Abstract
Recent studies suggest that certain acid-sensing ion channels (ASIC) are expressed in vascular smooth muscle cells (VSMCs) and are required for VSMC functions. However, electrophysiological evidence of ASIC channels in VSMCs is lacking. The purpose of this study was to test the hypothesis that isolated cerebral artery VSMCs express ASIC-like channels. To address this hypothesis, we used RT-PCR, Western blotting, immunolabeling, and conventional whole cell patch-clamp technique. We found extracellular H+-induced inward currents in 46% of cells tested (n = 58 of 126 VSMCs, pH 6.5–5.0). The percentage of responsive cells and the current amplitude increased as the external H+ concentration increased (pH6.0, n = 28/65 VSMCs responsive, mean current density = 8.1 ± 1.2 pA/pF). Extracellular acidosis (pH6.0) shifted the whole cell reversal potential toward the Nernst potential of Na+ (n = 6) and substitution of extracellular Na+ by N-methyl-d-glucamine abolished the inward current (n = 6), indicating that Na+ is a major charge carrier. The broad-spectrum ASIC blocker amiloride (20 μM) inhibited proton-induced currents to 16.5 ± 8.7% of control (n = 6, pH6.0). Psalmotoxin 1 (PcTx1), an ASIC1a inhibitor and ASIC1b activator, had mixed effects: PcTx1 either 1) abolished H+-induced currents (11% of VSMCs, 5/45), 2) enhanced or promoted activation of H+-induced currents (76%, 34/45), or 3) failed to promote H+ activation in nonresponsive VSMCs (13%, 6/45). These findings suggest that freshly dissociated cerebral artery VSMCs express ASIC-like channels, which are predominantly formed by ASIC1b.
Keywords: amiloride, proton, acid-sensing ion channel
acid-sensing ion channel (ASIC) proteins are members of the degenerin/epithelial sodium (Na+) channel (Deg/ENaC) family (3, 20, 25, 28). A signature feature of ASIC channels is activation by extracellular protons/acidosis and inhibition by the diuretic amiloride (3, 20, 25, 28). Because of their characteristic H+ gating, ASIC channels have been considered to act as chemosensors responding to extracellular acidosis in peripheral neurons, central neurons, glomus cells, and taste cells (3, 20, 25, 28). ASIC channels are also closely related to the mechanosensory degenerin proteins in the nematode Caenorhabditis elegans and are required for normal function of specific populations of mechanosensitive sensory neurons in mice. Thus, certain ASIC proteins are thought to act as mechanosensors in mammals. ASIC channels are predominantly expressed in neurons and sensory epithelia; however, recent studies suggest that ASIC proteins are expressed in vascular smooth muscle cells (VSMCs) where they regulate VSMC migration in response to chemical and wounding stimuli and influence vascular tone by mediating pressure-induced constriction in cerebral vessels (11, 12, 15, 17).
ASIC channels, and other members of the Deg/ENaC family, share a common structure: intracellular NH2 and COOH termini separated by two membrane-spanning domains and a large extracellular domain (3, 20, 25, 28). At least three different genes encode ASIC proteins ASIC1–ASIC3. ASIC1 and ASIC2 have splice variants, a and b. ASIC proteins merge to form homo- and heteromeric channels that predominately conduct Na+ but are also permeable to Ca2+, Li+, K+, and H+ (3, 20, 25, 28). In addition to gating by extracellular protons, ASIC currents are characterized by a quickly inactivating transient followed by a slower inactivating sustained current, of varying magnitude. Sensitivity to pH and psalmotoxin 1 (PcTx1, a tarantula venom toxin) are features that distinguish certain ASIC channels. For example, in the mouse, ASIC2a homomeric channels are activated at a lower pH (pH0.5 = 4.9) compared with ASIC1a (pH0.5 = 6.8), ASIC3 (pH0.5 = 6.6), and ASIC heteromers (pH0.5 = 6.1–6.4) (4, 28). ASIC1a and ASIC1b channels are identified by their inhibition and activation responses to PcTx1 (8). Thus, pH gating and PcTx1 sensitivity can help narrow the identity of ASIC channels.
While functional evidence supports a role for ASIC channels in VSMCs, electrophysiological evidence of these channels is lacking. Therefore, the goal of the present study was to demonstrate ASIC-like currents in freshly isolated VSMCs using conventional patch-clamp technique. We examined the effect of acidic external solutions with pH ranging from 7.0 to 5.0 on endogenous whole cell membrane currents in enzymatically dissociated murine cerebral artery VSMCs. The results of this study demonstrate that extracellular acidosis induces inward currents in a population of the cerebral VSMCs. These currents are induced by pH at or below 6.5 and are mainly carried by Na+. All H+-gated currents were inhibited by 20 μM amiloride. Seventy-six percent of the H+-gated currents were enhanced or promoted by PcTx1, an ASIC1b activator. Our findings suggest that cerebral VSMCs contain ASIC-like channels, predominantly formed by ASIC1b.
METHODS
All protocols and procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center.
Preparation of VSMCs.
C57BL6 mice (6–12 wk of age, Jackson Laboratory, Bar Harbor, ME) were anesthetized with isoflurane and immediately decapitated. Cerebral arteries were dissected from the brain and collected into an ice-cold cell isolation solution containing (in mM) 142 NaCl, 5 KCl, 0.1 CaCl2, 2 MgCl2, 10 HEPES, 5 d-glucose, and 6 mannitol, pH 7.4. The vessels were then subjected to enzymatic digestion by sequential incubation at 30°C in two different enzyme solutions: 1) 0.5 mg/ml papain (Worthington, Lakewood, NJ) and 1 mg/ml dithioerythritol (Fluka, St. Louis, MO) for 5 min; and 2) 1.5 mg/ml type IV collagenase (Worthington) and 0.5 mg/ml elastase (Worthington) for 10 min. After digestion, the enzyme solution was washed off using ice-cold cell isolation solution, and single VSMCs were released by gently triturating the digested vessels with a fire-polished Pasteur pipette. Isolated VSMCs were maintained in low Ca2+ external solution at 4°C and used within 12 h after isolation.
Reverse transcriptase polymerase chain reaction of ASIC message.
