Abstract
The sodium-bicarbonate cotransporter NBCn1 (SLC4A7) is an acid-base transporter that normally moves Na+ and HCO3− into the cell. This membrane protein is sensitive to cellular and systemic pH changes. We examined NBCn1 expression and localization in the brain and its response to chronic metabolic acidosis. Two new NBCn1 antibodies were generated by immunizing a rabbit and a guinea pig. The antibodies stained neurons in a variety of rat brain regions, including hippocampal pyramidal neurons, dentate gyrus granular neurons, posterior cortical neurons, and cerebellar Purkinje neurons. Choroid plexus epithelia were also stained. Double immunofluorescence labeling showed that NBCn1 and the postsynaptic density protein PSD-95 were found in the same hippocampal CA3 neurons and partially colocalized in dendrites. PSD-95 was pulled down from rat brain lysates with the GST/NBCn1 fusion protein and was also coimmunoprecipitated with NBCn1. Chronic metabolic acidosis was induced by feeding rats with normal chow or 0.4 M HCl-containing chow for 7 days. Real-time PCR and immunoblot showed upregulation of NBCn1 mRNA and protein in the hippocampus of acidotic rats. NBCn1 immunostaining was enhanced in CA3 neurons, posterior cortical neurons, and cerebellar granular cells. Intraperitoneal administration of N-methyl-d-aspartate caused neuronal death determined by caspase-3 activity, and this effect was more severe in acidotic rats. Administering N-methyl-d-aspartate also inhibited NBCn1 upregulation in acidotic rats. We conclude that NBCn1 in neurons is upregulated by chronic acid loads, and this upregulation is associated with glutamate excitotoxicity.
Keywords: acid/base, pH, ion transporter
because many ion channels and receptors responsible for neuronal excitability are sensitive to pH, acid-base homeostasis in the brain has to be carefully regulated. In particular, neurons must have mechanisms to extrude acids from the cytosol as intracellular acidification can affect neuronal excitability (3). Acid extrusion in the soma of neurons is mediated by Na/H exchange (NHE) and Na-HCO3 transport. NHE is primarily governed by the Na/H exchanger NHE5 (1), which moves H+ from the cytosol in exchange for external Na+, whereas Na-HCO3 transport is primarily governed by the Na+-driven Cl/HCO3 exchanger (2), which moves Na+ and HCO3− into the cytosol in exchange for internal Cl−.
In contrast to soma, acid extrusion at synapses is not well understood. Experiments using synaptosomes show the presence of NHE (25, 34). We have previously demonstrated that the electroneutral Na-HCO3 cotransporter NBCn1 (SLC4A7), which moves Na+ and HCO3− into the cell without Cl−, is localized to both the soma and dendrites in primary cultures of rat hippocampal neurons (10). NBCn1 localization in dendrites partially overlaps with PSD-95 localization, indicating the presence of the transporter at excitatory synapses in addition to other areas of the cell membrane. In the brain, NBCn1 is detected in a variety of regions by immunoblot (7) and choroid plexus epithelia by immunohistochemistry (30) The β-galactosidase staining of mice with the LacZ gene trap shows prominent expression of NBCn1 in neurons in many brain regions (5). It is unclear whether NBCn1 is present at synapses, where the effect of pH on neuronal activity is tremendous (8).
NBCn1 is sensitive to cellular and systemic pH changes. In the thick ascending limb of the kidney, the transporter is markedly upregulated during chronic metabolic acidosis (23, 26). This observation led the authors of the papers to propose a model in which excessive H+ load caused by acidic luminal pH stimulates tubule cells to enhance HCO3− influx via NBCn1. On the other hand, recent reports show that NBCn1 expression is reduced during chronic metabolic acidosis mediated by urinary tract obstruction (41) as well as by calcineurin inhibition (24). The effect of chronic metabolic acidosis on NBCn1 in the kidney is thus still controversial. In the brain, NBCn1 appears to be upregulated under acidic environments. Kanaan et al. (18) have reported that the acid-extruding proteins such as NBCn1 and NHE in the neonatal rat cortex are stimulated by chronic elevated Pco2. It is not known whether NBCn1 in the brain is similarly affected by chronic metabolic acidosis.
In the present study, we examined NBCn1 localization in the brain and its response to systemic acid load by asking two questions: whether NBCn1 can be localized to excitatory synapses in hippocampal neurons and whether transporter expression is stimulated by chronic metabolic acidosis. To address these questions, we performed immunohistochemistry of rodent brains with NBCn1 antibodies. Chronic metabolic acidosis was induced in rats, and NBCn1 expression levels were determined. We also examined the effect of N-methyl-d-aspartate (NMDA) administration to these rats on neuronal death. Our data show that NBCn1 is present at excitatory synapses in addition to other areas of the cell membrane and its expression is dynamically affected by systemic acid load.
MATERIALS AND METHODS
Antibodies
The affinity-purified NBCn1 antibodies were made by a custom production service (Covance; Princeton, NJ). The peptides corresponding to the COOH-terminal 14 amino acids of rat NBCn1 (GenBank accession number NM_058211) were cross linked to bovine serum albumin and injected into a rabbit and a guinea pig. The antibodies were affinity-purified using Protein A/G-Sepharose column. For antibody specificity, the affinity-purified antibodies were preabsorbed with the antigenic peptides (0.1 mg/ml) at room temperature for 1 h before incubating the immunoblot membranes with primary antibodies. Presera were also used for controls in parallel experiments.
Immunoblot
Rat brains were dissected and hippocampi were isolated as described by Chen et al. (7) with slight modification. Tissues were homogenized in 300 mM mannitol, 5 mM HEPES (pH 7.2), 0.1 mg/ml phenylmethanesulfonyl fluoride, and 1× protease inhibitor cocktail I (Calbiochem; San Diego, CA). The homogenates were centrifuged at 3,000 g for 10 min and then at 100,000 g at 4°C for 1 h to obtain membrane pellets. The pellets were dissolved in phosphate-buffered saline (PBS), and protein concentration was determined using the Bradford reagents (Sigma-Aldrich; St. Louis, MO). Equal amounts of proteins were loaded on a 4–10% SDS polyacrylamide gel, separated, and blotted to a nitrocellulose membrane. The blot was incubated with the rabbit NBCn1 antibody (1:500) overnight in PBS containing 0.05% Tween 20 and 5% nonfat dry milk. The blot was washed and then incubated with the horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000; Millipore) for 1 h. The immunoreactivity was visualized by ECL chemiluminescence (GE Healthcare; Chicago, IL). For PSD-95 and NR2A, the blot was incubated with the mouse PSD-95 antibody (1:500; Millipore) or rabbit NR2A (1:500; Millipore) for 2 h and then with the horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (1:5,000; Millipore) for 1 h. The blot was stripped and reprobed with the β-actin antibody (1:500; Millipore). Quantitative measurements of NBCn1 and β-actin were achieved by using ImageJ software (NIH). The mean pixel intensity of NBCn1 or β-actin was measured by positioning a box around the protein band and subtracting background. A control blot with a series of different amounts of rat brain lysates showed the linear function of the NBCn1 intensity (supplemental data).
