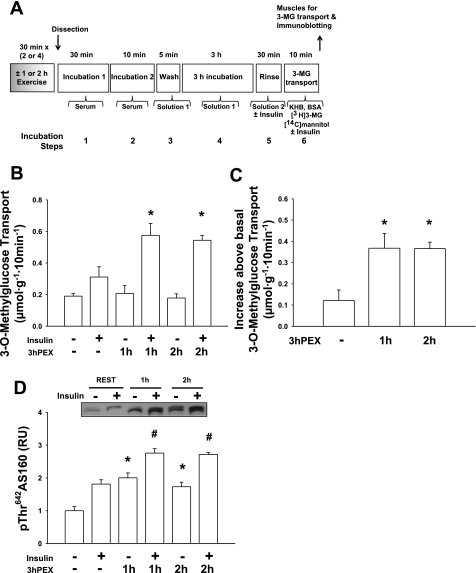
Fig. 3.
Comparison of 1- or 2-h bout of in vivo exercise. Following 1 h of exercise bout (2 × 30 min), 2 h of exercise bout (4 × 30 min), or sedentary, rat epitrochlearis muscles were incubated in serum and were subsequently incubated in buffer solution for 3 h. A: experimental design. B: rate of 3-MG transport; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. PEX, postexercise (in vivo exercise). *P < 0.05 (significantly different from 3 h REST within the insulin-treated muscles). C: Δinsulin (increase above basal, calculated by subtracting the values for muscles incubated without insulin from the respective values of paired muscles incubated with insulin) for the rate of 3-MG transport; data were analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST with insulin). D: p642ThrAS160; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST, within the muscles that were incubated without insulin); #P < 0.05 (significantly different from 3 h REST, among the muscles that were incubated with insulin). Data are means ± SE, n = 6/group.
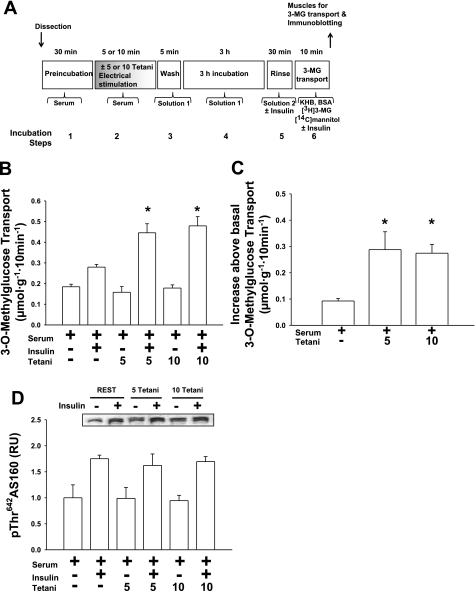
Fig. 4.
Comparison of 5 or 10 tetani in vitro electrical stimulation. Isolated rat epitrochlearis muscles were incubated in serum before and during in vitro electrical stimulation (5 vs. 10 tetani) or resting control and were subsequently incubated in buffer solution for 3 h. A: experimental design. B: rate of 3-MG transport; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST in the insulin-treated muscles). C: Δinsulin (increase above basal, calculated by subtracting the values for muscles incubated without insulin from the respective values of paired muscles incubated with insulin) for the rate of 3-MG transport; data were analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST). D: p642ThrAS160; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. Data are means ± SE, n = 5–6/group.
Additivity experiments.
