Abstract
Low birth weight (LBW) is associated with type 2 diabetes and depression, which may be related to prenatal stress and insulin resistance as a result of chronic hypothalamic-pituitary-adrenal (HPA) axis hyperactivity. We examined whether treatment with a selective serotonin reuptake inhibitor [escitalopram (ESC)] could downregulate HPA axis activity and restore insulin sensitivity in LBW rats. After 4–5 wk of treatment, ESC-exposed LBW (SSRI-LBW) and saline-treated control and LBW rats (Cx and LBW) underwent an oral glucose tolerance test or a hyperinsulinemic euglycemic clamp to assess whole body insulin sensitivity. Hepatic phosphoenolpyruvate carboxykinase (PEPCK) mRNA expression and red skeletal muscle PKB Ser473 phosphorylation were used to assess tissue-specific insulin sensitivity. mRNA expression of the hypothalamic mineralocorticoid receptor was fivefold upregulated in LBW (P < 0.05 vs. Cx), accompanied by increased corticosterone release during restraint stress and total 24-h urinary excretion (P < 0.05 vs. Cx), whole body insulin resistance (P < 0.001 vs. Cx), and impaired insulin suppression of hepatic PEPCK mRNA expression (P < 0.05 vs. Cx). Additionally, there was a tendency for reduced red muscle PKB Ser473 phosphorylation. The ESC treatment normalized corticosterone secretion (P < 0.05 vs. LBW), whole body insulin sensitivity (P < 0.01) as well as postprandial suppression of hepatic mRNA PEPCK expression (P < 0.05), and red muscle PKB Ser473 phosphorylation (P < 0.01 vs. LBW). We conclude that these data suggest that the insulin resistance and chronic HPA axis hyperactivity in LBW rats can be reversed by treatment with an ESC, which downregulates HPA axis activity, lowers glucocorticoid exposure, and restores insulin sensitivity in LBW rats.
Keywords: selective serotonin reuptake inhibitors
stress may be involved in the development of major Western lifestyle disorders such as cardiovascular disease and the metabolic syndrome (53, 59, 61). Particularly in humans born with low birth weight (LBW) (e.g., birth weight <2,500 g), stress-related psychiatric illness and metabolic disturbances seem to coexist, and the prevalence of conditions associated with psychological stress, such as melancholic depression, is increased in subjects born with LBW (35). In addition, these individuals also have a higher prevalence of type 2 diabetes (23, 57, 80) that is potentially due to early development of insulin resistance. Hence, the LBW condition can be considered as both a predepressive and a prediabetic state. However, the exact mechanisms responsible for these changes and whether they are associated are still debated.
Recently, impairments in hippocampal structure and function have been proposed to account for some of the phenotypic characteristics of LBW (21, 40, 63). Hippocampus regulates the overall circadian tonus of the hypothalamic-pituitary-adrenal (HPA) axis, and accordingly, the processes involved in corticosteroid regulation in LBW have been studied extensively. At this point the HPA axis in LBW has been studied at various developmental stages using a variety of experimental paradigms, and unfortunately the results are conflicting, reporting both hypo- and hyperactivity (35, 37). However, Vieau et al. (71) tried to deal with this problem and demonstrated how HPA axis tonus changed throughout life in LBW rats subjected to fetal malnutrition. At weaning the HPA axis displayed severe hypoactivity, but at the age of 4 mo HPA axis regulation was again close to normal, whereas at 8 mo a vast overcompensation had emerged, resulting in significantly elevated corticosteroid levels. A similar postnatal developmental pattern regarding the HPA axis tonus in LBW has been demonstrated in sheep subjected to fetal glucocorticoid exposure (65).
In elderly LBW subjects, some human studies have found signs of increased HPA axis activity (54, 58, 59), whereas others have found the opposite (36).
Although HPA axis hyperactivity might not be consistently present in the early or late phases of life in LBW subjects, it is still likely to play an important role for the adult LBW phenotype, and several studies in both young adult LBW primates (20) as well as young adult LBW humans support this notion (39, 55).
The hippocampo-hypothalamic system of adult LBW subjects is considered to be vulnerable, and if additionally exposed to excessive levels of adrenal steroids the neurobiological sequelae are likely to deteriorate further, increasing the risk for diseases such as depression (73). In addition, corticosteroids are known to be capable of causing insulin resistance, obesity, impaired blood-lipid profile, and hypertension (51). Therefore, fetal injuries within certain hippocampo-hypothalamic circuits in the brain leading to increased secretion of corticosteroids are plausible to be the common denominator responsible for the development of both type 2 diabetes and depression in LBW subjects.
The antidepressant selective serotonin reuptake inhibitors (SSRIs) are capable of downregulating HPA axis activity in rats and humans (32, 34, 52), and some studies further suggest that SSRIs can improve glucose metabolism in depressed nondiabetic patients as well as in depressed patients displaying obesity and/or type 2 diabetes (43, 69, 74). Since depression, obesity, and type 2 diabetes are generally associated with abnormal regulation of glucocorticoid metabolism (3, 6, 67, 75), we therefore hypothesized that downregulation of HPA axis activity using a SSRI compound would lower glucocorticoid levels and thereby improve glucose metabolism and insulin action in LBW.
In adult, 40-day-old, LBW rats subjected to prenatal dexamethasone exposure, we aimed to study the effects of SSRI [escitalopram (ESC)] treatment on the hippocampo-hypothalamic corticosteroid feedback system, pituitary ACTH secretion and adrenal corticosterone production as well as whole body insulin action, hepatic mRNA expression of gluconeogenetic enzymes, and insulin sensitivity of fat and skeletal muscle tissue. The present rat model displays the combination of insulin resistance and HPA axis hyperactivity (17), features similar to those of the adult LBW man.
MATERIALS AND METHODS
Animals.
Experiments were approved by the Danish Animal Experiments Inspectorate and complied with The European Convention for the Protection of Vertebrate Animals Used for Experiments and Other Scientific Purposes. Pregnant Sprague-Dawley dams (Taconic) were housed in a light- (light from 6 AM to 6 PM) and temperature-controlled (22°C) environment and had free access to food and water. Pregnant dams (n = 39) were treated daily from day 14 to day 21of gestation, with 0.15 mg/kg body wt dexamethasone sc in a 4% ethanol-saline (0.9% NaCl) solution or with 4% ethanol-saline (0.9% NaCl). At birth, pups (n = 273) regardless of the prenatal treatment (e.g., saline or dexamethasone) were transferred to nontreated, healthy, and lactating foster mothers (n = 39) in comparably sized groups of 6–8 pups/litter. Pups were weaned at the age of 3 wk, and only male offspring were included for further studies (n = 133).
For 4–5 wk, 40-day-old male rats were injected intraperitoneally twice a day with either ESC-saline solution (diluted oral droplets of Cipralex obtained at the pharmacy) at a concentration of 1.4 mg/ml, pH = 4.95 (10 mg/kg body wt), or saline (pH = 4.95). A washout period of 16–18 h after the last injection was allowed prior to all experiments. Rats were euthanized during isoflurane-anesthesia or pentobarbital (clamped animals), and all tissues were snap-frozen and stored at −80°C until further analysis. The first group of saline-treated control (Cx) and LBW rats (n = 37) underwent the oral glucose tolerance test (OGTT), the second group (n = 30) underwent the hyperinsulinemic euglycemic clamp, the third group was used for basal muscle and liver tissue analysis (n = 23), and in the fourth group (n = 27) brain tissue was collected. In a pilot study, the effects of ESC on OGTT and corticosterone secretion were assessed in Cx rats (n = 16).
Restraint stress test.