Immunohistochemistry
Immunoperoxidase immunostaining.
Staining was done as described by Wang et al. (40) with slight modification. Briefly, rats were perfused with ice-cold 4% paraformaldehyde, and brains were dissected and postfixed in the same solution overnight. Brains were embedded in paraffin and sliced with 5-μm thickness. Sections were deparaffinized with xylene and hydrated in a series of ethanols (95%, 70%, 50%, 30%, and 0% for 2 min each). The sections were heated in 10 mM sodium citrate buffer (pH 6.0) in a microwave oven for 10 min to retrieve antigen, and endogenous peroxidase was quenched with 0.3% hydrogen peroxide for 30 min. The sections were washed in PBS containing 0.1% Tween 20 for 15 min and blocked with 10% normal goat serum for 1 h. The sections were incubated with the rabbit NBCn1 antibody (1:30) at 4°C overnight and, after being washed, were incubated with biotinylated goat anti-rabbit IgG (1:67) (Vector Laboratories; Burlingame, CA) and then with the avidin-horseradish peroxidase complex Vectastatin Elite ABC kit (1:300) (Vector Laboratories) for 1 h each. The sections were stained using the DAB substrate kit (Zymed Laboratories; San Francisco, CA) and mounted with Histofix (Histolab; Göteborg, Sweden). The slides were observed under the Zeiss Axiovert 135 microscope (Oberkochem, Germany) using a Plan Neofluar ×16 and ×40 lens (numerical aperture 0.75).
Immunofluorescence.
Brains from mice (ICR) were fixed with 4% paraformaldehyde, dehydrated in 30% sucrose, and embedded in OCT. Brains were sliced with 30-μm thickness, and sections were stored in 30% ethylene glycol and 30% glycerin at 4°C. Sections were washed in PBS containing 0.1% Tween 20, blocked with 10% normal donkey serum at 37°C for 1 h, and then incubated with the guinea pig NBCn1 antibody (1:20) at 4°C overnight. The secondary antibody was the goat Texas red-conjugated anti-guinea pig IgG (1:200; Jackson ImmunoResearch; West Grove, PA) for 1 h. Immunofluorescence of HEK-293 cells expressing rat NBCn1 was done as previously described (10). For confocal fluorescence microscopy, primary antibodies were the rabbit NBCn1 antibody (1:100) and the mouse PSD-95 antibody (1:100), and secondary antibodies were Alexa 488 anti-rabbit IgG (1:500; Invitrogen; Carlsbad, CA) and Alexa 594 anti-mouse IgG (1:500; Invitrogen) for 1 h. The sections were mounted onto gelatin-coated slides with Vectashield (Vector Laboratories) and covered with coverslips. Immunofluorescence images were visualized using a Zeiss Axiovert 135M and an Olympus Fluoview FV1000 confocal microscope using a UPLFLN ×40 and ×100 lens (numerical aperture 1.3). Images were acquired and analyzed using Olympus software.
Quantification of Immunohistochemical Images
Quantification was made using the protocol we previously described (11) with slight modification. Briefly, images (×40) were analyzed using ImageJ software (NIH). Images were converted to a gray scale, and the pixel intensity was calibrated by setting the display value ranging from 0 (white) to 255 (black). The threshold level for detection was selected by viewing histograms and adjusted to distinguish the intensity of the labeling and the nonspecific background. The threshold level remained constant for all images to validate comparisons between images for controls and acidotic samples. The pixel intensity of the labeling was determined by randomly positioning boxes (700 square pixels) around the labelings at different locations on the image. For hippocampal pyramidal neurons and dentate gyrus granular neurons, cell body membranes and proximal dendrites were selected for analysis. For posterior cortical neurons and cerebellar neurons, cell bodies were selected. Background was determined by positioning boxes in the area where there was no neuron.
Glutathaione S Transferase Pull-Down Assay
Glutathaione S transferase (GST)/NBCn1 was constructed by subcloning the nucleotides for the COOH-terminal 26 amino acids of rat NBCn1 into pGEX-4T (GE Healthcare). GST-NBCn1 and GST proteins were purified from bacteria using glutathione-Sepharose 4B beads (GE Healthcare) according to the manufacturer's protocol and resuspended in PBS containing 0.5% Nonidet P-40 and protease inhibitors. The equal amounts of GST fusion protein and GST only were incubated with brain rat lysates for 4 h. Beads were washed with ice-cold PBS containing 0.5% Nonidet P-40, and proteins were dissociated from beads by adding the SDS sample buffer. Samples were subjected to immunoblot with the PSD-95 antibody.
Coimmunoprecipitation
Rat brain lysates were preincubated with Protein A/G gel agarose (Calbiochem) in the buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, 0.5% Triton X-100, and 0.2% sodium deoxycholate) at 4°C for 20 min. Beads were removed by gentle centrifugation at low speed, and lysates were incubated with the mouse PSD-95 antibody at 4°C with gentle rotation overnight. Controls were mouse IgG (Millipore). The beads were washed five times with ice-cold lysis buffer, and the bound proteins were eluted with the SDS-PAGE sample buffer. Immunoprecipitated protein complexes were resolved on 7.5% SDS-PAGE and subjected to immunoblot analysis with the NBCn1 antibody.
Real-Time PCR
Total RNAs from rat hippocampi were isolated using TRIzol (Invitrogen) and in vitro transcribed with random hexamers. Primers for real-time PCR were designed based on rat NBCn1. Primer sequences were GACTCCATAAGGGAGAATGTTCGAGAAGCT (forward) and CATCTCCGTGAAGAGGTCGTGGGGAATGTG (reverse). An internal control was rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers for GAPDH were TGGCCTTCCGTGTTCCTACC (forward) and TGTAGGCCATGAGGTCCACCAC (reverse). Real-time PCR was done with 200 nM of primers and 100 nM of a SYBR Green fluorescence probe (Sigma-Aldrich). PCR was performed at 95°C, 60°C, and 68°C (30 s each) for 40 cycles with the TaqMan PCR core reagent kit using iCycler (Bio-Rad; Hercules, CA). The annealing temperature was determined based on the melting temperature for primers. A serial dilution of the plasmid containing NBCn1 or GAPDH was amplified to generate a standard curve. The cycle threshold (i.e., the PCR cycle number at which exponential fluorescence is first detected) was obtained and fitted to the standard curve to obtain the amount of NBCn1 or GAPDH template. The NBCn1 value was normalized to the GAPDH value.