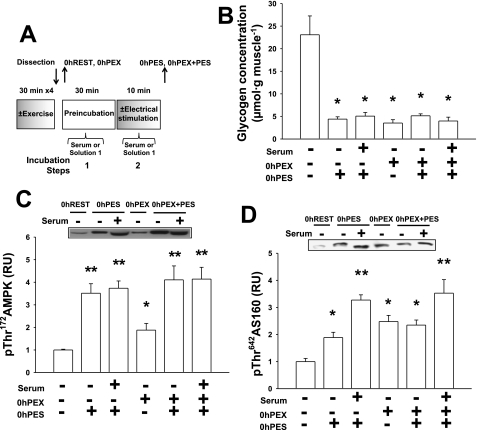
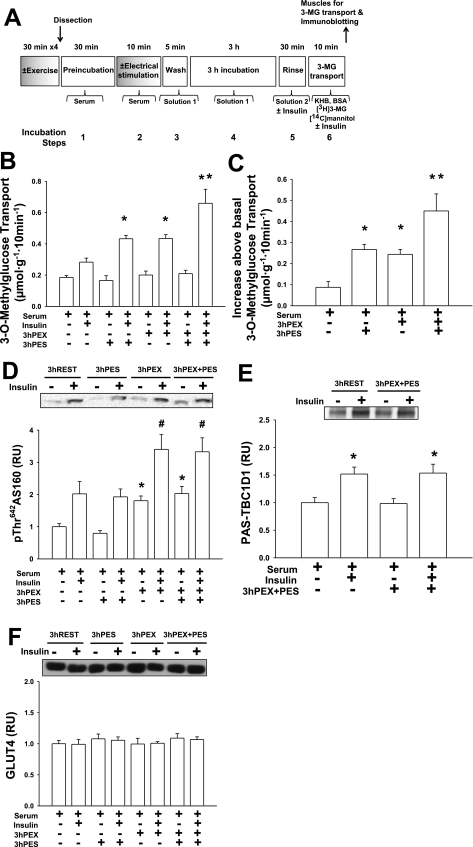
In experiments comparing the combined effects of exercise and electrical stimulation on the subsequent increase in insulin-stimulated glucose transport, four groups were studied: REST, PES, PEX, and PEX + PES. Before anesthetization and muscle incubation steps, rats in the PEX or PEX + PES groups performed 2 h swim exercise. Immediately after exercise or rest, both epitrochlearis muscles were rapidly removed and were either frozen immediately (0 h PEX or 0 h REST; Fig. 6A) or were electrically stimulated to contract in vitro before being frozen (0 h PEX + PES or 0 h PES; Fig. 6A). In other rats, immediately after exercise or rest, both epitrochlearis muscles were dissected out and incubated in serum before and during electrical stimulation (10 tetani) or rest, followed by a 5-min wash in solution 1, a 3-h incubation step in solution 1, and a 30-min rinse in solution 2 (± insulin) before incubation with 3-MG as described below (3 h REST, 3 h PES, 3 h PEX and 3 h PEX + PES; Fig. 5A).
Fig. 6.
Immediately after exercise + electrical stimulation (0 h PEX + PES). Following 2 h of exercise bout (4 × 30 min) or sedentary, isolated rat epitrochlearis muscles were frozen immediately or were incubated in serum or serum-free buffer before and during in vitro electrical stimulation or resting control and were frozen immediately after. A: experimental design. B: muscle glycogen concentration: data were analyzed with Kruskal-Wallis nonparametric 1-way ANOVA on ranks and the Dunn's post hoc test. *P < 0.05 (significantly different from 0 h REST). C: p172ThrAMPK; data were analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 0 h REST); **P < 0.05 (significantly different from 0 h REST and 0 h PEX). D: p642ThrAS160; data were analyzed with 1-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 0 h REST); **P < 0.05 (significantly different from 0 h REST, 0 h PES-buffer, 0 h PEX, and 0 h PEX/PES-buffer). Data are means ± SE, n = 6–8/group.
Fig. 5.
3 h postexercise + electrical stimulation (3 h PEX + PES). Following 2 h of exercise bout (4 × 30 min) or sedentary, isolated rat epitrochlearis muscles were incubated in serum before and during in vitro electrical stimulation or resting control and were subsequently incubated in buffer solution for 3 h. A: experimental design. B: rate of 3-MG transport; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST with insulin); **P < 0.05 (significantly different from 3 h REST, 3 h PEX, and 3 h PES muscles that were insulin treated). C: Δinsulin (increase above basal, calculated by subtracting the values for muscles incubated without insulin from the respective values of paired muscles incubated with insulin) for the rate of 3-MG transport; data were analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST); **P < 0.05 (significantly different from 3 h REST, 3 h PEX, and 3 h PES). D: p642ThrAS160; data for muscles incubated without insulin and those incubated with insulin were each analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST, comparison within muscles that were incubated without insulin); #P < 0.05 (significantly different from 3 h REST, comparison among muscles that were incubated with insulin). E: PAS-TBC1D1; data were analyzed with 2-way ANOVA and the Student-Newman-Keuls post hoc test. *P < 0.05 (significantly different from 3 h REST). F: GLUT4 abundance. Data are means ± SE, n = 8–14/group.
3-MG incubation, muscle homogenization, and 3-MG transport determination.
After incubation in solution 2, muscles were transferred to flasks containing KHB + 0.1% BSA + 8 mM 3-MG (including 3-[3H]MG at a final specific activity of 0.25 mCi/mmol) and 2 mM mannitol (including [14C]mannitol at a final specific activity of 6.25 μCi/mmol) with or without 50 μU/ml insulin at 30°C. After 10 min, muscles were rapidly blotted, trimmed, freeze-clamped, and stored (−80°C) until processed.