The restraint stress test was performed as described previously (17) at 9 AM. Tail blood samples were drawn into heparinized capillary tubes at t = 0, 15, 30, 45, 60, 75, and 90 min after restrain was initiated. Blood was transferred to NaF-coated tubes, centrifuged at 2,000 rpm, and stored at −20°C until further analysis.
Twenty-four-hour urine sampling.
Rats were housed for 24 h from 10 AM in metabolic cages, and urine was collected as described previously (17). Debris was removed by centrifugation and urine kept at −20°C until further analysis.
OGTT.
After a 16-h overnight fast, 2.5 mg glucose/kg body wt was administered by gavage. Tail blood samples were obtained and placed into NaF-coated tubes at 0, 15, 30, 60, and 120 min after the glucose challenge. Plasma was stored at −20°C until further analysis.
Insulin sensitivity index.
The insulin sensitivity index (ISI) was calculated according to Matsuda and DeFronzo (44): FG, fasting plasma glucose concentration (mg/dl); FI, fasting plasma insulin concentration (mU/l). Mean OGTT glucose and mean OGTT insulin are the average concentrations of blood glucose and plasma insulin, respectively, during the OGTT. The ISI represents the composite whole body insulin sensitivity, reflecting both hepatic and peripheral tissue insulin sensitivity.
Metabolites and hormones.
Plasma and urine concentrations of insulin and corticosterone were measured by a rat insulin ELISA kit (DRG Instruments) and a rat corticostrone radioimmunoassay (Amersham), respectively. Blood glucose concentrations were measured using a One-Touch Ultra blood glucose analyzer.
Hepatic mRNA expressions.
Hepatic mRNA was isolated by use of a RNA easy column kit (Qiagen, Valencia, CA). Complementary DNA was synthesized, and mRNA levels were assessed by quantitative real-time PCR (qPCR; TaqMan) using an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). The primer sets used were glucose-6-phosphatase (G-6-Pase): forward primer AGG GTA AAA GAA AAG AGC GTT G and reverse primer GTA GAC ATG GCT TGC ATA TGG T; phosphoenolpyruvate carboxykinase (PEPCK): forward primer CAG GAA GTG AGG AAG TTT GTG G and reverse primer ATG ACA CCC TCC TCC TGC AT; and GAPDH: forward primer TCACCACCATGGAGAAGGC and reverse primer GCTAAGCAGTTGGTGGTGCA. Pilot studies revealed that GAPDH was not differentially regulated in these rats, and threshold cycle values of for G-6-Pase and PEPCK were normalized relative to GAPDH (Supplemental Table S1; Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site).
Real-time qPCR of hippocampus and hypothalamus.
The rats were decapitated, and hypothalamus and hippocampus were dissected and frozen on dry ice powder. Total RNA was isolated using the ABI PRISM 6100 Nucleic Acid Prepstation (Applied Biosystems) and reverse transcribed using random primers and Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA), as described previously (22). The real-time qPCR reactions were carried out by using the Mx3000P (Stratagene, La Jolla, CA) and SYBR Green. The gene expression of glucocorticoid receptor (GR), mineralocorticoid receptor (MR), 11β-hydroxysteroid dehydrogenase-1 (HSD-1), corticotropin-releasing hormone (CRH) receptor-1 and -2 (CRH-R1 and CRH-R2, respectively), CRH-binding protein (CRH-BP), the apoptosis genes Bax and Bcl-2, and eight different reference genes [18S subunit ribosomal RNA (18S), β-actin, cyclophilin A, Gapdh, hydroxymethylbilane synthase, hypoxanthine guanine phosphoribosyl transferase 1, ribosomal protein L13A, and tyrosine 3-monooxygenase-tryptophan 5-monooxygenase activation protein-ζ] was investigated. The reference genes were selected as described by Bonefeld et al. (13). Stability comparison of the expression of the reference genes was conducted with the Normfinder software (http://www.mdl.dk) (Supplemental Table S1) (2).
Hyperinsulinemic euglycemic clamp.
One week prior to the euglycemic hyperinsulinemic clamp experiment, indwelling catheters were placed as described previously (61) into the jugular vein for infusions and into the left carotid artery for blood collections, filled with a polyvinylpyrrolidine heparine solution, and closed.
After 12-h overnight fast, catheters were flushed with saline, and the 150-min euglycemic hyperinsulinemic clamp was initiated with a primed continuous insulin infusion in awake and unrestrained rats (primed: 200 mU/kg body wt; continuous: 4 mU·kg−1·min−1) (Actrapid; Novo Nordisk, Bagsvaerd, Denmark). Blood glucose levels were kept at ∼100 mg/dl by a variable infusion of 20% d-glucose. Plasma glucose concentrations were measured using a Glucose Analyzer II (Beckman Instruments, Fullerton, CA).
To estimate rates of insulin-stimulated glucose transport activity in red (RG) and white gastrocnemius (WG) muscle tissue and epididymidal fat pads, a single dose of 20 μCi 2-[1-14C]deoxyglucose was administered at t = 120 min. Plasma-specific activity of 2-[1-14C]deoxyglucose was measured at t = 121, 123, 125, 130, 135, 140, and 150 min, and the concentrations of plasma glucose were used to estimate glucose uptake activity in tissues, as described previously (17).
Clamp tissue glucose uptake assay.
The tissues were homogenized 1:10 (wt/vol) in demineralized H2O and placed in a heat block at 100°C for 10 min. Samples were cooled to room temperature and centrifuged for 5 min. In supernatants, the total activity of 2-[1-14C]deoxyglucose as well as the phosphorylated (intracellular) and unphosphorylated (extracellular) fractions of 2-[1-14C]deoxyglucose were counted following separation by use of anion exchange chromatography columns (cat. no. 731-6211; Bio-Rad Laboratories, Hercules, CA).
Skeletal muscle tissue glycogen levels.
Muscles were heated for 120 min at 99°C in 2.0 N HCl. Afterward, samples were neutralized with 2.0 N NaOH and centrifuged at 10,000 g for 1 min. Sample glucose levels were assessed using hexokinase reagent (CIMA Scientific, De Soto, TX), as described previously (12).
RG PKB protein expression and Ser473 phosphorylation.
Muscle tissue was homogenized as described previously (79). Phospho-Ser473 and total PKB antibodies were obtained from Cell Signaling Technology (Beverly, MA). Western blots were developed using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ) and quantified using the UVP BioImaging System (UVP, Upland, CA).
Total crude membrane GLUT4 contents.
Crude membranes were prepared from ∼30 mg of RG and WG muscles, and aliquots of protein were resolved as described previously by use of a polyclonal anti-COOH-terminal peptide GLUT4 antibody (16). Protein bands were visualized by chemiluminescence (Pierce Super Signal) and subsequently quantified by use of the UVP BioImaging System.
Statistical analysis.
Data are given as means ± SE. Data were analyzed with the one-way ANOVA test and Newmann-Keuls post hoc test. P < 0.05 was considered statistically significant.
RESULTS
Body weights, fat content, and food consumption.
Birth weights of the LBW rats were 17% less than the control rats (Cx: 6.40 ± 0.05 g, n = 72, vs. LBW: 5.32 ± 0.04 g, n = 66; P < 0.001). In a subgroup of random animals, no differences were seen with regard to the weight of epididymidal fat pads (Cx: 3.25 ± 0.18, n = 8; LBW: 2.71 ± 0.12 g, n = 8; SSRI-LBW: 2.89 ± 0.24, n = 7; P = 0.1279). At the end of the treatment period, body weights were the same for all groups (Cx: 352.9 ± 5.4 g, n = 44; LBW: 356.1 ± 10.4 g, n = 38; SSRI-LBW: 346.7 ± 9.8 g, n = 35; P = 0.7474). Furthermore, in a subgroup of rats (data not shown), food consumption and body weight development during treatment and rates of spontaneous activity, blood pressure, and body temperature as measured by telemetry at the end of the treatment period did not differ between any of the experimental groups.
