Abstract
Located at the anterior portion of the nose, the paired vomeronasal organs (VNO) detect odors and pheromones. In vomeronasal sensory neurons (VSNs) odor responses are mainly mediated by phospholipase C (PLC), stimulation of which elevates diacylglycerol (DAG). DAG activates a transient receptor potential channel (TRPC2) leading to cell depolarization. In this study, we used a natural stimulus, urine, to elicit odor responses in VSNs and found urine responses persisted in TRPC2−/− mice, suggesting the existence of a TRPC2-independent signal transduction pathway. Using perforated patch-clamp recordings on isolated VSNs from wild-type (WT) and TRPC2−/− mice, we found a PLC inhibitor blocked urine responses from all VSNs. Furthermore, urine responses were reduced by blocking DAG lipase, an enzyme that produces arachidonic acid (AA), in WT mice and abolished in TRPC2−/− mice. Consistently, direct stimulation with AA activated an inward current that was independent of TRPC2 channels but required bath Ca2+ and was blocked by Cd2+. With the use of inside-out patches from TRPC2−/− VSNs, we show that AA activated a channel that also required Ca2+. Together, these data from WT and TRPC2−/− mice suggest that both DAG and its metabolite, AA, mediate excitatory odor responses in VSNs, by activating two types of channels, a TRPC2 and a separate Ca2+-permeable channel.
Keywords: arachidonic acid, signal transduction, urine, odor responses, perforated patch clamp
vomeronasal organs (VNO) are part of the accessory olfactory system present in many vertebrates. They detect chemical signals: pheromones and general odorants (reviewed in Ref. 2). Pheromones are substances “secreted to the outside by an individual and received by a second individual of the same species, in which they release a specific reaction” (20). In rodents, pheromones are usually concentrated in urine and mediate such behavior as gender recognition, mating, male-male aggression, and territorial defense.
In vomeronasal sensory neurons (VSNs), sensory stimulation mainly activates phospholipase C (PLC) pathway, resulting in the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (14, 18, 23, 52). DAG can be further hydrolyzed to arachidonic acid (AA) by a DAG lipase. The activation of the PLC pathway results in an elevation of intracellular Ca2+ and excitation. However, the mechanism underlying this Ca2+ increase is controversial. IP3-mediated internal Ca2+ release does not appear to play a role because of its slow activation kinetics (31) and the finding that odor responses were not affected by thapsigargin, a sarcoplasmic-endoplasmic reticulum calcium ATPase (SERCA) inhibitor (44). Rather it appears that DAG activates a Ca2+-permeable channel, (transient receptor potential channel TRPC2) (31) that is highly expressed in VSN microvilli (28, 34). TRPC2−/− mice showed defects in pheromone detection and mating-related behaviors, supporting a role for TRPC2 channels in odor detection of VNO (26, 46).
However, both behavioral and electrophysiological studies suggest that the activation of TRPC2 channels is not the only excitatory mechanism involved in VSN odor transduction. The “Bruce Effect” (selective pregnancy failure) is mediated by pheromones acting through the VNO. It is influenced by the surgical lesion of VNO but not by the ablation of TRPC2 gene (21). In electrovomeronasogram recordings from TRPC2−/− mice, responses to urine or putative pheromones were diminished but not abolished (21, 26). These data suggest the existence of a TRPC2-independent pathway. Consistently, whole cell recordings showed that a DAG analogue (1-stearoyl-2-arachidonoyl-sn-glycerol, SAG) activated a diminished conductance in TRPC2−/− mouse VSNs (31). Since SAG (similar to DAG) can be hydrolyzed to AA, we propose that the responses in TRPC2−/− mice are mediated by an AA-activated channel, distinct from TRPC2 channels.
To elucidate the involvement of a second odor transduction mechanism in VSNs, we performed perforated patch-clamp experiments on isolated VSNs of wild-typw (WT) and TRPC2−/− mice. We found that urine responses were decreased (WT) or abolished (TRPC2−/−) by blocking AA synthesis with a DAG lipase inhibitor. Direct stimulation with AA activated an inward current in either WT or TRPC2−/− VSNs. Thus we propose that when an odor activates PLC, there are two pathways transducing the signal: the activation of TRPC2 channels by DAG and the activation of calcium-permeable channels by a metabolite of DAG, AA.
MATERIALS AND METHODS
Experimental animals.
We used 1- to 5-mo-old, male and female mice of two strains, WT (C57BL/6, Charles River, Wilmington, MA) and TRPC2−/− mice (constructed from C57BL/6 strain, a kind gift from Catherine Dulac, Harvard University) (46). Mice were maintained in the Animal Care Facility at the University of Vermont according to IACUC guidelines and their use in this study was approved by UVM's IACUC committee.
Urine collection.
Urine was collected by gently pressing the abdomens of mice. A urine mixture was made by combining the urine from at least 30 male and female mice. The urine mixture was filtered through a 0.2-μm syringe filter (Fisher, Pittsburgh, PA) and stored at −80°C for up to 3 mo. Before use, the urine mixture was diluted in Ringer solution (1:500) and the pH adjusted to 7.4.
Preparation of isolated VSNs.
The VSNs of WT and TRPC2−/− mice were acutely dissociated by a modified version of a previously reported method (44). Briefly, mice were euthanized with CO2 followed by cervical dislocation. The VNOs were dissected out, cut into small pieces, and incubated with 50 μg/ml papain (Calbiochem, La Jolla, CA) in a divalent cation-free Ringer solution for 15 min at room temperature. Cells were gently pipetted, filtered through a 250-μm nylon mesh (Small Parts, Miami Lakes, FL), and transferred into a fresh Ringer solution containing 10 μg/ml leupeptin (USB, Cleveland, OH). Isolated cells were kept at room temperature and usually used within 4 h of preparation.
Odor and drug application.
A SF-77B fast perfusion stepper system (Warner Instruments, Hamden, CT) was used to apply drugs focally. It allowed for application of up to nine different solutions with our reservoir setup. The switch time between solutions was about 20 ms.
Electrophysiology.
Perforated patch clamp was performed using gramicidin, pores of which are permeable to Na+ and K+ but not to Cl− (35). With the perforated patch-clamp configuration, diffusible second messengers remain in the cell. This recording method allows for the repeated testing of odor responses. Gramicidin was dissolved in DMSO (100 mg/ml) and mixed into the intracellular solution (0.22 mg/ml) by vortex for 10 min and brief sonication. Isolated VSNs were placed in a recording chamber on a Nikon Eclipse TE200 inverted microscope. Glass pipettes were pulled from LE16 glass tubing (Dagan, Minneapolis, MN) with a P-97 flaming/brown micropipette puller (Sutter Instrument, Novato, CA). Pipettes with a resistance of 6–10 MΩ were filled at the tips with the intracellular solution minus gramicidin and then back-filled with the gramicidin-containing intracellular solution. After gigaseals were formed on isolated VSNs, diffusion of gramicidin to the membrane was monitored by the appearance of voltage-activated currents as electrical access to the interior of the cell was established (access resistance Ra was usually <20 MΩ). After voltage control was established, either voltage- or current-clamp recordings were performed. For the voltage-clamp experiments, cells were held at −80 mV and were exposed to 1:500 urine or 50 μM AA (see Solutions) for 0.5 s through the fast perfusion stepper system (Warner SF-77B). The urine- and AA-activated currents were determined by subtracting the base current before stimulation from the peak responses. There were 2-min intervals between consecutive stimulations to avoid adaptation. In most of cells, the urine- and AA-activated currents were stable, with little rundown. Immediately after recordings of the urine- and AA-activated currents in Ringer solution, the same cell was usually exposed to bath-applied drugs [for example, U73122, RHC80267, 2-aminoethoxydiphenyl borate (2-APB)] and stimulated with urine/AA again to evaluate the effect of drugs. The responses in drugs were normalized to these in Ringer (100%). For current clamp recordings, current was injected so that the membrane potential was close to −60 mV to allow for comparison of responses between cells. Action potentials (APs) were elicited by urine (1:500) application (1 s) through the perfusion system.