Chronic Metabolic Acidosis
Adult Sprague-Dawley rats (weighing 160–185 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed under a 12:12 h light/dark cycle. Metabolic acidosis was induced as described by Klein et al. (22). Briefly, 4–6 rats were fed with a 50:50 mixture of normal chow and 0.8 M HCl (wt/vol) with free access to water for 7 days. Controls were pair-fed with a 50:50 mixture of normal chow and water. Urine was collected during the final 24 h of the protocol. At the end of the feeding period, rats were euthanized and their plasma was collected for plasma chemistry analysis of arterial blood pH, Pco2, and HCO3−. Arterial blood gas was measured immediately after animal euthansia to minimize a potential artifact of high Pco2. The measurement was done by using a blood gas analyzer Opti 1 (AVL Scientific; Roswell, GA). Urine osmolality was determined by using a vapor pressure osmometer (Wescor; Logan, UT). Experiments were done three times. For NMDA experiment, rats fed with normal and acidic chow for 7 days were intraperitoneally injected with NMDA (75 mg/kg body wt) or none in PBS. Hippocampi were collected 3 days after injection and subjected to caspase-3 activity assay. All experiments were conducted under the NIH guideline for research on animals and the protocols were approved by the Institutional Animal Care and Use Committee at Emory.
Caspase-3 Activity
Caspase-3 activity was measured as described by Park et al. (27). Briefly, rat hippocampi were isolated 3 days after NMDA injection and lysed with homogenization buffer. Lysates were incubated with the caspase substrate N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide (Ac-DVED-pNA) at 37°C overnight using a Caspase-3 Assay Kit (Sigma-Aldrich) according to the manufacturer's protocol. The production of p-nitroanilide released from the substrate was measured by the absorbance at 405 nm. Controls were incubations of purified caspase-3 without lysates in the absence and presence of the inhibitor Ac-DVED-CHO.
Statistical Analysis
Data were reported as means ± SE. Levels of significance were assessed using the unpaired, one-tailed Student's t-test for comparison between control and acidotic rats. The P value of <0.05 was considered significant. Data were analyzed using Microsoft Office Excel 2003 and Origin 8.1 software (OriginLab; Northampton, MA).
RESULTS
Characterization of New NBCn1 Antibodies
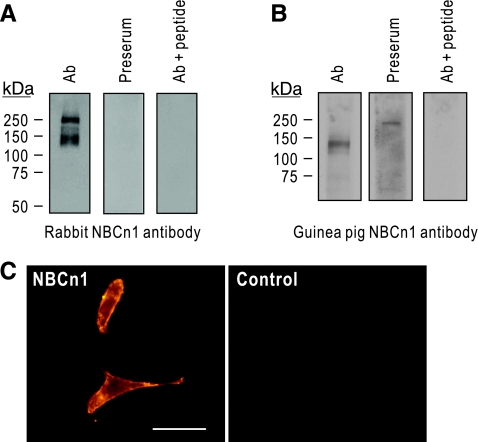
We generated new anti-NBCn1 antibodies by immunizing a rabbit and a guinea pig with the peptides corresponding to the cytoplasmic COOH-terminal 14 amino acids of rat NBCn1. This amino acid sequence is uniquely present in NBCn1 and its antigenic ability has been previously confirmed by other investigators (4, 10, 12, 29). To further confirm the specificity, we performed immunoblot of membrane preparations from rat brain by probing with the affinity-purified rabbit NBCn1 antibody (Fig. 1A). The antibody recognized a major band of 130 kDa. The antibody also detected a band of ∼250 kDa that is probably a dimeric formation of the transporter, similar to that for the electrogenic Na-HCO3 cotransporter NBCe1 (19). The detection of this higher molecular weight band varies depending on the amount of SDS in the sample buffer (Han Soo Yang, unpublished observation). Preimmune serum did not detect the bands. The specificity of the antibody was confirmed by preabsorption with the peptides, which did not detect immunoreactive bands. Figure 1B shows the characterization of the affinity-purified guinea pig NBCn1 antibody. The antibody recognized a band of 130 kDa in a plasma membrane preparation from rat hippocampus. Preimmune serum or preabsorption with the peptides did not detect the band. The band in preimmune serum is nonspecific. Furthermore, the antibody labeled plasma membrane of cells transfected with the neuronal splice variant NBCn1-E (Fig. 1C). Control cells transfected with vector only did not show immunofluorescence.
Fig. 1.
Characterization of new NBCn1 antibodies. A: immunoblot of membrane preparation from rat whole brain. Immunoblot was performed with the affinity-purified rabbit NBCn1 antibody. The antibody detected a major band of 130 kDa. The detection of the band of 250 kDa varied depending on the amount of SDS in the sample buffer. B: immunoblot of membrane preparation from rat hippocampus with the affinity-purified guinea pig NBCn1 antibody. The band in preserum is nonspecific. C: immunocytochemistry of HEK-293 cells transfected with NBCn1. Experiments were done with the guinea pig antibody. Controls were cells transfected with vector only. Bar: 50 μm.
NBCn1 Expression in Rat Brain
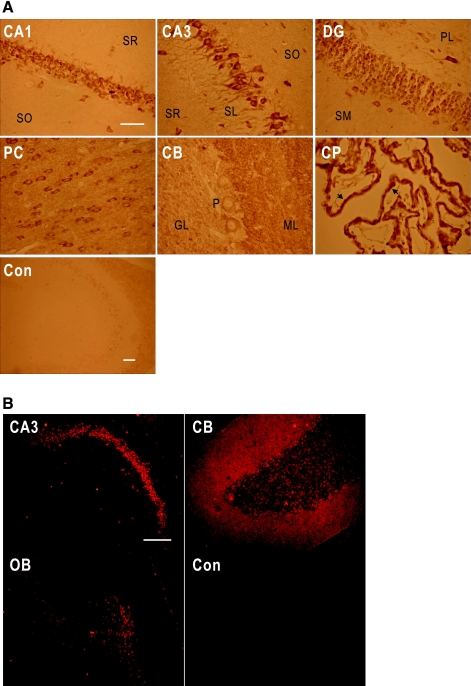
We examined NBCn1 localization in the rat brain by immunoperoxidase immunohistochemistry using the rabbit antibody. NBCn1 staining was strong in the hippocampal pyramidal layer and the dentate gyrus granular layer (Fig. 2A). The staining in the pyramidal layer was throughout the CA1-CA4 regions (data not shown). In CA3 neurons, the staining was prominent in cell bodies and proximal dendrites in stratum lucidum. In dentate gyrus, the staining was detected in granular neurons and processes in the polymorphic layer. In other brain regions, NBCn1 staining was detected in the multiple layers of posterior cortex, cerebellar Purkinje neurons, and choroid plexus. The staining in Purkinje neurons was restricted to membrane, whereas the staining in the molecular layer was in puncta. The staining in choroid plexus was localized to the basolateral membrane of the epithelium, consistent with the report by others (30). Controls without the primary antibody showed negligible staining. Figure 2B shows immunofluorescence immunochemistry using the guinea pig NBCn1 antibody. The NBCn1 immunofluorescence patterns were similar and comparable to the chromogenic labeling patterns in many brain regions.
Fig. 2.