Restraint stress test.
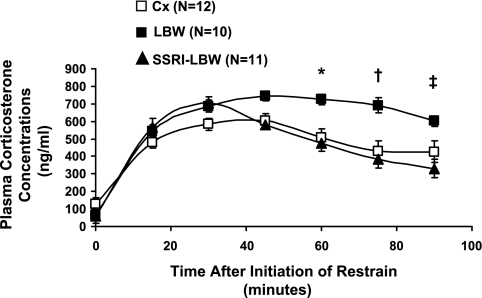
Basal concentration of plasma corticosterone (the main glucocortidoid in the rat) did not differ (Fig. 1). Upon restraint stress in the Cx group, plasma corticosterone concentrations increased approximately fourfold to a peak at 30 min followed by a gradual decline throughout the test. A similar initial increase was observed in the LBW group; however, plasma corticosterone concentrations remained approximately fourfold elevated until the end of the test. The area under the curve from 60 to 90 min (AUC60–90) was 56% larger in the LBW group than in the Cx group (Cx: 12,470 ± 1,270 ng/min vs. LBW: 19,440 ± 1,240 ng/min, P < 0.05; Table 1), suggesting impaired adrenal negative feedback inhibition in the LBW rats. The ESC treatment completely reversed this impairment of the adrenal stress response such that both the time course for plasma corticosterone concentrations and the AUC60–90 were similar to the Cx control group (SSRI-LBW: 11,180 ± 1,030 ng/min).
Fig. 1.
Restraint stress test. Plasma corticosterone concentrations during restraint. Data are given as means ± SE. *P < 0.05 vs. selective serotonin reuptake inhibitor-low-birth-weight (SSRI-LBW) and saline-treated control (Cx) rats; †P < 0.01 vs. SSRI-LBW and Cx; ‡P < 0.01, LBW vs. SSRI-LBW and Cx.
Table 1.
Characterization of hippocampus and HPA axis
| Cx (n = 5–13) | LBW (n = 8–14) | SSRI-LBW (n = 8–16) | |
|---|---|---|---|
| Adrenal corticosterone release | |||
| RST AUC 60-90 min, ng/min | 12,470 ± 1,270 | 19,440 ± 1,240* | 11,180 ± 1,030 |
| 24-h Urinary corticosterone excretion, nmol·24 h−1·kg body wt−1 | 10.1 ± 0.6 | 13.9 ± 1.2† | 8.1 ± 0.5 |
| Pituitary ACTH release | |||
| Plasma ACTH concentrations, pg/ml | |||
| 9 AM | 15.96 ± 0.65 | 18.13 ± 1.40 | 16.87 ± 1.01 |
| 9 PM | 13.18 ± 0.37‡ | 15.53 ± 1.42 | 12.89 ± 0.37‡ |
| Hypothalamic corticosteroid feedback | |||
| HSD-1 mRNA expression, %Cx | 100.0 ± 20.4 | 82.5 ± 16.1 | 73.6 ± 13.7 |
| MR mRNA expression, %Cx | 100.0 ± 21.4 | 501.7 ± 160.4§ | 205.8 ± 60.0 |
| GR mRNA expression, %Cx | 100.0 ± 15.8 | 123.9 ± 15.8 | 121.6 ± 46.5 |
| Hippocampal corticosteroid feedback | |||
| HSD-1 mRNA expression, %Cx | 100.0 ± 15.5 | 122.2 ± 25.3 | 67.5 ± 8.6 |
| MR mRNA expression, %Cx | 100.0 ± 10.0 | 123.2 ± 13.3# | 85.5 ± 8.9 |
| GR mRNA expression, %Cx | 100.0 ± 14.1 | 122.5 ± 11.1 | 88.1 ± 10.9 |
Data are means ± SE. HPA, hypothalamic-pituitary-adrenal; RST, restraint stress test; AUC, area under the curve; Cx, saline-treated control; SSRI, selective serotonin reuptake inhibitor; LBW, low birth weight; HSD-1, 11β-hydroxysteroid dehydrogenase-1; MR, mineralocorticoid receptor; GR, glucocorticoid receptor. P values for comparisons reflect the results for the post hoc analysis that has been carried out only when the 1-way of analysis of variance (ANOVA) test results in a P value <0.05. (Fig. 1). Post hoc test P values:
P < 0.01 vs. Cx and vs. SSRI-LBW;
P < 0.05 vs. Cx and P < 0.01 vs. SSRI-LBW;
P < 0.05 vs. 9 AM;
P < 0.05 vs. Cx;
P = 0.0725 vs. Cx.
Twenty-four-hour urine corticosterone excretion.
Urinary corticosterone excretion was increased by 38% in the LBW compared with the Cx rats (Cx: 12,470 ± 1,270 ng/min vs. LBW: 19,440 ± 1,240 ng/min, P < 0.05; Table 1), suggesting defects in the basal circadian rhythm of corticosteroid production in this group. This increase was completely reversed in the SSRI-LBW group (SSRI-LBW: 11,180 ± 1,030 ng/min).
Plasma ACTH levels.
Although not significant, plasma ACTH levels at both 9 AM and 9 PM tended to be higher in the LBW group than in the Cx group (Table 1). During the day, plasma ACTH levels decreased moderately, by ∼12 to 14% in the Cx and LBW groups, although this was significant only for the Cx group (P < 0.05 vs. 9 AM) and not for the LBW group (P = 0.286 vs. 9 AM), suggesting a possible defect in the diurnal control of ACTH secretion.
In contrast, plasma ACTH concentrations in the SSRI-LBW group were similar to the Cx rats at both 9 AM and 9 PM and decreased by ∼24% during the day (P < 0.01 vs. 9 AM; Table 1).
The hypothalamic and hippocampic glucocorticoid feedback axis.
The mRNA expression of HSD-1 and the GR in the hippocampus and hypothalamus was similar in all three groups (Table 1). In contrast, hypothalamic mRNA expression of the MR was approximatelty fivefold upregulated in LBW compared with Cx (Cx: 100 ± 20.4% vs. LBW: 501.7 ± 160.4%, P < 0.05). This was completely normalized in the SSRI-LBW group (205.8 ± 60.0, P < 0.05 vs. LBW), reflecting a downregulation of the MR as a result of the ESC treatment.
There was a similar strong, albeit not statistically significant, tendency for upregulation of mRNA expression of the MR in the hippocampus of the LBW group (Cx: 100.0 ± 10.0% vs. LBW: 123.2 ± 13.3%, P = 0.072). However, in the SSRI-LBW group the hippocampic MR mRNA expression was normal and even tended to be lower than in the Cx group (SSRI-LBW: 85.5 ± 8.9% of Cx, n = 7), suggesting a potential modulation of the glucocorticoid feedback system within the LBW hippocampus as a result of ESC treatment. Furthermore, the hypothalamic and hippocampal mRNA expression patterns of CRH-R1, CRH-R2, and CRH-BP did not display any differences (data not shown). Similarly, the hippocampal mRNA expression patterns of the apoptosis genes Bcl-2 and Bax were also unaffected by the phenotype and treatments (data not shown).
OGTT.
