Abstract
Local delivery of glucose into a critical glucose-sensing region within the brain, the ventromedial hypothalamus (VMH), can suppress glucose counterregulatory responses to systemic hypoglycemia. Here, we investigated whether this suppression was accomplished through changes in GABA output in the VMH. Sprague-Dawley rats had catheters and guide cannulas implanted. Eight to ten days later, microdialysis-microinjection probes were inserted into the VMH, and they were dialyzed with varying concentrations of glucose from 0 to 100 mM. Two groups of rats were microdialyzed with 100 mM glucose and microinjected with either the KATP channel opener diazoxide or a GABAA receptor antagonist. These animals were then subjected to a hyperinsulinemic-hypoglycemic glucose clamp. As expected, perfusion of glucose into the VMH suppressed the counterregulatory responses. Extracellular VMH GABA levels positively correlated with the concentration of glucose in the perfusate. In turn, extracellular GABA concentrations in the VMH were inversely related to the degree of counterregulatory hormone release. Of note, microinjection of either diazoxide or the GABAA receptor antagonist reversed the suppressive effects of VMH glucose delivery on counterregulatory responses. Some GABAergic neurons in the VMH respond to changes in local glucose concentration. Glucose in the VMH dose-dependently stimulates GABA release, and this in turn dose-dependently suppresses the glucagon and epinephrine responses to hypoglycemia. These data suggest that during hypoglycemia a decrease in glucose concentration within the VMH may provide an important signal that rapidly inactivates VMH GABAergic neurons, reducing inhibitory GABAergic tone, which in turn enhances the counterregulatory responses to hypoglycemia.
Keywords: γ-aminobutyric acid, microdialysis, ventromedial hypothalamus, counterregulation, bicuculline methiodide, diazoxide
hypoglycemia remains the most common adverse side effect of insulin therapy, and it has emerged as a major obstacle to achieving near-normal glucose control in patients with diabetes. The brain has evolved mechanisms for sensing and regulating glucose homeostatic mechanisms. The metabolic demands of the body are continually communicated to the brain, and it in turn regulates efferent signals involved in energy intake, expenditure, and storage to provide for these needs. Neurons that are involved in sensing metabolic stasis integrate a variety of metabolic, humoral, and neural signals from the periphery, including changes in glucose (23, 25, 26, 29–31), fatty acids (19, 20), and hormones and metabolites (29–31) and utilize this information to regulate their membrane potential and firing rate. For glucose-sensing neurons, it has been suggested that glucokinase may act as the rate-limiting step in glucosensing, while the pathways that mediate responses to metabolites like lactate, ketone bodies, and fatty acids are less well characterized (18). Each set of afferent signals arrives from peripheral glucosensors (15–17, 24) as well as central fuels sensors (26, 28, 29) with different temporal profiles, and these inputs are likely summated to produce a given neural firing pattern that acts to modulate glucose counterregulatory mechanisms.
Hence, the central nervous system plays an important role in regulating glucose homeostasis, especially during periods of hypoglycemia (2). However, the precise mechanism by which the brain detects falls in blood glucose levels and activates counterregulatory responses is not well understood. The hypothalamus, and in particular the ventromedial hypothalamus (VMH), has been implicated as playing a critical role in this regard (6, 7), and previous studies have shown that the local delivery of glucose into the VMH can dramatically suppress both the glucagon and the epinephrine responses to hypoglycemia (4, 5). However, the mechanism underlying this phenomenon has not been established.
While various hormones, metabolic fuels, and neuropeptides may act to relay changes in metabolic status to glucose-sensitive neurons in the VMH, these signals likely regulate the release of neurotransmitters that eventually communicate with peripheral organs to alter their metabolism. Recently, our laboratory reported that the inhibitory neurotransmitter γ-aminobutyric acid (GABA) acts to modulate the magnitude of both glucagon and epinephrine responses to hypoglycemia and that the release of GABA can be modulated by ATP-sensitive potassium (KATP) channels (9, 11). Specifically, opening and closing KATP channels in the VMH can decrease or increase VMH GABA levels, respectively, and this in turn corresponds to amplification or suppression of counterregulatory responses to hypoglycemia.