NBCn1 localization in rat brain. A: immunoperoxidase immunochemistry for NBCn1 in rat brain. Brain slices were stained with the rabbit NBCn1 antibody. Images were taken from pyramidal CA1 and CA3 regions (CA1 and CA3), dentate gyrus (DG), posterior cortex (PC), cerebellum (CB), and choroid plexus (CP). The staining without the primary antibody served as a control (Con). SR, stratum radiatum; SO, stratum oriens; SL, stratum lucidum; SM, stratum moleculare; ML, molecular layer; GL, granular layer; P, Purkinje cells. Arrows are basolateral membranes of CP epithelia. Bars represent 50 μm. The bar for CA1 applies to the other 5 panels. B: immunofluorescence immunochemistry for NBCn1 in rat brain. Brain slices were labeled with the guinea pig NBCn1 antibody and then with the Texas red-conjugated goat anti-guinea pig IgG. Images were taken from CA3, CB, and olfactory bulb (OB). Bar: 100 μm.
NBCn1 Localization in Hippocampal CA3 Neurons
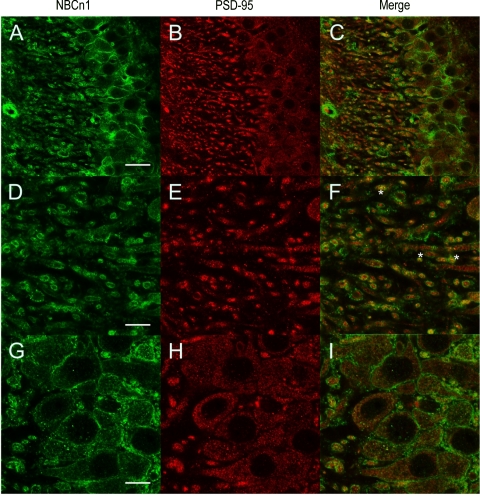
In primary cultures of rat hippocampal neurons (10, 11), NBCn1 localization partially overlaps with PSD-95 localization in dendrites (10). To test this colocalization, we performed double labeling of NBCn1 and PSD-95 in the hippocampus of the mouse brain. Figure 3 shows confocal images of Alexa 488-labeled NBCn1 (green) and Alexa 594-labeled PSD-95 (red) in CA3 neurons. NBCn1 immunofluorescence was prominently detected in dendritic processes. Cell body membranes were also positive for NBCn1. In contrast, PSD-95 immunofluorescence was found in puncta in dendrites, consistent with the report by others (17). The PSD-95 immunofluorescence in the cell body was dispersed in the cytosol but not on plasma membranes. The double label of PSD-95 and NBCn1 showed that punctuate PSD-95 labelings were frequently overlapped with NBCn1 labelings in dendrities, thus giving a yellow color when two images were merged (Fig. 3F). However, the overlap between the two labelings was negligible in cell bodies (Fig. 3I). These data indicate that NBCn1 is found at excitatory synapses in addition to other areas of the cell membrane.
Fig. 3.
Confocal immunofluorescence images of hippocampal CA3 neurons for NBCn1 and PSD-95. Hippocampal slices were labeled with the rabbit NBCn1 antibody (A, D, G) and the mouse PSD-95 antibody (B, E, H). NBCn1 was detected with the Alexa 488 anti-rabbit IgG (green) and Alexa 594 anti-mouse IgG (red). Images in A–C were taken in ×40 magnification (bar: 50 μm). Images of proximal dendrites (D–F) and cell bodies (G–I) were taken in ×100 magnification (bar: 10 μm). Asterisks (*) in F are examples for NBCn1/PSD-95 colocalization.
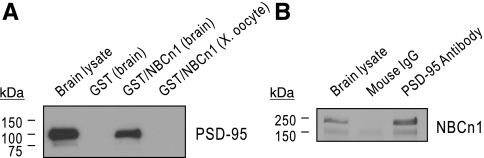
To further demonstrate colocalization of NBCn1/PSD-95, we used biochemical approaches in which the physical interaction between NBCn1 and PSD-95 was tested. NBCn1 has a PDZ-binding motif (the sequence is ETSL) at the COOH-terminal end of the protein that can interact with other cellular proteins (28, 31, 32). This led us to postulate that NBCn1 would cluster with PSD-95 in the postsynaptic density. In the pull-down assay (Fig. 4A), rat brain lysates were incubated with the GST/NBCn1 fusion protein containing the COOH-terminal 26 amino acids or GST only. Immunoblot revealed that PSD-95 was pulled down from brain lysates with GST/NBCn1 but not with GST only. The incubation of GST/NBCn1 with lysates from Xenopus oocytes (negative control) did not show the band. In coimmunoprecipitation experiments (Fig. 4B), rat brain lysates were incubated with the mouse anti-PSD-95 antibody and immunoprecipitates were examined with the NBCn1 antibody. NBCn1 was detected in the immunoprecipitates of lysates that were incubated with the PSD-95 antibody but not with the mouse IgG. A faint band in the mouse IgG is due to nonspecific binding even after vigorous washes of protein A/G beads. Together with the GST pull-down assay, this coimmunoprecipitation demonstrates that NBCn1 interacts with PSD-95 in the brain.
Fig. 4.
Interaction between NBCn1 and PSD-95. A: GST pull-down assay. Brain lysates were incubated with the GST/NBCn1 fusion protein containing the COOH-terminal 26 amino acids of rat NBCn1, or GST only. Proteins bound to GST/NBCn1 or GST were dissociated and examined with the PSD-95 antibody by immunoblot. Xenopus oocyte lysates were used as a negative control. B: coimmunoprecipitation of NBCn1 with the mouse PSD-95 antibody. Brain lysates were incubated with the PSD-95 antibody and immunoprecipitates were examined with the NBCn1 antibody by immunoblot. Mouse IgG was used as a control.
Induction of Chronic Metabolic Acidosis
To examine whether NBCn1 expression in the brain is affected by systemic acid loads, we performed experiments in which chronic metabolic acidosis was induced in rats by feeding 0.4 M HCl-containing chow for 7 days (22). Rats were allowed to have free access to water, and control rats were pair-fed with normal chow. Table 1 shows plasma and urine chemistries of those rats. When compared with control rats, HCl-fed rats had significantly decreased arterial blood pH (pH 7.32 for controls and pH 7.18 for HCl-fed rats, P < 0.05, n = 10 for each, combined from two separate experiments) and low serum [HCO3−] (24.2 mM for controls and 16.3 mM for HCl-fed rats, P < 0.05). These decreases in pH and [HCO3−] were similar to our previous report (35). The arterial blood Pco2 was insignificantly affected. The reason for this insignificant change is unclear, but we note that a similar HCl feeding protocol increases Pco2 (35). HCl-fed rats had significantly decreased urine pH due to increased acid excretion in the kidney. HCl-fed rats had increased urine output and low osmolality, probably due to high water intake caused by acidic food.
Table 1.