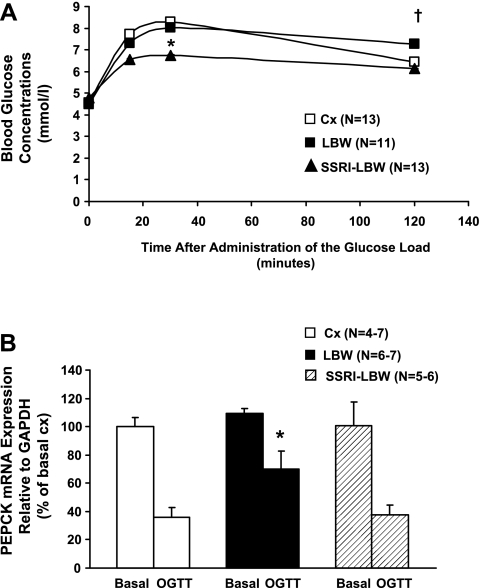
The LBW group had elevated blood glucose concentrations at 120 min of the OGTT (P < 0.01, LBW: 7.26 ± 0.04 mmol/l, n = 11, vs. Cx: 6.44 ± 0.03 mmol/l, n = 13; Fig. 2A), but SSRI treatment reversed this elevation (P < 0.05, SSRI-LBW: 6.12 ± 0.02 mmol/l, n = 13, vs. LBW; Fig. 2A). Whereas glucose AUC and fasting blood glucose were similar in the LBW and Cx groups, the OGTT glucose AUC was reduced by 15% in the SSRI-LBW group compared with the LBW group (P < 0.01 vs. LBW and P < 0.05 vs. Cx; Table 2).
Fig. 2.
A: oral glucose tolerance test (OGTT). Full blood glucose concentrations are shown before and after administration of 2.5 g glucose/kg body wt given by gavage. Data are given as means ± SE. *P < 0.01, SSRI-LBW vs. LBW; †P < 0.05, LBW vs. SSRI-LBW and Cx. B: hepatic phosphoenolpyruvate carboxykinase (PEPCK) mRNA expressions in the basal (i.e., fasted overnight for 12 h) and the postprandial state [i.e., tissues taken out 90 min after glucose gavage (OGTT)]. Data are given as means ± SE. *P < 0.05 vs. SSRI-LBW and Cx.
Table 2.
OGTT
| Cx (n =13) | LBW (n =11) | SSRI-LBW (n =13) | |
|---|---|---|---|
| Fasting glucose concentrations, mmol/l | 4.55 ± 0.13 | 4.37 ± 0.11 | 4.72 ± 0.13 |
| Fasting insulin concentrations, mU/l | 7.83 ± 1.12 | 26.93 ± 5.24* | 11.83 ± 2.17 |
| OGTT total AUCglucose, mmol·l glucose−1·min | 871 ± 20 | 898 ± 23 | 767 ± 29† |
| OGTT total AUCinsulin, mU·l insulin−1·min | 1,542 ± 39 | 3,738 ± 79‡ | 1,996 ± 37 |
| ISI (expressed as %Cx) | 100.0 ± 10.3 | 51.1 ± 6.3§ | 85.0 ± 8.1 |
Data are means ± SE. OGTT, oral glucose tolerance test; ISI, insulin sensitivity index. P values for comparisons reflect the results for the post hoc analysis that has been carried out only when the 1-way ANOVA test results in a P value <0.05. Post hoc test P values:
P < 0.01 vs. Cx and P < 0.05 vs. SSRI-LBW;
P < 0.05 vs. Cx and P < 0.01 vs. LBW;
P < 0.01 vs. Cx and SSRI-LBW;
P < 0.01 vs. Cx and P < 0.05 vs. SSRI-LBW.
Both fasting and postprandial plasma insulin concentration were two- to threefold increased in the LBW rats (P < 0.01 vs. Cx; Table 2) but were reduced significantly in ESC-exposed LBW rats (P < 0.01 vs. LBW; Table 2).
Accordingly, the whole body ISI in the LBW group was ∼50% lower compared with the Cx group (P < 0.01; Table 2), which is consistent with whole body insulin resistance in the LBW group. However, ESC treatment almost normalized the ISI of the SSRI-LBW group (P < 0.05 vs. LBW; Table 2), reflecting restoration of whole body insulin sensitivity.
Hepatic PEPCK and G-6-Pase mRNA expressions.
Basal hepatic PEPCK mRNA levels were similar in all three experimental groups and were suppressed by ∼60% in the Cx group 90 min after administration of the oral glucose load (P < 0.01 vs. basal; Fig. 2B). In contrast, LBW rats suppressed PEPCK mRNA expression by only ∼30% after oral glucose administration (P < 0.05 vs. Cx), strongly suggesting hepatic insulin resistance and inability for glucose-induced suppression of hepatic gluconeogenesis in the LBW liver.
The glucose-induced suppression of hepatic PEPCK mRNA expression was totally restored in the SSRI-LBW group (P < 0.05 vs. LBW-saline; Fig. 2B).
Basal G-6-Pase levels tended to be elevated in the LBW rats compared with the Cx (Cx: 100 ± 7% vs. LBW: 169 ± 33%, P = 0.068). However, ESC-treated rats had completely normal basal G-6-Pase expression levels (SSRI-LBW: 86 ± 23%; no difference). Postprandial G-6-Pase expressions were suppressed by ∼95% in all groups (data not shown).
Euglycemic hyperinsulinemic clamp.
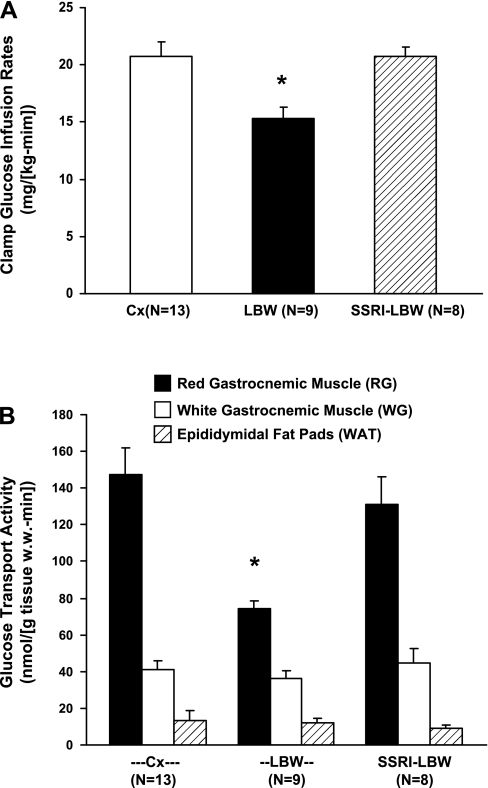
All animals were clamped at similar blood glucose (Cx: 102.7 ± 1.10 mg/dl, n = 13; LBW: 100.0 ± 3.01 mg/dl, n = 9; SSRI-LBW: 99.1 ± 0.52 mg/dl, n = 8; P = 0.3233) and plasma insulin concentrations (Cx: 133.2 ± 12.6 mU/l, n = 13; LBW: 136.1 ± 10.7 mU/l, n = 9; SSRI-LBW: 128.5 ± 12.3 mU/l, n = 8; P = 0.9239). As suggested by the reduction in ISI, the LBW rats displayed 26% lower rates of whole body insulin-stimulated glucose uptakes compared with the Cx group (Cx: 20.7 ± 0.9 mg·kg−1·min−1 vs. LBW: 15.3 ± 1.0 mg·kg−1·min−1, P < 0.01; Fig. 3A). In the ESC-treated LBW rats, this insulin resistance was completely normalized (SSRI-LBW: 20.7 ± 1.3 mg·kg−1·min−1, P < 0.01 vs. LBW).
Fig. 3.
A: mean rates of glucose infusion from 90 to 150 min after initiation of the hyperinsulinemic euglycemic clamp experiment. Data are given as means ± SE. *P < 0.01 vs. SSRI-LBW and Cx. B: rates of insulin-stimulated glucose transport activities in red (RG) and white (WG) gastrocnemic muscle tissue as well as in epididymidal fat pads. Data are given as means ± SE. *P < 0.01 vs. Cx and P < 0.05, SSRI-LBW.