Cerebral vessels (n = 2 mice), brain, and cerebellum were dissected from brain surface and collected into ice-cold PBS and stored at −70°C until use. Total RNA was isolated using RNASTAT-60 (Tel-Test, Friendswood, TX), then DNase treated using TURBO DNA-free (Ambion, Austin, TX). One microgram of RNA was reverse transcribed (iScript, Bio-Rad, Hercules, CA). Three microliters of RT reaction was used for PCR amplification (IQ Supermix, Bio-Rad). Samples were amplified using Stratagene Robocycler. Oligonucleotide sequences and predicted PCR product sizes are provided in Table 1. Samples were held at 94°C for 2 min, then cycled at 94°C for 30 s, 55°C for 30s, and 68°C for 30s, for 35 cycles, then held at 68°C for 7 min. PCR products were separated on 1% agarose. PCR products were sequenced to confirm identity (Davis Sequencing, Davis, CA).
Table 1.
RT-PCR oligonucleotide sequences
| Primer | Primer Sequence 5′–3′ | Predicted Product Size, bp |
|---|---|---|
| ASIC1a | 230 | |
| S | GGTCTTGCCCACATCTTCTCC | |
| AS | TGGAGACTTGGCTAAAGCGAAACT | |
| ASIC1b | 351 | |
| S | CACCGGCAGCAGCAGGACAG | |
| AS | CTGGGACAACCGCACGGCAT | |
| ASIC2a | 398 | |
| S | CGCCAACACCTCTACTCTCC | |
| AS | ATGCTGAACTGCTTCGGTTT | |
| ASIC2b | 528 | |
| S | TATGCTGCTCTCCTGCAAG | |
| AS | ACACTCCTTGTGCTGCTCAGG | |
| ASIC3 | 412 | |
| S | CCCAGCTCTGGACGCTATG | |
| AS | CTTCCTGGAGCAGAGAGTTG |
ASIC, acid-sensing ion channel; S, sense; AS, antisense.
Western blot analysis.
For Western blotting experiments, cerebral vessels were isolated from mice and homogenized in 2× sample buffer. Whole lysates were separated using standard SDS-PAGE electrophoresis protocols on 7.5% gels (Bio-Rad, Criterion II). Proteins were transferred to nitrocellulose membranes and probed using goat anti-ASIC1a (1:500, Santa Cruz Biochemicals), goat anti-ASIC1b (1:500, Santa Cruz), rabbit anti-ASIC2a/b (1:1,000) (15), rabbit anti-ASIC2a [1:500, mDEG1, Alpha Diagnostic International (ADI)], rabbit anti-ASIC2b (1:500, mDEG2, ADI) or goat anti-ASIC3 (1:500, Santa Cruz). For antigen blockade studies, antibodies were preincubated with antigen (10 μg in 50 μl PBS) overnight at 4°C, then centrifuged, before incubating with membrane. Antibody labeling was visualized using donkey anti-rabbit or -goat, IR700 or IR800 dye (1:2,000, LI-COR Biosciences, Lincoln, NE). Blots for ASIC3 were reprobed with mouse-anti-β-actin (1:10,000, Abcam) and donkey anti-mouse IR800 to confirm equivalent protein loading. All membranes, including antibody and antibody-plus-antigen-treated membranes, were scanned using Odyssey Infrared Scanner (LI-COR Biosciences) under identical conditions.
Immunolabeling.
ASIC localization was determined in dissociated cerebral VSMCs using laser-scanning confocal microscopy. VSMCs were fixed in 4% paraformaldehyde (10 min) and briefly air-dried to glass slides. Samples were blocked in 5% normal donkey serum in PBS for 1 h and were then incubated with the primary antibodies overnight at 4°C. Samples were triple-labeled with mouse anti-α-SM actin (1:100, Sigma) and the following combinations of ASIC antibodies: 1) rabbit anti-ASIC2a/b (1:100) and goat anti-ASIC1a (1:100, Santa Cruz), 2) rabbit anti-ASIC2a/b and goat anti-ASIC1b (1:100, Santa Cruz), 3) guinea pig anti-ASIC3 (1:100, Chemicon) and goat anti-ASIC1b, and 4) guinea pig anti-ASIC3 and goat anti-ASIC1a. After rinsing, VSMCs were incubated with the appropriate secondary antibodies labeled with Alexa 488 (1:1,000, Invitrogen), Cy3 (1:200, Jackson Immunologicals), or Cy5 (1:200). VSMC samples where the antibody was preincubated with the antigen as noted above served as negative controls. Antibody labeling was examined using a Leica TCS-SP2 confocal microscope. Imaging conditions (laser power, gain, offset, pinhole) were adjusted so fluorescence signal from the antigen control was barely visible. For ASIC1a and ASIC3 labeling, the signal fluorescence signal was only slightly above the no primary control, indicative of weak labeling. Images were prepared for publication in Adobe Photoshop CS3.
Patch-clamp recording.
For patch-clamp recording, single VSMCs were plated onto the coverslip of the recording chamber at room temperature, incubated 20 min to allow for cell attachment, and used within 2 h. In some instances, cells were coincubated with collagenase (1 mg/ml) for 20 min before attachment. Only cells remaining elongated after incubation were used for analysis. Membrane current was recorded using conventional whole cell patch-clamp techniques with an Axopatch 200B amplifier (Axon Instruments) interfaced with a PC through Digidata 1440A digitizer (Axon Instruments). Data were sampled at 5 kHz and filtered at 1 kHz using a low-pass Bessel filter. pCLAMP10.0 (Axon Instruments) was used for data acquisition. Patch pipettes were pulled from FLG15 filamentous glass capillaries (Prism, Dagan) using a DMZ-Universal Puller (Zeitz Instruments). The resistances of patch pipettes used ranged from 3.0 to 6.0 MΩ. Fast capacitance transients were compensated before forming the whole cell configuration. Membrane capacitances of VSMCs used in calculating current density ranged from 10 to 15 pF. Series resistances were around 10–15 MΩ and were not compensated.
Command voltage for the voltage-clamp mode was −40 mV. The external and internal solutions were designed to create a Cl− Nernst potential (ECl) near the command voltage to minimize the interference from the spontaneous activity of Ca2+-activated Cl− currents. The external solution used in most experiments contained the same ingredients as aforementioned cell isolation solution except for the concentration of Ca2+, which was either reduced to 0 or increased to 1.5 mM. For low pH external solutions, HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] was replaced with MOPS [3-(N-morpholino) propanesulfonic acid]. For Na+-free solutions, Na+ was replaced with equimolar N-methyl-d-glucamine (NMDG+). The internal (pipette) solution contained (in mM) 106 K-gluconate, 25 KCl, 4 NaCl, 1 MgCl2, 20 MOPS, 0.1 EGTA, 4 ATP-Na2, and 0.05 GTP-Tris, with pH adjusted to 7.2 using KOH. In the control solution (pH 7.4), spontaneous current activity of cells voltage clamped at −40 mV is relatively quiescent. The pH of all acidic external solutions was monitored to ensure stability.