Frozen muscles were homogenized in 1 ml ice-cold homogenization buffer (2 mM Na3VO4, 2 mM EDTA, 2 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethanesulfonyl fluoride, and 1 μg/ml leupeptin in T-PER) using glass-on-glass tubes (Kontes, Vineland, NJ). Homogenates were subsequently rotated at 4°C for 1 h before being centrifuged (15, 000 g for 10 min at 4°C). Aliquots of the supernatant from muscles used for the 3-MG transport measurement were pipetted into vials with scintillation cocktail for scintillation counting, and 3-MG transport was determined as previously described (5). A portion of supernatant was used to determine protein concentration according to the manufacturer's instructions (Pierce Biotechnology catalog no. 23227). The remaining supernatant was stored at −80°C until further analyzed.
Immunoprecipitation.
Homogenized muscle (200–300 μg protein) was precleared and immunoprecipitated with anti-AS160 or anti-TBC1D1 at 4°C with protein G-agarose beads. After gentle rotation overnight, the immunoprecipitation mix was centrifuged at 4,000 g, and the supernatant was aspirated. After washing (four times with 500 μl PBS), the protein bound to the beads was eluted with 2× SDS loading buffer and boiled before loading on a polyacrylamide gel.
Immunoblotting.
Samples (immunoprecipitates or homogenates) were boiled with SDS loading buffer, separated by PAGE, and electrophoretically transferred to nitrocellulose. Samples were then rinsed with Tris-buffered saline plus Tween (TBST; 0.14 mol/l NaCl, 0.02 mol/l Tris base, pH 7.6, and 0.1% Tween), blocked with 5% nonfat dry milk in TBST for 1 h at room temperature, washed 3 × 5 min at room temperature, and treated with the primary antibodies (1:1,000 in TBST + 5% BSA) overnight at 4°C. Blots were then washed 3 × 5 min with TBST, incubated with the secondary antibody, goat anti-rabbit IgG HRP conjugate (1:20,000 in TBST + 5% milk), for 1 h at room temperature, washed again 3 × 5 min with TBST, and developed with West Dura Extended Duration Substrate reagent. Protein bands were quantified by densitometry (Alpha Innotech, San Leandro, CA). The mean values for basal muscles (REST without insulin) on each blot were normalized to equal 1.0, and then all samples on the blot were expressed relative to the normalized basal value.
Muscle glycogen concentration.
Muscles used for measurement of glycogen were weighed and then homogenized in ice-cold 0.3 M perchloric acid. An aliquot of the homogenate was stored at −80°C for later determination of glycogen concentration by the amyloglucosidase method (27).
Statistical analysis.
Statistical analyses were performed using Sigma Stat version 2.0 (San Rafael, CA). Data were expressed as means ± SE. P ≤ 0.05 was considered statistically significant. Data from the REST vs. PES (0 h and 3 h) experiments were analyzed with two-way ANOVA (main effects of PES and insulin). Data from the serum vs. insulin experiment were analyzed using one-way ANOVA. Data from experiments to examine the effects of increasing the amount of exercise or electrical stimulation were analyzed using one-way ANOVA for muscles with and without insulin. Data from the additivity experiments were analyzed with: 1) one-way ANOVA for those muscles that were frozen immediately after exercise and/or electrical stimulation (0 h PEX + PES) or 2) two-way ANOVA (main effects of PEX and PES) for those muscles that were frozen 3 h after exercise and/or electrical stimulation (3 h PEX + PES) for muscles with and without insulin. For all ANOVA, the Student-Newman-Keuls post hoc test was used to identify the source of significant variance. When data failed the Levene Median test for equal variance, the Kruskal-Wallis nonparametric ANOVA on ranks, was used with Dunn's post hoc test.
RESULTS
Immediately postelectrical stimulation.
Glucose transport determined immediately after the electrical stimulation (0 h PES) was significantly greater (P < 0.05) compared with 0 h REST values, regardless of the incubation media (serum-free buffer or serum) (Fig. 1B). Electrical stimulation (0 h PES) also resulted in significantly greater p308ThrAkt, p172ThrAMPK, PAS-AS160, p642ThrAS160, PAS-TBC1D1, and p237SerTBC1D1 compared with the 0 h REST group, regardless of the incubation media (serum-free buffer or serum) (P < 0.05; Fig. 1, C–H).