Plasma and urine chemistries of control rats versus HCl-fed rats
| Control Rats | HCl-Fed Rats | |
|---|---|---|
| Plasma pH | 7.32 ± 0.03 | 7.18 ± 0.02* |
| Plasma [HCO3−], mM | 24.2 ± 1.1 | 16.3 ± 1.4* |
| Pco2, mmHg | 45.9 ± 3.6 | 44.0 ± 1.9 |
| Urine pH | 7.76 ± 0.13 | 6.19 ± 0.07* |
| Urine volume, ml/day | 16.0 ± 1.6 | 40.5 ± 1.4* |
| Urine osmolality, mosmol/kg H2O | 1,039 ± 94 | 752 ± 25* |
Data are means ± SE, n = 10 rats/group combined from 2 separate experiments.
Significantly different from pair-fed control rats at P < 0.05.
Upregulation of NBCn1 mRNA and Protein by Chronic Metabolic Acidosis
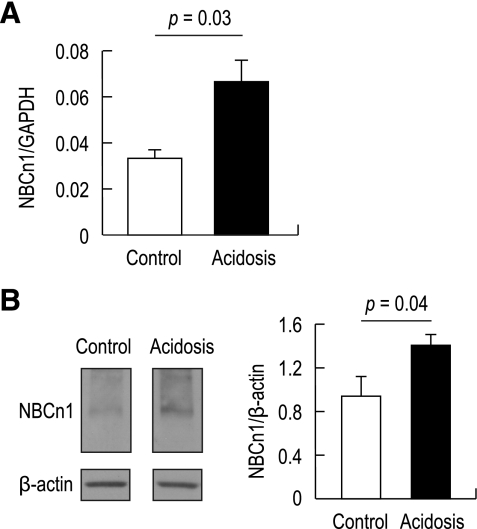
Real-time PCR and immunoblot were used to analyze NBCn1 expression in the hippocampus. When compared with controls, acidotic rats had a twofold increase in NBCn1 mRNA (P = 0.03) normalized to GAPDH mRNA (Fig. 5A). Immunoblot of membrane preparations from the hippocampus also revealed a marked increase in NBCn1 protein expression by chronic metabolic acidosis (Fig. 5B). Analyzed by densitometric measurements of NBCn1 normalized to β-actin, the increase was 1.5-fold (P = 0.04, n = 5 for control rats and n = 6 for HCl-fed rats).
Fig. 5.
Effects of chronic metabolic acidosis on NBCn1 mRNA and protein levels in the hippocampus. A: real-time PCR. The PCR cycle threshold for NBCn1 or GAPDH was determined and fitted to the standard curve made from a serial dilution of NBCn1 and GAPDH templates. NBCn1 was then normalized to GAPDH (P = 0.03, n = 3 for each). B: immunoblot of hippocampal lysates. Left, immunoblots of membrane preparations from control and acidotic hippocampi were probed with the NBCn1 antibody. The blots were reprobed with the β-actin antibody. Right, mean pixel intensity of immunoreactive bands was quantitated, and NBCn1 was normalized to β-actin (P = 0.04, n = 5 for controls and 6 for HCl-fed rats).
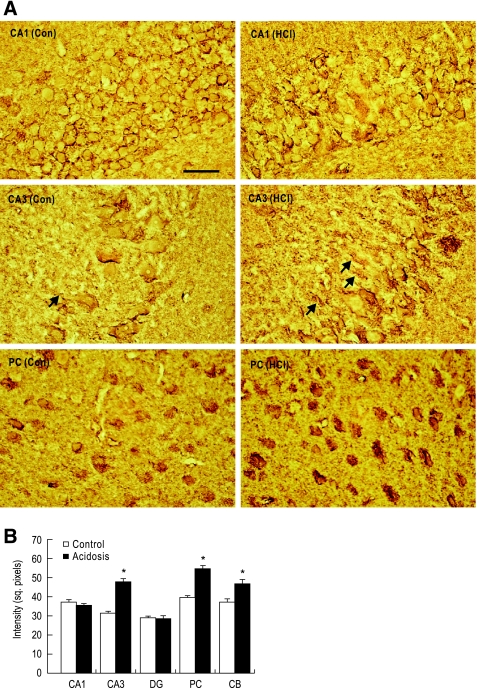
We then examined NBCn1 upregulation patterns by immunohistochemistry of rat brain slices. In the hippocampus, the NBCn1 staining was similar in CA1 neurons of the two groups of rats, but increased in CA3 neurons (Fig. 6A). The increase occurred primarily along cell body membranes and proximal dendrites in stratum lucidum. The staining in dentate gyrus granular neurons remained unaffected (figure not shown). We also observed significant upregulation of the staining in other neurons such as posterior cortical neurons and cerebellar granule cells. The upregulation of NBCn1 in the posterior cortical neurons occurred primarily in the soma. The numbers of NBCn1-positive neurons appear to be similar in control rats and acidotic rats. Quantitative measurements revealed 20–70% increases in NBCn1 expression in neurons where the upregulation occurred (Fig. 6B).
Fig. 6.
NBCn1 expression in brains of control rats versus acidotic rats. A: immunohistochemistry for NBCn1. Brain slices were stained with the rabbit NBCn1 antibody. Arrows are proximal dendrites. Bar: 50 μm. B: quantitation of NBCn1 expression. The mean pixel intensity of NBCn1 was determined by collecting signals in cell bodies or dendrites. More than 20 signals were counted. *P < 0.05.
Association of NBCn1 Upregulation With Neuronal Death
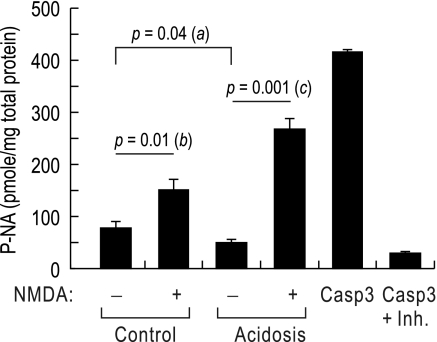
Our recent study (11) shows that knocking down NBCn1 in cultured hippocampal neurons reduces neuronal death. To examine whether NBCn1 upregulation caused by chronic metabolic acidosis is associated with neuronal death, we induced glutamate excitotoxicity in rats and determined caspase-3 activity (Fig. 7). Rats fed with normal and HCl-containing chow for 7 days were intraperitoneally injected with NMDA (75 mg/kg body wt), and caspase-3 activity in the hippocampus was determined 3 days later. Active caspase-3 is cleaved from procaspase between 2 and 7 days after NMDA treatment (14). In the absence of NMDA, acidotic rats had slightly lower caspase-3 activity than control rats (P = 0.04; a in the figure; n = 4 for each), probably due to the protective effect of acidic pH on cell death. Administering NMDA increased caspase-3 activity by 1.9-fold in control rats (from 80.0 ± 12.2 to 151.8 ± 20.3 pmol/mg; the unit is pmole of p-nitroanilide production per mg of total protein; n = 4 for each, P = 0.01; b in the figure). In acidotic rats, however, the same NMDA treatment increased caspase-3 activity by 5.4-fold (from 49.5 ± 6.2 to 267.2 ± 21.1 pmol/mg; n = 4 for each, P = 0.001; c in the figure). Therefore, the caspase-3 activity was significantly increased in acidotic rats after administering NMDA. These results indicate that acidotic rats are more vulnerable to glutamate excitotoxicity.