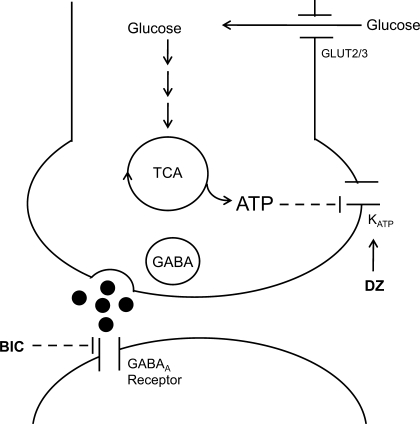
The present study expands upon these findings by showing that changes in the local availability of glucose within the VMH may play an important role in regulating local GABA release, which in turn modulates the magnitude of glucose counterregulatory responses during hypoglycemia (Fig. 1).
Fig. 1.
Simplified schema showing the potential mechanism by which glucose stimulates the release of GABA. Glucose enters GABAergic neurons in the ventromedial hypothalamus (VMH) through glucose transporters and is metabolized through glycolytic and oxidative pathways to generate ATP. An increase in ATP production then closes off ATP-sensitive potassium (KATP) channels, depolarizes the neuron, and allows for GABA release. Diazoxide (DZ) will open the KATP channel, hyperpolarize the neuron, and prevent GABA release. Bicuculline methiodide (BIC) acts on postsynaptic GABAA receptors to block GABAergic inhibition.
RESEARCH DESIGN AND METHODS
Animals.
Male Sprague-Dawley rats (Charles River, Richmond, VA) weighing 300–325 g were individually housed in the Yale Animal Resources Center in temperature- (22–23°C) and humidity-controlled rooms. The animals were fed rat chow (Agway Prolab 3000, Syracuse, NY) and water ad libitum and were acclimatized to handling and a 12:12-h light-dark cycle (lights on between 0700 and 1900) for a period of 1 wk before experimental manipulation.
Mice expressing enhanced green fluorescent protein (eGFP) reporter gene under the control of the GAD65 promoter were generously donated to us by Dr. Gábor Szabó (Institute of Experimental Medicine, Budapest, Hungary) (1). These animals were bred in our animal facilities and at ∼3 mo of age were used for the immunohistochemical studies.
Principles of laboratory animal care were followed, and experimental protocols were approved by the Institutional Animal Care and Use Committee at Yale University.
Surgery.
Approximately 8–10 days prior to the experiment, the animals underwent aseptic surgery to have vascular catheters implanted into the left carotid artery and right jugular vein. These catheters were tunneled subcutaneously and exteriorized at the back of the neck. Subsequently, the animals are placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA), and stainless steel guide cannulas (Eicom, Kyoto, Japan) for microdialysis and microinjection (from bregma: 2.6 mm posterior, 3.8 mm lateral, and 8.9 mm ventral, at an angle of 16°) were bilaterally inserted down to the level of the VMN and secured in place with screws and dental acrylic. The animals were then allowed to recover for ∼1 wk in their home cages before being studied.
Microdialysis.
Animals were fasted overnight in the microdialysis cages to allow sufficient time for acclimation. The next day, the animals were connected to infusion pumps, and combined microdialysis-microinjection probes were inserted. Artificial extracellular fluid (aECF) was perfused through the microdialysis probe at a constant rate of 1.5 μl/min for 2.5–3 h to allow for GABA levels to stabilize prior to the start of microdialysate and baseline blood sample collection. Microdialysate samples were collected at 10-min intervals for the duration of the study. The animals then had either 1) aECF vehicle (n = 7), 2) 15 mM d-glucose (n = 7), 3) 30 mM d-glucose (n = 6), 4) 100 mM d-glucose (n = 8), or 5) 100 mM of the nonmetabolizable l-glucose (n = 8) microdialyzed through the probes. Relative probe efficiency for glucose delivery was determined by in vitro recovery to be between 4 and 7%.
Microinjection.
