Abstract
The bone morphogenetic protein (BMP) pathway is known to be involved in limb myogenesis during development, but whether it is involved in postnatal muscle regeneration is unclear. We have found that adult inhibitor of differentiation (Id)-mutant (Id1+/−Id3−/−) mice display delayed and reduced skeletal muscle regeneration after injury compared with either wild-type littermates or Id3-null mice. Immunoblotting of wild-type muscle lysates revealed that, not only were Id1 and Id3 highly upregulated within 24 h after injury, but other upstream components of the BMP pathway were as well, including the BMP receptor type II and phosphorylated Smad1/5/8 (pSmad1/5/8). Inhibition of BMP signaling in injured skeletal muscle by Noggin injection reduced pSmad1/5/8, Id1, and Id3 protein levels. The mouse myoblast-derived cell line C2C12 also expressed Id1, Id3, BMP receptor type II, and pSmad1/5/8 during proliferation, but all were reduced upon differentiation into myotubes. In addition, these cells secreted mature BMP-4, and BMP signaling could be inhibited with exogenous Noggin, causing a reduction in pSmad1/5/8, Id1, and Id3 levels. Confocal immunofluorescence microscopy revealed that activated Pax7+ myoblasts coexpressed nuclear pSmad1/5/8, Id1, and Id3 in injured mouse skeletal muscle sections. Although we did not observe differences in the numbers of quiescent Pax7+ satellite cells in adult uninjured hindlimb muscles, we did observe a significant reduction in the number of proliferating Pax7+ cells in the Id-mutant mice after muscle injury compared with either wild-type or Id3-null mice. These data suggest a model in which BMP signaling regulates Id1 and Id3 in muscle satellite cells, which directs their proper proliferation before terminal myogenic differentiation after skeletal muscle injury in postnatal animals.
Keywords: bone morphogenetic protein signaling, inhibitor of differentiation-1, inhibitor of differentiation-3, Pax7, satellite cells
postnatal muscle regeneration requires the coordinated growth and proliferation of many cell populations. These include resident endothelial cells that restore capillary bed function during sprouting angiogenesis (5), as well as other stem cell populations from the bone marrow compartment (57). Hematopoietic and CD45−/VEGFR2+ cells (known as endothelial progenitor cells or circulating angiogenic cells) have been reported to contribute to angiogenesis through the paracrine secretion of proangiogenic factors, as well as incorporate into new vessels, although luminal incorporation appears to be a relatively rare event (48, 55). In addition, other bone marrow-derived inflammatory cells, such as monocytes and macrophages, appear to be necessary for removal of necrotic debris and may exert other functions that allow for proper and efficient muscle regeneration (8, 37, 46, 51, 54, 57). Resident muscle stem cells or satellite cells must also coordinate their activities with the above cell types as they undergo the processes of activation, proliferation, self-renewal, and terminal myogenic differentiation (4, 45, 50).
We hypothesized that inhibitor of differentiation (Id) expression, mediated by bone morphogenetic protein (BMP) signaling, may play an important role in satellite cell proliferation during postnatal muscle regeneration following injury for the reasons discussed below. Satellite cells are a heterogeneous population of adult muscle stem cells, located beneath the basal lamina of skeletal muscle fibers (4, 45, 50). In resting muscle, these quiescent mononuclear cells express the paired box transcription factor Pax7 and, in some muscle types, Pax3. On muscle injury, these Pax7+ cells become activated and begin to proliferate into fusion-competent myoblasts that have been shown to express the muscle regulatory transcription factor (MRF) Myf5 (4, 25, 45). Myoblasts are able to fuse with damaged muscle fibers, as well as themselves, to regenerate skeletal muscle in postnatal animals. Several of the signaling pathways involved in the activation and proliferation of satellite cells have been identified (25). A subpopulation of Pax7+ satellite cells that does not express Myf5 has been proposed to be responsible for repopulating the stem cell niche in the regenerated muscle and once again become quiescent (26). Recently, it has been shown that Id3 is expressed in quiescent satellite cells, and it has been proposed that it is a direct transcriptional target of Pax7, suggesting Id3 may be involved directly in the maintenance of the quiescent state (27).
The inhibitor of differentiation/DNA binding proteins consist of four family members (Id1, 2, 3, and 4) involved in the growth and differentiation of multiple cell types throughout development. The mode of action of these helix-loop-helix (HLH) proteins is to sequester and form inactive heterodimers with basic-HLH (bHLH) transcription factors of the ubiquitously expressed E class (47). This sequestering is thought to prevent the E proteins from binding with tissue-specific bHLH transcription factors, such as MyoD and Myf5, during myogenic differentiation, as the binding affinity of Id proteins appears to be higher for the E proteins than the tissue-specific bHLH proteins (47). Although overexpression of Id1 and Id3 both has been shown to inhibit the in vitro differentiation of the myogenic cell line C2C12, where they both are known to be expressed (2, 21), it is not known if either is involved in postnatal muscle regeneration in animals.
It has been reported that Id1-null and Id3-null mice show no major developmental abnormalities (12, 33). In contrast to single gene knockouts, a double knockout of Id1 and Id3 caused embryonic lethality at embryonic day E13.5 due to brain and cardiovascular defects (33) and is consistent with functional redundancy between these genes (20). Interestingly, it was found that mice carrying one wild-type (WT) allele of Id1 and null for Id3 (Id1+/−Id3−/−, subsequently referred to here as Id-mutant) were phenotypically normal but could not sustain the growth of tumor xenografts or form neovessels in Matrigel plugs due to angiogenesis defects (33). The angiogenesis defects were subsequently shown to be due to reductions in the mobilization of VEGFR2+ presumptive endothelial progenitor cells, as well as other bone marrow progenitor cell populations, and could be corrected by transplanting WT bone marrow into Id-mutant host mice (32).
We have found that Id-mutant mice have altered revascularization and greater loss of muscle mass after creation of severe hindlimb ischemia compared with WT littermates (unpublished observation). Transplantation of WT bone marrow improved limb perfusion in the Id-mutant hosts, but did not prevent the development of gangrene and tissue loss (unpublished observation). These observations led us to hypothesize that Id1 and/or Id3 genes have additional critical functions in peripheral tissues required for skeletal muscle regeneration. The proposal that Id3 mediates quiescence in Pax7+ cells (27) also supported this notion that Id genes may function during skeletal muscle regeneration. Therefore, we have examined the effect of skeletal muscle injury on Id1 and Id3 expression and the role of Id gene expression on muscle regeneration in adult mice using a nonischemic model of skeletal muscle injury produced by cardiotoxin injection. Herein we report upregulation of Id1 and Id3 protein expression in muscle satellite cells, mediated by BMP signaling following muscle injury, and show that Id-mutant mice, but not Id3-null mice, have reduced numbers of proliferating Pax7+ satellite cells after injury.