Clamp glucose uptake in RG and WG muscle and epididymal fat.
Rates of insulin-stimulated glucose uptake in RG muscle as assessed by 2-[1-14C]deoxyglucose were reduced by ∼47% in the LBW group (n = 9) compared with Cx (n = 13, P < 0.01) (Fig. 3B) but were fully recovered by ESC exposure in SSRI-LBW group (P < 0.05 vs. LBW).
There were no differences in insulin-stimulated glucose uptake in WG muscle tissue or in epididymal adipose tissue between the three groups, and the contents of muscle glycogen and GLUT4 protein were similar in all groups, with RG muscle tissue displaying the lowest glycogen concentrations (P < 0.05 vs. WG) and the highest GLUT4 protein expression (P < 0.05 vs. WG) (Table 3).
Table 3.
Muscle glucose metabolism
| Cx (n = 5–14) | LBW (n = 8–9) | SSRI-LBW (n = 6–8) | |
|---|---|---|---|
| Glycogen and GLUT4 protein content in RG and WG gastrocnemius muscle tissue | |||
| Basal glycogen WG, μmol/g muscle wet wt | 38.8 ± 1.3 | 40.5 ± 1.7 | 42.9 ± 1.8 |
| Basal glycogen RG, μmol/g muscle wet wt | 31.4 ± 2.2* | 33.8 ± 1.7* | 32.4 ± 2.3* |
| Total GLUT4 content WG, arbitrary units | 9.89 ± 3.36 | 10.32 ± 0.90 | 9.97 ± 0.58 |
| Total GLUT4 content RG, arbitrary units | 13.93 ± 0.71* | 16.38 ± 1.92* | 13.22 ± 0.98* |
| PKB Ser473phosphorylation in RG muscle tisssue | |||
| Basal phosphorylation, arbitrary units | 29,568 ± 3,239 | 31,691 ± 4,901 | 22,459 ± 4,429 |
| Clamp phosphorylation, arbitrary units | 69,596 ± 10,600† | 56,729 ± 7,641† | 76,127 + 8,287†‡ |
| Insulin-stimulated increase from basal, % | 135 ± 4 | 79 ± 24 | 239 ± 43§ |
Data are means ± SE. RG, red gastrocnemius; WG, white gastrocnemius. P values for comparisons reflect the results for the post hoc analysis that has been carried out only when the 1-way ANOVA test results in a P value <0.05. Post hoc test P values:
P < 0.05 vs. WG;
P < 0.05 vs. basal;
P < 0.10 vs. LBW;
P < 0.05 vs. LBW.
Red muscle tissue PKB Ser473 phosphorylation.
Basal PKB Ser473 phosphorylation in RG muscle was similar in all three groups (Table 3). In the Cx group, insulin stimulation was accompanied by a 135% increase in RG PKB Ser473 phosphorylation (P < 0.05 vs. basal; Table 3). This insulin stimulation was decreased by ∼60% in the LBW rats, and although this did not reach statistical significance, it suggests impaired intracellular insulin signaling in RG muscle in the LBW rats. Insulin caused an ∼240% increase in RG PKB Ser473 phosphorylation in the SSRI-LBW rats (P < 0.01 vs. LBW; Table 3). Together, these observations strongly suggest that ESC administration leads to a tissue-specific improvement in insulin signaling in RG muscle of LBW rats.
Pilot study assessing the effects of ESC in Cx rats (SSRI-Cx).
The SSRI-Cx rats (n = 14–16) had similar food intake and weight gain during treatment (data not shown). After treatment, body weights (355.4 ± 6.3 g), fasting plasma concentrations of glucose (4.58 ± 0.12 mmol/l), fasting plasma concentrations of insulin (11.85 ± 1.62 mU/l), and glucose levels during OGTT (AUC: 869 ± 23 mmol glucose/min; 2-h concentrations: 6.34 ± 0.28 mmol/l) were not different from Cx. Surprisingly, however, OGTT insulin concentrations were elevated significantly in SSRI-Cx animals (AUC: 3,032 ± 74 mU·l−1·min, P < 0.01 vs. Cx), and the ISI was lower than in Cx (ISI: 73.6 ± 6.3% of Cx, P < 0.05 vs. Cx), indicating that ESC causes insulin resistance in SSRI-Cx rats. Furthermore, ESC did not change 24-h urinary corticosterone excretion (11.0 ± 2.8 nmol·24 h−1·kg−1; n = 5) but tended to increase restraint stress corticosterone AUC from 60 to 90 min (16,648 ± 1,745 ng/min, n = 10, P = 0.1201 vs. Cx), indicating that ESC affects HPA axis differently in Cx rats compared with LBW.
DISCUSSION
In clinical studies of preeclampsia and intrauterine growth retardation, conditions known to cause LBW in humans, umbilical cord blood samples have revealed elevated glucocorticoid concentrations (25, 26). Furthermore, various animal models for LBW such as maternal malnutrition (11), maternal stress (41, 42), and late gestation uterine ligation (8) have all been shown to significantly increase fetal glucocorticoid levels. Hence, enhanced fetal glucocorticoid stimulation seems to be the common denominator for both human and animal LBW conditions, and therefore, as in our previous studies, we opted to study a model of LBW where fetal growth retardation was induced directly by prenatal treatment with dexamethasone, a glucocorticoid analog capable of freely passing the blood-placenta barrier, and to induce a robust glucocorticoid stimulation of the fetuses (17). Similar to LBW humans, this model is characterized by early postnatal development of impaired glucose metabolism and hypertension (17, 47, 68).
In this model of LBW, we studied the effects of ESC treatment on insulin sensitivity and HPA axis activity in a rat model of LBW (17, 64). This model has previously been shown to be insulin resistant and to have HPA axis hyperactivity, pituitary hyperplasia of the ACTH-producing cells, and increased ACTH secretion (17, 64). The HPA axis hyperactivity can be attributed to defects at both hypothalamic and hippocampal levels (64) causing augmented corticosteroid release during both stress and basal conditions, respectively. In accord with our previous studies, the LBW rats presented prolonged adrenal corticosterone release during restraint, reflecting disturbances in the hypothalamic control of the HPA axis. In all of the groups we found a diurnal decrease in ACTH levels, which tended to be diminished in the LBW groups compared with the Cx and the ESC-treated LBW groups. In addition, 24-h urine excretion of corticosterone was increased significantly and accompanied by disturbances of the circadian rhythm of ACTH secretion, indicating concurrent defects in the tonic hippocampal coordination of pituitary-adrenal activity.
O'Regan et al. (49) found the circadian oscillations of ACTH to be at peak in the evening. This difference between their data and ours is unclear, but their levels of ACTH were ∼15- to 20-fold higher than in other studies, including ours (70), which could be due to differences in the timing of the blood sampling, differences in assays used, and/or stress during the blood sampling procedure.
In the hypothalamus and hippocampus, corticosteroids exert a direct inhibitory feedback by binding to the MR and GR, whereas HSD-1 assists this process by converting inactive deoxycorticosteroid with low affinity for these receptors to active corticosteroid. Because the MR exhibits an ∼10-fold higher affinity for endogenous glucocorticoids than the GR, the MR is considered to be the most important receptor for mediating the glucocorticoid feedback in the brain (4, 50). Shoener et al. (64) reported increased mRNA levels of HSD-1 and decreased expression of the MR in hippocampus of prenatally exposed dexamethasone rats displaying increased plasma levels of ACTH and corticosterone. In contrast, Banjanin et al. (5) found increased MR expression in hippocampus but no changes in circulating corticosteroid or ACTH in prenatally glucocorticoid-treated male guinea pigs. In the LBW hippocampus, we found no change in HSD-1 or GR expression but a tendency toward increased MR expression, although this finding was not significant. In the hypothalamus, a similar pattern emerged with no change in GR or HSD-1 but a more than fivefold increase in MR mRNA levels in the LBW rats. The reasons for these differences are not clear, but Shoener et al. (64) used only 18S rRNA as a reference for the real-time qPCR experiments, and normalization with different reference genes may affect the obtained data differently (13). Also, circadian oscillations and different experimental settings easily influence the measurements and emphasize the difficulties in direct comparisons of such data between studies.