Muscles in both the 0 h REST and 0 h PES groups that were incubated with serum vs. no serum during the initial incubation steps were characterized by a small but significant (P < 0.05) increase in glucose transport (Fig. 1B). Incubation in serum had no effect on p172ThrAMPK and p237SerTBC1D1 (Fig. 1, D and H). In contrast, muscles that were incubated in serum before and during electrical stimulation (or resting controls) had greater values for p308ThrAkt, PAS-AS160, and p642ThrAS160 (P < 0.05) compared with the muscles that were incubated in serum-free buffer before and during the electrical stimulation step (Fig. 1, C, E, and F). Incubation in serum resulted in greater PAS-TBC1D1 (compared with incubation in serum-free buffer) in muscles that were not stimulated to contract (P < 0.05), but incubation in serum had no effect on PAS-TBC1D1 (compared with incubation in serum-free buffer) in muscles that were stimulated to contract (Fig. 1G). Incubating muscles in insulin at a concentration equivalent to that in the serum (21 μU/ml) resulted in levels of p308ThrAkt, PAS-AS160, p642ThrAS160, and PAS-TBC1D1 that were greater compared with muscles incubated in serum-free buffer (P < 0.05) but not different from muscles incubated in serum (Supplemental Fig. S1). Thus the effects of serum alone on these outcomes could be accounted for by the effects produced by the insulin concentration that was found in the serum.
Postelectrical stimulation (3 h) without serum.
As previously reported (8, 12, 16), prior electrical stimulation in serum-free buffer (3 h PES) had no effect on glucose transport in basal or insulin-stimulated muscles (measured 3 h after electrical stimulation) compared with 3 h REST (Supplemental Fig. S2, B). As expected, prior electrical stimulation had no persistent effect on the phosphorylation of any of the proteins studied (p308ThrAkt, p172ThrAMPK, PAS-AS160, p642ThrAS160, PAS-TBC1D1, and p237SerTBC1D1) in basal or insulin-stimulated muscles at 3 h PES compared with 3 h REST (Supplemental Fig. S2, C–H). Insulin treatment (immediately before and during the incubation with 3-MG) significantly elevated 3-MG transport, p308ThrAkt, PAS-AS160, p642ThrAS160, and PAS-TBC1D1 (P < 0.05; Supplemental Fig. S2, B–C and E–G) compared with no insulin treatment but had no effect on p172ThrAMPK or p237SerTBC1D1 (Supplemental Fig. S2, D and H).
Postelectrical stimulation (3 h) with serum.
As previously reported (12, 16), prior electrical stimulation in the presence of serum (3 h PES) resulted in greater insulin-stimulated glucose transport (measured 3 h after electrical stimulation) compared with 3 h REST (P < 0.05; Fig. 2B). In contrast, prior electrical stimulation had no persistent effect on the phosphorylation of any of the proteins studied (p308ThrAkt, p172ThrAMPK, PAS-AS160, p642ThrAS160, PAS-TBC1D1, and p237SerTBC1D1) in basal or insulin-stimulated muscles at 3 h PES compared with 3 h REST (Fig. 2, C–H). Insulin treatment (immediately before and during the incubation with 3-MG) significantly elevated 3-MG transport, p308ThrAkt, PAS-AS160, p642ThrAS160, and PAS-TBC1D1 (P < 0.05; Fig. 2, B–C and E–G) compared with no insulin treatment but had no effect on p172ThrAMPK or p237SerTBC1D1 (Fig. 2, D and H).
Increasing the amount of exercise or electrical stimulation.
Increasing the amount of exercise (1 vs. 2 h) or electrical stimulation (5 vs. 10 tetani) did not result in higher values for the subsequent p642ThrAS160 and insulin-stimulated glucose transport (Figs. 3 and 4), providing evidence that the protocol used for each stimulus (exercise or electrical stimulation) for the additivity experiments was maximally effective.
Additivity experiments.