EXPERIMENTAL PROCEDURES
Animals.
WT C57BL/6, Id-mutant (Id1+/−Id3−/−), Id3-null (Id1+/+Id3−/−), and littermate control mice (Id1+/+Id3+/+) (kind gift of R. Benezra), 8–16 wk of age, were used in this study (33). Mice were housed in an environmentally controlled room in a restricted access barrier facility and fed chow and water. PCR primers for genotyping of mice were described before.
Cardiotoxin muscle injury.
Mice were anesthetized using 2% isoflurane inhalation, delivered under a constant oxygen flow rate of 1 l/min. Left tibialis anterior (TA) muscles were injected at three positions, with a total of 0.03 ml of 0.5 mg/ml cardiotoxin from Naja mossambica mossambica in PBS using a 28-gauge needle (Sigma); uninjected right TA muscle served as control tissue. Some mice were coinjected with 0.03 ml of 0.5 mg/ml cardiotoxin containing 1.5 μg of recombinant mouse Noggin (Peprotech) also in PBS. All protocols were approved in accordance with the Committee on Animal Research at the University of California, San Francisco.
Tissue and lysate preparation.
Mice were killed by CO2 inhalation followed by cervical dislocation. The TA from the right and left sides was harvested and weighed for assessment of muscle mass. Middle sections of muscle were frozen on dry ice/isopentane in Tissue-Tek optimal cutting temperature compound for immunostaining analysis, or sections were frozen in liquid nitrogen and stored at −80°C for later use. TA muscle lysates were prepared in ice-cold RIPA buffer (1% Triton X-100, 1% deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.05 M Tris·HCl, 1 mM EDTA, pH 7.0) containing 1 mM PMSF using a Dounce homogenizer. Protein concentrations in the lysates were determined using the bicinchoninic acid assay, with bovine serum albumin as the standard (Pierce). C2C12 cell lysates were prepared in the same manner. Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes using the NUPAGE system (Invitrogen). Western blotting was carried out using standard procedures. Briefly, after the membranes were blocked in 5% dry milk in Tris-buffered saline, pH 7.5, they were incubated overnight in primary antibodies at 4°C. The ECL+ system (GE Healthcare) was used to detect horseradish peroxidase (HRP)-conjugated secondary antibodies.
Cell culture.
C2C12 mouse myoblasts were obtained from the American Type Culture Collection and grown in growth media that consisted of Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin and maintained at 37°C with 5% CO2. Differentiation media (DM) were used to induce myogenic differentiation and consisted of DMEM containing 2% horse serum, 100 U/ml penicillin G, and 100 μg/ml streptomycin as cells reached ∼80% confluence. Media were changed every other day. In some experiments, 50 ng/ml of BMP-2 or BMP-4 was included in the DM. Various concentrations of Noggin were added to growth media in other experiments. For heparin-agarose (Sigma) pull-down studies, 10 ml of conditioned-DM were collected every day, and media were changed every other day and centrifuged at 3,000 g for 5 min to remove cell debris before being stored at 4°C.
Antibodies and proteins.
Rabbit monoclonal antibodies to Id1 (37–2) and Id3 (17–3) were used in Western blotting and immunofluorescence (IF) microscopy (Biocheck, Foster City, CA). Rabbit polyclonal antibodies were as follows: anti-hypoxia-inducible factor-1α (Novus), anti-VEGF, anti-MyoD (Santa Cruz Biotechnology), anti-phospho-Smad1/5/8 (pSmad1/5/8), anti-Smad1, anti-Smad5 (Cell Signaling), and anti-Ki67 (Novocastra). The goat polyclonal anti-sera to BMP receptor type II (BMPR-II) was from Santa Cruz Biotechnology. A rat monoclonal antibody to laminin B2 was from Chemicon. Mouse monoclonal antibodies were as follows: anti-β-actin, anti-BMP-4 (Santa Cruz Biotechnology), anti-Pax7, anti-myogenin, and anti-myosin heavy chain (MF20). (These monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the National Institute of Child Health and Human Development, and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.) Recombinant mouse Noggin, human BMP-2, and human BMP-4 were purchased from Peprotech.
IF microscopy.
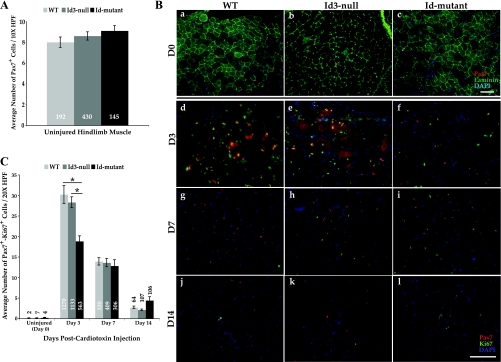
IF and hematoxylin and eosin (H&E) staining were performed on 8- to 10-μm-thick frozen muscle sections stored at −80°C. Three separate areas at least 60 μm apart were stained per muscle to provide a representative sample. For indirect IF, tissue sections were fixed in 4% paraformaldehyde at 4°C overnight and washed in PBS. Sections underwent heat-induced antigen retrieval in 10 mM sodium citrate and 0.05% Tween 20, pH 6.0. Endogenous peroxidases were quenched with 3% hydrogen peroxide in methanol for 10 min at room temperature before sections were blocked using the M.O.M. kit, as instructed (Vector Laboratories). Primary antibodies were diluted in PBS and incubated on sections at 4°C overnight in a humidified container. After washing in PBS, sections were incubated with secondary antibodies labeled with biotin (Vector Laboratories) and/or HRP (Santa Cruz Biotechnology) for 10 min at room temperature. Slides were next incubated for 10 min at room temperature with strepavidin-conjugated fluorochromes. Finally, sections were incubated with cyanine 3-TSA reagent for 5 min at room temperature to detect HRP-conjugated secondary antibodies (Perkin Elmer) and then mounted in vectashield medium containing 4,6-diamidino-2-phenylindole (Vector Laboratories). To stain for pSmad1/5/8, Cy3-labeled secondary antibodies were directly applied to sections without HRP or biotin-strepavidin amplification for 10 min at room temperature. For confocal images, a Zeiss LSM 510 microscope (UCSF Cancer Center) was used to obtain optical sections of 0.8 μm in thickness. Other IF images were obtained using a Zeiss Axioscope 2 Plus fluorescence microscope. A ×10 or ×20 objective lens was used to obtain the images shown in Fig. 5B. The area represented by the ×10 objective is 0.32 mm2 in Fig. 5B, a–c, and is 0.12 mm2 for the ×20 objective in Fig. 5B, d–l, per high-power field. For quantifying the numbers of Pax7+ and Pax7-Ki67+ cells per ×10 or ×20 high-power field, at least 5–10 photomicrographs were taken per mouse muscle section in areas showing the highest levels of cardiotoxin-induced damage before positive cells were counted.