Just prior to the start of the insulin infusion, the rats were microinjected with 0.1 μl of aECF vehicle over the course of 1 min by use of a CMA 402 syringe pump (CMA Microdialysis, North Chelmsford, MA). To assess whether glucose in the VMH suppressed counterregulatory responses by stimulating GABA release, we microinjected the GABAA receptor antagonist bicuculline methiodide (BIC, 12.5 pmol/side) into the VMH of one subgroup of animals microdialyzed with 100 mM d-glucose (n = 7; Fig. 1). The dose of BIC used here is a subconvulsive dose determined in a previously described pilot study (11). Last, to ascertain whether glucose acts through an KATP channel to regulate GABA release and modulate the counterregulatory responses, we microinjected the KATP channel opener diazoxide (1 nmol/side, n = 6) into the VMH of another group of rats that were being microdialyzed with 100 mM glucose to see whether we could reverse the effects of glucose on GABA and counterregulatory hormone release (Fig. 1).
Hypoglycemic clamp.
Following microinjection, a constant infusion of regular human insulin (50 mU·kg−1·min−1; Eli Lilly, Indianapolis, IN) and a variable 20% glucose infusion were started, and plasma glucose levels were lowered and maintained at 45 mg/dl for 90 min. Blood samples were collected at 30-min intervals throughout the study for measurement of plasma glucagon, catecholamine, and corticosterone responses and at 60-min intervals for plasma insulin concentrations. Following each sample collection, the red blood cells were resuspended in an equivalent volume of artificial plasma (22) and reinfused back into the animal to prevent volume depletion and anemia. At the end of the study, following collection of the final blood and microdialysate samples, the animals were euthanized with an overdose of pentobarbital sodium, and the brains were removed and frozen on dry ice. Subsequently, accuracy of probe placement was determined histologically by visual inspection of coronal brain sections. Only data obtained from those animals with correctly positioned microdialysis probes were analyzed.
Hormone and microdialysate analysis.
Plasma catecholamine concentrations were analyzed by HPLC using electrochemical detection, and plasma hormone concentrations were determined using commercially available radioimmunoassay kits. VMH GABA concentrations from microdialysate samples were determined using LC-MS-MS after butylation, as described previously (3).
Immunohistochemical analyses.
GAD65-eGFP mice (n = 6) were deeply anesthetized with pentobarbital sodium and transcardially perfused with 0.01 M phosphate-buffered saline followed by cold 10% neutral buffered formalin. The brains were then removed and immersed in 30% sucrose in phosphate-buffered saline for 18–36 h at 4°C. Coronal sections (30 μm) were then taken through the brain by means of a cryostat and stored at −20°C in cryoprotectant until histochemical processing. Briefly, after blocking, free-floating sections were incubated overnight with mouse anti-GFP (Chemicon) and rabbit anti-sulfonylurea type 1 receptor (SUR1; Abcam) in fresh donkey serum. The following day, the sections were washed and incubated in biotinylated donkey anti-rabbit antibody for 1 h. Then, the sections were washed again and incubated with a cocktail of Alexa fluor 594-conjugated streptavidin and Alexa fluor 488-conjugated antiserum. Tissues were then mounted onto glass slides and imaged using a Zeiss LSM510 confocal microscope.
Statistical analysis.
Treatment effects were analyzed using one- or two-way analysis of variance for independent or repeated measures as appropriate followed by post hoc analysis using the Statistica suite of analytical software for personal computers by StatSoft. P < 0.05 was set as the criterion for statistical significance.