For excised patch recordings, pipettes with a resistance between 8 and 15 MΩ were filled with the intracellular solution. After a tight seal was formed on the soma or a dendritic knob (>1 GΩ), inside-out patches were obtained by passing through the water-air interface. Single-channel recordings were carried out in a symmetric solution (see Solutions). The [Ca2+]free in the bath solution was adjusted to 10 nM-50 μM free Ca2+ using 1 mM EGTA and various amounts of CaCl2 according to Winmaxc 2.5 software (http://www.stanford.edu/∼cpatton).
Electrophysiological data were acquired using MultiClamp 700A, Digidata 1322A, and pCLAMP 8.2 software (Axon Instruments, Union City, CA). The analog data were sampled at 10 kHz and filtered at 4 kHz with an eight-pole Bessel low-pass filter and digitized. The single channel data were further filtered at 50 Hz with the eight-pole Bessel filter for display purpose only.
Ca2+ imaging.
Isolated VSNs were placed onto Concanavalin A-coated microscope coverslips fitted in a recording chamber. Cells were loaded with a Ca2+-sensitive dye fura 2-AM (Molecular Probes, Eugene, OR) for 30 min at room temperature (see Solutions). The dish was washed with a constant flow of Ringer solution for 10 min before testing to completely wash off the fura 2-AM. Calcium imaging was performed with a Zeiss Axioskop 2FS or a Nikon Eclipse TE200 microscope. The VSNs were identified using a ×40 phase objective and images taken with an Orca100 digital camera. Cells were illuminated every 8 s for 50–200 ms at 340 and 380 nm. The average intensity in the selected regions of interest was captured with either Open Lab 3.5.1 software (Improvision, Lexington, MA) or SimplePCI 6.0 software (Compix, Cranberry Township, PA). The Ca2+-dependent fluorescence signal was expressed as a F340/F380 ratio.
Solutions.
All solutions were adjusted to pH 7.4. Ringer solution consisted of the following (in mM): 138 NaCl, 10 Hepes, 10 glucose, 5 KCl, 2 MgCl2 and 2 CaCl2. Ca2+-free Ringer consisted of the following (in mM): 138 NaCl, 10 Hepes, 10 glucose, 5 KCl, 2 MgCl2, 0.02 CaCl2, and 5 EGTA. For cell dissociation, the divalent cation-free Ringer solution consisted of the following (in mM): 140 NaCl, 10 Hepes, 10 glucose, and 5 KCl. Mouse intracellular solution consisted of the following (in mM): 110 K-gluconate, 30 KCl, 10 NaCl, 10 Hepes, 0.023 CaCl2, 1 MgCl2, and 1 EGTA. High K+ Ringer solution consisted of the following (in mM): 80 NaCl, 65 KCl, 10 Hepes, 10 glucose, and 2 CaCl2. Inside-out patch recording solution consisted of the following (in mM): 110 K-gluconate, 30 KCl, 10 NaCl, 10 Hepes, 0.023 CaCl2, 1 MgCl2, and 1 EGTA. AA was dissolved in DMSO (50 mM stock) and subsequently diluted to 50 μM in Ringer with sonication. The fura 2-AM solution was made to a final concentration of 4 μM with 0.0083% pluronic F127 added. All chemicals and drugs were bought from Sigma-Aldrich (St. Louis, MO) if not specified.
Statistical analyses.
Quantitative data are expressed as means ± SE. Statistical significance was assessed using a paired or an unpaired Student's t-test, and P < 0.05 was considered as significant.
RESULTS
Diluted urine elicited responses in mouse VSNs.
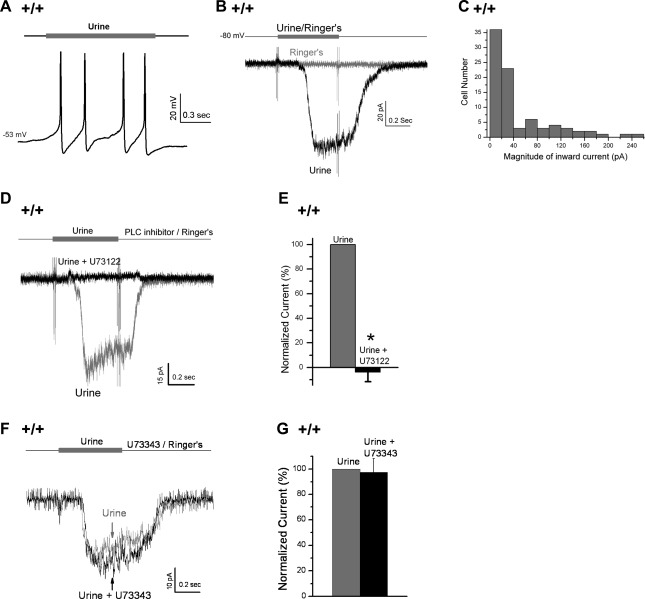
For mice one of the richest sources for pheromones is urine, so to maximize odor responses, we used a mixture of male and female urine to stimulate VSNs (Fig. 1). In all of these experiments, perforated patch clamp was used to record responses. Using current clamp, we found that a 2-s stimulation with dilute urine (1:500) activated repetitive APs in WT VSNs, although the number of APs elicited by urine varied from cell to cell (n = 9, Fig. 1A). To examine the current responses to urine, VSNs were voltage clamped, held at −80 mV, and stimulated with dilute urine (1:500) for 0.5 s (Fig. 1B). In ∼60% of the cells tested (85 of 145), urine elicited inward currents of −47.0 ± 6.0 pA (means ± SE of 85 cells; maximum: −256.0 pA) (Fig. 1, B and C), and the responses were dose dependent (data not shown). To confirm that these urine responses were PLC-mediated odor responses, we tested the effect of a PLC inhibitor U73122 (10 μM). Consistent with previous reports (5, 31, 50, 55), we found that U73122 abolished the urine-induced currents in all WT VSNs tested [n = 6, t(5) = 13.6, P < 0.001, paired Student's t-test; Fig. 1, D and E], and the inhibitory effect was reversible by washing off U73122 (n = 5). As a control, the inactive isomer of U73122, U73343, did not affect the urine-induced responses [n = 7, t(6) = 0.23, P = 0.83, paired Student's t-test; Fig. 1, F and G], similar to experiments previously reported (31). Therefore, this blockade was not due to nonspecific effects of U73122. Conversely, a novel PLC activator, 50 μM m-3M3FBS (Calbiochem, La Jolla) (1) activated an inward current similar to urine-induced currents (data not shown). Thus we used diluted urine to effectively stimulate PLC-mediated odor responses in our preparation of VSNs.