Fig. 5.
Id-mutants appear to harbor a satellite cell proliferation defect after cardiotoxin-induced skeletal muscle injury. A: indirect immunofluorescence was used to stain Pax7+ satellite cells and laminin in uninjured TA muscle from WT (light shaded bars), Id3-null (medium shaded bars), and Id-mutant (dark shaded bars) mice. Photomicrographs (as in Ba–c) were taken with a ×10 objective, and the number of Pax7+ cells was quantified per high power field (×10 HPF = 0.32 mm2). The total number of Pax7+ cells quantified per group is shown in white numbers inside each bar. Error bars represent SE. B: uninjured TA muscle (a–c, ×10 fields) and cardiotoxin-damaged TA muscle (d–l, ×20 fields = 0.12 mm2) at D3 (d–f), D7 (g–i), and D14 (j–l) postinjury from WT (left), Id3-nulls (middle), and Id-mutants (right) were costained for Pax7 (red), laminin (a–c), or Ki67 (d–l; green) and DAPI (blue). Bars = 100 μm. C: quantification of the number of double-positive Pax7-Ki67 cells in uninjured TA muscle (D0) and at D3, D7, and D14 post-cardiotoxin injury from WT littermates (light shaded bars), Id3-nulls (medium shaded bars), and Id-mutants (dark shaded bars). D0: WT, n = 3; Id3-null, n = 5; Id-mutant, n = 3; D3: WT, n = 5; Id3-null, n = 4; Id-mutant, n = 3; D7: WT, n = 3; Id3-null, n = 3; Id-mutant, n = 3; D14: WT, n = 3; Id3-null, n = 5; Id-mutant, n = 3 (ANOVA). *P < 0.001. The total number of double-positive cells counted per group is shown inside or above each bar. Error bars represent SE.
Morphometric analysis.
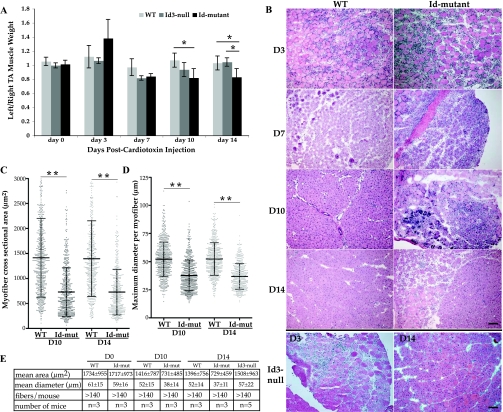
The average myofiber cross-sectional area (μm2) and average maximum myofiber diameter (μm) were determined after manually outlining individual fibers from digitally captured H&E images using NIH ImageJ 1.42q. Only regenerated fibers with prototypic centralized nuclei were measured at days 10 or 14 postcardiotoxin injury. Mature, uninjured (day 0) fibers were measured similarily and were defined as those with peripherally located nuclei. At least 140 fibers were measured per mouse, as indicated in Fig. 1E. GraphPad Prism 5 was used to display morphometric data shown in Fig. 1, C and D.
Fig. 1.
Inhibitor of differentiation (Id)-mutant mice display an altered muscle regeneration phenotype. A: ratio of cardiotoxin-injected left tibialis anterior (TA) muscle weight over uninjured right TA weight at days (D) 3, 7, 10, and 14 postinjury in wild-type (WT) littermates (light shaded bars), Id3-null mice (medium shaded bars), and Id-mutants (dark shaded bars). D0 represents control, uninjured muscle mass ratios. D0: WT, n = 4; Id3-null, n = 2; Id-mutant, n = 4; D3: WT, n = 5; Id3-null, n = 4; Id-mutant, n = 3; D7: WT, n = 3; Id3-null, n = 3; Id-mutant, n = 3; D10: WT, n = 5, Id3-null, n = 3, Id-mutant, n = 3; D14: WT, n = 5; Id3-null, n = 3; Id-mutant, n = 5 (error bars represent SD; ANOVA). *P < 0.05. B: representative hematoxylin and eosin staining of cardiotoxin-injured left TA muscle at D3, D7, D10, and D14 from WT (left) and Id-mutants (right). Representative staining of cardiotoxin-injured left TA muscle from Id3-null mice at D3 and D14 is shown as the bottom 2 panels, respectively. Bar = 100 μm. C: average regenerated myofiber cross-sectional areas (μm2) in WT and Id-mutant (Id-mut) left TA muscle at D10 and D14 postinjury. Each point represents an individual fibers area measurement, with the thick center bar being the average. The standard deviation is also indicated by the thinner bars (T-test) **P < 0.00001. D: average maximum diameter in micrometers (μm) of fibers as explained in C (T-test). **P < 0.00001. E: table showing the average myofiber cross-sectional area ± SD (mean area), average maximum myofiber diameter ± SD (mean diameter), number of fibers measured per mouse (fibers/mouse), and the number of mice used in each group from uninjured TA muscle (D0), D10, and D14 postcardiotoxin injury.
Statistical analysis.
For comparison between two groups, P values from Student's T-test < 0.05 were considered statistically significant; results are expressed as means ± SE or SD, as noted in the figure legends. For comparisons between three or more groups, one-way ANOVA with the Bonferroni post hoc correction was performed, and P values < 0.05 were considered statistically significant.
RESULTS
Muscle regeneration phenotype in Id-mutants.