RESULTS
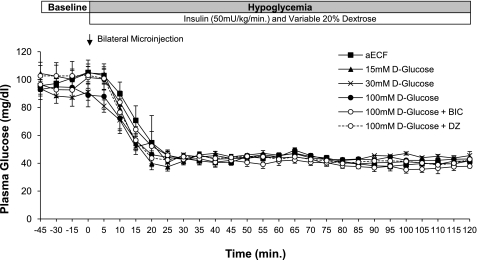
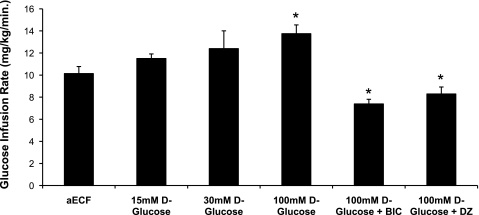
Plasma glucose (Fig. 2) and insulin (range: 3,362 ± 282 to 3,993 ± 464 μU/ml) concentrations between the six treatment groups were maintained at similar levels during the hypoglycemic clamping period. Nevertheless, average glucose infusion rates over the final 90 min of the hypoglycemic clamp varied significantly among the different treatment groups (Fig. 3). As the concentration of glucose in the dialysate was raised from 0 to 100 mM, we observed a gradual increase in the amount of exogenous glucose required to maintain steady hypoglycemia at our target of 45 mg/dl. This increase reached statistical significance when the concentration of d-glucose in the dialysate was 100 mM. Of note, prior microinjection of either the GABAA receptor antagonist BIC or the KATP channel opener diazoxide into the VMH significantly suppressed glucose infusion rates in animals dialyzed with 100 mM d-glucose (P < 0.05).
Fig. 2.
Plasma glucose concentrations during baseline sampling period (t = −45–0 min) and during hypoglycemic clamp. Data are represented as means ± SE.
Fig. 3.
Average glucose infusion rates during the final 90 min of the hypoglycemic clamping period. Data are represented as means ± SE. *P < 0.05 vs. controls.
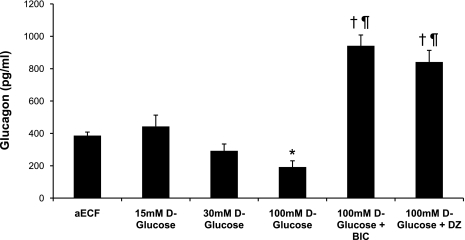
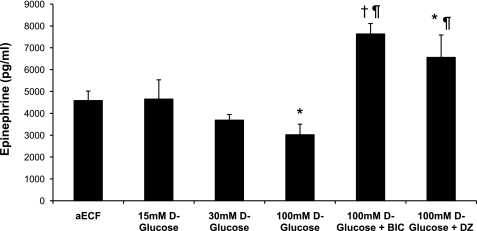
Changes in glucose infusion rates correlated with changes in peak plasma glucagon and epinephrine levels (Figs. 4 and 5). Plasma glucagon and epinephrine responses were similar between aECF controls and animals receiving 15 mM d-glucose or 100 mM nonmetabolizable l-glucose (data for l-glucose group shown in Table 1). In contrast, when the concentration of d-glucose in the dialysate was increased to 30 mM, the glucagon and epinephrine responses were suppressed by 24 and 19%, respectively, although this failed to reach statistical significance. This suppression was even more striking (50 and 34%, respectively) when the concentration of d-glucose was raised to 100 mM (P < 0.05). In contrast, animals microinjected with either BIC or diazoxide exhibited amplified glucagon and epinephrine responses despite having 100 mM d-glucose being continuously delivered (P < 0.02; Figs. 4 and 5 and Table 1). No significant differences in plasma norepinephrine or corticosterone (data not shown) were observed between treatment groups.
Fig. 4.
Peak plasma glucagon responses obtained during the hypoglycemic clamp. Data are shown as means ± SE. *P < 0.05 vs. controls; †P < 0.01 vs. controls; ¶P < 0.001 vs. 100 mM d-glucose.
Fig. 5.
Peak plasma epinephrine responses obtained during the hypoglycemic clamp. Data are shown as means ± SE. *P < 0.05 vs. controls; †P < 0.02 vs. controls; ¶P < 0.001 vs. 100 mM d-glucose.
Table 1.