The entire recording chamber was continuously perfused with the normal external solution at about 2 ml/min throughout the experiments. VSMCs were perfused locally using a DAD-12 superfusion system (ALA Scientific Instruments) with control or treatment solutions continuously delivered through a 100-μm quartz capillary. For all experiments except those evaluating the effect of 1.5 mM external Ca2+ (Fig. 4), cells were maintained in 0.1 mM Ca2+ and were then briefly exposed to Ca2+-free pH 7.4 external solution (15 s) before acid treatment with the 15-s low pH stimulus. For experiments using external solutions with 1.5 mM Ca2+, VSMCs were isolated under low-Ca2+ conditions but transitioned into the 1.5 mM solution by slow perfusion (0.2 ml/min, for 20 min) to gradually raise Ca2+ concentration in the chamber, before perfusing at 2 ml/min.
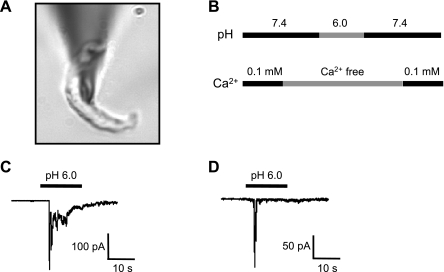
Fig. 4.
A: shape of a representative isolated cerebral VSMC. B: protocol for exposure to extracellular protons. VSMCs were briefly exposed to nominally Ca2+-free solution before exposure to extracellular H+ to prevent contraction. C and D: representative traces of the two main types of extracellular H+-evoked currents found in cerebral VSMCs.
Pharmacological disruption of ASIC channels.
To determine whether the H+-gated currents were sensitive to the broad-spectrum ASIC channel blocker amiloride (20 μM, Biomol International, Enzo Life Sciences, Plymouth Meeting, PA), VSMCs that responded to extracellular H+ were incubated with the amiloride for 1 min and then were retreated with extracellular H+ in the presence of the inhibitor. PcTx1 (100 nM, synthetic peptide, Peptide Institute, Osaka, Japan) was used to determine whether H+-gated currents involved ASIC1a or ASIC1b. PcTx1 is a selective inhibitor of ASIC1a channels and an activator of ASIC1b channels (8). Stock solutions (5 mM for amiloride, 0.1 mM for PcTx1) were prepared in water. The final pH of these solutions was less than 0.01 pH units different from their respective control.
Data analysis.
Patch-clamp data were analyzed off-line using Clampfit 10 (Axon Instruments) and Origin 7.0 (OriginLab). The pH-response curve was obtained by fitting the peak current density data to the Hill equation:
where I is the peak current density, [H+]0.5 is the H+ concentration required to cause half of the maximal current (Imax), and n is Hill coefficient. pH0.5, defined as the pH causing half of the maximal current, was then calculated from [H+]0.5. Desensitization time constants (τdecay) were obtained by fitting a 0.02-s segment of trace after the peak current showing monotonic decline to a single exponential function. To facilitate curve fitting for calculating τdecay, noise, and transients in the raw data were minimized by the data reduction function of Clampfit with a factor of 10 and substitution of average.
All data are presented as means ± SE, and n is the number of cells examined. VSMCs that failed to recover their basal current level after low pH wash-off were excluded from analysis. Data were compared using paired t-test, ANOVA, or repeated-measures ANOVA with Student-Newman-Keuls post hoc test (InStat3 GraphPad Software, La Jolla, CA) where appropriate. Statistical difference was considered significant at P < 0.05.
RESULTS
Expression of ASIC proteins in cerebral VSMCs.
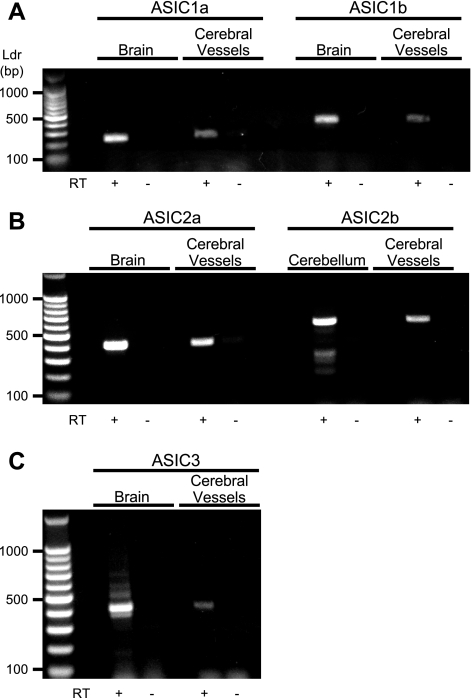
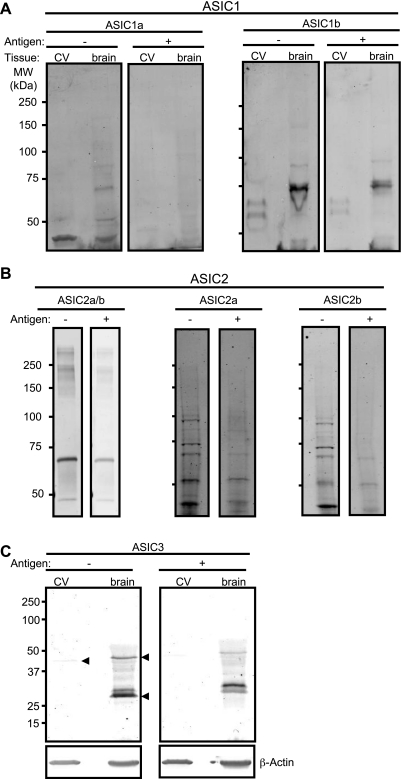
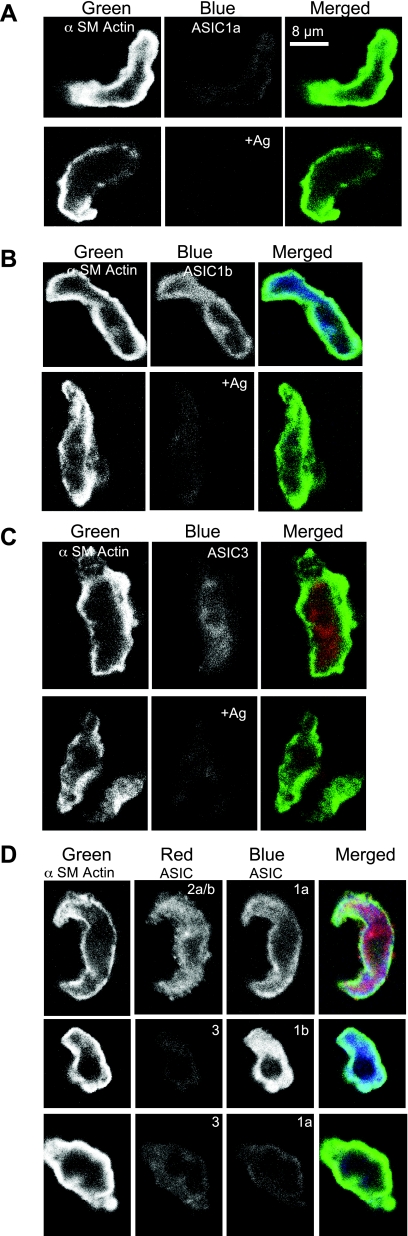
As shown in Fig. 1, we detected message for ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC in control tissue (brain or cerebellum) and cerebral vessels. Western blotting detection of ASIC1a, ASIC1b, ASIC2a, ASIC2b, and ASIC3 is shown in Fig. 2. Antibody labeling was diminished with antigen coincubation. Strong signals for ASIC1b and ASIC2a, but faint signals for ASIC1a, ASIC2b, and ASIC3, were detected. The presence of multiple bands was noted for all antibodies, consistent with previous studies (17). Slower migrating bands may represent glycosylated proteins or multimers. Faster migrating bands may represent degradation products (6). Alternatively, the additional bands may represent nonspecific products. Triple-staining of α-smooth muscle (SM) actin and ASIC pairs (1a/2ab, 1a/3, and 1b/3) is shown in Fig. 3.Nearly all cells examined contained signal above the negative control (antigen + antibody coincubation); however, the signal intensity varied among subunits. Labeling for ASIC1b and ASIC2a/b was strong and closely associated with α-SM actin, which localizes near the membrane in dissociated VSMCs. In a previous study, we described a similar localization pattern of ASIC2a and ASIC2b proteins in isolated cerebral VSMCs (15); however, labeling was stronger for ASIC2a compared with ASIC2b. Staining for ASIC1a was usually weak, with a few exceptions. A very weak and diffuse staining pattern was observed for ASIC3. The high percentage of labeling for all subunits, regardless of signal intensity, suggests a tendency for a given VSMC to express all ASIC subunits.
Fig. 1.
RT-PCR detection of acid-sensing ion channel (ASIC) mRNA expression in mouse cerebral vessels. A: ASIC1. B: ASIC2. C: ASIC3. Whole brain or cerebellum was used as a positive control where noted. The presence of reverse transcriptase is indicated (RT+). Samples without RT served as negative controls. Base pair ladder (Ldr) is shown at left.
Fig. 2.
Western blot detection of ASIC proteins in isolated cerebral vessels (CV). Representative blots for ASIC1a and ASIC1b (A), ASIC2a/b, ASIC2a, and ASIC2b (B), and ASIC3 (C) are shown. β-Actin was used as a loading control for ASIC3. +/− denotes presence/absence of antigen. Arrowheads in C identify bands that are diminished in corresponding antigen control. MW, molecular weight.
Fig. 3.
Immunolocalization of ASIC proteins in isolated cerebral vascular smooth muscle cells (VSMCs). Dissociated cerebral VSMCs were labeled for α-smooth muscle (α-SM) actin (far left in white, green in merged image on right), a VSMC marker, and ASIC proteins. A–C: representative examples of antibody and antibody + antigen (+Ag) controls for ASIC1a (A), ASIC1b (B), and ASIC3 (C). D: VSMCs labeled for ASIC2a/b and ASIC1a, ASIC3 and ASIC1b, and ASIC3 and ASIC1a. Nearly all VSMCs examined contained labeling for ASIC1a (14/16), ASIC1b (13/13), ASIC2a/b (31/31), and ASIC3 (13/14).
Inward current response to extracellular H+.
To determine whether extracellular H+ activates inward currents in freshly isolated cerebral artery VSMCs (Fig. 4A, holding potential −40 mV, pH 7.4), single voltage-clamped VSMCs were briefly exposed to an external solution of pH 6.0. To prevent potential Ca2+ entry through ASIC channels, Ca2+ was temporarily removed during H+ exposure following the protocol shown in Fig. 4B. Two predominant patterns of H+-gated currents were found, and representative traces are shown in Fig. 4, C and D. The most common pattern was characterized by a fast transient followed by slower sustained waveforms of varying magnitude and duration (Fig. 4C); other cells were characterized by a prominent fast transient (Fig. 4D).
pH dependence of H+-induced current in VSMCs.
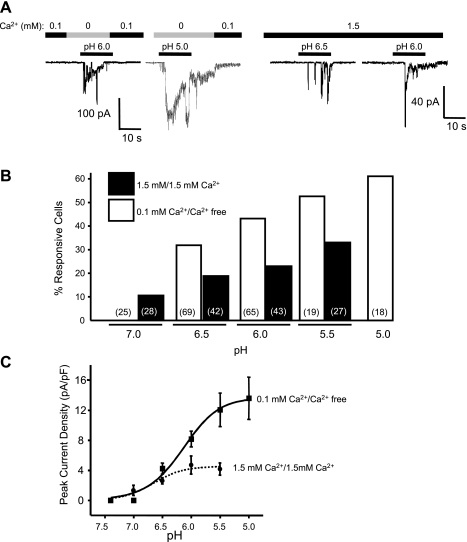
To determine whether current activation was dependent on extracellular [H+], cells were exposed to varying concentrations of H+ (pH 7.0, 6.5, 6.0, 5.5, and 5.0). As shown in the representative trace in Fig. 5A, amplitude of the H+-induced current was dependent on extracellular [H+], suggesting a concentration-dependent activation of H+-gated current. A similar trend was observed in the presence of 0.1 or 1.5 mM external Ca2+. The percentages of VSMCs responding to pH 7.0–5.0 are summarized in Fig. 5B. Exposure to external solution with a pH 7 failed to evoke an inward current in any of the VSMCs tested (n = 25 VSMCs evaluated); however, increasing the concentration of extracellular H+ increased the likelihood of a pH-gated response. The relationship between extracellular pH and peak current density is summarized in Fig. 5C. Fitting the peak current density data to the Hill equation gives a pH0.5 of 6.15 and 6.65 under 0.1 and 1.5 mM Ca2+, respectively. The leftward shift of the curve with 1.5 mM Ca2+ suggests that increased extracellular Ca2+ may increase the sensitivity of the H+-gated channel to H+. However, extracellular Ca2+ reduces maximal current density, suggesting slow permeation or channel block by Ca2+.