Cardiotoxin was injected into the left hindlimb TA muscles of WT, Id3-null, and Id-mutant mice. Groups of mice were killed at 3, 7, 10, and 14 days postinjection. The uninjured right TA, and cardiotoxin-injected left TA, were carefully dissected and weighed (Fig. 1A). Analysis of mass ratios of the injured to uninjured TA muscle within each mouse revealed that the Id-mutants did not recover as well as either the WT littermates or Id3-null mice at days 10 and 14 postinjury (Fig. 1A). The difference between the WT and Id-mutants reached statistical significance by day 10 postinjury, and this was maintained at day 14, while the difference between the Id3-null and Id-mutants reached significance by day 14 (P < 0.05). Histology of the muscle was assessed by H&E staining at each time point for WT and Id-mutants (Fig. 1B). WT, Id3-null, and Id-mutant TA muscle showed large numbers of infiltrating and proliferating mononuclear cells at day 3 post-cardiotoxin injection (Fig. 1B, rows 1 and 2, and row 5, left panel). By day 7, the number of mononuclear cells was dramatically reduced in the WT muscle, but was still apparent in the Id-mutants (Fig. 1B, row 2). In addition, many regenerating muscle fibers with prototypic centralized nuclei were visible in both the WT and Id-mutant sections. Very few mononuclear cells were visible in the WT muscle at day 10 or 14, and the regenerating myofibers were still clearly visible (Fig. 1B, rows 3 and 4, left panels). Mononuclear cells were evident in the Id-mutants at both 10 and 14 days postinjury, although their numbers were reduced by day 14, regenerating muscle fibers were abundant (Fig. 1B, rows 3 and 4, right panels). The Id3-null muscle was similar to WT at days 7, 10 (data not shown), and 14 (Fig. 1B, row 5, right panel). To determine whether there were significant differences in the sizes of regenerated myofibers, the cross-sectional area and maximum myofiber diameters were quantified. The average myofiber cross-sectional area in Id-mutant fibers was ∼50% of WT fibers at both 10 and 14 days post-cardiotoxin injury (P < 0.00001) (Fig. 1, C and E). The average maximum myofiber diameter was also reduced ∼30% in the Id-mutant mice compared with WT at both days 10 and 14 (P < 0.00001) (Fig. 1, D and E). In contrast, the regenerated myofibers in the Id3-null mice did not show reductions in either average cross-sectional area or maximum diameter at day 14 post-cardiotoxin injury compared with WT (Fig. 1E). Therefore, based on the muscle mass recovery and histological and morphometric phenotypes, it appeared that the Id-mutant mice harbored a muscle regeneration defect compared with either Id3-null or WT mice.
Upregulation of Id1 and Id3 proteins upon muscle injury.
We hypothesized that Id1 and/or Id3 genes would be upregulated after acute muscle injury in peripheral tissues. To test this, cardiotoxin was injected into the left TA muscles of WT mice, as above. Mice were killed, and lysates were prepared from both uninjured right, and injured left, TA muscles at 6 h, and 1, 3, 7, 10, and 14 days postinjection. Equal amounts of total protein in these muscle lysates were subjected to Western blot analysis to determine Id1 and Id3 protein expression levels (Fig. 2, A and B). Both Id1 and Id3 became detectable in the injured left TA muscle lysates by day 1 post-cardiotoxin injection (Fig. 2A, panels 5 and 6 from top, respectively, mice 4–6). Neither Id1 nor Id3 were visible in the injured left TA at 6 h (mice 1–3), nor were they visible in any of the uninjured right TA lysates (Fig. 2A, mice 1–6). The expression of Id1 appeared to reach a peak at day 3 and declined rapidly thereafter (Fig. 2, B, panel 5, and C, triangles). Id3 expression also peaked at day 3, but expression persisted longer than Id1 and was reduced, but still detectable, by day 10 and was faint by day 14 postinjection (Fig. 2, B, panel 6, and C, circles). These results confirm that Id1 and Id3 proteins are undetectable in adult skeletal muscle, but are expressed following muscle injury.
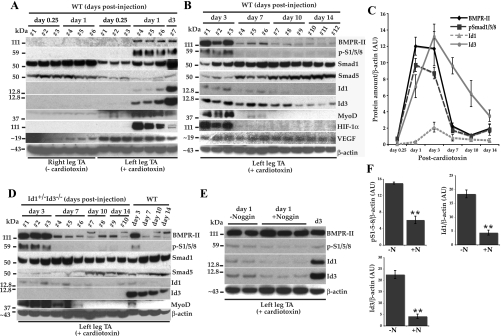
Fig. 2.
Immunoblots showing the appearance of selected proteins in the bone morphogenetic protein (BMP)-Id pathway in mouse TA muscle lysates after cardiotoxin-induced muscle injury. A: TA muscle lysates prepared from WT mice at day 0.25 (6 h) and 1 and 3 days (d3) from cardiotoxin-injected left or uninjured right tissues. The order of the blots from top to bottom is the same as B. The apparent molecular weight in kilodaltons (kDa) of makers is shown to the left of each panel. Seven WT mice are represented here. B: WT injured TA muscle lysates at D3, D7, D10, and D14 post-cardiotoxin injury. Tissue lysates from 12 mice are shown. C: densitometry of blots shown in A and B for BMP receptor type II (BMPR-II), phospho (p) Smad1/5/8, Id1, and Id3 normalized to β-actin amounts in arbitrary units (AU). Each time point represents the mean values from 3 mice ± SE. We used the values obtained from the D3 samples shown in the last lane of A to normalize the densitometric values from A to those obtained from the blots shown in B. For all 4 proteins, the densitometric values at day 0.25 were between 0 and 1. D: Id-mutant injured TA muscle lysates at D3, D7, D10, and D14 postcardiotoxin are shown along with samples from WT mice for comparison. Lysates from 10 Id-mutants are shown. E: immunoblots showing the reduction in pSmad1/5/8 (p-S1/5/8; second panel down), Id1 (third panel down), and Id3 (fourth panel down) proteins in WT left TA muscle after coinjection of cardiotoxin alone (lanes 1 and 2) or with recombinant mouse Noggin (lanes 3–5) 1 day postinjury, along with a D3 WT lysate for comparison (lane 6). F: quantification of p-S1/5/8, Id1, and Id3 protein levels by densitometry of the immunoblots shown in E. Top left graph indicates p-S1/5/8 normalized to β-actin amounts, while top right graph indicates Id1 normalized to β-actin amounts, either without Noggin (−N; left bars) or with Noggin coinjection (+N; right bar). Bottom graph indicates Id3 amounts. Error bars represent SE; T-test. **P ≤ 0.005. β-Actin served as a loading control in all immunoblots. HIF-1α, hypoxia-inducible factor-1α.