Glucose infusion rates, peak plasma hormone concentrations, and extracellular GABA concentrations
| aECF | 100 mM d-Glucose | 100 mM l-Glucose | aECF + BIC | |
|---|---|---|---|---|
| Glucose infusion rate, mg·kg–1·min–1 | 10 ± 1 | 14 ± 1* | 11.0 ± 1 | 7 ± 1* |
| Glucagon, pg/ml | 344 ± 21 | 192 ± 39* | 406 ± 84 | 904 ± 82* |
| Epinephrine, pg/ml | 4,585 ± 434 | 3,022 ± 483* | 4,883 ± 881 | 7,633 ± 476* |
| GABA, nM | 11 ± 3‡ | 25 ± 4 | 10 ± 2† | 12 ± 6‡ |
Data are presented as means ± SE. Glucose infusion rates, peak plasma hormone concentrations, and extracellular GABA concentrations during the hypoglycemic clamping period for animals microdialyzed with artificial extracellular fluid (aECF) and microinjected with aECF (A), microdialyzed with 100 mM d-glucose and microinjected with aECF (B), microdialyzed with 100 mM l-glucose and microinjected with aECF (C), or microdialyzed with aECF and microinjected with bicuculline (aECF + BIC; D).
P < 0.05 vs. aECF controls;
P < 0.05 vs. baseline GABA concentrations;
P < 0.03 vs. baseline GABA concentrations.
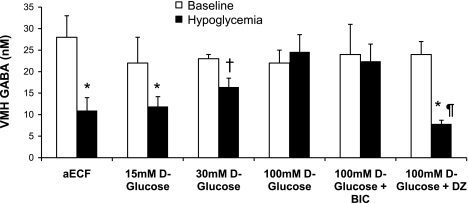
Consistent with our previously published observations (8, 9), changes in plasma counterregulatory hormone concentrations were inversely associated with changes in VMH extracellular GABA concentrations (Fig. 6). In our control animals, as plasma glucose levels fell to a nadir of 45 mg/dl, VMH GABA concentrations decreased 54% from baseline levels (P < 0.05), and this corresponded with activation of counterregulatory responses. Local delivery of 30 and 100 mM d-glucose into the VMH attenuated the drop in VMH GABA levels and prevented full activation of counterregulatory responses despite the presence of peripheral hypoglycemia. The drop in VMH GABA was completely abolished with 100 mM d-glucose, and VMH GABA remained at baseline levels despite peripheral hypoglycemia. The increase in extracellular GABA concentrations was positively correlated with the increase in dialysate glucose concentrations (R2 = 0.37, P < 0.001) and inversely correlated with plasma glucagon and epinephrine concentrations (R2 = 0.34 and 0.20, respectively, P < 0.02). As expected, microinjection of the GABAA receptor antagonist into the VMH did not prevent the glucose-induced rise in extracellular GABA levels, whereas microinjection of the KATP channel opener diazoxide did. Regardless, counterregulatory responses were amplified in both of these latter instances.
Fig. 6.
Average extracellular GABA concentrations in the VMH at baseline (open bars) and during the 90-min hypoglycemic clamping period (filled bars), shown as means ± SE. *P < 0.03 vs. baseline; †P < 0.05 vs. baseline; ¶P < 0.001 vs. 100 mM d-glucose.
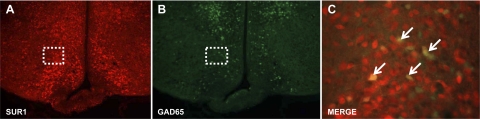
Immunohistochemical analyses revealed that ∼14% of VMH neurons that were positive for the SUR1 subunit of the KATP channel colabeled with the GAD65-eGFP signal (Fig. 7).
Fig. 7.
Representative hypothalamic sections showing immunofluorescent labeling for the type 1 sulfonylurea receptor (SUR1) subunit of the KATP channel in red (A) and in green fluorescent protein (GFP), which is expressed under the control of the GAD65 promoter in green (B). ×100 magnification of the highlighted field within the VMH showing colabeling of the SUR1 subunit on GAD65-GFP-positive neurons, as indicated by arrows (C).
DISCUSSION
We have previously reported (4, 5) that the local delivery of glucose into the VMH can dramatically suppress glucose counterregulation in response to systemic hypoglycemia. The current study provides in vivo physiological evidence that the effect of glucose is mediated via stimulation of inhibitory GABAergic neurons in the VMH and suggests that the reduction in GABA tone within the VMH observed during hypoglycemia is essential for full activation of the glucagon and epinephrine responses.