The purpose of these experiments was to examine the possibility that prior in vitro contraction (in serum) enhances insulin-stimulated glucose transport through a mechanism distinct from that after exercise. Insulin-stimulated glucose transport in muscles from rats that exercised immediately before electrical stimulation of the isolated muscles (PEX + PES) was compared with that of muscles that were subjected to PEX or PES treatment alone (Fig. 5B). Total force production during electrical stimulation (11,102.3 ± 755.5 vs. 8,969.8 ± 1,129.2 g·min−1·g muscle−1, P < 0.05), but not peak force (478.0 ± 26.1 vs. 424.5 ± 52.4 g/g muscle, P = 0.122), was significantly reduced in PEX + PES muscles compared with PES muscles. Either exercise (3 h PEX; Fig. 5C) or electrical stimulation (3 h PES; Fig. 5C) induced a subsequent increase in insulin-stimulated glucose transport, with no significant differences between the groups. Importantly, muscles that underwent electrical stimulation after being dissected from exercised rats (3 h PEX + PES; Fig. 5C) had greater insulin-stimulated glucose transport compared with muscles from the 3 h PEX or 3 h PES group. The greater insulin-stimulated glucose transport in 3 h PEX + PES muscles was not accompanied by altered p642ThrAS160, PAS-TBC1D1, or GLUT4 abundance in the 3 h PEX + PES group vs. the PEX group (Fig. 5, D–F). Furthermore, glycogen concentration, p172ThrAMPK, and p642ThrAS160 were not different for the 0 h PEX + PES group compared with the 0 h PES group (Fig. 6, B–D).
DISCUSSION
The results of this study demonstrated that in vivo exercise causes a sustained increase in AS160 phosphorylation (PAS or 642Thr) and a subsequently increased insulin-stimulated glucose transport in skeletal muscle, confirming earlier research that implicated the persistent increase in AS160 phosphorylation after exercise as a possible mechanism for the prolonged improvement in insulin sensitivity (1, 15). These earlier observations led to the hypothesis in the current study that in vitro contraction by isolated skeletal muscle in the presence of serum (a procedure that results in increased insulin-stimulated glucose transport) (12, 13, 16), but not in vitro contraction in the absence of serum (which does not enhance insulin-stimulated glucose transport) (8, 12, 13, 16), would be characterized by persistently elevated AS160 phosphorylation. Although AS160 phosphorylation (PAS and 642Thr) was increased immediately after in vitro contraction, the elevated AS160 phosphorylation had reversed at 3 h postcontraction whether or not serum was present during the electrical stimulation. Thus, in contrast to in vivo exercise, increased AS160 phosphorylation (PAS or 642Thr) cannot account for the increased insulin sensitivity found at 3 h after contraction in serum. Finally, insulin-stimulated glucose transport determined for muscles that had performed both in vivo exercise and in vitro electrical stimulation (PEX + PES) exceeded the values found in muscles after either in vivo exercise (PEX) or in vitro electrical stimulation (PES) alone. These results raised the possibility that the in vivo exercise and in vitro electrical stimulation protocols may not have caused elevated insulin-stimulated glucose transport via an identical mechanism.
To identify the mechanism for improved insulin sensitivity after contraction in serum, which did not induce a sustained increase in AS160 PAS or 642Thr phosphorylation, we also evaluated the phosphorylation of TBC1D1, a paralog protein of AS160. Similar to AS160, TBC1D1 in mouse (35) or rat (2, 14, 15) skeletal muscle becomes phosphorylated in response to insulin, in vitro contraction, or in vivo exercise. In the current study, 237Ser phosphorylation of TBC1D1 was increased immediately after in vitro contraction with or without serum. However, at 3 h PES, TBC1D1 237Ser phosphorylation had reversed to resting control values. TBC1D1 PAS phosphorylation was also not elevated at 3 h postexercise. Thus the enhanced insulin sensitivity 3 h after in vitro contraction in serum or 3 h after in vivo exercise occurred in the absence of elevated phosphorylation (PAS or 237Ser) of TBC1D1. Interestingly, Treebak et al. (37) reported that prior exercise by humans that increased insulin sensitivity was accompanied by a persistent increase in phosphorylation of AS160 on 318Ser, 341Ser, and 751Ser but not on 642Thr or PAS sites. The sustained effect of in vivo exercise or in vitro contraction on AS160 phosphosites other than 642Thr has not been evaluated for rat skeletal muscle. It remains possible that in vitro contraction of rat epitrochlearis muscles in serum and/or in vivo exercise was accompanied by a persistent increase in phosphorylation of AS160 on one or more sites other than 642Thr.
The most important new finding was the additive effect of in vivo exercise and in vitro electrical stimulation protocols on subsequent insulin-stimulated glucose transport. The insulin-stimulated increase in glucose transport (Δinsulin; calculated by subtracting glucose transport in muscles incubated without insulin from glucose transport in muscles incubated with insulin) in the PEX + PES group was significantly greater than that of the PEX or PES group. Furthermore, the effect was essentially additive as evidenced by comparing the Δinsulin value in the PEX + PES group (0.362 μmol·g−1·10 min−1) with the sum of the Δinsulin in the PEX group (0.156 μmol·g−1·10 min−1) plus the Δinsulin in the PES group (0.179 μmol·g−1·10 min−1). In contrast, increasing the amount of exercise (from 1 to 2 h) or electrical stimulation (from 5 to 10 tetani) did not increase the insulin-stimulated glucose transport, providing evidence that 1 h exercise or 5 tetani were maximally effective exercise or electrical stimulation protocols, respectively. In this context, the additive effect of exercise and in vitro contraction on insulin-stimulated glucose transport was consistent with the idea that these protocols used distinct mechanisms to enhance insulin sensitivity.