Fig. 5.
pH dependence of acid-induced inward current in freshly isolated cerebral VSMCs. A: representative traces of currents evoked by various concentrations of H+ ([H+]) when conditioned with 0.1 mM (left) and 1.5 mM Ca2+ (right). B: a tendency of pH dependence was also seen in the percentage of cells exhibiting acid-induced inward currents. C: plot of pH-current relation shows pH dependence of the peak current density (peak currents normalized to their respective membrane capacitances) in responsive cells. A pH-response curve was obtained by fitting the data to the Hill equation. The pH50 was 6.15 and 6.65 for conditioning with 0.1 and 1.5 mM Ca2+, respectively.
Effect of different extracellular [Ca2+] on cell viability.
Since VSMCs were initially studied under 0.1 mM Ca2+, we wanted to ensure that the cells were healthy. To address this possibility, we compared the membrane potential and activity of spontaneous Ca2+-activated K+ current (BK) of VSMCs kept either in 0.1 mM or slowly transitioned into 1.5 mM Ca2+. Membrane potential (−31.9 ± 5.2 vs. −36.1 ± 5.1 mV, n = 5), frequency (3.64 ± 0.26 vs. 3.76 ± 0.30 Hz, n = 5), and mean peak current (67.4 ± 11.8 vs. 59.5 ± 11.1 pA, n = 5) of BK currents was not affected by extracellular Ca2+. The negative membrane potential and robust BK activity also suggest that the plasma membranes were intact.
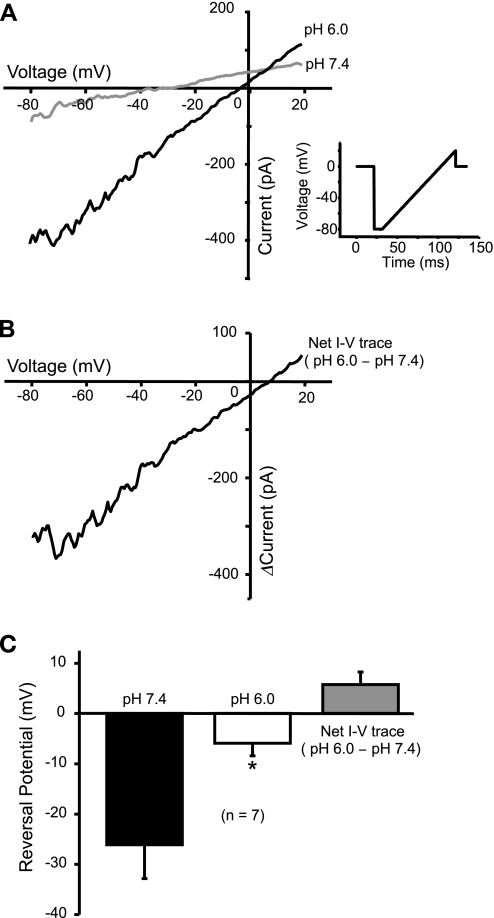
Extracellular acidosis shifts the voltage-current relationship.
Since in our study cells were voltage-clamped at near ECl (−40 mV), the charge carrier for the acid-induced inward current is likely to be Na+. To address this possibility, we used a voltage ramp to examine the change in current-voltage relationship caused by extracellular H+. The voltage-ramp protocol and waveform are shown in Fig. 6A. Exposure to pH 6.0 increased the slope of the whole cell current-voltage curve (Fig. 6B), indicating more channels opening during acidosis. Exposure to pH 6.0 also shifted the whole cell reversal potential to the right. The reversal potential probably does not shift completely to +60 mV because other channels, such as BK, are also activated during the voltage ramp. To compensate for this, we evaluated the net effect of H+ by subtracting the current response at pH 7.4 from that at pH 6.0. The net reversal potential is slightly positive (Fig. 6B). On the basis of the external and internal solutions, the equilibrium potential of Na+ (ENaI) is about +60 mV while EK is around −80 mV. Thus, the rightward shift in reversal potential suggests an increase in the membrane permeability favoring Na+ during acidosis.
Fig. 6.
Current-voltage (I-V) relation changed by extracellular acidosis. A: I-V curves in pH 7.4 and pH 6.0 were acquired using a voltage-ramp protocol. Exposure to pH 6.0 increased the slope of I-V curve, indicating an increase in conductance during acidosis. The whole cell reversal potential (when I = 0) is also shifted to the right toward equilibrium potential of Na+ (ENa). Inset A: voltage-ramp protocol and waveform used in this experiment to acquire I-V curve. B: the net effect of pH 6.0 on I-V curve is shown, which is obtained by deducting the curve at pH 7.4 from that at pH 6.0. C: statistic shows a significant shift of the whole cell reversal potential to the right (*P < 0.05). The average net reversal potential is slightly positive (n = 5).
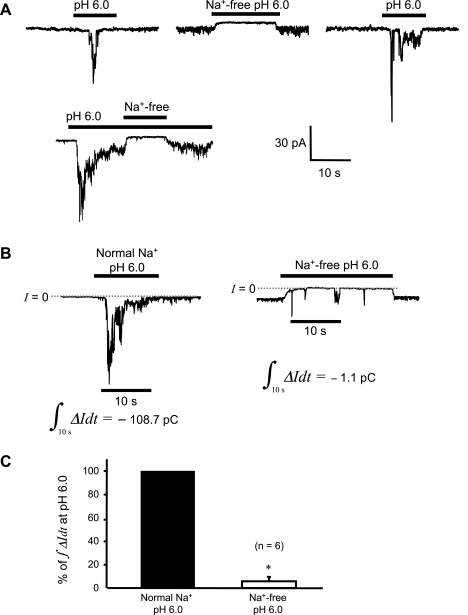
Depleting external Na+ abolishes acid-induced current.