Fig. 1.
Diluted urine elicited odor responses in wild-type (WT, +/+) vomeronasal sensory organs (VSNs). A: in current-clamp mode, a 2-s stimulation of the 1:500-diluted urine depolarized the membrane potential and elicited repetitive APs in a WT neuron (resting membrane potential = −53 mV, n = 9). B: in comparison, in voltage-clamp mode, a 0.5-s application of the 1:500-diluted urine induced an inward current in a cell held at −80 mV (n = 85/145), whereas the application of Ringer (control) had no effect on the same cell. C: magnitude of urine-induced inward currents from the 85 urine-responsive cells. D: phospholipase C (PLC) inhibitor (10 μM U73122) abolished the urine response in a cell. E: U73122 significantly reduced the urine-induced currents (n = 6, P < 0.001, paired Student's t-test). F: U73343 (10 μM, the inactive analog of U73122) failed to abolish the urine response in a cell. G: U73343 did not affect the urine-induced currents (n = 8, P = 0.83, paired Student's t-test). For each cell, the currents were normalized to the peak responses in Ringer. Data are expressed as means ± SE. *P < 0.05.
Urine activated a TRPC2-independent odor transduction pathway.
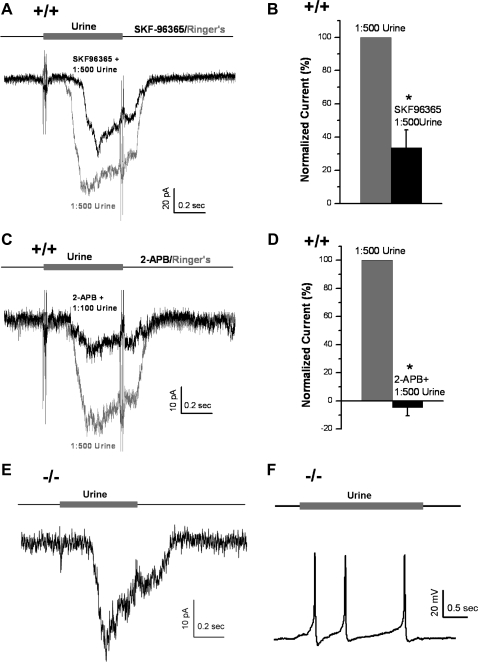
TRPC2 channels are activated by DAG (31) and have been considered essential for odor transduction of VSNs (26, 46). However, odors also appear to activate a TRPC2-independent signal transduction pathway in VSNs. To test for this possibility, we first blocked TRPC2 channels in WT VSNs by using two “putative” TRP channel blockers, SKF-96365 (Fig. 2, A and B) and 2-APB (Fig. 2, C and D). We found that SKF-96365 (100 μM) only decreased but did not eliminate urine responses (1:500) in the majority of WT VSNs [n = 6/8, t(7) = 6.0, P < 0.001, paired Student's t-test; Fig. 2, A and B], supporting the existence of a TRPC2-independent pathway in odor responses. Only in 2 of 8 cells did SKF-96365 abolish odor responses. This inconsistency among cells suggests that there could be heterogeneities between different populations of neurons (see discussion). The other TRP inhibitor, 2-APB (50 μM), had similar but stronger inhibitory effect than SKF-96365 on odor responses (Fig. 2, C and D): 2-APB almost completely blocked responses to 1:500 urine [Fig. 2D, n = 5, t(4) = 18.9, P < 0.001, paired Student's t-test], but it failed to abolish responses to a higher concentration of urine (1:100, Fig. 2C). It should be noted that both drugs block many types of TRP channels and can have nonspecific effects. The SKF96365 effectively blocks a variety of TRP channels at low concentrations, including TRPC1 (15 μM) (51), TRPC3 (10 μM) (30), TRPC6 (IC50 = 4.2 μM), TRPM8 (20 μM, IC50 = 0.8 μM) (32), and TRPP1 (10 μM) (49). The relatively stronger inhibition by 2-APB could be due to its nonspecific effects, such as the inhibition of the store-operated Ca2+ entry and the IP3-induced Ca2+ release (4). Because of the limitations of these drugs, these data do not exclude other possibilities, including partial inhibition of TRPC2 channels by these drugs, nonspecific effects, and inhibition of other Ca2+-permeable channels.
Fig. 2.
Urine activated a transient receptor potential channel (TRPC2)-independent pathway in WT and TRPC2−/− VSNs. A and B: putative TRPC2 channel blocker (100 μM SKF-96365) significantly decreased but did not block the urine (1:500) responses in WT neurons (n = 8, P < 0.001, paired Student's t-test). C and D: second putative TRPC2 channel blocker (50 μM 2-APB) inhibited the urine responses (1:500) in WT neurons (n = 5, P < 0.001, paired Student's t-test). However, 2-APB failed to completely block the odor responses to a more concentrated urine solution (1:100). E: in voltage-clamp mode, a 0.5-s stimulation of urine (1:500) induced an inward current at −80 mV in a TRPC2−/− VSN (−/−, n = 6). F: in current-clamp, a 2-s stimulation of urine (1:500) depolarized the membrane potential and elicited repetitive action potentials in a TRPC2−/− VSN (resting membrane potential = −55 mV, n = 12). *P < 0.05.
To determine whether a TRPC2-independent pathway really existed or was just an artifact of the nonspecific pharmacological blocking agents, we turned to using VSNs from TRPC2−/− mice. Dilute urine (1:500) activated an inward current of −9.7 ± 2.6 pA at −80 mV in voltage-clamped TRPC2−/− VSNs (means ± SE of 11 cells; Fig. 2E) that was smaller than responses in WT VSNs [t(94) = 3.4, P = 0.001, Student's t-test], probably due to the loss of the TRPC2 pathway. The percentage of TRPC2−/− VSNs responded to urine was around 50% (15/32 cells), slightly lower than WT VSNs (∼60%, Fig. 1B). Furthermore, by using current clamp, we found that urine (1:500) also stimulated APs in TRPC2−/− VSNs (n = 12; Fig. 2F), similar to responses in WT VSNs (Fig. 1A). The number of urine-induced APs varied widely from cell to cell so it was not possible to determine whether the number of APs elicited by urine was less in the TRPC2−/− VSNs compared with WT. These results are consistent with previous electrovomeronasogram recordings that showed TRPC2−/− VNO responds to MHC peptides or putative pheromones (21, 26). Thus urine-elicited odor responses persist in TRPC2−/− VSNs, and must function through a TRPC2-independent pathway.