Welberg et al. (78) have previously demonstrated increased CRH expression within the periventricular nucleus of the hypothalamus of a similar model of dexamethasone-induced LBW. Although we did not measure CRH expression in our study, we measured mRNA expression levels of the CRH receptors and the CRH-BP. CRH-R1 and CRH-R2 are widely expressed in the brain, and in states of hyperactive central CRH systems a concomitant dysregulation of the magnitude and duration of CRH receptor signaling is thought to contribute to an enhanced CRH neurotransmission (30). Further disturbances in CRH-BP, also a modulator of CRH activity, are also likely to play a role. In the rat hypothalamus and hippocampus, CRH-R1, CRH-R2, and CRH-BP are differentially expressed throughout different nuclei. In particular, hippocampal and hypothalamic R1 and R2 receptors take part in initiation (CRH-RH1) and recovery/maintenance of the stress response (CRH-RH2) (19, 24) as well as the regulation of serotonergic neurotransmission (CRH-R1) (53). However, here we found no differences in the hypothalamic or hippocampal mRNA expression pattern of these proteins in either of the experimental groups. Since chronic stress has been proposed to cause atrophy of the hippocampus, we also measured hippocampal mRNA expression patterns of two proteins involved in cellular apoptosis (i.e., Bax and Bcl-2), but we did not find any changes. Structural hippocampal change following a restraint prenatal stress paradigm has been observed, but only in females (62). Whether this is relevant in the context of the present work awaits future studies.
In LBW rats, the mRNA expressions of MR, which is quantitatively the most important protein for mediating negative corticosteroid feedback in the brain, were higher in hypothalamus and tended to be higher in the hippocampus compared with Cx. Despite these changes, LBW rats exhibited increased corticosteroid secretion and signs of disturbed ACTH secretion. Together, these alterations may suggest a compensatory upregulation of the MR due to impairments within the hippocampo-hypothalamic corticosteroid feedback regulation. These abnormalities were completely restored in the ESC-treated LBW animals, which displayed normal expressions of the MR in hypothalamus and hippocampus and had a normal 24-h urinary corticosterone excretion as well as a normal diurnal control (9 AM to 9 PM) of pituitary ACTH release. These findings strongly support the hypothesis that ESC treatment restores the HPA axis control in the LBW phenotype.
The mRNA expression data do not necessarily predict protein levels or protein function, due to possible posttranslational modifications or phosphorylations/nitrolylations of the protein. In addition, there are several limitations with regard to the quantitative PCR on dissected hippocampal and hypothalamic tissues. Although the method is superior in quantitative detection of mRNA expression, it may not provide information about qualitative differences, as exemplified by the anatomic distribution of the mRNA. Therefore, additional studies such as in situ hybridizations and/or proteomic studies would be required to provide a more comprehensive understanding of the neurobiological effects of the SSRI treatment on the HPA axis in this LBW model.
In accord with our previous findings, the present studies show that the 70-day-old LBW rats were insulin resistant, as reflected by both reduced ISI and reduced insulin-stimulated rates of whole body glucose uptake. The increased hepatic PEPCK mRNA levels during the OGTT strongly suggest hepatic insulin resistance and increased postprandial rates of gluconeogenesis (17, 47). In addition, basal hepatic G-6-Pase mRNA levels also tended to be upregulated, consistent with hepatic dysregulation of glucose metabolism.
In addition to the hepatic insulin resistance, we also found that the LBW rats had muscle-specific insulin resistance, as determined by the 2-[1-14C]deoxyglucose technique. This could be accounted for by an ∼50% decrease in rates of insulin-stimulated glucose uptake in RG muscle, whereas glucose uptake in fat tissue and WG muscle appeared to be normal. Total GLUT4 content in RG was similar to the Cx, suggesting defects at the level of insulin signaling possibly due to decreased phosphatidylinositol 3-kinase activation, as has been shown in other conditions of muscle insulin resistance (27). Defects in insulin signaling in the muscle at the level of PKB, which is involved in initiating translocation of GLUT4, are possible given the strong trend toward decreased insulin stimulation of PKB Ser473 phosphorylation in RG muscle in LBW (LBW: 79% increase vs. Cx: 135% increase).
We have shown previously that hepatic insulin resistance is the prominent phenotype of 40-day-old rats in the present LBW model (17), and the current studies show that additional muscle insulin resistance develops later in the lives of these LBW rats. In contrast, muscle insulin resistance appears to be the most consistent finding in humans born with LBW (31, 33). Although additional hepatic insulin resistance has been suggested (15, 66), it is still unclear whether humans born with LBW have increased activity of hepatic gluconeogenesis.
ESC treatment of LBW rats completely restored whole body insulin sensitivity, as reflected by an augmented ISI as well as by normalization of insulin-stimulated glucose disposal during the clamp. Additionally, ESC treatment completely normalized hepatic postprandial PEPCK expression and basal G-6-Pase expressions, indicating normalization of hepatic gluconeogenesis in the ESC-treated LBW rats.
ESC administration improved glucose uptake and insulin stimulation of red muscle PKB Ser473 phosphorylation, indicating that ESC treatment specifically improved the defects in insulin signaling in red muscle of the LBW rats. The ESC treatment did not influence basal muscle glycogen concentrations or GLUT4 content in red or white muscle tissue.
In animal models, it is well established that, by increasing the expression of PEPCK and to a lesser extent G-6-Pase, corticosteroids are potent activators of hepatic gluconeogenesis (7, 29). Moreover, corticosteroids are known to cause insulin resistance in skeletal muscle by reducing both insulin-stimulated Ser473 and Thr308 PKB phosphorylation (18, 60). Furthermore, rat skeletal muscles exposed to high-dose dexamethasone have elevated GLUT4 protein levels despite reduced GLUT4 translocation to the cell surface (76, 77). These observations are in agreement with our findings, which strongly suggest impaired glucose transport in RG muscle tissue, possibly due to impaired PKB activation, although this was not significant. In accord with this, our LBW rats have a chronic low-grade corticosteroid overexposure, which may explain the less pronounced phenotype compared with the effects observed in skeletal muscle tissue exposed to high-dose dexamethasone (76, 77). Nevertheless, the ESC administration normalized circulating corticosteroid levels and enhanced insulin sensitivity in LBW rats, indicating a possible regulatory role for adrenal steroids for muscle insulin signaling in the LBW rats.
Although HPA axis hyperactivity may be responsible for the insulin resistance in LBW, alterations in HSD-1 (and GR) expression in peripheral tissues may also contribute (28, 45). Increased splanchnic cortisol production [especially due to hepatic HSD-1 activity (9, 10)] has been suggested to play a role for the development of insulin resistance and obesity in humans (46, 72). In the present study, HSD-1 expression in peripheral tissues was not measured, but in the same LBW model, Nyirenda et al. (47) found no change in HSD-1 expression in liver tissues of adult rats. Furthermore, the present LBW model displays no or only modest changes in hepatic GR mRNA expression (17, 47). Although minor changes in peripheral glucocorticoid signaling may contribute to the insulin resistance in the present LBW model, the demonstrated HPA axis hyperactivity is still likely to be of greater importance.