To further test whether Na+ is the major permeant ion for the acidosis-activated channels, we examined the effect of equimolar impermeant N-methyl-d-glucamine (NMDG+) replacement on the acid-induced current. Currents evoked by acidic solution with normal external [Na+] (Fig. 7A) were abolished or reduced to small transients when challenged again with the same low pH in the absence of external Na+ (Fig. 7B). A quantitative analysis of these data is shown in Fig. 7C. External Na+ removal decreased the integral of inward current (∫ΔIdt) over the 10-s time interval during acid exposure, to 5.8 ± 3.9% of control (n = 6 cells). These findings suggest that Na+ is the predominant charge carrier of these acid-induced currents.
Fig. 7.
H+-gated inward current is dependent on external Na+. A: segments from a representative continuous recording, showing that removal of external Na+ obliterated current induced by pH 6.0. B: integration of the inward current over a 10-s time interval during acid exposure gives the amount of charge transported during that interval. The interval picked starts from 2 s before the peak current. A substantial reduction in charge movement is seen in the example shown (pC = pA·s). C: the average charge movement in the absence of external Na+ is significantly reduced to only 5.8 ± 3.9% of the control, a decrease of >94% (*P < 0.05).
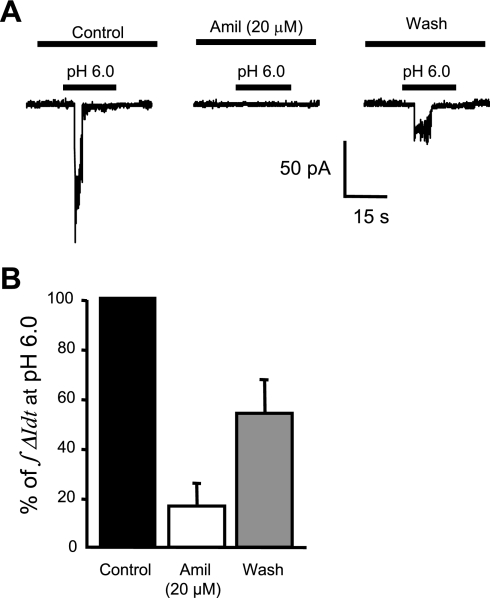
Broad-spectrum ASIC blocker, amiloride, and inhibition of H+-gated current.
To further identify the ion channels responsible for these H+-gated currents, we next examined the effect of amiloride, a broad-spectrum ASIC channel blocker. Amiloride (20 μM) was used as a broad-spectrum inhibitor of ASIC channels. A representative trace for amiloride inhibition of H+-gated currents is shown in Fig. 8A. Cells that responded to the first pH 6.0 stimulus were washed for 3 min and then pretreated with amiloride (20 μM) for 1 min before and during the second exposure to pH 6.0. The effect of amiloride on the acid-induced current response, quantified as the area under the curve (∫ΔIdt), is shown in Fig. 8B. Amiloride inhibited proton-gated inward currents to 16.5 ± 8.7% of control (n = 6). The inhibitory effect of amiloride was partially reversed after a 4-min wash (Fig. 8B).
Fig. 8.
Pharmacological inhibition of acid-induced currents by the broad-spectrum ASIC inhibitor amiloride (Amil) in isolated cerebral VSMCs. Representative trace (A) and group data in response to 20 μM amiloride (B) are shown. Cells were pretreated with the amiloride 1 min before and during the second exposure to pH 6.0.
Effect of PcTx1 on acid-induced currents in VSMCs.
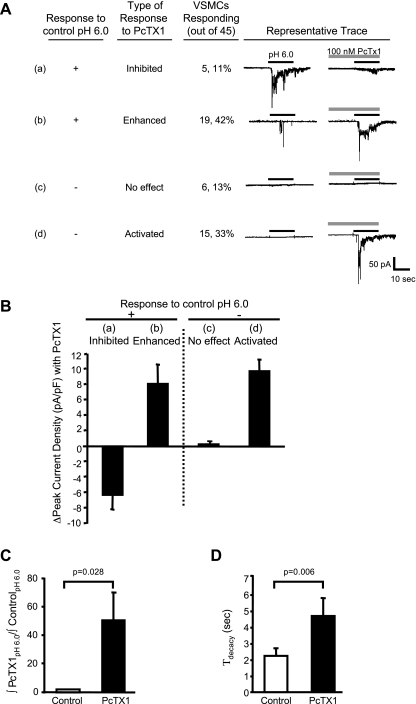
PcTx1 was used to determine whether ASIC1a or ASIC1b channels mediate H+-gated currents. To determine the contribution of ASIC1a and ASIC1b, VSMCs were pretreated with PcTx1 (100 nM) for 30 s and were then exposed to acid (pH 6.0, ± PcTx1). PcTx1 either inhibited or potentiated H+-gated currents. In other words, there were few H+-gated currents that were unaffected by PcTx1 (<10%). PcTx1 inhibited [H+]-induced currents only in a small subpopulation of VSMCs (5 out of 45 cells, 11%, Fig. 9Aa). PcTx1 enhanced acid-induced currents in nearly half the cells (19 out of 45 cells, 42%, Fig. 9Ab). Furthermore, PcTx1 activated currents in a subpopulation of cells, which had previously failed to respond to pH 6.0 (15 out of 45 cells, 33%, Fig. 9Ad). In VSMCs where PcTx1 enhanced or activated currents (34 out of 45 cells), PcTx1 increased peak current density 8–9 pA/pF (Fig. 9B) and increased the area under the curve 50-fold (Fig. 9C) in response to pH 6.0. The decay time constant (τdecay) was also increased twofold following preincubation with PcTx1. The potentiated response to H+ (observed in 3/4 of the VSMC population) and an increased decay time constant with PcTx1 suggests a role for ASIC1b (5). The finding of a large population of ASIC1b channels was consistent with our Western blotting and immunolabeling studies.
Fig. 9.