Taken together, the pharmacological blocking data and urine responses in TRPC2−/− mice, provide strong support for the existence of dual pathways, a TRPC2 pathway and a TRPC2-independent pathway in odor transduction of WT VSNs.
AA mediated the TRPC2-independent pathway.
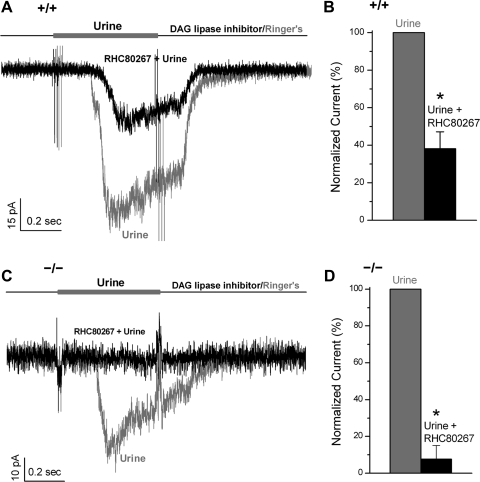
Since DAG can be converted to AA through a DAG lipase, we tested whether the TRPC2-independent pathway was mediated by AA. We blocked the synthesis of AA from DAG with a DAG lipase inhibitor, 50 μM RHC80267, a concentration that is sufficient to completely block DAG lipase (IC50 of RHC80267 for DAG lipase is around 4 μM) (47). We found that RHC80267 decreased urine-induced currents by ∼60% in WT VSNs [n = 7, t(6) = 6.9, P < 0.001, paired Student's t-test; Fig. 3, A and B], as we recently reported (56). By contrast, RHC80267 almost completely abolished urine responses in TRPC2−/− VSNs [n = 9, t(8) = 17.8, P < 0.001, paired Student's t-test; Fig. 3, C and D]. Consistently, we found that RHC80267 blocked the urine-induced APs in current-clamped TRPC2−/− VSNs (n = 7, data not shown). These data agree with a Ca2+-imaging study that shows RHC80267 greatly diminished the urine-induced intracellular Ca2+ increase (44) and strongly support that AA mediates the TRPC2-independent pathway in VSNs.
Fig. 3.
Diacylglycerol (DAG) lipase inhibitor decreased urine responses in WT and TRPC2−/− VSNs, supporting a role for arachidonic acid (AA) in the odor responses. A and B: DAG lipase inhibitor (50 μM RHC80267) significantly decreased odor responses in WT VSNs by ∼60% (n = 7, P < 0.001), suggesting that AA mediates at least part of urine responses in WT VSNs. C and D: with DAG lipase inhibited with RHC80267 more than 90% of urine responses in TRPC2−/− neurons were blocked (n = 6, P < 0.001, paired Student's t-test), suggesting that AA mediates most if not all urine responses in TRPC2−/− VSNs. *P < 0.05.
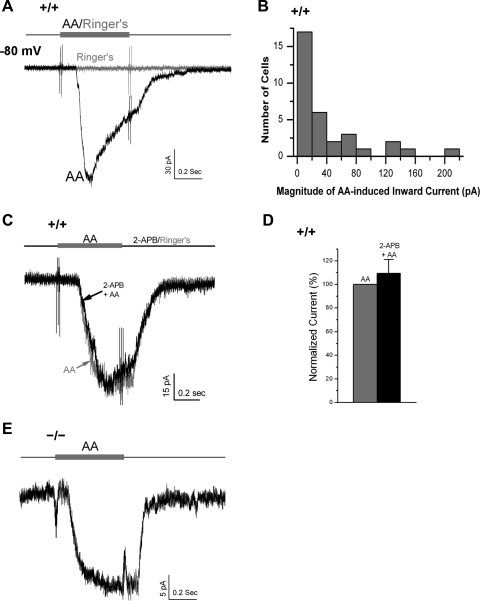
To directly assess the role of AA, we applied 50 μM AA to WT and TRPC2−/− VSNs (Fig. 4). AA elicited inward currents in approximately half of WT VSNs tested (33/68). The average current at −80 mV was −41.0 ± 8.8 pA (means ± SE of 33 cells, maximum = −215.0 pA; Fig. 4, A and B). The TRP channel blocker 2-APB (50 μM) failed to affect the AA-activated currents in WT VSNs [n = 5, t(4) = 0.81, P = 0.47, paired Student's t-test; Fig. 4, C and D], suggesting that AA did not activate TRPC2 channels. Consistently, the AA-activated currents were also found in TRPC2−/− VSNs (n = 5/17 cells, at −80 mV with voltage clamp; Fig. 4E), and AA could activate APs in TRPC2−/− VSNs (current-clamp, data not shown). Therefore, AA mediates the TRPC2-independent odor transduction pathway in VSNs.
Fig. 4.
AA activated a TRPC2-independent current in both WT and TRPC2−/− VSNs. A: 50 μM AA elicited an inward current in about half of the WT VSNs tested (33/68, −80 mV), whereas the application of Ringer had no effect. B: AA-induced currents from the 33 responsive WT VSNs. C and D: AA-induced inward current in WT VSNs was not blocked by the putative TRPC2 channel blocker 50 μM 2-APB (n = 5, P = 0.47, paired Student's t-test). E: AA activated a similar inward current in TRPC2−/− VSNs (n = 5), suggesting that AA activated a TRPC2-independent channel.
AA activated an inward Ca2+ or Ca2+-dependent current.
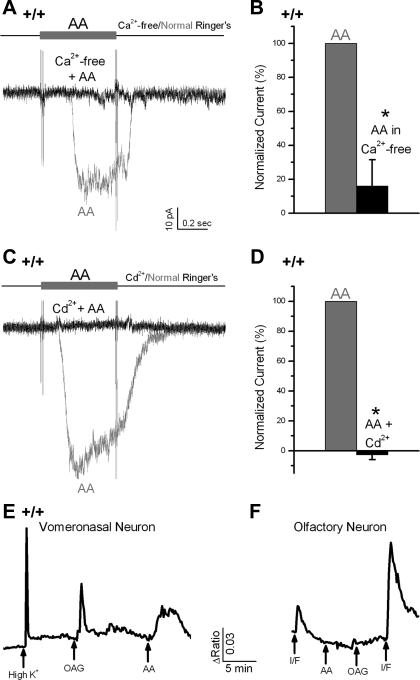
The AA-induced inward currents appeared to be at least partially carried by Ca2+ or were Ca2+ dependent, since they were abolished in a Ca2+-free Ringer that contained 5 mM EGTA [n = 5, t(4) = 5.4, P = 0.006, paired Student's t-test; Fig. 5, A and B], or in 1 mM Cd2+, a blocker for several Ca2+ channels and some Ca2+-activated nonselective cation (CaNS) channels (7, 11, 36) [n = 8, t(7) = 30.6, P < 0.001, paired Student's t-test; Fig. 5, C and D].
Fig. 5.