Several possible scenarios might account for the additive increase in insulin-stimulated glucose transport. One possibility would be that exercise and electrical stimulation recruited discrete groups of muscle fibers. However, the tetanic stimulation protocol would be expected to recruit all muscle fibers, consistent with the nearly complete depletion of glycogen with electrical stimulation. Glycogen concentration was not lower in the PEX + PES group compared with the PES or PEX groups, arguing against exercise and electrical stimulation recruiting entirely separate groups of muscle fibers. A second possible scenario would be if exercise compared with in vitro contraction altered different molecular processes that regulate GLUT4 traffic (e.g., exocytosis vs. endocytosis; recruitment vs. docking, tethering, or insertion of GLUT4, etc.). Although the enhanced insulin-stimulated glucose transport in the rat epitrochlearis after in vivo exercise can be accounted for by greater cell surface GLUT4 content (20), the specific steps of GLUT4 vesicle trafficking that are influenced by either exercise or in vitro contraction are uncertain. Another putative scenario would be if discrete pools of GLUT4 were recruited by insulin to the cell surface membranes after exercise vs. postcontraction, but it is currently not technically feasible to experimentally test this idea in skeletal muscle.
We assessed several specific potential mechanisms for the additive effects of exercise and in vitro contraction on insulin action in the PEX + PES group. Increased GLUT4 protein expression can lead to enhanced insulin-stimulated glucose transport in isolated skeletal muscle (19), but the additive effect on insulin-stimulated glucose transport was not because the PEX + PES group had greater GLUT4 abundance than any of the other groups studied. It has been suggested that glycogen depletion may elevate insulin sensitivity (26), but the additive effect of in vivo exercise and in vitro contraction on the insulin-stimulated glucose transport in the PEX + PES group compared with PEX or PES alone was not attributable to differences in glycogen depletion. Finally, activation of AMPK has been reported to be associated with a subsequent increase in insulin sensitivity (13), but the PES and PEX + PES groups had similar levels of AMPK phosphorylation. It is notable that the sustained increase in p642ThrAS160 in the 3 h PEX + PES group was essentially the same as in the 3 h PEX group (Fig. 5D), indicating that electrical stimulation did not reverse the persistent increase in AS160 phosphorylation found after exercise alone. Thus the prolonged increase in AS160 phosphorylation in the PEX + PES group may have contributed to a portion of the enhanced insulin-stimulated glucose transport in these muscles.
Which phosphosite(s) of TBC1D1 is(are) recognized by the PAS antibody, and which kinases are potentially relevant for changes in PAS-TBC1D1? PAS can recognize TBC1D1 phosphorylated on 596Thr (11), and insulin leads to increased p596Thr by an Akt-dependent mechanism based on results with skeletal muscle, cells, or recombinant Akt in cell-free assays (11, 28, 30). Insulin was reported to not cause an increase of p237Ser TBC1D1 in skeletal muscle or cultured cells (11, 28), consistent with the current results. Furthermore, in a cell-free assay, Akt can phosphorylate TBC1D1 on p596Thr, but not on p237Ser. Accordingly, it seems likely that PAS-TBC1D1 in insulin-stimulated muscle in this study is reflecting Akt-induced phosphorylation of TBC1D1 on 596Thr.
Interpreting the effects of contraction on PAS-TBC1D1 is complicated because: 1) in vitro contraction can activate both Akt and AMPK in rat epitrochlearis muscle (4, 14); 2) in a cell-free assay, either Akt or AMPK can increase PAS-TBC1D1 (35); and 3) in a cell-free assay, AMPK can phosphorylate TBC1D1 on both 596Thr and 237Ser (11). Several lines of evidence support an important role for AMPK in the contraction-stimulated increase in PAS-TBC1D1: 1) the AMPK inhibitor compound C eliminated contraction-stimulated PAS-TBC1D1 without reducing phosphorylation of Akt in isolated rat epitrochlearis muscles (14); 2) wortmannin eliminated the contraction effect on phosphorylation of glycogen synthase kinase-3 (GSK3), an Akt substrate, without reducing the contraction effects on AMPK phosphorylation or PAS-TBC1D1 in isolated rat epitrochlearis muscles (14); and 3) genetic disruption of AMPK signaling eliminated the contraction-induced increases in PAS-TBC1D1, p237Ser, and p596Thr in isolated mouse skeletal muscle (28). However, it remains uncertain which TBC1D1 phosphosite(s) was(were) reflected by PAS-TBC1D1 in the electrically stimulated muscles.