Fig. 7.
Caspase-3 activity in control versus acidotic rats treated with N-methyl-d-aspartate (NMDA). Control rats and acidotic rats (n = 4 for each) were intraperitoneally injected with NMDA (75 mg/kg body wt), and hippocampi were isolated 3 days later and lysed. Lysates were incubated with the caspase substrate Ac-DVED-p-nitroanilide at 37°C overnight. The production of p-nitroanilide was measured by the absorbance at 405 nm. The unit is pmole of p-nitroanilide per milligram of total protein. Positive and negative controls were incubations of purified caspase-3 without lysates in the absence/presence of the inhibitor Ac-DVED-CHO.
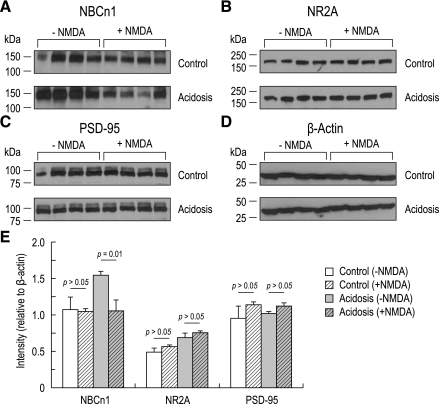
We then examined NBCn1 expression by immunoblotting a membrane preparation from the hippocampus 3 days after treatment (Fig. 8A). Administering NMDA to control rats produced negligible effect on NBCn1 expression. NBCn1 was upregulated by chronic metabolic acidosis, consistent with the above results. However, administering NMDA to acidotic rats almost abolished NBCn1 upregulation. Analyzed by quantitative measurements of NBCn1 normalized to β-actin (Fig. 8E), the NBCn1 expression in acidotic rats was significantly lower after NMDA administration (P = 0.01). We also examined NR2A and PSD-95 expression to determine whether these proteins were affected by administering NMDA. Immunoblot probed with NR2A and PSD-95 antibodies revealed negligible difference before and after NMDA administration in both control rats and acidotic rats (Fig. 8E).
Fig. 8.
NBCn1, NR2A, PSD-95, and β-actin expression levels before and after NMDA administration. A–D: immunoblot of the hippocampus prepared from controls and acidotic rats 3 days after NMDA treatment (n = 4). The blots were probed with antibodies to NBCn1 (A), NR2A (B), PSD-95 (C), and β-actin (D). E: quantitative measurements of immunoreactive bands. The bands were quantitated and normalized to β-actin.
DISCUSSION
Overview
The major findings from the present study are the following: 1) NBCn1 is expressed in neurons in a variety of brain regions; 2) NBCn1 is partially colocalized to PSD-95 in dendrites of CA3 neurons and they interact with each other; 3) NBCn1 is upregulated by chronic metabolic acidosis; 4) NMDA induces more severe neuronal death in chronic metabolic acidosis; and 5) NMDA inhibits acidosis-induced NBCn1 upregulation. These findings are the first demonstration that the Na-HCO3 cotransporter NBCn1 in the brain is upregulated by chronic metabolic acidosis. Our data are valuable for understanding the physiological and pathological roles of HCO3−-dependent acid extrusion during systemic acid loads and its involvement in neuronal cell death.
Expression and Localization of NBCn1 in the Brain
We generated new antibodies using the last 14 amino acids of rat NBCn1. This antigenic peptide sequence we used to generate antibodies is uniquely present in NBCn1 and exhibits extremely low sequence similarity to other bicarbonate transporters. The specificity of the antibodies is confirmed by the competition experiments (Fig. 1, A and B). The antigenic ability of this peptide sequence has been previously supported (4, 10, 12, 29), demonstrating no cross-reactivity to other Na-HCO3 transporters. Both rabbit and guinea pig antibodies recognize a band of 130 kDa.
Our immunohistochemical data show that NBCn1 is expressed in neurons in a variety of brain regions (Fig. 2). Those neurons include hippocampal pyramidal neurons, dentate gyrus granular neurons, posterior cortical neurons, and cerebellar Purkinje neurons. NBCn1 is also localized to the basolateral membrane of the choroid plexus epithelia. By β-galactosidase staining of mice that have the LacZ gene trap in slc4a7 (NBCn1) gene, Boedtkjer et al. (5) recently demonstrated NBCn1 promoter activity in many neurons and choroid plexus epithelia. The antibody-dependent localization patterns we observe are overall in good agreement with the β-galactosidase staining patterns. Nonetheless, we find several differences between the two approaches. First, the staining intensity is not consistent in some cells. For example, our antibody approach shows strong staining in the basolateral membrane of the choroid plexus epithelium, whereas the β-galactosidase staining is relatively weak and diffuse in the cytosol. This disparity is in contrast to the staining intensity in hippocampal neurons, where both NBCn1 antibody and β-galactosidase staining are equally strong. Second, the antibody stains certain regions of the brain where β-galactosidase expression is almost negligible. In the cerebellum, the molecular layer is recognized by the antibody only. Both antibody and β-galactosidase stain Purkinje neurons, which project dendritic arbors to the molecular layer. We think that the β-galactosidase fusion protein may have difficulty being delivered to dendrites in this layer.
Confocal images of CA3 neurons (Fig. 3) show NBCn1 being localized to both cell body membranes and dendritic processes. The somatic localization of the transporter is an interesting finding because HCO3−-dependent acid extrusion in the cell body is known to be governed by the Na+-driven Cl-HCO3 exchanger (2). The electroneutral Na-HCO3 cotransporter and the Na+-driven Cl/HCO3 exchanger are functionally similar with each other except for their ability to move Cl−. We note that distinguishing one from another is difficult particularly when these two HCO3−-dependent acid extruders are present in the same cell.
NBCn1/PSD-95 Interaction
NBCn1 can partially colocalize with PSD-95 in dendrites of CA3 neurons (Fig. 3). The GST/NBCn1 fusion protein pulls down PSD-95 from brain lysates and NBCn1 coimmunoprecipitates with PSD-95 (Fig. 4). These data demonstrate that NBCn1 can be present at excitatory synapses. PSD-95 serves as a scaffold and interacts with other proteins to form large molecular complexes in the postsynaptic density of glutamatergic synapses (20). The interaction occurs between PSD-95 and the PDZ-binding motifs that are mostly located at the COOH-terminal end of the proteins (28, 31, 32). The interaction recruits signaling proteins, cluster with functionally associated proteins into microdomains, and tether proteins to the cytoskeleton connected to the plasma membrane. Our findings of NBCn1/PSD-95 interaction and colocalization provide evidence that NBCn1 is one of those associated proteins (36). Furthermore, some PSD-95-bound proteins such as NMDA receptors (13), nitric oxide synthase (16), and K+ channels (9) are sensitive to pH. This leads us to postulate that “pH-regulating” NBCn1 and “pH-sensitive” binding partners may cluster, and the clustering may enable NBCn1 to affect the activity of its binding partners by modulating local pH near the partners. Such interactions may provide a mechanism by which the binding partners exhibit different levels of activity at different synapses.