Upregulation of upstream BMP pathway proteins upon muscle injury.
The Id proteins are thought to be the downstream transcriptional targets in the BMP pathway in some cell types (19, 35). BMPs, which are part of the transforming growth factor-β superfamily, are soluble ligands that bind to tetrameric BMP receptors on the cell surface (34). The functional receptors consist of both type I and type II molecules. Ligand-receptor binding leads to the phosphorylation of the receptor-activated Smad1, 5, or 8 proteins that act as transcription factors, along with the co-Smad, Smad 4, that turn on downstream targets, such as the Id genes. Therefore, we also sought to determine whether any of the components of this pathway were also upregulated in the cardiotoxin-injured TA muscle lysates. pSmad1/5/8 became visible in the injured TA muscle by day 1, was still visible at day 3, and became undetectable on days 7, 10, and 14 postinjury (Fig. 2, A and B, panel 2, and C, squares). The BMPR-II also appeared only in the injured TA muscle lysates with a similar time course compared with the pSmad1/5/8. BMPR-II appeared at day 1, was still visible at day 3, and was reduced at days 7, 10, and 14 (Fig. 2, A and B, panel 1, and C, diamonds). Total Smad1 and Smad5 were both present in uninjured skeletal muscle lysates (Fig. 2, A and B, panels 3 and 4, respectively, lanes 1–6). Total Smad1 was reduced at 6 h after cardiotoxin injury and was recovering by days 1 and 3 and back to preinjury levels by day 7 (Fig. 2, A and B, panel 3). Total Smad5 was also reduced at 6 h postinjury, further reduced at day 1, and was returning to preinjury levels at about day 10 (Fig. 2, A and B, panel 4). VEGF, which has also been reported to induce Id gene expression in certain systems (32), was upregulated within 6 h of cardiotoxin injection (Fig. 2A, panel 9) and persisted throughout the 14 days postcardiotoxin injection (Fig. 2B, panel 9). Hypoxia inducible factor-1α became detectable by day 1, peaked at day 3, and was undetectable thereafter (Fig. 2, A and B, panel 8). Hypoxia has been reported to cause an increase in BMP-Id pathway expression in a mouse model of pulmonary hypertension in lung lysates (13). We also examined the expression of the MRF MyoD after cardiotoxin injury. MyoD became detectable at 6 h postinjury and appeared to reach maximal expression at days 1 and 3 before becoming undetectable at days 10 and 14 (Fig. 2, A and B, panel 7). In addition to the WT mice, we looked at TA muscle lysates from Id-mutant mice at days 3, 7, 10, and 14 post-cardiotoxin injection by Western blotting (Fig. 2D). Both BMPR-II and pSmad1/5/8 were highly expressed at day 3 as in the WT mice (Fig. 2D, panels 1 and 2). Total Smad1 levels were similar to WT, although total Smad5 levels appeared to be slightly reduced at day 7 compared with WT (Fig. 2D, panels 3 and 4, respectively). Id1 showed a similar expression pattern to WT, although its expression seemed to persist longer than in most WT mice, while Id3 was undetectable in these Id-mutant mice (Id1+/−Id3−/−) (Fig. 2D, panels 5 and 6). MyoD expression was apparent at both days 3 and 7 in the Id-mutant mice, suggesting a slightly prolonged expression of this transcription factor compared with WT mice (Fig. 2D, panel 7). Together, these data suggested that BMP signaling was a possible candidate pathway, leading to Id1 and Id3 upregulation after skeletal muscle damage.
Inhibition of BMP signaling in limb tissue.
To determine whether BMP signaling was causing the upregulation of Id1 and/or Id3 in the cardiotoxin-injured TA muscle, we sought to inhibit BMP signaling in the WT mouse hindlimb by coinjection of the BMP antagonist Noggin. Cardiotoxin and recombinant mouse Noggin were coinjected into the left TA muscles, and 24 h later the mice were euthanized. Western blotting of the TA muscle lysates revealed that pSmad1/5/8, Id1, and Id3 were reduced in the muscles of mice receiving Noggin compared with control mice that were injected with cardiotoxin alone (Fig. 2E, panels 2–4, respectively). Quantitation of the Western blots revealed that pSmad1/5/8 was reduced ∼2.5-fold (P = 0.005), Id1 was reduced ∼4-fold (P = 0.003), and Id3 was reduced 5-fold (P = 0.002) (Fig. 2F). Protein levels of BMPR-II were not reduced by Noggin coinjection compared with control mice (Fig. 2E, panel 1). These data suggest that BMP signaling is leading to the increase in pSmad1/5/8, Id1, and Id3 seen by 24 h after cardiotoxin-induced muscle injury.
The BMP-Id pathway in the myoblast-derived cell line C2C12.
Based on the time course of Id1 and Id3 expression that we observed in the mouse muscle lysates, we suspected that much of the protein was probably being expressed by activated and proliferating satellite cells or myoblasts. It is well established that days 2–4 after cardiotoxin-induced muscle injury are the peak of the proliferative phase of skeletal muscle regeneration (50). This is also when we observed the peak of Id1 and Id3 protein expression. Both Id1 and Id3 are also know to be expressed in the mouse myoblastic cell line, C2C12 (2, 21). These cells grow as myoblasts at low confluency in high serum and differentiate into myotubes upon reaching confluence in low serum (3). Recently, isolated mouse primary satellite cells have also been shown to express Id2, Id3, and BMP-4 transcripts (27). In addition, it is known that certain BMPs (BMP-2 and BMP-4) can inhibit the differentiation of C2C12 cells and cause them to transdifferentiate into osteoblast-like cells, away from a myogenic fate (9, 23). Multipotent primary mouse and human satellite cells also have the ability to differentiate toward a myogenic, osteoblastic, or adipogenic lineage, depending on the extracellular signals they receive (1, 41, 60). These previous BMP-related observations have made it difficult to reconcile how the Id genes might be activated in cells proceeding along a myogenic lineage, if BMP signaling leads to an osteoblastic cell fate. Another confounding observation is that it is well established that overexpression/misexpression of certain BMPs in skeletal muscle causes ectopic bone formation or heterotopic ossification (17, 22).