AA-induced inward current was a Ca2+ or Ca2+-dependent current. A and B: AA-induced current was abolished in a Ca2+-free Ringer (n = 5, P < 0.001, paired Student's t-test). C and D: responses to AA were blocked by the addition of 1 mM Cd2+ to bath (n = 9, P < 0.001, paired Student's t-test). E and F: Ca2+ imaging, with fura-2 as the Ca2+ indicator, was performed to measure the intracellular Ca2+ of the vomeronasal and olfactory neurons. In a WT VSN (5E), the intracellular Ca2+ ([Ca2+]i) was increased by depolarization (with a high K+ Ringer for 3 s), 20 μM 1-oleoyl-2-acetyl-sn-glycerol (OAG, 30 s, n = 34/60), or 50 μM AA (30 s, n = 6/15). In contrast, in an OSN (5F), application of cAMP pathway activators (I/F: 100 μM IBMX and 30 μM Forskolin, 8 s), but not AA or OAG (30 s) induced the increases of [Ca2+]i. *P < 0.05.
These data support the hypothesis that AA increased intracellular Ca2+ concentration ([Ca2+]i) by an influx of bath Ca2+. To support this hypothesis, Ca2+ imaging was performed. Similar to a previous report in rats (44), we found that AA induced transient increases of [Ca2+]i in WT mouse VSNs (Fig. 5E, 6/15). In addition, a DAG analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) (34/60) or depolarization with K+ induced increased [Ca2+]i as expected (Fig. 5E). To test for nonspecific effects of the drugs, olfactory sensory neurons (OSNs) were used as a control. The application of 100 μM 3-isobutyl-1-methylxanthine (IBMX, which inhibits phosphodiesterase) with 30 μM 7-deacetyl-7-[O-(N-methylpiperazino)-γ-butyryl]-forskolin dihydrochloride (Forskolin, which activates adenylyl cyclase) for 8 s to increase intracellular cAMP induced a Ca2+ transient as expected in OSNs. In contrast, both AA and OAG failed to affect the [Ca2+]i in OSNs (Fig. 5F, n = 3). Thus such AA-sensitive Ca2+-permeable channels are probably specific for VSNs.
AA application elicited channel activity.
To determine what channels were activated by AA, we recorded from excised inside-out patches, from both the dendritic knob and soma of WT (Fig. 6) and TRPC2−/− VSNs (Fig. 7), with a symmetrical solution (containing from <0.01 to 50 μM free Ca2+, see materials and methods).
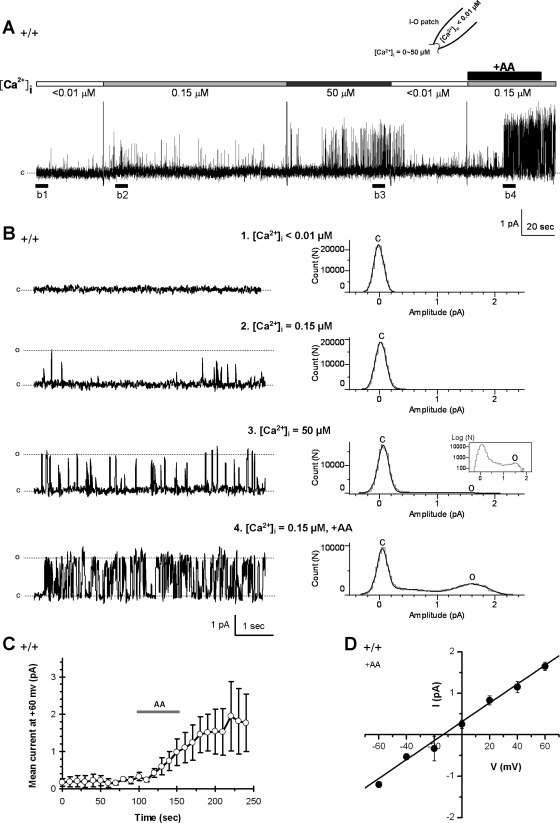
Fig. 6.
AA activated a Ca2+-dependent channel in WT VSNs. A: in an excised inside-out patch from a WT VSN, no spontaneous channel activity was observed with <0.01 μM [Ca2+]i (holding potential = +60 mV). The increase of [Ca2+]i to 0.15 or 50 μM increased the channel activities without the presence of AA. The same patch was then exposed to 50 μM AA (intracellular side) for 1 min, which greatly potentiated the channel activities (n = 20/36). The dashed line indicates the close state (c). B: left, panels 1–4, expanded areas of single-channel recordings on excised patch in underlined region of A; right, all-point amplitude histograms are constructed from the corresponding traces in the left and show the regulatory effect of both Ca2+ and AA on the channel opening. C: close state; O: the opening state. C: excised patches were exposed to AA for 1 min and mean currents at +60 mV were calculated in 10-s windows. The mean currents vs. time plot (n = 4) shows a relative rapid stimulatory effect of AA on the channel opening, whereas the stimulatory effect remained after AA was washed off. D: AA-activated channels had a single channel conductance of 23.1 ± 1.6 pS (means ± SE of 4 patches).
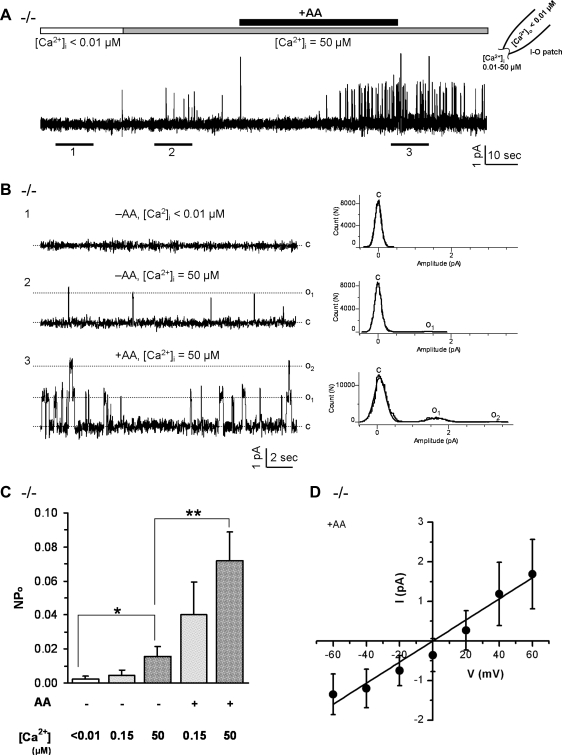
Fig. 7.