NBCn1 Upregulation in the Brain During Chronic Metabolic Acidosis
Chronic metabolic acidosis is an acid-base disorder in which arterial pH is below normal (<pH 7.3) due to a decrease in plasma [HCO3−]. The decrease in plasma [HCO3−] occurs as the kidney reduces its capacity to excrete H+ (e.g., reduced ammonium production in the proximal tubules) or retain HCO3− in the distal tubules. The kidney undergoes a variety of adaptive processes during chronic metabolic acidosis to restore plasma pH, and one of the adaptive processes is to alter acid-base transporter expression and activity (39). Consistent with this adaptive process, NBCn1 protein expression in the rat kidney is significantly increased during chronic metabolic acidosis (23, 26). The reason for such a dramatic effect is proposed to counter intracellular H+ overload caused by an increased NH4+ uptake from the lumen. The elevation effect is also observed in the thick ascending limb cell line ST-1 when cells are incubated for 24 h in a pH 6.8 medium (unpublished observations). Thus, at least in the kidney and a kidney cell line, NBCn1 expression is elevated in response to acidosis or acidic incubation. Our data in the present study provide novel evidence that chronic metabolic acidosis also upregulates NBCn1 in the brain. A significant upregulation is detected in CA3 neurons, as well as posterior cortical neurons and cerebellar neurons (Fig. 6). This upregulation is comparable to an increased level of the transporter in primary cultures of rat hippocampal neurons when cultures are incubated at low pH adjusted by [HCO3−] (11). Similarly, Kanaan et al. (18) have reported that NBCn1 and NHE are increased by chronic elevation of Pco2 in the neonatal rat cortex. Kiwull-Schone et al. (21) have shown that NHE3 mRNA is upregulated in rat brain in response to metabolic acidosis. These reports support the idea that brain acid-base transporters, particularly acid-extruding membrane proteins, are upregulated by chronic metabolic and/or respiratory acidosis.
Given the fact that NBCn1 in the kidney is upregulated to counter intracellular H+ overload, the transporter upregulation in the brain is probably a compensatory process that helps maintain intracellular pH in the presence of a chronic acid challenge. Dendritic pH changes are greater than somatic pH changes in rat Purkinje neurons (43). High Pco2-induced acidification is faster in more distal regions of the dendrites (33). These reports suggest that intracellular pH can vary locally in a neuron. We think that NBCn1 upregulation may allow neurons to adjust local intracellular pH, particularly dendritic pH, in response to acidic environments. Nonetheless, we do not exclude the possibility of an extracellular pH effect. NBCn1 may affect extracellular pH at synapses, particularly in the synaptic cleft, where a small change in H+ movement across the membrane can cause a large effect on local pH (8).
Interestingly, our data show that not all of the NBCn1-expressing neurons are affected by metabolic acidosis. A substantial upregulation of NBCn1 is found in CA3 neurons but neither in CA1 neurons nor dentate gyrus granular neurons. The upregulation occurs along the cell body membrane and proximal dendrites of CA3 neurons, whereas it is mostly limited to the cytosol in posterior cortical neurons. Thus NBCn1 upregulation in response to systemic acidosis occurs in a cell-specific manner.
Effect of NBCn1 Upregulation on Glutamate-Mediated Neuronal Death
NMDA-mediated excitotoxicity involves Ca2+ influx via the NMDA receptors and multiple signaling cascades resulting in cell death (for review see Ref. 13). NMDA receptors are inhibited by low extracellular pH with the half-maximum inhibition at pH 7.3 in cultured neurons (37), and therefore one might expect that the ability of NMDA to promote neuronal death would be reduced in acidotic rats. However, we instead observe more NMDA-mediated neuronal death in these rats. A significantly higher caspase-3 activity is observed after NMDA administration (Fig. 7), indicating that acidotic rats are more vulnerable to excitotoxicity. The reason for this increased neuronal death is unclear, but we note that there might be a disparity between in vivo and in vitro results. Urenjak et al. (38) demonstrate that NMDA-evoked currents are not reduced in rats with acid-base challenge. We speculate that acidosis may cause ATP depletion and membrane depolarization, which would remove magnesium block of NMDA receptors.
Our data show a close association of NBCn1 upregulation and NMDA-mediated neuronal death. NBCn1 upregulation is inhibited in acidotic rats with severe neuronal death (Fig. 8). A possible explanation for this result is that NMDA receptors reduce HCO3−-dependent acid extrusion by inhibiting NBCn1 upregulation and subsequently cause intracellular pH to drop to the level where neuronal death begins to occur. NMDA receptors induce intracellular acidification in cultured hippocampal neurons (42). NMDA receptors can stimulate acid-sensing ion channels that are activated at low pH and induce cell death by raising intracellular Ca2+ (15). These effects of NMDA receptors may be mediated by altering NBCn1 protein expression. We note that NBCn1 knockout mice develop slow degeneration of retinal photoreceptors and cochlear hair cells probably due to the inability of these neurons to recover from intracellular acidification (6).
Alternatively, NMDA receptors may selectively kill neurons in which NBCn1 is upregulated. In this case, NBCn1 upregulation is deleterious to neurons when NMDA receptors are activated. Our observation of lack of NBCn1 upregulation in NMDA-administered acidotic rats may thus reflect selective death of those neurons that have undergone the transporter upregulation. This interpretation is consistent with our previous observation (11) that knocking down NBCn1 in hippocampal neuronal cultures reduces excitotoxicity caused by magnesium depletion.
In summary, our data demonstrate that NBCn1 is upregulated by chronic metabolic acidosis. Our data also provide new evidence that chronic metabolic acidosis can cause neuronal damage, particularly when glutamate levels are increased. NBCn1 upregulation may play a key role in glutamate-mediated brain damage. It will be interesting to investigate the mechanism of how acid load induces the upregulation of the transporter in future experiments.
GRANTS
This work was supported by National Institutes of Health R01-GM-078502 and American Heart Association (to I. Choi), NIH R01-DK-41707 and P01-DK-61521 (J. Sands), and NIH R01-DK-62081 (J. Klein).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Chad Denson and Guangping Chen for animal acidosis studies.