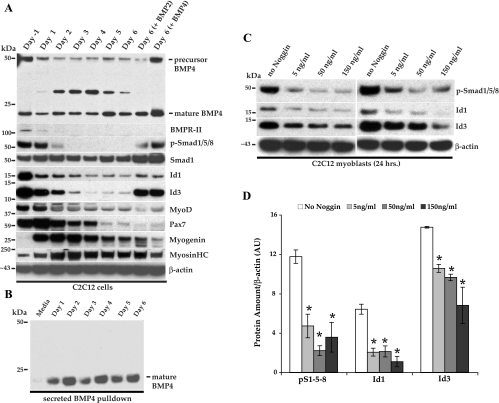
C2C12 cells were grown to near confluence in high serum and allowed to differentiate into myotubes for 6 days under low-serum conditions (Supplemental Fig. I; the online version of this article contains supplemental data) in either the presence or absence of 50 ng/ml of recombinant BMP-2 or BMP-4. Lysates were prepared and subjected to Western blotting as above (Fig. 3A). We observed that both Id1 and Id3 were most highly expressed during active proliferation under high serum conditions (Fig. 3A, day −1, panels 5 and 6). Expression of Id1 and Id3 persisted under low-serum conditions in the presence of either BMP-2 or BMP-4 (Fig. 3A, panel 5 and 6, last 2 lanes), as has been previously reported for Id1 (23), although the Id3 protein appeared to persist to a greater degree. Interestingly, we also found that BMPR-II and pSmad1/5/8 were most highly expressed during active proliferation and, subsequently, both proteins were downregulated during differentiation into myotubes (Fig. 3A, panels 2 and 3). In the presence of BMP-2 or BMP-4, BMPR-II protein still became undetectable, while pSmad1/5/8 expression persisted for the 6 days of culture in low serum (Fig. 3A, panels 2 and 3, last 2 lanes). Total Smad1 levels did not dramatically change and were present throughout the 6 days of culture (Fig. 3A, panel 4). However, addition of BMP-2 or BMP-4 to the cultures did appear to cause a slight increase in total Smad1 levels (Fig. 3A, panel 4, last 2 lanes). Our particular isolate of C2C12 cells express large amounts of the satellite cell marker and paired box transcription factor Pax7 that is lost during differentiation, as expected (Fig. 3A, panel 8). Since BMP-2 and BMP-4 are known to direct myoblasts toward an osteoblastic fate, we were not surprised that Pax7 expression was also lost in those cultures (Fig. 3A, panel 8, last 2 lanes). As expected, the cells also downregulated the myogenic bHLH transcription factors MyoD (Fig. 3A, panel 7) and upregulated myogenin (Fig. 3A, panel 9), as well as expressed increasing amounts of myosin heavy chain, a marker of terminal differentiation (Fig. 3A, panel 10). Unexpectedly, these cells expressed both the precursor and mature forms of BMP-4 in cell lysates throughout the 6 days of differentiation (Fig. 3A, panel 1). These data suggested to us that autocrine BMP-4 signaling might be responsible for the expression of Id1 and Id3 seen in C2C12 cells during active proliferation in high serum. Recently, Id3 expression has been shown to be regulated by autocrine BMP-4 signaling in human ovarian cancer cells (49). In order to determine whether mature BMP-4 was being secreted into the cell culture media, we took advantage of the heparin-binding domain in BMP-4 and performed heparin-agarose pulldown studies (Fig. 3B). C2C12 cells were grown under low-serum conditions for 6 days as above. The media were harvested every day, and changed every second day, and subjected to heparin-agarose binding overnight. Proteins binding to the agarose beads were released and separated by SDS-PAGE and subjected to Western blotting. Mature BMP-4 was secreted into the low-serum media throughout the 6-day time course (Fig. 3B). This observation is somewhat paradoxical, since exogenous BMP-4, at concentrations of >25–400 ng/ml, are well known to cause this cell line to transdifferentiate into osteoblast-like cells. We sought to determine the concentration of BMP-4 that these cells were actively secreting. We subjected known amounts of mature, recombinant BMP-4, along with our pull-down samples to Western blotting, and from this determined that C2C12 cells secreted ∼5 pg/ml of mature BMP-4 over a 24-h period (Supplemental Fig. II). To determine whether BMPs secreted by these cells were responsible for the expression of Id1 and Id3, we treated actively proliferating cells to varying concentrations of the BMP-4 antagonist Noggin overnight (Fig. 3C). All concentrations of Noggin tested caused a statistically significant reduction in the amounts of pSmad1/5/8 and both Id1 and Id3 (P < 0.05) (Fig. 3, C, panels 1, 2, and 3, respectively, and D). As the cells differentiate into myotubes, they downregulate Id1 and Id3, as well as the BMPR-II, which may be why BMP signaling through phosphorylation of Smad1/5/8 is downregulated, even though BMP-4 continues to be secreted into the media. Another possibility is that an antagonist(s) of BMP-4 is being secreted by these cells during differentiation, which could also inhibit BMP signaling and pSmad1/5/8 appearance. In support of this idea, a group recently reported that C2C12 cells upregulate Noggin RNA and protein expression in response to exogenous BMP-2 stimulation (56). This and other possibilities remain to be further clarified. However, our data clearly indicate that C2C12 myoblasts secrete mature BMP-4 that may lead to the expression of both Id1 and Id3 through phosphorylation of Smad1/5/8 and that BMP signaling can be inhibited by incubation of cells with Noggin.
Fig. 3.
Immunoblots of C2C12 cells showing selected BMP-Id pathway components during differentiation from myoblasts into myotubes. A: lysates were prepared from subconfluent cells in growth media (GM; day −1) and after D1, D2, D3, D4, D5, and D6 in differentiation media (DM). Lysates were also prepared from cells grown for 6 days in DM + 50 ng/ml BMP-2 or BMP-4 (last 2 lanes, respectively). HC, heavy chain. B: heparin-agarose was used to pull down mature BMP-4 from 10 ml of DM alone (media) or conditioned DM media on D1, D2, D3, D4, D5, and D6 postdifferentiation. Media were changed every second day and harvested every day. C: C2C12 cells were grown overnight in GM+/− various concentrations of mouse Noggin, after which lysates were prepared for blotting. The results of 2 independent experiments are shown. D: densitometric quantification of immunoblots from 3 independent experiments are shown in arbitrary units (AU). The amounts of p-S1/5/8 normalized to β-actin are shown in the first 4 bars with increasing concentrations of Noggin. The amounts of Id1 and Id3 normalized to β-actin are indicated next after incubation with the indicated amounts of Noggin. Error bars represent SE (ANOVA). *P < 0.05, comparing no Noggin group with each concentration of Noggin.