AA activated a Ca2+-dependent channel in TRPC2−/− VSNs. A: in a representative inside-out patch obtained from a TRPC2−/− VSN, increases of bath (intracellular) Ca2+ concentration from <0.01 to 50 μM slightly increased the single-channel activity of a channel, whereas the exposure to 50 μM AA for ∼1 min further potentiated the opening of the channel (+60 mV, n = 17). B: left, panels 1–3, the expanded tracings from underlined regions of A show the stimulatory effect of both Ca2+ and AA on channel activities. (right, panels 1–3), the corresponding all-point amplitude histograms show the effect of Ca2+ and AA on single channel currents. C: close state; O1: the opening state of one channel; O2: the opening state of two channels. C: averaged opening probabilities (NPo) under different concentration of Ca2+ and AA, show that both Ca2+ and AA stimulated the activity of the channel. Data are averaged from 11 (−AA, <0.01 μM Ca2+), 6 (−AA, <0.15 μM Ca2+), 11 (−AA, 50 μM Ca2+), 6 (+AA, 0.15 μM Ca2+) and 13 (+AA, 50 μM Ca2+) instances from 13 inside-out patches, respectively. Data are expressed as means ± SE. *P < 0.05, **P < 0.01 with Student's t-test. D: averaged current voltage (I-V) relationship of the single channel currents of the AA-activated channels, indicates a single-channel conductance of 27.1 ± 3.8 pS (means ± SE, n = 9).
In excised patches from WT VSNs, single channel activity was examined at different concentrations of Ca2+ and AA (Fig. 6, A and B). In the absence of AA, no channel openings were observed in <0.01 μM [Ca2+]i (the free Ca2+ concentration in bath, i.e., the intracellular side of the excised patch), whereas some channel activity was apparent at 50 μM [Ca2+]i (n = 8, Fig. 6, A and B). The addition of 50 μM AA for ∼1 min to the intracellular side of the membrane (bath) greatly increased channel activities ([Ca2+]i = 0.15 μM, n = 20/36, Fig. 6, A and B). These data suggest that while increased [Ca2+]i activated channels, addition of AA could also activate channels. However, the AA-activated channels required some free intracellular Ca2+ to open. The mean current versus time plot (Fig. 6C) showed that AA stimulatory effect persisted even after AA was washed off (data are means ± SE of 4 excised patches), suggesting that AA may need intercalate into the membrane to regulate channels or require enzymatic degradation for termination. The Ca2+, AA-activated channels had a single channel conductance of 23.1 ± 1.6 pS (means ± SE of 4 excised patches; Fig. 6D), close to that of CaNS channels found in hamster VSNs (27).
To exclude the possibility that AA activated TRPC2 channels, we performed excised patch-clamp recordings with TRPC2−/− VSNs (Fig. 7) using the methods described above. As with WT VSNs, TRPC2−/− VSNs contained a Ca2+-sensitive channel that was potentiated by AA (Fig. 7, A and B, n = 17). The stimulatory effect of AA was sustained even after AA was washed off (Fig. 7A), similar to our observation in WT VSNs (Fig. 6A). Analysis of open probability (NPo) confirmed that these channels were slightly Ca2+ sensitive even without AA stimulation [Fig. 7C, panels 1 vs. 3, t(20) = 2.3, P = 0.03]; AA potentiated the channel opening at low [Ca2+]i [0.15 μM Ca2+, panels 2 vs. 4, t(10) = 2.0, P = 0.07] and greatly potentiated these channel openings at high [Ca2+]i [50 μM Ca2+, panels 3 vs. 5, t(22) = 3.0, P = 0.006]. The AA-activated channels from TRPC2−/− mice had an average conductance of 27.1 ± 3.8 pS (means ± SE of 9 excised patches, Fig. 7D), close to the channel conductance found in WT VSNs [Fig. 6D, t(11) = 0.71, P = 0.49], implying that AA-activated channels are of same type in WT and TRPC2−/− VSNs. One difference was that the reversal potential of AA-activated channels shifted to positive potentials in TRPC2−/− neurons (Figs. 6D and 7D). Together, these data suggest the presence of a channel activated by AA and slightly affected by Ca2+ in VSNs, which is clearly not the TRPC2 channel.
DISCUSSION
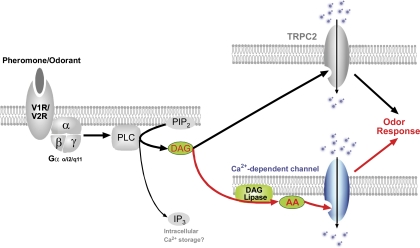
Using perforated patch clamp on WT and TRPC2−/− VSNs, we demonstrated that urine responses persist in TRPC2−/− VSNs and are mediated through PLC. Based on the available data, we propose that odor responses are transduced through two parallel pathways in a subset of VSNs (Fig. 8): the binding of odor to its receptor results in the activation of PLC and the production of DAG and AA. DAG activates a cation influx through TRPC2 channels, whereas AA activates a Ca2+-permeable channel that might have a slight calcium dependence. The activation of the two pathways may help to amplify the signal and thus ensure an ultra high sensitivity for select chemical signals such as pheromones (25). In addition, this redundancy may have served as a survival strategy during evolution since a defect in one pathway would not result in a total loss of pheromone detection. Or it could be that odors stimulating these dual pathways in VSNs have a biological importance to the function of the animal so that these responses would be “enhanced” by activating a second signaling pathway.
Fig. 8.
Proposed signal transduction of odor responses in VSNs. Odor (urine) stimulation activates the PLC pathway and elevates DAG, which is converted to AA by a DAG lipase. DAG activates TRPC2, whereas AA activates a Ca2+-dependent channels. Both pathways play the excitatory role in the odor responses.
In 2002, two laboratories independently developed TRPC2−/− mice (26, 46). Both groups deleted parts of the protein including the channel region although not the same regions of the protein. They both reported loss of aggression in male TRPC2−/− mice. However, the cellular responses of VSNs to urine differed in the two strains: using electrode array recordings, Dulac's lab (46) reported that the VSNs from TRPC2−/− mice did not respond to urine; whereas the VSNs from TRPC2−/− mice generated in Axel's lab still responded to urine but the response was decreased using the electrovomeronasogram method (26). Our results on VSNs from TRPC2−/− mice (from Dulac's lab) clearly demonstrate that many of these cells can still respond to dilute urine, although the overall responses tend to be smaller than those from WT mice, thus confirming the observation from Axel's lab (26) and demonstrating TRPC2 knockout does not result in complete loss of cellular response to stimuli. Our differing results from Dulac's lab (46) can probably be explained by the different methodology used, as the electrode array recordings might be not sensitive enough to detect the smaller responses in TRPC2−/− mice. Many studies using TRPC2−/− mice as a genetic tool have assumed that odor-induced responses are completely disrupted in TRPC2−/− mice (6, 22), which is not true according to our data and that from others (21, 26).
Our results for the involvement of AA in odor transduction are supported by those from Spehr et al. (44) who used calcium imaging and found that the DAG lipase inhibitor, RHC80267, greatly decreased the responses to urine. However, conflicting results were reported by Lucas et al. (31). They did not see any effect of RHC80267 on urine-induced inward currents, whereas we saw a partial (or complete) block in all of our VSNs with the same inhibitor. This discrepancy between our results and theirs could be due to the difference in recording techniques. They used whole cell patch clamp to record currents to 1:100 diluted urine. We used the perforated patch-clamp configuration to record 1:500 urine-induced responses. Under our recording conditions, diffusible components of the signaling pathway should remain intact and allow for a more vigorous response. Indeed this could explain why we can obtain responses from more dilute urine, as none of the diffusible second messengers were diluted with the intracellular solution. However, another reason could be that not all VSNs have both pathways (see below).