The prolonged effect of exercise on AS160 phosphorylation is rather remarkable, with elevated AS160 phosphorylation found at 3 h after in vivo exercise in the current study and as long as 27 h postexercise in an earlier publication (15). This result would be expected if there were sustained effects of exercise on the activity of AS160 kinases (increased) and/or AS160 phosphatases (decreased). However, neither of the key AS160 kinases (Akt, as evidenced by p308ThrAkt, p473SerAkt, and Akt activity; or AMPK, as evidenced by p172ThrAMPK) is activated at 3 h postexercise (1, 15), indicating that their prolonged activation was not responsible for sustained AS160 phosphorylation. To the best of our knowledge, the persistent effect of exercise on protein phosphatases has not been reported, but there is not a prolonged postexercise increase in phosphorylation of other Akt substrates (GSK3 or TBC1D1) or AMPK substrates (acetyl-CoA carboxylase or TBC1D1) (1, 15). The specific protein phosphatase(s) that regulate AS160 have not been identified, but, because of the similarity in the sequences of AS160 and TBC1D1, it seems likely that there is overlap between those that dephosphorylate AS160 and TBC1D1. Thus the lack of sustained elevation in TBC1D1 phosphorylation provides indirect evidence against a persistent, postexercise inhibition of the relevant protein phosphatase(s) that regulate AS160 phosphorylation. Our current working hypothesis is that prior in vivo exercise causes a long-lasting increase in AS160 phosphorylation by altering the access of relevant kinases or phosphatases to AS160 rather than by persistently modifying the activation of these enzymes. Access might be regulated by altering the colocalization of AS160 with the relevant enzyme(s). Alternatively, AS160 might undergo posttranslational modifications or bind to other proteins that affect AS160's susceptibility to the actions of these kinases or phosphatases. Regardless of the cellular mechanism, it is notable that, when muscles were dissected out immediately after in vivo exercise and incubated in vitro for several hours, the level of AS160 phosphorylation remained increased above the resting control values. Thus persistence of the elevated AS160 phosphorylation after exercise did not require that muscle remain in vivo. Gulve et al. (17) demonstrated a persistent increase in insulin-stimulated glucose transport when rat epitrochlearis muscles were dissected out immediately after exercise and then incubated for 3 h in the absence of serum. We have also found increased AS160 phosphorylation when muscles were dissected out of rats immediately postexercise and incubated for 3 h in the absence of serum (unpublished results). These findings indicate that the sustained effects of exercise on both AS160 phosphorylation and insulin-stimulated glucose transport can persist for several hours independent of the direct influence of systemic factors (including continued neural and humoral inputs). The isolated epitrochlearis muscle includes multiple different cell types, so it remains possible that cells other than myocytes may play some regulatory role in the persistent elevation of AS160 phosphorylation and insulin-stimulated glucose transport.
The current study focused on the potential role of sustained AS160 phosphorylation in the persistent increase in glucose transport after exercise or in vitro contractions, and it was not designed to identify what factor(s) in serum is(are) essential for serum's effect on postcontraction insulin sensitivity. Previously, Gao et al. (16) provided strong evidence that the elevated insulin-stimulated glucose transport found in isolated rat epitrochlearis muscles 3 h postcontraction in serum depends on a serum protein with a mass >10 kDa. Although they did not identify the specific protein, they found that neither insulin nor IGF-I could recapitulate the ability of serum to enhance glucose transport 3 h after in vitro contraction.
AS160 phosphorylation was increased immediately after either in vivo exercise or in vitro contractions, but these two treatments differed in their ability to induce a sustained increase in AS160 phosphorylation determined at 3 h posttreatment. There were numerous differences between the two treatments, including that in vivo exercise occurred in the presence of many systemic factors that were absent during in vitro contractions (e.g., intact efferent and afferent innervation and the vascular delivery of hormones, fuels, and other molecules). Another major difference was the duration of the treatments: 1 h for exercise vs. 5–10 min for in vitro contractions. The treatments were also fundamentally different with regard to the mode and pattern of muscle recruitment: voluntary activation of motoneurons using an undefined recruitment pattern leading to dynamic, in vivo contractions vs. electrically stimulated, static and tetanic contractions that were performed in vitro. It is unclear which of these or the many other differences between in vivo exercise and in vitro contractions were important for the protocols' differing effects on sustained AS160 phosphorylation and which might be relevant for the additive effects on insulin-stimulated glucose transport.