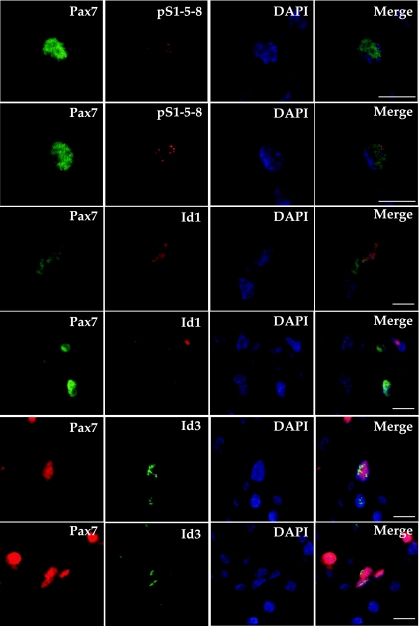
Activated Pax7+ satellite cells coexpress pSmad1/5/8, Id1, and Id3 in the mouse hindlimb.
Based on our in vitro and in vivo observations, we hypothesized that proliferating satellite cells in the WT mouse hindlimb were expressing pSmad1/5/8, Id1, and Id3 after activation caused by cardiotoxin damage. Satellite cells are well known to express the transcription factor Pax7 in both their quiescent and actively proliferating states (4, 45, 50). Pax7 expression is lost during the differentiation of myoblasts into myotubes and mature myofibers. We performed IF microscopy on TA muscle sections from WT mice at day 3 after cardiotoxin injection using Pax7 to identify myogenic cells along with antibodies to pSmad1/5/8, Id1, or Id3 (Fig. 4). Id-mutant (Id1+/−Id3−/−) mouse tissue served as a control for the specificity of the rabbit monoclonal Id3 antibody used in the indirect IF microscopy (Supplemental Fig. III). Confocal microscopy revealed that many Pax7+ cells (Fig. 4, column 1) also expressed pSmad1/5/8 (Fig. 4, column 2, top 2 panels). Many Pax7+ cells also expressed Id1 (Fig. 4, column 2, middle 2 panels), and Id3 (Fig. 4, column 2, bottom 2 panels, and Supplemental Fig. III). 4,6-Diamidino-2-phenylindole staining, along with confocal microscopy, revealed that all four antigens were expressed within the nuclei of immunoreactive cells (Fig. 4, columns 3 and 4). Quantification of coexpressing cells revealed that pSmad1/5/8 immunoreactivity was present in ∼36% of Pax7+ cells (146 of 409 Pax7+ cells), Id1 staining was visible in ∼47% of Pax7+ cells (303 of 706 Pax7+ cells), and Id3 staining was present in ∼29% of Pax7+ cells (208 of 727 Pax7+ cells) at day 3 postcardiotoxin injury. These data indicate that many activated Pax7+ satellite cells express pSmad1/5/8, Id1, and Id3 in regenerating mouse hindlimb skeletal muscle. Many of these coexpressing Pax7+ cells are located at the periphery of regenerating myofibers, while others are located in areas of more intense damage/regeneration, where few original myofibers remain intact at day 3 postinjury. Supplemental Fig. IV shows lower magnification nonconfocal micrographs to allow the visualization of Pax7+-pSmad1/5/8, -Id1, and -Id3 coexpressing cells near adjacent myofibers in areas of less intense cardiotoxin-induced damage.
Fig. 4.
Confocal micrographs of activated Pax7+ satellite cells showing coexpression of p-S1/5/8, Id1, or Id3. Cardiotoxin-damaged TA muscle sections at D3 postinjury were costained with antibodies to Pax7 (left) and either p-S1/5/8 (top 2 rows), Id1 (middle 2 rows), or Id3 (bottom 2 rows). The optical thickness represented is 0.8 μm. All 4 antigens show nuclear colocalization with the nuclear stain 4,6-diamidino-2-phenylindole (DAPI). Bars = 10 μm.
Myoblast proliferation defect in Id-mutant mice.
Because of the timing of the upregulation of Id1 and Id3 after cardiotoxin muscle damage, which peaked at about day 3, we hypothesized that these proteins might be responsible for inhibiting the differentiation of the so-called transit amplifying population of Pax7+ satellite cells, thus giving them ample time to proliferate before their fusion into myotubes and eventual terminal differentiation into regenerated skeletal muscle fibers. If our hypothesis was correct, we thought that the loss of Id1 and/or Id3 in the Pax7+ cells of our Id-mutant mice (Id1+/−Id3−/−) would lead to alterations in their proliferative ability and could potentially be responsible, in part, for the muscle recovery phenotype we had observed. We first determined whether the Id-mutant mice harbored reduced numbers of quiescent satellite cells by IF staining for Pax7 and laminin in undamaged TA hindlimb skeletal muscle (Fig. 5B, a–c). After counting the number of Pax7+ cells on multiple micrographs from many mice, we determined that there was no detectable difference in the number of quiescent Pax7+ satellite cells comparing the WT, Id3-null, and Id-mutant groups (Fig. 5, A and B, a–c). We then stained cardiotoxin-damaged TA muscle sections from day 3, 7, and 14 postinjection (Fig. 5, B, d–l, and C). Sections were costained with antibodies to Pax7 and the proliferation marker Ki67. Immunofluoresence microscopy photographs were taken of the damaged muscle sections at each time point, and the number of double-positive (Pax7+-Ki67+) cells was determined from multiple sections derived from many animals (Fig. 5, B and C). We found that there was a statistically significant reduction (P < 0.001) in the number of proliferating satellite cells in the Id-mutants (n = 5) compared with either WT littermates (n = 5) or Id3-null mice (n = 4) at day 3 post-cardiotoxin injection (Fig. 5, B and C). The average number of proliferating satellite cells was reduced by ∼40% at day 3 in the Id-mutant muscle compared with either the WT littermates or Id3-null mice (Fig. 5C). The differences in the numbers of double-positive cells at days 7 and 14 were not statistically significant among the three groups of mice (Fig. 5C). To determine whether another type of hindlimb muscle injury would elicit a similar phenotype, we also performed Pax7 staining on WT and Id-mutant TA muscle tissue that was derived from mice that had undergone a severe ischemic injury produced by femoral artery excision (FAE) (Supplemental Fig. V). We observed a significant reduction in the numbers of Pax7+ cells at day 5 post-ischemic injury in the Id-mutant tissue compared with WT controls, similar to the reduction seen at day 3 after the cardiotoxin injury (Supplemental Fig. V). These data indicate that Id3 is not necessary to maintain quiescent Pax7+ satellite cells in adult postnatal skeletal muscle, since the Id3-null mice and the Id-mutant mice (Id1+/−Id3−/−) harbored equal numbers compared with WT mice in the undamaged TA muscle sections. However, there is a significant reduction in the number of proliferating Pax7+ cells in the Id-mutant mouse tissue after muscle damage induced by cardiotoxin injection compared with either Id3-null or WT mouse tissue.