Glucose concentrations in VMH interstitial fluid in rats normally range from ∼0.75 mM under fasting conditions to ∼1.5–2 mM under fed conditions and decrease to levels below 0.3 mM during acute hypoglycemia (12). On the basis of our in vitro recovery studies, we determined the efficiency of our microdialysis probes to be between 4 and 7% for glucose. Thus, we estimate that we raised local VMH extracellular glucose levels between 1 and 7 mM glucose during our glucose clamp studies, and these levels encompass both the normal and pathophysiological ranges. It is noteworthy that, although glucose appeared to stimulate GABA release in a dose-dependent manner, extracellular GABA levels in the VMH never rose beyond normal baseline levels. This suggests that the effect of glucose on GABA release might be limited by a ceiling effect that plateaus by the time “euglycemia” is achieved. These data are consistent with the in vitro data from Song et al. (29), who showed that, when glucose levels in the culture medium are gradually increased from 0 to 2.5 mM, glucose-excited neurons gradually increase their firing rate. However, from 2.5 to 5 mM of glucose, glucose-excited neurons do not increase their firing rate any further, indicating that, at least in vitro, at 2.5 mM of glucose these neurons have reached their maximal firing frequency. Although the specific identity of the VMH GABAergic neurons that are being manipulated in our studies remains uncertain, our findings are most consistent with the possibility that they represent a subpopulation of glucose-excited neurons. Our previous work (9, 14, 21) demonstrated that the pharmacological opening or closing of KATP channels within the VMH during a standardized reduction in blood glucose can effectively decrease or increase local extracellular GABA concentrations, respectively, and modulate counterregulatory responses to hypoglycemia. In the current study, we assessed whether glucose acts through a similar mechanism by microinjecting diazoxide, a KATP channel opener, into the VMH while simultaneously microdialyzing this area with 100 mM d-glucose. Whereas 100 mM d-glucose alone prevented the fall in extracellular GABA levels and suppressed counterregulatory responses during hypoglycemia, diazoxide overcame the effect of continuous local glucose delivery. VMH GABA levels fell and counterregulatory responses were amplified. Moreover, immunohistochemical analysis showed the presence of the SUR1 subunit of the KATP channel on GABAergic neurons within the VMH. While microinjection of diazoxide can potentially open KATP channels located on other types of neurons in the VMH, the fact that we can still modulate VMH GABA release and alter counterregulatory responses suggests that GABAergic neurons involved in glucose sensing may lie downstream or even at the end of the glucose-sensing pathway. Taken together, these results suggest that the KATP channel and its modulatory effect on GABA release in the VMH may parallel the mechanism for insulin secretion in pancreatic β-cells, thereby forming a crucial link between glucose-sensing mechanisms in the hypothalamus and activation of counterregulatory responses during hypoglycemia.
To further investigate whether stimulated release of GABA is the primary mechanism by which glucose inhibits counterregulatory responses to hypoglycemia, we microinjected the GABAA receptor antagonist BIC into the VMH while simultaneously delivering glucose to this region of the brain using microdialysis. Our data indicate that, while glucose continued to maintain VMH GABA levels at normal baseline levels during systemic hypoglycemia, blocking GABAA receptors in the VMH with BIC amplified the counterregulatory responses. It is noteworthy that, regardless of whether VMH GABA levels are “low” (i.e., aECF + BIC group) or “high” (i.e., 100 mM d-glucose + BIC group), antagonism of GABAA receptors in the VMH always amplifies the counterregulatory responses to a similar degree. This would suggest that GABA release within the VMH and the activation of VMH GABAA receptors generate a critical downstream signal determining the magnitude of the counterregulatory response to hypoglycemia. Our data are consistent with the hypothesis that GABAergic neurons may be an integral part of the metabolic fuel-sensing mechanism within the VMH that monitors changes in fuel availability and, in turn, respond to these fluctuations by modulating glucose homeostatic mechanisms accordingly.