Effect of psalmotoxin 1 (PcTx1), an ASIC1a inhibitor and ASIC1b activator, on H+-induced currents in isolated cerebral VSMCs. A: representative traces of acid-induced current response to preconditioning with 100 nM PcTx1 for 30 s. Traces of VSMCs (24 out of 45) that responded to the control H+ stimulus are shown in a and b. In these cells, inward currents were either inhibited (a) or enhanced (b). Currents were enhanced (19/45) nearly four times as often as they were inhibited (5/45). Traces of VSMCs (21/45) that did not respond to an initial extracellular H+ stimulus are shown in c and d. In these cells, PcTx1 had no effect (c) or resulted in activation of currents (d). Currents were activated (15/45) nearly twice as often as they were unaffected (6/45). Thus, PcTx1 predominantly potentiated H+-gated currents in VSMCs. B: group data summarizing the effect of PcTx1 on the change in peak current density. C and D: in the VSMCs where PcTx1 enhanced or activated current, PcTx1 increased total current density relative to control (∫PcTx1pH6.0/∫controlpH6.0) (C) and delayed desensitization (τdecay, decay time constant) of the inward current (D).
DISCUSSION
ASIC proteins coassemble to form homo- and heteromeric cation channels, typically with Na+ as the major charge carrier. Most ASIC channels are gated by external H+ with pH between 7.2 and 4.0 and are sensitive to amiloride. ASIC1a channels are inhibited by PcTx1 and ASIC1b channels are activated by PcTx1 (3, 8, 20, 25, 28). Previous studies support a role for ASIC channels in VSMC migration and myogenic constriction (11, 12, 15, 17). However, which ASIC subunits are expressed in VSMC and the presence of ASIC-like currents in freshly isolated VSMCs has not been determined. The major findings of this study are that freshly dissociated cerebral VSMCs 1) express ASIC1b and ASIC2, as well as ASIC1a and ASIC3 to a lesser extent; and 2) contain H+-gated inward currents carried by Na+. An amiloride-blockable rapid transient followed with or without a sustained current characterizes the current. PcTx1 inhibited a small population of currents; however, a larger population of currents was potentiated or activated by PcTx1, an activator of ASIC1b. The findings demonstrate that ASIC-like, H+-gated inward currents are present in freshly dissociated cerebral artery VSMCs and the majority of the currents are likely carried by ASIC1b.
Not all VSMCs respond to extracellular H+.
Immunolocalization studies suggest that nearly all VSMCs express ASIC proteins. Surprisingly, only a portion of VSMCs [43% (28/65 cells) at pH 6.0] tested in this study responded to extracellular H+ with an inward current. There are several possible explanations for this inconsistency between protein expression and channel activity. First, the presence of H+-gated currents may be dependent on VSMC heterogeneity; VSMCs were obtained from brain surface vessels, including the vertebral arteries, basilar artery, circle of Willis, cerebellar artery, and posterior, middle, and anterior cerebral arteries and their branches. Second, there may be too few channels at the surface membrane to produce measurable currents. Third, VSMCs may have been subjected to varying extent of digestion of the surrounding matrix that may regulate channel activity (22). Fourth, most ASIC channels may be electrically silent or desensitized under the conditions of this study. We favor the latter explanation; PcTx1 revealed H+-gated currents in 71% (15/21) of the VSMCS that failed to respond to the control test pH 6.0.
Importance of extracellular calcium in ASIC channel gating.
In the current study, VSMCs were initially maintained in low external Ca2+ (0.1 mM) because initial attempts at raising Ca2+ to 1–2 mM increased the tendency of VSMCs to contract. Contraction was undesirable because it induced cell shortening and rounding and membrane “blebbing” (14). The use of kinase-dependent and Ca2+-channel dependent contraction inhibitors, such as PKC and myosin light chain kinase inhibitors and verapamil, were avoided because of the potential to interfere with degenerin channel function (2, 5, 24, 29). Therefore, when these studies were initiated, reducing extracellular Ca2+ was the best option.
While lowering extracellular Ca2+ minimized the potential interference of contraction, it also created some limitations. First, it raised a concern of whether or not currents were present under more physiological [Ca2+]. Although the magnitude of the currents was reduced under 1.5 mM Ca2+, the H+-gated currents were still present and evoked in a concentration-dependent manner. Second, reducing calcium increased the possibility of activating Ca2+ store-operated nonselective cation current. However, we did not observe any current that was evoked by just removing the 0.1 mM Ca2+ from solution. Even after those cells were kept in 0.1 mM Ca2+ for more than 30 min, BK activity, which has been considered an indicator of internal Ca2+ load, did not decrease, suggesting that Ca2+ stores remained intact and internal Ca2+ levels did not change. Third, low calcium introduced an additional variable since extracellular Ca2+ plays an important role in H+ gating of ASIC channels. How changes in extracellular Ca2+ may contribute to the responses is discussed in the following section. Regardless of these limitations, our findings suggest that H+-gated currents can be elicited under more physiological levels of external Ca2+.
Increasing extracellular Ca2+ from 0.1 to 1.5 mM resulted in a down- and leftward shift of the pH dose-response curve, which is likely caused by the opposing effects of Ca2+ in stabilizing ASIC channels and blocking the current during H+ activation. Extracellular Ca2+ during preconditioning stabilizes ASIC channels in the closed state by competing with and preventing H+ induced desensitization. Therefore, reduction of preconditioning extracellular Ca2+ should increase the number of desensitized channels (1, 9, 10) and decrease the number of available channels. The slight leftward shift of the pH-response curve is consistent with mild increase in the sensitivity to acid (Fig. 5C, 6.15 vs. 6.65 pH0.5) with an increase in preconditioning extracellular Ca2+. This increase in sensitivity may reflect an increased number of channels available for opening, consistent with previous reports of the effect of Ca2+ on ASIC (1, 9, 10, 19). In contrast, extracellular Ca2+ during the pH stimulus blocks H+-gated current (10, 19, 23, 26, 30). The mean peak current density was lower in the presence of 1.5 mM Ca2. A similar finding has been reported by others and suggests that extracellular Ca2+ during activation may block ASIC channels, and hence reducing the overall conductance of the channels (10, 23, 26, 30). Thus, the changes in the pH dose-response curve with increasing extracellular Ca2+ may be due to contrasting effects of Ca2+ on H+-gating sensitivity and open channel blockade.
H+-gated currents are permeable to Na+.