In some studies, SSRIs cause obesity and hypercholesterolemia, whereas in others, SSRIs have been reported to exert no or even positive metabolic effects (1, 38, 56). To assess whether the metabolic changes observed in the SSRI-LBW rats could represent a direct drug effect independent of the HPA axis and LBW, a pilot study was performed to determine the effects of ESC in the Cx rats. We found that ESC treatment decreased insulin sensitivity as measured by the ISI index and plasma insulin levels and tended to increase corticosterone release during stress. Although these findings are puzzling, it clearly suggests a differentiated and phenotype-dependent drug response. Similar mechanisms might explain the inconsistent results from previous studies regarding the metabolic effects of SSRI compounds. Although further studies are needed to clarify these issues, our pilot data strongly indicate that the metabolic alterations in liver and muscle of the SSRI-LBW rats are unlikely to represent a primary drug effect; instead, these changes seem to be secondary to the reduction of HPA axis activity.
In conclusion, our data support the hypothesis that insulin resistance associated with LBW is caused by HPA axis hyperactivity and resulting elevation of corticosteroid levels. These studies are the first to demonstrate that an SSRI compound downregulates the increased HPA axis activity in adult rats born with LBW and reverses the resulting insulin resistance in both liver and muscle.
Present pharmaceutical approaches focus on a possible primary defect involved in LBW insulin resistance. Although the SSRI compounds of today might not be considered suitable for the treatment of LBW humans, other more appropriate HPA axis modulating pharmaceuticals might instead prove to be useful for the future treatment of HPA axis disturbances in humans born with LBW. Hence, this treatment strategy may reduce the risk for developing type 2 diabetes and in addition prevent the progression of other LBW-related disorders such as melancholic depression, which is known to be overrepresented in humans born with LBW.
GRANTS
These studies were supported by The Clinical Institute, Aarhus University, Denmark; The Novo Nordisk Foundation; The Danish Diabetes Association; The Danish Heart Association; The Danish Medical Research Council, “Fonden til Laegevidenskabens Fremme”; The A. P. Moller and Spouse Chastine Mc-Kinney Moller Foundation for General Charity; the Torben and Alice Frimodts Foundation; and US National Institutes of Health Grants R01-AG-23686 (K. F. Petersen) and P01-DK-69229 (K. F. Petersen). K. F. Petersen is a recipient of a Distinguished Clinical Scientist Award from the American Diabetes Association.
DISCLOSURES
The authors have nothing to declare.
Supplementary Material
ACKNOWLEDGMENTS
Linda Edge, Elin Carstensen, Elsebeth Hornemann, and Hanne Petersen are thanked for excellent help and technical assistance. Facility manager Ulla Dansberg and the staff of the Bartholin-building Animal Facility, Aarhus University, are thanked for skilful animal care and assistance. Prof. J. Rungby, Prof. Ulf Simonsen, and Dr. N. E. Magnusson (Department of Pharmacology, Aarhus University) are all thanked for stimulating discussions.
Dr. Bjarke Ebert, Dr. Chrisana Grande, and Dr. Francisca Y. Ochoa, H. Lundbeck, are thanked for kindly helping with the analysis of rat plasma ACTH. Dr. Nils E. Magnusson (Dept. of Pharmacology, University of Aarhus, Denmark, and Baker IDI Heart and Diabetes Institute, Melbourne, Australia) is thanked for providing primers for Bcl-2 and Bax.
Present address of E. S. Buhl: Dept. of Internal Medicine, Regional Hospital Silkeborg, Central Denmark Region, Silkeborg, Denmark.
REFERENCES
- 1.An Z, Moore MC, Winnick JJ, Farmer B, Neal DW, Lautz M, Smith M, Rodewald T, Cherrington AD. Portal infusion of escitalopram enhances hepatic glucose disposal in conscious dogs. Eur J Pharmacol 607: 251–257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab 87: 5587–5593, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Atkinson HC, Wood SA, Castrique ES, Kershaw YM, Wiles CC, Lightman SL. Corticosteroids mediate fast feedback of the rat hypothalamic-pituitary-adrenal axis via the mineralocorticoid receptor. Am J Physiol Endocrinol Metab 294: E1011–E1022, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol 558: 305–318, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barden N. Implication of the hypothalamic-pituitary-adrenal axis in the physiopathology of depression. J Psychiatry Neurosci 29: 185–193, 2004 [PMC free article] [PubMed] [Google Scholar]
- 7.Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285: E685–E692, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Baserga M, Hale MA, McKnight RA, Yu X, Callaway CW, Lane RH. Uteroplacental insufficiency alters hepatic expression, phosphorylation, and activity of the glucocorticoid receptor in fetal IUGR rats. Am J Physiol Regul Integr Comp Physiol 289: R1348–R1353, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Basu R, Basu A, Grudzien M, Jung P, Jacobson P, Johnson M, Singh R, Sarr M, Rizza RA. Liver is the site of splanchnic cortisol production in obese nondiabetic humans. Diabetes 58: 39–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu R, Edgerton DS, Singh RJ, Cherrington A, Rizza RA. Splanchnic cortisol production in dogs occurs primarily in the liver: evidence for substantial hepatic specific 11beta hydroxysteroid dehydrogenase type 1 activity. Diabetes 55: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Blondeau B, Lesage J, Czernichow P, Dupouy JP, Breant B. Glucocorticoids impair fetal β-cell development in rats. Am J Physiol Endocrinol Metab 281: E592–E599, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 20: 586–590, 1974 [PubMed] [Google Scholar]
- 13.Bonefeld BE, Elfving B, Wegener G. Reference genes for normalization: a study of rat brain tissue. Synapse 62: 302–309, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Brøns C, Jensen CB, Storgaard H, Alibegovic A, Jacobsen S, Nilsson E, Astrup A, Quistorff B, Vaag A. Mitochondrial function in skeletal muscle is normal and unrelated to insulin action in young men born with low birth weight. J Clin Endocrinol Metab 93: 3885–3892, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Buhl ES, Jessen N, Schmitz O, Pedersen SB, Pedersen O, Holman GD, Lund S. Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50: 12–17, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Buhl ES, Neschen S, Yonemitsu S, Rossbacher J, Zhang D, Morino K, Flyvbjerg A, Perret P, Samuel V, Kim J, Cline GW, Petersen KF. Increased hypothalamic-pituitary-adrenal axis activity and hepatic insulin resistance in low-birth-weight rats. Am J Physiol Endocrinol Metab 293: E1451–E1458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buren J, Lai YC, Lundgren M, Eriksson JW, Jensen J. Insulin action and signalling in fat and muscle from dexamethasone-treated rats. Arch Biochem Biophys 474: 91–101, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Coste SC, Murray SE, Stenzel-Poore MP. Animal models of CRH excess and CRH receptor deficiency display altered adaptations to stress. Peptides 22: 733–741, 2001 [DOI] [PubMed] [Google Scholar]
- 20.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 117: 1058–1067, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieni S, Rees S. Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol 55: 41–52, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Elfving B, Bonefeld BE, Rosenberg R, Wegener G. Differential expression of synaptic vesicle proteins after repeated electroconvulsive seizures in rat frontal cortex and hippocampus. Synapse 62: 662–670, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet Med 15: 220–227, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol 583: 215–225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab 77: 1174–1179, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Goland RS, Tropper PJ, Warren WB, Stark RI, Jozak SM, Conwell IM. Concentrations of corticotrophin-releasing hormone in the umbilical-cord blood of pregnancies complicated by pre-eclampsia. Reprod Fertil Dev 7: 1227–1230, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Alfaidy N, Holloway AC, Whittle WL, Lye SJ, Gibb W, Challis JR. Effects of cortisol and oestradiol on hepatic 11beta-hydroxysteroid dehydrogenase type 1 and glucocorticoid receptor proteins in late-gestation sheep fetus. J Endocrinol 176: 175–184, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581–611, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets 5: 453–479, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermann TS, Rask-Madsen C, Ihlemann N, Domínguez H, Jensen CB, Storgaard H, Vaag AA, Kober L, Torp-Pedersen C. Normal insulin-stimulated endothelial function and impaired insulin-stimulated muscle glucose uptake in young adults with low birth weight. J Clin Endocrinol Metab 88: 1252–1257, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Inder WJ, Prickett TC, Mulder RT, Donald RA, Joyce PR. Reduction in basal afternoon plasma ACTH during early treatment of depression with fluoxetine. Psychopharmacology (Berl) 156: 73–78, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Jaquet D, Gaboriau A, Czernichow P, Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab 85: 1401–1406, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Jensen JB, Jessop DS, Harbuz MS, Mørk A, Sánchez C, Mikkelsen JD. Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. J Neuroendocrinol 11: 465–471, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Kajantie E. Fetal origins of stress-related adult disease. Ann NY Acad Sci 1083: 11–27, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Kajantie E, Feldt K, Raikkonen K, Phillips DI, Osmond C, Heinonen K, Pesonen AK, Andersson S, Barker DJ, Eriksson JG. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J Clin Endocrinol Metab 92: 4094–4100, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 572: 31–44, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kauffman RP, Castracane VD, White DL, Baldock SD, Owens R. Impact of the selective serotonin reuptake inhibitor citalopram on insulin sensitivity, leptin and basal cortisol secretion in depressed and non-depressed euglycemic women of reproductive age. Gynecol Endocrinol 21: 129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J Clin Endocrinol Metab 85: 4611–4618, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Lodygensky GA, Seghier ML, Warfield SK, Tolsa CB, Sizonenko S, Lazeyras F, Huppi PS. Intrauterine growth restriction affects the preterm infant's hippocampus. Pediatr Res 63: 438–443, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van RO. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev 27: 119–127, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le MM. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 15: 110–116, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maheux P, Ducros F, Bourque J, Garon J, Chiasson JL. Fluoxetine improves insulin sensitivity in obese patients with non-insulin-dependent diabetes mellitus independently of weight loss. Int J Obes Relat Metab Disord 21: 97–102, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 45.McMillen IC, Warnes KE, Adams MB, Robinson JS, Owens JA, Coulter CL. Impact of restriction of placental and fetal growth on expression of 11beta-hydroxysteroid dehydrogenase type 1 and type 2 messenger ribonucleic acid in the liver, kidney, and adrenal of the sheep fetus. Endocrinology 141: 539–543, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Morton NM, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res 36: 146–164, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 101: 2174–2181, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab 287: E863–E870, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Oitzl MS, van Haarst AD, de Kloet ER. Behavioral and neuroendocrine responses controlled by the concerted action of central mineralocorticoid (MRS) and glucocorticoid receptors (GRS). Psychoneuroendocrinology 22, Suppl 1: S87–S93, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Olefsky JM, Kimmerling G. Effects of glucocorticoids on carbohydrate metabolism. Am J Med Sci 271: 202–210, 1976 [DOI] [PubMed] [Google Scholar]
- 52.Paile-Hyvärinen M, Wahlbeck K, Eriksson JG. Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: a single-blind randomised placebo controlled trial. BMC Fam Pract 4: 7, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penalva RG, Flachskamm C, Zimmermann S, Wurst W, Holsboer F, Reul JM, Linthorst AC. Corticotropin-releasing hormone receptor type 1-deficiency enhances hippocampal serotonergic neurotransmission: an in vivo microdialysis study in mutant mice. Neuroscience 109: 253–266, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab 83: 757–760, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension 35: 1301–1306, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Raeder MB, Bjelland I, Emil VS, Steen VM. Obesity, dyslipidemia, and diabetes with selective serotonin reuptake inhibitors: the Hordaland Health Study. J Clin Psychiatry 67: 1974–1982, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, Bleker OP. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351: 173–177, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Phillips DI. Is there a gender difference in the associations of birthweight and adult hypothalamic-pituitary-adrenal axis activity? Eur J Endocrinol 152: 249–253, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab 86: 245–250, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 48: 2119–2130, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry 7: 810–813, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Schober ME, McKnight RA, Yu X, Callaway CW, Ke X, Lane RH. Intrauterine growth restriction due to uteroplacental insufficiency decreased white matter and altered NMDAR subunit composition in juvenile rat hippocampi. Am J Physiol Regul Integr Comp Physiol 296: R681–R692, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Shoener JA, Baig R, Page KC. Prenatal exposure to dexamethasone alters hippocampal drive on hypothalamic-pituitary-adrenal axis activity in adult male rats. Am J Physiol Regul Integr Comp Physiol 290: R1366–R1373, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Sloboda DM, Moss TJ, Li S, Doherty D, Nitsos I, Challis JR, Newnham JP. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab 292: E61–E70, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Stefan N, Weyer C, Levy-Marchal C, Stumvoll M, Knowler WC, Tataranni PA, Bogardus C, Pratley RE. Endogenous glucose production, insulin sensitivity, and insulin secretion in normal glucose-tolerant Pima Indians with low birth weight. Metabolism 53: 904–911, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Stulnig TM, Waldhäusl W. 11beta-Hydroxysteroid dehydrogenase Type 1 in obesity and Type 2 diabetes. Diabetologia 47: 1–11, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol 145: 529–539, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Svacina S. Our experience with antidepressant treatment in the obese and type 2 diabetics. Prague Med Rep 106: 291–296, 2005 [PubMed] [Google Scholar]
- 70.Uehara A, Habara Y, Kuroshima A, Sekiya C, Takasugi Y, Namiki M. Increased ACTH response to corticotropin-releasing factor in cold-adapted rats in vivo. Am J Physiol Endocrinol Metab 257: E336–E339, 1989 [DOI] [PubMed] [Google Scholar]
- 71.Vieau D, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Molendi-Coste O, Laborie C, Breton C, Deloof S, Lesage J. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology 32, Suppl 1: S16–S20, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Walker BR, Andrew R. Tissue production of cortisol by 11beta-hydroxysteroid dehydrogenase type 1 and metabolic disease. Ann NY Acad Sci 1083: 165–184, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus 16: 239–249, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Weber-Hamann B, Gilles M, Lederbogen F, Heuser I, Deuschle M. Improved insulin sensitivity in 80 nondiabetic patients with MDD after clinical remission in a double-blind, randomized trial of amitriptyline and paroxetine. J Clin Psychiatry 67: 1856–1861, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Weber-Hamann B, Kopf D, Lederbogen F, Gilles M, Heuser I, Colla M, Deuschle M. Activity of the hypothalamus-pituitary-adrenal system and oral glucose tolerance in depressed patients. Neuroendocrinology 81: 200–204, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Weinstein SP, Paquin T, Pritsker A, Haber RS. Glucocorticoid-induced insulin resistance: dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin- and non-insulin-related stimuli. Diabetes 44: 441–445, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism 47: 3–6, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104: 71–79, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Wojtaszewski JF, Hansen BF, Urso B, Richter EA. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J Appl Physiol 81: 1501–1509, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Xiao X, Zhang ZX, Cohen HJ, Wang H, Li W, Wang T, Xu T, Liu A, Gai MY, Ying S, Schmitz O, Yi Z. Evidence of a relationship between infant birth weight and later diabetes and impaired glucose regulation in a Chinese population. Diabetes Care 31: 483–487, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.