The two pathways may be differently expressed in different populations of VSNs. Two populations of VSNs exist: apical and basal neurons. They express different types of pheromone receptors and G proteins: apical neurons express V1R/ Gαi2, whereas basal neurons express V2R/Gαo (8, 13, 33, 39). The two populations of VSNs may differ in odor transduction cascades. For example, 2-heptanone is a putative pheromone that exclusively activates the apical VSNs. Odor responses to 2-heptanone were decreased but not eliminated in TRPC2−/− mice compared with WT mice (21, 26), suggesting that these apical neurons use both the TRPC2 pathway and the TRPC2-independent pathway to mediate odor responses. By contrast, major histocompatibility complex (MHC) class I peptides only activate basal VSNs. Odor responses to these peptides were not affected by TRPC2 ablation; we would suggest that these basal neurons (or only these MHC-I peptide-responding basal neurons) predominately use the AA-activated odor transduction pathway. In addition, we found that ∼15% of VSNs tested (9/56) responded to urine but not to AA (data not shown). Since AA failed to elicit an inward current in these cells, the AA-mediated pathway must be missing, although these cells still responded to urine. Thus the urine response would have to be mediated by the TPRC2 pathway. This suggests that the AA-mediated pathway may be only present in a subset of neurons.
The hypothesis that VSNs would have functionally more than one signal transduction pathway is not unique to this tissue since many tissues respond to more than one chemical stimuli with a variety of effects. Because the VNO plays a large role in many behavioral responses that are modulated by hormones, it seems possible for the VNO to be a target of hormones. Interestingly, many hormones are mediated by the PLC pathway so it would seem possible that these hormones could work via a PLC pathway in the vomeronasal system and that the downstream transduction elements (channels) might show some overlap between the chemical-sensing pathway and the hormonal-modulatory pathway. For example, in other types of neurons, progesterone potentiates calcium release through IP3 receptors in hippocampal neurons (16). Activation of corticotropin-releasing factor 2 inhibits Purkinje neuron P-type calcium currents via a PKC pathway (48). Cholecystokinin increases intracellular calcium in vagal afferent terminals using part of PLC pathway (38). Oxytocin (24) and vasopressin (40), two hormones recently found to play of role in pair bonding (12) can increase intracellular Ca2+ via PLC. Although oxytocin has been shown to affect the firing pattern in the accessory olfactory bulb, where the VSNs synapse (10), none of these hormones have been investigated in the vomeronasal sensory epithelium. Certainly one hormone, gonaodotropin-releasing hormone (GnRH), has been shown to alter voltage-activated currents in olfactory neurons (9, 57) and appears to target the VNO in voles (53). GnRH neurons share a common origin with olfactory and vomeronasal neurons during the development, both arising from the nasal placode (41, 54). GnRH activates a G protein-couple 7-transmembrane-domain receptor GnRHr (19, 42, 58), belonging to the same category as the odorant receptors and pheromone receptors. GnRHr couples to Gαi (17, 29) and Gαq/11 (15, 37, 45), which activate phospholipase Cβ (PLCβ) (15), the same signal transduction machinery that is also coupled to pheromone receptors in VSNs (3), so modulation of VSNs by GnRH seems likely.
Contradicting to a previous report (14), our voltage-clamp recordings showed that VSNs quickly desensitize when exposed to urine: a half second application of urine could show adaptation, as urine-induced currents decreased before the urine was washed off (for example, see Figs. 1D, and 2, A, C, and E). It was recently suggested that stimulus-induced Ca2+ entry, together with calmodulin, activates a negative feedback pathway to cause adaptation of VSNs (43). In addition, the large-conductance calcium-activated potassium (BK) channels may also play a role in adaptation. We previously showed that BK channels are expressed in vomeronasal neurons and are activated by the Ca2+ entry and AA (56). Activation of BK channels in turn could decrease firing frequency of VSNs and could cause adaptation (56).
In hamster VSNs, Liman (27) reported the presence of Ca2+-activated cation channels (CaNS) that are permeable to monovalent cations (Na+, K+) and divalent cations (Ca2+, low permeability). The presence of CaNS channels in VSNs was recently confirmed in mice (43). The genetic identity of CaNS channels remains unknown, although it has been speculated CaNS channels belong to TRP channel family (27). The uncertainty of their genetic identity and channel properties makes it difficult to study this pathway definitively at present. Whereas the AA-activated channels needed some level of Ca2+ to be present, they did not appear to allow Na+ ions to pass (see Fig. 5A), but we did not investigate the ion permeability of this channel systematically. We did observe a shift in the reversal potential of AA-activated channels to more positive potentials in TRPC2−/− VSNS (Figs. 6D and 7D). A possible explanation could be the multimerization of TRPC2 with the AA-activated channels, as more and more TRP channels are found to form heteromultimers with other TRP channels or non-TRP channels. It is possible that TRPC2 forms multimers with the AA-sensitive channels, and by doing so, TRPC2 regulates the permeaselectivity of the AA-sensitive channels. In the future, it would be essential to further characterize the AA-activated channels, including their ion selectivity, voltage dependence, and blockage by nucleotides (27), etc.
In summary, we have found that the odor-activated PLC pathway leads to the opening of two distinct channels through two parallel pathways in mouse VSNs: one is the DAG-activated TRPC2 channel pathway; the other one involves AA-activated Ca2+-permeable channels. The redundancy of the two pathways in odor responses may have evolved as a survival strategy, or it may play a role in signal amplification.
GRANTS
This work was supported by NIH-DC-006939, NIH-P20RR-16435, and NSF-EPS-0236976 grants.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Vincent E. Dionne for the critical reading of the manuscript.
Current address of P. Zhang: Dept. of Biology, Brandeis University, Waltham, MA 02454.