In conclusion, the increased insulin-stimulated glucose transport observed 3 h after in vivo exercise was accompanied by increased AS160 phosphorylation (PAS or 642Thr), but not TBC1D1 PAS phosphorylation, supporting the idea that sustained phosphorylation of AS160 plays a role in the improved postexercise insulin sensitivity. In contrast, the elevated insulin sensitivity after in vitro electrical stimulation in serum was not because of a prolonged increase in phosphorylation (PAS or 642Thr) of AS160 or TBC1D1 (PAS or 237Ser). Clearly, in vivo exercise and in vitro contraction differ in their ability to induce a prolonged increase in AS160 phosphorylation. Furthermore, in vivo exercise and in vitro electrical stimulation had additive effects on subsequent insulin-stimulated glucose transport, suggesting that exercise and in vitro contraction may also differ in the mechanisms that are responsible for their effects on insulin sensitivity. If this interpretation proves to be correct, the identification of their respective mechanisms will provide an opportunity for their distinct mechanisms to be targeted either separately or together to oppose skeletal muscle insulin resistance.
GRANTS
This research was supported by National Institutes of Health Grants AG-010026 and DK-071771 (to G. D. Cartee). This work utilized the Chemistry Laboratory Core of the Michigan Diabetes Research and Training Center funded by DK-020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
REFERENCES
- 1.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Blair DR, Funai K, Schweitzer GG, Cartee GD. A myosin II ATPase inhibitor reduces force production, glucose transport and phosphorylation of AMPK and TBC1D1 in electrically stimulated rat skeletal muscle. Am J Physiol Endocrinol Metab 296: E993–E1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonen A, Tan MH, Clune P, Kirby RL. Effects of exercise on insulin binding to human muscle. Am J Physiol Endocrinol Metab 248: E403–E408, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol Endocrinol Metab 268: E902–E909, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol 75: 972–978, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exercise Sport Sci Rev 37: 188–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol Endocrinol Metab 258: E390–E393, 1990 [DOI] [PubMed] [Google Scholar]
- 9.Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol Endocrinol Metab 256: E494–E499, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Dumke CL, Kim J, Arias EB, Cartee GD. Role of kallikrein-kininogen system in insulin-stimulated glucose transport after muscle contractions. J Appl Physiol 92: 657–664, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes 58: 1096–1104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Gulve EA, Holloszy JO. Contraction-induced increase in muscle insulin sensitivity: requirement for a serum factor. Am J Physiol Endocrinol Metab 266: E186–E192, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gulve EA, Cartee GD, Zierath JR, Corpus VM, Holloszy JO. Reversal of enhanced muscle glucose transport after exercise: roles of insulin and glucose. Am J Physiol Endocrinol Metab 259: E685–E691, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Hamada T, Arias EB, Cartee GD. Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise. J Appl Physiol 101: 1368–1376, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hansen PA, Gulve EA, Marshall BA, Gao J, Pessin JE, Holloszy JO, Mueckler M. Skeletal muscle glucose transport and metabolism are enhanced in transgenic mice overexpressing the Glut4 glucose transporter. J Biol Chem 270: 1679–1684, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol 85: 1218–1222, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 284: E453–E467, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Howlett KF, Sakamoto K, Hirshman MF, Aschenbach WG, Dow M, White MF, Goodyear LJ. Insulin signaling after exercise in insulin receptor substrate-2-deficient mice. Diabetes 51: 479–483, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J 391: 87–93, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedachi T, Fujita H, Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295: E1191–E1204, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Nolte LA, Gulve EA, Holloszy JO. Epinephrine-induced in vivo muscle glycogen depletion enhances insulin sensitivity of glucose transport. J Appl Physiol 76: 2054–2058, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Passoneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement. Anal Biochem 60: 405–412, 1974 [DOI] [PubMed] [Google Scholar]
- 28.Pehmoller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA, Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14–3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab 297: E665–E675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20: 260–270, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab 295: E29–E37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab 256: E138–E144, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Treebak JT, Frosig C, Pehmoller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H, Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia 52: 891–900, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wojtaszewski JF, Hansen BF, Gade, Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes 49: 325–331, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest 104: 1257–1264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.