Abstract
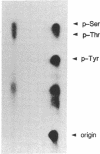
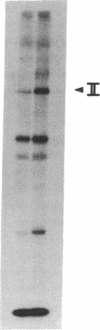
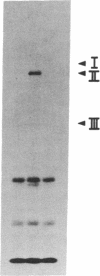
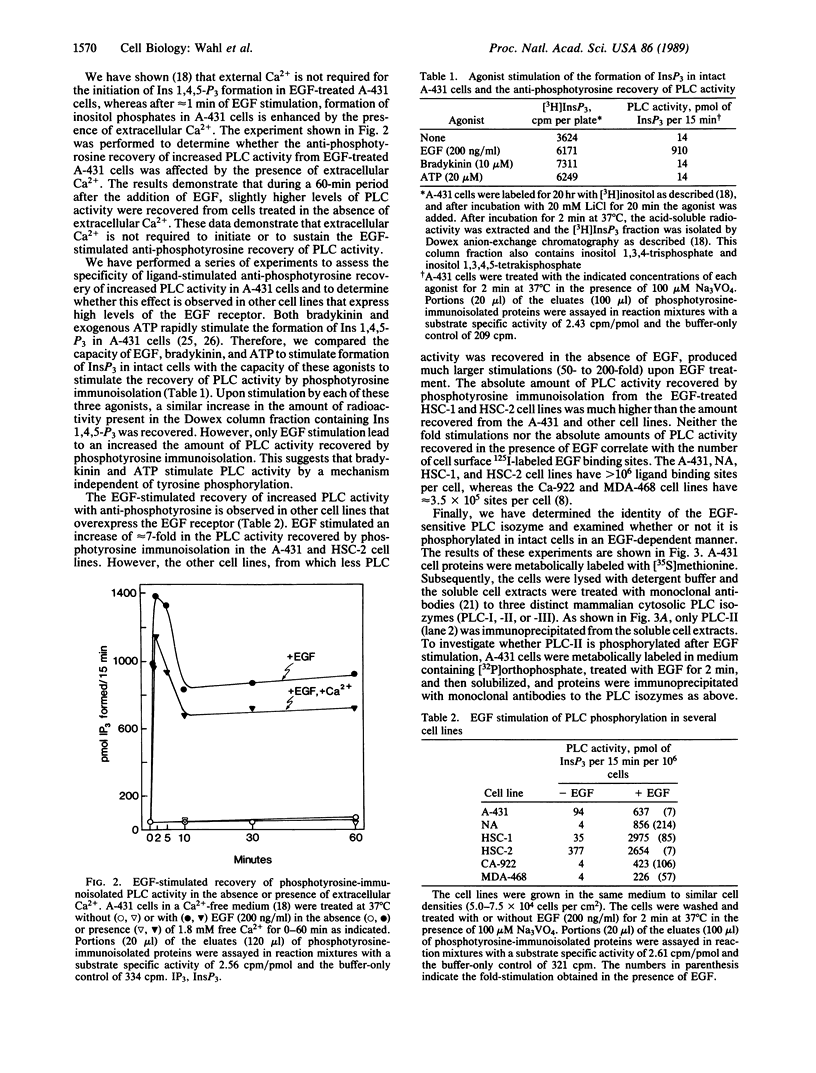
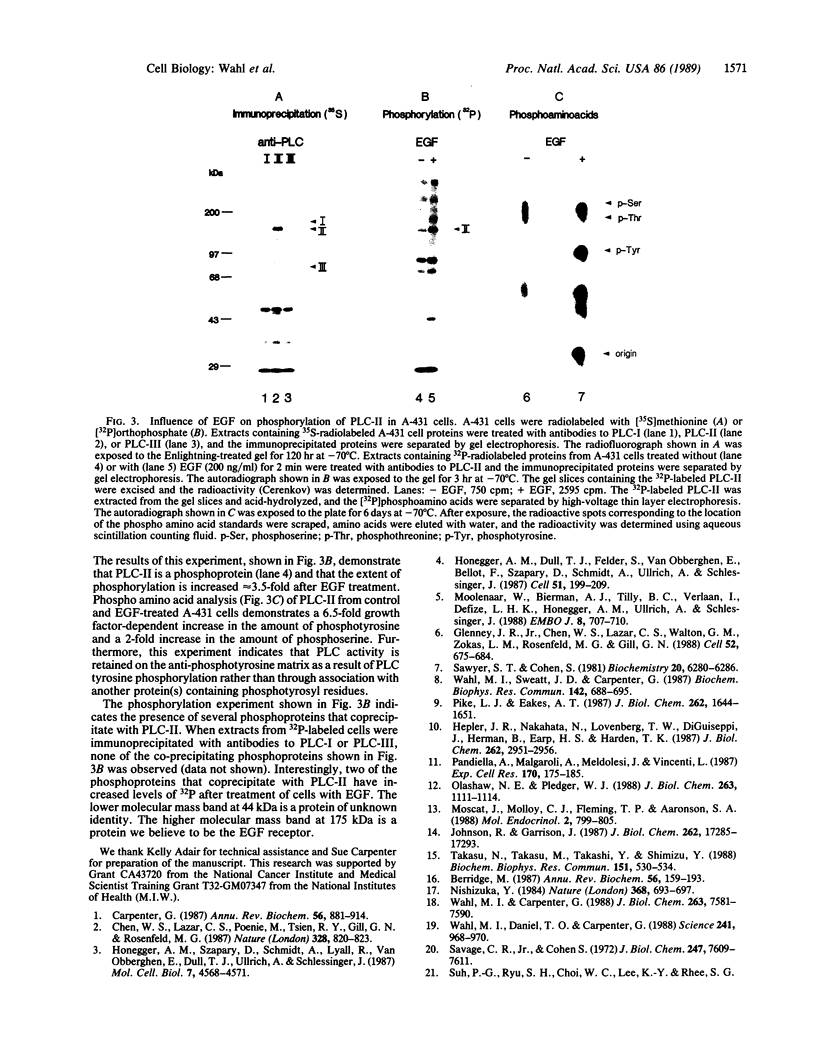
Epidermal growth factor (EGF) rapidly stimulates the formation of inositol 1,4,5-trisphosphate in a variety of cell types. Previously we have found that in intact cells stimulation of phospholipase C (PLC) activity by EGF is correlated with the retention of increased amounts of PLC activity by a phosphotyrosine immunoaffinity matrix, suggesting that the EGF-receptor tyrosine kinase phosphorylates PLC. We now define parameters of the mechanism by which EGF addition to A-431 cells stimulates phosphotyrosine immunoisolation of PLC activity and demonstrate that EGF addition to A-431 cells increases tyrosine phosphorylation of PLC. EGF rapidly and reversibly stimulated the anti-phosphotyrosine recovery of increased PLC activity when cells were treated with growth factor at 3 degrees C, indicating that receptor internalization is not required and that the phosphorylation event occurs prior to formation of inositol 1,4,5-trisphosphate. Also, the EGF stimulation of anti-phosphotyrosine recovery of PLC activity occurred in the absence of extracellular Ca2+. Stimulation of PLC activity in intact cells by other agonists, such as bradykinin or ATP, did not result in increased anti-phosphotyrosine recovery of PLC activity, suggesting two separate mechanisms exist in A-431 cells for hormone-stimulated formation of inositol phosphates. Finally, using monoclonal antibodies that specifically recognize three distinct PLC isozymes, we show that an approximately 145-kDa PLC isozyme (PLC-II) is present in A-431 cells and that EGF treatment of A-431 cells stimulates phosphorylation of PLC-II on both tyrosine and serine residues.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M. Internalization and degradation of receptor-bound human choriogonadotropin in Leydig tumor cells. Fate of the hormone subunits. J Biol Chem. 1982 Nov 25;257(22):13306–13311. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Chen W. S., Lazar C. S., Walton G. M., Zokas L. M., Rosenfeld M. G., Gill G. N. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell. 1988 Mar 11;52(5):675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Garrison J. C. Epidermal growth factor and angiotensin II stimulate formation of inositol 1,4,5- and inositol 1,3,4-trisphosphate in hepatocytes. Differential inhibition by pertussis toxin and phorbol 12-myristate 13-acetate. J Biol Chem. 1987 Dec 25;262(36):17285–17293. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Bierman A. J., Tilly B. C., Verlaan I., Defize L. H., Honegger A. M., Ullrich A., Schlessinger J. A point mutation at the ATP-binding site of the EGF-receptor abolishes signal transduction. EMBO J. 1988 Mar;7(3):707–710. doi: 10.1002/j.1460-2075.1988.tb02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Molloy C. J., Fleming T. P., Aaronson S. A. Epidermal growth factor activates phosphoinositide turnover and protein kinase C in BALB/MK keratinocytes. Mol Endocrinol. 1988 Sep;2(9):799–805. doi: 10.1210/mend-2-9-799. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Olashaw N. E., Pledger W. J. Epidermal growth factor stimulates formation of inositol phosphates in BALB/c/3T3 cells pretreated with cholera toxin and isobutylmethylxanthine. J Biol Chem. 1988 Jan 25;263(3):1111–1114. [PubMed] [Google Scholar]
- Pandiella A., Malgaroli A., Meldolesi J., Vicentini L. M. EGF raises cytosolic Ca2+ in A431 and Swiss 3T3 cells by a dual mechanism. Redistribution from intracellular stores and stimulated influx. Exp Cell Res. 1987 May;170(1):175–185. doi: 10.1016/0014-4827(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Takasu N., Takasu M., Yamada T., Shimizu Y. Epidermal growth factor (EGF) produces inositol phosphates and increases cytoplasmic free calcium in cultured porcine thyroid cells. Biochem Biophys Res Commun. 1988 Feb 29;151(1):530–534. doi: 10.1016/0006-291x(88)90626-2. [DOI] [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., Wirtz K. W., de Laat S. W., Moolenaar W. H. Inositol phosphate metabolism in bradykinin-stimulated human A431 carcinoma cells. Relationship to calcium signalling. Biochem J. 1987 May 15;244(1):129–135. doi: 10.1042/bj2440129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Sweatt J. D., Carpenter G. Epidermal growth factor (EGF) stimulates inositol trisphosphate formation in cells which overexpress the EGF receptor. Biochem Biophys Res Commun. 1987 Feb 13;142(3):688–695. doi: 10.1016/0006-291x(87)91469-0. [DOI] [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]