REFERENCES
- 1.Bae YS, Lee TG, Park JC, Hur JH, Kim Y, Heo K, Kwak JY, Suh PG, Ryu SH. Identification of a compound that directly stimulates phospholipase C activity. Mol Pharmacol 63: 1043–1050, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci 29: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bigiani A, Mucignat-Caretta C, Montani G, Tirindelli R. Pheromone reception in mammals. Rev Physiol Biochem Pharmacol 154: 1–35, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Brann JH, Fadool DA. Vomeronasal sensory neurons from Sternotherus odoratus (stinkpot/musk turtle) respond to chemosignals via the phospholipase C system. J Exp Biol 209: 1914–1927, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature 450: 899–902, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Chang W, Chen TH, Gardner P, Shoback D. Regulation of Ca2+-conducting currents in parathyroid cells by extracellular Ca2+ and channel blockers. Am J Physiol Endocrinol Metab 269: E864–E877, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell 83: 195–206, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Eisthen HL, Delay RJ, Wirsig-Wiechmann CR, Dionne VE. Neuromodulatory effects of gonadotropin releasing hormone on olfactory receptor neurons. J Neurosci 20: 3947–3955, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang LY, Quan RD, Kaba H. Oxytocin facilitates the induction of long-term potentiation in the accessory olfactory bulb. Neurosci Lett 438: 133–137, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Haj-Dahmane S, Andrade R. Calcium-activated cation nonselective current contributes to the fast afterdepolarization in rat prefrontal cortex neurons. J Neurophysiol 78: 1983–1989, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci 361: 2187–2198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell 90: 763–773, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science 289: 1569–1572, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hsieh KP, Martin TF. Thyrotropin-releasing hormone and gonadotropin-releasing hormone receptors activate phospholipase C by coupling to the guanosine triphosphate-binding proteins Gq and G11. Mol Endocrinol 6: 1673–1681, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Hwang JY, Duncan RS, Madry C, Singh M, Koulen P. Progesterone potentiates calcium release through IP3 receptors by an Akt-mediated mechanism in hippocampal neurons. Cell Calcium 45: 233–242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai A, Takagi H, Horibe S, Fuseya T, Tamaya T. Coupling of gonadotropin-releasing hormone receptor to Gi protein in human reproductive tract tumors. J Clin Endocrinol Metab 81: 3249–3253, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Inamura K, Kashiwayanagi M, Kurihara K. Blockage of urinary responses by inhibitors for IP3-mediated pathway in rat vomeronasal sensory neurons. Neurosci Lett 233: 129–132, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun 189: 289–295, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Karlson P, Luscher M. Pheromones': a new term for a class of biologically active substances. Nature 183: 55–56, 1959 [DOI] [PubMed] [Google Scholar]
- 21.Kelliher KR, Spehr M, Li XH, Zufall F, Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur J Neurosci 23: 3385–3390, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448: 1009–1014, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Krieger J, Schmitt A, Lobel D, Gudermann T, Schultz G, Breer H, Boekhoff I. Selective activation of G protein subtypes in the vomeronasal organ upon stimulation with urine-derived compounds. J Biol Chem 274: 4655–4662, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Chen TH, Hsu WH. Oxytocin-stimulated insulin release in a clonal beta-cell line RINm5F: involvement of phospholipase C-dependent and -independent pathways. Zhongguo Yao Li Xue Bao 17: 390–394, 1996 [PubMed] [Google Scholar]
- 25.Leinders-Zufall T, Lane AP, Puche AC, Ma W, Novotny MV, Shipley MT, Zufall F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405: 792–796, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA 99: 6376–6381, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liman ER. Regulation by voltage and adenine nucleotides of a Ca2+-activated cation channel from hamster vomeronasal sensory neurons. J Physiol 548: 777–787, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA 96: 5791–5796, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limonta P, Moretti RM, Marelli MM, Dondi D, Parenti M, Motta M. The luteinizing hormone-releasing hormone receptor in human prostate cancer cells: messenger ribonucleic acid expression, molecular size, and signal transduction pathway. Endocrinology 140: 5250–5256, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Liu D, Scholze A, Zhu Z, Kreutz R, Wehland-von-Trebra M, Zidek W, Tepel M. Increased transient receptor potential channel TRPC3 expression in spontaneously hypertensive rats. Am J Hypertens 18: 1503–1507, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron 40: 551–561, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Malkia A, Madrid R, Meseguer V, de la Pena E, Valero M, Belmonte C, Viana F. Bidirectional shifts of TRPM8 channel gating by temperature and chemical agents modulate the cold sensitivity of mammalian thermoreceptors. J Physiol 581: 155–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell 90: 775–784, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Menco BP, Carr VM, Ezeh PI, Liman ER, Yankova MP. Ultrastructural localization of G-proteins and the channel protein TRP2 to microvilli of rat vomeronasal receptor cells. J Comp Neurol 438: 468–489, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochem Biophys Acta 274: 313–322, 1972 [DOI] [PubMed] [Google Scholar]
- 36.Nakajima T, Hazama H, Hamada E, Wu SN, Igarashi K, Yamashita T, Seyama Y, Omata M, Kurachi Y. Endothelin-1 and vasopressin activate Ca2+-permeable non-selective cation channels in aortic smooth muscle cells: mechanism of receptor-mediated Ca2+ influx. J Mol Cell Cardiol 28: 707–722, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Naor Z, Harris D, Shacham S. Mechanism of GnRH receptor signaling: combinatorial cross-talk of Ca2+ and protein kinase C. Front Neuroendocrinol 19: 1–19, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Rogers RC, Hermann GE. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides 29: 1716–1725, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron 19: 371–379, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: involvement of multiple intracellular transduction signals. J Neuroendocrinol 16: 221–236, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature 338: 161–164, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev 18: 180–205, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Spehr J, Hagendorf S, Weiss J, Spehr M, Leinders-Zufall T, Zufall F. Ca2+-calmodulin feedback mediates sensory adaptation and inhibits pheromone-sensitive ion channels in the vomeronasal organ. J Neurosci 29: 2125–2135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spehr M, Hatt H, Wetzel CH. Arachidonic acid plays a role in rat vomeronasal signal transduction. J Neurosci 22: 8429–8437, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojilkovic SS, Catt KJ. Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res 50: 161–205, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295: 1493–1500, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Sutherland CA, Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem 257: 14006–14010, 1982 [PubMed] [Google Scholar]
- 48.Tao J, Zhang Y, Huang H, Jiang X. Activation of corticotropin-releasing factor 2 receptor inhibits Purkinje neuron P-type calcium currents via G(o)alpha-dependent PKC epsilon pathway. Cell Signal 21: 1436–1443, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Vandorpe DH, Chernova MN, Jiang L, Sellin LK, Wilhelm S, Stuart-Tilley AK, Walz G, Alper SL. The cytoplasmic C-terminal fragment of polycystin-1 regulates a Ca2+-permeable cation channel. J Biol Chem 276: 4093–4101, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Chen P, Quan W, Halpern M. Suprasternal gland secretion of male short-tailed opossum induces IP3 generation in the vomeronasal organ. Biochim Biophys Acta 1770: 725–732, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434: 898–904, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wekesa KS, Anholt RR. Pheromone regulated production of inositol-(1,4,5)-trisphosphate in the mammalian vomeronasal organ. Endocrinology 138: 3497–3504, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Wirsig-Wiechmann CR, Wiechmann AF. The prairie vole vomeronasal organ is a target for gonadotropin-releasing hormone. Chem Senses 26: 1193–1202, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86: 8132–8136, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang JJ, Huang GZ, Halpern M. Firing properties of accessory olfactory bulb mitral/tufted cells in response to urine delivered to the vomeronasal organ of gray short-tailed opossums. Chem Senses 32: 355–360, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Zhang P, Yang C, Delay RJ. Urine stimulation activates BK channels in mouse vomeronasal neurons. J Neurophysiol 100: 1824–1834, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W, Delay RJ. Gonadotropin-releasing hormone modulates voltage-activated sodium current and odor responses in Necturus maculosus olfactory sensory neurons. J Neurosci Res 85: 1656–1667, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, Millar RP, Sealfon SC. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol 45: 165–170, 1994 [PubMed] [Google Scholar]