This work may have important implications for type 1 diabetic patients undergoing intensive insulin therapy. A variety of studies using positron emission tomography analyses, magnetic resonance spectroscopy, and arteriovenous differences have suggested that intensive insulin treatment and a history of antecedent hypoglycemia may cause adaptations in brain fuel metabolism designed to limit the adverse effects of hypoglycemia on brain function in type 1 diabetic patients compared with their nondiabetic counterparts. It has been hypothesized that this may be due to increases in glucose transport or to an adaptation to the use of alternate fuels. If similar metabolic adaptations extend to glucose-sensing cells in the VMH, they might be expected to promote GABA output and in turn contribute to defective counterregulation in this patient population. This hypothesis is consistent with our observations in diabetic and recurrently hypoglycemic animals, where increases in VMH GAD65 and GABA levels are associated with impaired counterregulatory mechanisms (8, 10).
In conclusion, release of GABA in the VMH is modulated by the local availability of glucose. During periods of fuel deficit, GABAergic tone in the VMH decreases, which allows for the full activation of counterregulatory responses. Local delivery of glucose into the VMH attenuates the fall in local GABA levels, and this in turn suppresses glucose counterregulation. Our data are consistent with the hypothesis that a subpopulation of VMH glucose-excited neurons may be GABAergic neurons that express SUR1 KATP channels and that these inhibitory VMH neurons regulate downstream neural pathways via GABAA receptors. These neurons are thus likely to have an important role in linking brain fuel sensing to peripheral glucose homeostasis.
GRANTS
This work was generously supported by research grants from the Juvenile Diabetes Research Foundation (JDRF) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-20495 and DK-45735). O. Chan is a recipient of a Career Development Award from the JDRF, and S. A. Paranjape and L. Zhou are recipients of postdoctoral fellowships from the JDRF.
DISCLOSURES
No conflicts of interest are reported by the author(s).
ACKNOWLEDGMENTS
We thank Aida Groszmann, Andrea Belous, and Ralph Jacob for invaluable technical assistance with these studies.
REFERENCES
- 1.Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci Lett 380: 60–65, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT. Role of brain in counterregulation of insulin-induced hypoglycemia in dogs. Diabetes 38: 7–16, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bolteus AJ, Garganta C, Bordey A. Assays for measuring extracellular GABA levels and cell migration rate in acute slices. Brain Res Brain Res Protoc 14: 126–134, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99: 361–365, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS. Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52: 663–666, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest 93: 1677–1682, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44: 180–184, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes 57: 1363–1370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS. ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 56: 1120–1126, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chan O, Paranjape SA, Czyzyk D, Seashore MR, Zhu W, Ding Y, Fan X, Sherwin RS. Role of Increased GABAergic inhibition within the ventromedial hypothalamus in the impaired counterregulatory response to hypoglycemia in diabetes. Diabetes 58, Suppl 1: A34, 2009 [Google Scholar]
- 11.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABAA receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 55: 1080–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 12.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 52: 2767–2773, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes 51: 2056–2065, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53: 2542–2551, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Fernandez M, Ortega-Saenz P, Pardal R, Lopez-Barneo J. Glucose sensing cells in the carotid body. Adv Exp Med Biol 536: 47–53, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 46: 1521–1525, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hevener AL, Bergman RN, Donovan CM. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 49: 8–12, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 55: 412–420, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 8: 579–584, 2005 [DOI] [PubMed] [Google Scholar]
- 21.McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS. Activation of ATP-sensitive K+ channels in the ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 54: 3169–3174, 2005 [DOI] [PubMed] [Google Scholar]
- 22.McDermott JC, Hutber A, Tan MH, Bonen A. The use of a cell-free perfusate in the perfused rat hindquarter: methodological concerns. Can J Physiol Pharmacol 67: 1450–1454, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature 222: 282–284, 1969 [DOI] [PubMed] [Google Scholar]
- 24.Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci 5: 197–198, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav 76: 403–413, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Routh VH. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes Metab Res Rev 19: 348–356, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Simpson IA, Vannucci SJ. Glucose transport into brain: effects of hypoglycemia. Diabetes Nutr Metab 15: 281–284, 2002 [PubMed] [Google Scholar]
- 28.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50: 2673–2681, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 54: 15–22, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Spanswick D, Smith MA, Groppi VE, Logan SD, Ashford ML. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390: 521–525, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3: 757–758, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21: 2–21, 1997 [DOI] [PubMed] [Google Scholar]