Three lines of evidence suggest that the H+-gated current is carried by Na+. First, the conditions of the internal and external solutions favor Na+ movements. Second, the rightward shift of the current-voltage relationship toward the Na+ equilibrium potential during acid exposure is consistent with Na+ as a major charge carrier. Third, replacement of external Na+ with impermeant NMDG abolishes H+-gated currents. In a few cells, H+-induced current was not completely abolished by removal of extracellular Na+. Instead, a few very short pulses of transient current were observed. These current spikes could be attributed to H+, which is also permeant to ASIC channels (7). Although the concentration of protons is low, an internal pH at 7.2 the Nernst potential of H+ is about +60 mV for an external pH of 6.0. At a holding potential of −40 mV, this may provide enough driving force to cause these short current transients.
Types of ASIC channels that may be present in cerebral VSMCs.
Most studies characterizing ASIC channel properties have been conducted in heterologous expression systems where ASIC channels are expressed at high levels. In the current investigation, we evaluated the endogenous currents in native cells where the channels are probably expressed at lower levels, and the coexpression of associated proteins that may alter channel function, such as stomatins, is high. Thus it is likely that the smaller currents observed in VSMCs may be due to lower membrane expression and coexpression of negative regulatory proteins.
A limitation of the current investigation is that we cannot determine which ASIC proteins mediate H+-gated currents in cerebral VSMCs with certainty. Although protein expression studies suggest that all ASIC proteins are expressed in cerebral VSMCs, specificity of the anti-ASIC antibodies may contribute to the results. However, we can get an idea of which subunits are likely to be involved based on PcTx1 sensitivity and pH activation. PcTx1 inhibition of H+-gated currents in 11% of VSMCs suggests that in a small population of cells, channels are formed by ASIC1a homomers (13). Furthermore, PcTx1 potentiation of currents in three-quarters of the VSMCs studied supports the presence of an ASIC1b channel in a large population of cerebral VSMCs. Despite the strong labeling for ASIC2 in these VSMCs, the half-maximal activation (pH0.5) between 6.65 and 6.15 suggests that ASIC2 homomers are not likely involved since the pH0.5 of ASIC2a homomeric channels is less than 5.0 (4). This finding suggests that ASIC2 is either interacting with ASIC1b to form a heteromeric channel or not contributing to channels mediating the H+-gated currents. It is unclear why all VSMCs seem to have a similar pattern of ASIC subunit expression, yet express one of two major type of H+-gated currents in an all or none fashion. A role for ASIC3 is difficult to ascertain since ASIC3 was barely detectable. Determining the role of specific ASIC proteins in H+-gated channels from VSMCs will require the use of genetically modified animals.
Physiological significance of extracellular acidosis in activating ASIC currents: a potential role in acid-mediated vasodilation?
The precise role of ASIC channels in VSMC is unclear. Previous studies suggest that ASIC channels contribute to vascular tone and VSMC migration; however, the contribution of VSMC ASIC channels in vascular responses to acidosis during ischemia, hypoxia, stroke, or vascular injury is unknown. Role of ASIC channels as direct mediators of acid-mediated dilation is unlikely since activation of ASIC channels should lead to membrane depolarization and vasoconstriction. There are several potential significant speculative roles of VSMC ASIC channels. First, ASIC channels may serve as a counter-force to acid-induced dilation and prevent excessive dilation. Second, ASIC channels may play a role in modulating neurovascular coupling. Cerebral vessels receive significant innervation with synapses forming between vessels and neurons, glia, and astrocytes. Since synaptic vesicles are acidic (pH ∼5.5), it is enticing to speculate that synaptic vesicle H+ release could activate ASIC channels and modulate/enhance effects of vasoconstrictor neurotransmitters/peptides (16, 18). If such a scenario were true, overactive ASIC channels might prevent appropriate control of cerebral blood flow (functional hyperemia), a problem in Alzheimer's disease, ischemic stroke, and hypertension. On the other hand, ASIC channels may not act as H+ receptors; other investigators have noted that H+ gating may just be a coincidence and not necessarily reflect the intended function of ASIC channels (21, 27). H+ ion may not be the natural ligand of these channels; it may be a substance such as a neuropeptide (21).
In the current investigation, we have demonstrated the presence of ASIC-like currents in freshly isolated cerebral VSMCs. The currents are gated by external protons, carried primarily by Na+ and blocked by amiloride. A heterogeneous population of ASIC channels mediates the currents; most currents are carried by ASIC1b (PcTx1 activated) and a smaller population is carried by ASIC1a (PcTx1 inhibited). Future studies are required to fully understand the importance of ASIC channels in VSMC function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-086996 (to H. A. Drummond) and HL-51971.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank laboratory colleagues for excellent assistance. We are especially grateful to Dr. Candice Askwith at Ohio State University for helpful comments.
REFERENCES
- 1.Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J Biol Chem 277: 41597–41603, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem 277: 50463–50468, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Benos DJ, Stanton BA. Functional domains within the degenerin/epithelial sodium channel (Deg/ENaC) superfamily of ion channels. J Physiol 520 Pt 3: 631–644, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem 277: 45734–45740, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Brockway LM, Zhou ZH, Bubien JK, Jovov B, Benos DJ, Keyser KT. Rabbit retinal neurons and glia express a variety of ENaC/DEG subunits. Am J Physiol Cell Physiol 283: C126–C134, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Grunder S. Permeating protons contribute to tachyphylaxis of the acid-sensing ion channel (ASIC) 1a. J Physiol 579: 657–670, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Kalbacher H, Grunder S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol 127: 267–276, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol 126: 71–79, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Weille J, Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca(2+) ions. Brain Res 900: 277–281, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Drummond HA, Jernigan NL, Grifoni SC. Sensing tension: epithelial sodium channel/acid-sensing ion channel proteins in cardiovascular homeostasis. Hypertension 51: 1265–1271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Fay FS, Delise CM. Contraction of isolated smooth-muscle cells–structural changes. Proc Natl Acad Sci USA 70: 641–645, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37: 75–84, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Schrank B, Waterston RH. Interaction between a putative mechanosensory membrane channel and a collagen. Science 273: 361–364, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Paukert M, Babini E, Pusch M, Grunder S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol 124: 383–394, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal AS, Hayslett JP, Desir GV. On the natriuretic effect of verapamil: inhibition of ENaC and transepithelial sodium transport. Am J Physiol Renal Physiol 283: F765–F770, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev 84: 1097–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Welsh MJ, Price MP, Xie J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J Biol Chem 277: 2369–2372, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci USA 103: 16556–16561, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Canessa CM. Single channel properties of rat acid-sensitive ion channel-1alpha, -2a, and -3 expressed in Xenopus oocytes. J Gen Physiol 120: 553–566, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]