REFERENCES
- 1.Baird NR, Orlowski J, Szabo EZ, Zaun HC, Schultheis PJ, Menon AG, Schull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J Biol Chem 274: 4377–4382, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Baxter KA, Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J Physiol 493: 457–470, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevensee MO, Boron WF. pH regulation in mammalian neurons. In: pH and Brain Function, edited by Kaila K, Ransom BR. New York: Wiley-Liss, 1998, p. 211–231 [Google Scholar]
- 4.Boedtkjer E, Praetorius J, Aalkjaer C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ Res 98: 515–523, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Boedtkjer E, Praetorius J, Fuchtbauer EM, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (Slc4a7) in mice. Am J Physiol Cell Physiol 294: C591–C603, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bok D, Galbraith G, Lopez I, Woodruff M, Nusinowitz S, BeltrandelRio H, Huang W, Zhao S, Geske R, Montgomery C, Van S, I, Friddle C, Platt K, Sparks MJ, Pushkin A, Abuladze N, Ishiyama A, Dukkipati R, Liu W, Kurtz I. Blindness and auditory impairment caused by loss of the sodium bicarbonate cotransporter NBC3. Nat Genet 34: 313–319, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chen LM, Choi I, Haddad GG, Boron WF. Chronic continuous hypoxia decreases the expression of SLC4A7 (NBCn1) and SLC4A10 (NCBE) in mouse brain. Am J Physiol Regul Integr Comp Physiol 293: R2412–R2420, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Claydon TW, Boyett MR, Sivaprasadarao A, Ishii K, Owen JM, O'Beirne HA, Leach R, Komukai K, Orchard CH. Inhibition of the K+ channel kv1.4 by acidosis: protonation of an extracellular histidine slows the recovery from N-type inactivation. J Physiol 526: 253–264, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DS, Saxena NC, Yang HS, Lee HJ, Moring AG, Lee A, Choi I. Molecular and functional characterization of the electroneutral Na/HCO3 cotransporter NBCn1 in rat hippocampal neurons. J Biol Chem 280: 17823–17830, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Yang HS, He P, Kim E, Rajbhandari I, Yun CC, Choi I. Sodium/bicarbonate cotransporter NBCn1/slc4a7 increases cytotoxicity in magnesium depletion in primary cultures of hippocampal neurons. Eur J Neurosci 29: 437–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol 293: R2136–R2146, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999 [PubMed] [Google Scholar]
- 14.Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7, Suppl 3: S3–S11, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48: 635–646, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Gorren AC, Schrammel A, Schmidt K, Mayer B. Effects of pH on the structure and function of neuronal nitric oxide synthase. Biochem J 331: 801–807, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci 16: 1380–1388, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanaan A, Douglas RM, Alper SL, Boron WF, Haddad GG. Effect of chronic elevated carbon dioxide on the expression of acid-base transporters in the neonatal and adult mouse. Am J Physiol Regul Integr Comp Physiol 293: R1294–R1302, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem 283: 26782–26794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kiwull-Schone H, Kiwull P, Frede S, Wiemann M. Role of brainstem sodium/proton exchanger 3 for breathing control during chronic acid base imbalance. Am J Respir Crit Care Med 176: 513–519, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Klein JD, Rouillard P, Roberts BR, Sands JM. Acidosis mediates the upregulation of UT-A protein in livers from uremic rats. J Am Soc Nephrol 13: 581–587, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kwon TH, Fulton C, Wang W, Kurtz I, Frokiaer J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO3 cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol 282: F341–F351, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Mohebbi N, Mihailova M, Wagner CA. The calcineurin inhibitor FK506 (Tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport. Am J Physiol Renal Physiol 297: F499–F509, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Nachshen DA, Drapeau P. The regulation of cytosolic pH in isolated presynaptic nerve terminals from rat brain. J Gen Physiol 91: 289–303, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J. Basolateral Na+-dependent HCO3- transporter NBCn1-mediated HCO3− influx in rat medullary thick ascending limb. J Physiol 555: 205–218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park HJ, Kim HJ, Park HJ, Ra J, Zheng LT, Yim SV, Chung JH. Protective effect of topiramate on kainic acid-induced cell death in mice hippocampus. Epilepsia 49: 163–167, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Park M, Ko SBH, Davidson N, Muallem G, Thomas PJ, Pushkin A, Lee K, Kim JY, Lee MG, Muallem S. The cystic fibrosis transmembrane conductance regulator interacts with and regulates the activity of the HCO3− salvage transporters human Na+-HCO3− cotransporter isoform 3. J Biol Chem 277: 50503–50509, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Perry C, Quissell DO, Reyland ME, Grichtchenko II. Electrogenic NBCe1 (SLC4A4), but not electroneutral NBCn1 (SLC4A7), cotransporter undergoes cholinergic-stimulated endocytosis in salivary ParC5 cells. Am J Physiol Cell Physiol 295: C1385–C1398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praetorius J, Nejsum LN, Nielsen S. A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells. Am J Physiol Cell Physiol 286: C601–C610, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Pushkin A, Abuladze N, Newman D, Muronets V, Sassani P, Tatishchev S, Kurtz I. The COOH termini of NBC3 and the 56-kDa H+-ATPase subunit are PDZ motifs involved in their interaction. Am J Physiol Cell Physiol 284: C667–C673, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Reiners J, van Wijk E, Marker T, Zimmermann U, Jurgens K, te BH, Overlack N, Roepman R, Knipper M, Kremer H, Wolfrum U. Scaffold protein harmonin (USH1C) provides molecular links between Usher syndrome type 1 and type 2. Hum Mol Genet 14: 3933–3943, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol 289: C1094–C1104, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Armass S, Martinez-Zaguilan R, Martinez GM, Gillies RJ. Regulation of pH in rat brain synaptosomes. I. Role of sodium, bicarbonate, and potassium. J Neurophysiol 71: 2236–2248, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24: 1–29, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Traynelis SF. pH modulation of ligand-gated ion channels. In: pH and Brain Function, edited by Kaila K, Ransom BR. New York: Wiley-Liss, 1998, p. 417–446 [Google Scholar]
- 38.Urenjak J, Zilkha E, Gotoh M, Obrenovitch TP. Effect of acidotic challenges on local depolarizations evoked by N-methyl-d-aspartate in the rat striatum. Life Sci 61: 523–535, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Wagner CA. Metabolic acidosis: new insights from mouse models. Curr Opin Nephrol Hypertens 16: 471–476, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Lee HJ, Cooper DS, Cebotaro L, Walden PD, Choi I, Yun CC. Coexpression of MAST205 inhibits the activity of Na+/H+ exchanger NHE3. Am J Physiol Renal Physiol 290: F428–F437, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G, Li C, Kim SW, Ring T, Wen J, Djurhuus JC, Wang W, Nielsen S, Frokiaer J. Ureter obstruction alters expression of renal acid-base transport proteins in rat kidney. Am J Physiol Renal Physiol 295: F497–F506, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Wang GJ, Randall RD, Thayer SA. Glutamate-induced intracellular acidification of cultured hippocampal neurons demonstrates altered energy metabolism resulting from Ca2+ loads. J Neurophysiol 72: 2563–2569, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Willoughby D, Schwiening CJ. Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol 544: 487–499, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.