DISCUSSION
In this study, we have shown that Id-mutant mice, unlike Id3-null mice, display a muscle regeneration phenotype using the cardiotoxin-induced TA muscle regeneration paradigm. The Id1 and Id3 proteins were markedly upregulated in the injured skeletal muscle of WT mice within 24 h, as were BMPR-II and pSmad1/5/8. Hindlimb injection of the BMP antagonist Noggin reduced the amounts of pSmad1/5/8 and both Id1 and Id3 at 24 h after cardiotoxin injury, suggesting that BMP signaling was responsible for their expression. These proteins were also expressed in the satellite cell-derived myogenic cell line C2C12 (3) when actively proliferating as myoblasts, but were all reduced on differentiation into myotubes. Immunofluorescent microscopy revealed that pSmad1/5/8, Id1, and Id3 were all detectable in the nuclei of many Pax7+ myoblasts in the injured mouse hindlimb at day 3 post-cardiotoxin injection. Finally, we showed that the Id-mutant mice, but not Id3-null mice, displayed a reduced number of proliferating Pax7+ myoblasts after injury, suggesting that Id1 and Id3 may be necessary during the transit amplification stage of satellite cell growth. Our results, however, do not rule out the possibility that Id1 and/or Id3 might also be expressed in the relatively small population of quiescent Pax7+ stem cells in undamaged muscle. Proteins derived from those cells may simply be below the limit of detection in the immunblots used here. In support of this possibility, a recent study has found that highly purified, flow cytometry sorted, presumably quiescent, satellite cells from mice expressed Id3 but not Id1 mRNA immediately after isolation (27). These authors went on to show that Id3 is a direct transcriptional target of Pax7 in satellite cells (27). Somewhat surprisingly, we found that the numbers of quiescent Pax7+ cells in undamaged hindlimb muscle from both adult Id3-null and Id-mutant mice were not reduced compared with adult WT muscle. Surprisingly, recent work using conditional gene inactivation and lineage tracing techniques have shown that expression of Pax7 is not necessary in adult satellite cells, where it can be inactivated without affecting in vivo muscle regeneration (29). These authors also showed that Pax7 expression was only necessary up to the juvenile stage when satellite cells become quiescent (29) and helps clarify previously published contradictory results (24, 40). Given these recent observations, it may not be totally surprising that we saw no reductions in the numbers of quiescent satellite cells in the Id3-null and Id-mutant mouse hindlimb muscle. Our data suggest that Id1/Id3 are involved in the amplification/proliferation of adult satellite cells and not their quiescence.
We had initially observed the increased loss of muscle mass in the Id-mutant mice in a model of severe acute tissue ischemia created by surgical FAE (unpublished observation). Tissue recovery from the FAE ischemia model is more complex than that from the cardiotoxin injury model, because it involves coordinated recruitment and enlargement of collateral arteries (arteriogenesis), in addition to regeneration of injured muscle tissue, and this requires a more extensive recovery period. In this model, we also observed the upregulation of Id1, Id3, and pSmad1/5/8 proteins in WT mouse skeletal muscle within 5 days after ischemic injury that persisted for at least 2 wk (J. L. Clever, unpublished observation). The cardiotoxin-injection studies were undertaken to determine whether this putative BMP-Id pathway also appeared in another skeletal muscle injury model. Both injury models are known to require local angiogenesis to restore proper tissue perfusion. This appears to require resident endothelial, inflammatory, other bone marrow-derived progenitors, and resident muscle progenitor cells (4, 5, 48, 54, 55, 57). In other unpublished observations, we had found that, even after transplantation of WT bone marrow into Id-mutant hosts, which significantly improved hindlimb perfusion after FAE, about one-third of chimeric mice continued to develop gangrene. These observations suggested to us that processes other than local angiogenesis were defective in the Id-mutant mice, since restoration of bone marrow progenitor function did not improve tissue loss rates (unpublished observation). Together, these observations lead to the studies reported here to examine skeletal muscle satellite cells in the Id-mutant mice. Because we observed the upregulation of components of the BMP-Id pathway in both injury models, we conclude that these proteins are induced and expressed in the hindlimb skeletal muscles after either a severe ischemic (unpublished observation) or a nonischemic cardiotoxin-induced injury. These data suggest that this is a generalized response to muscle injury, consistent with injury-induced activation and expansion of satellite cells.
However, the data reported here do not rule out other possible mechanisms that might contribute to the muscle regeneration phenotype we have seen in the Id-mutant mice. These may include alterations in the inflammatory milieu in the Id-mutant muscle tissue, as shown in Fig. 1B. Mononuclear cell infiltrates appeared to persist longer in the regenerating muscle of the Id-mutant mice compared with either Id3-null or WT mice. In fact, Id3-null mice have been reported to have defects in both B- and T-lymphocyte proliferation and maturation, respectively (18, 30, 42, 44, 53). About 10% of Id3-null mice between 6 and 10 mo of age harbor lymphocytic infiltrates in lachrymal and salivary glands, a pathological hallmark of Sjogren's syndrome, a human autoimmune disease (30). The frequency of the pathology appears to increase with the age of the mice and includes the development of auto-antibodies (30). However, these pathological abnormalities were reported to be relatively restricted to these two organ systems (30). Studies with CC chemokine receptor 2 knockout mice (CCR2−/−) have shown that bone marrow-derived cells contribute to hindlimb skeletal muscle regeneration (8, 39, 54). After muscle injury, the CCR2−/− mice displayed an altered inflammatory response that included decreased macrophage and bone marrow-derived cell accumulation (8, 54). In addition, they harbored reductions in myofiber size that were similar to the reductions we have observed in the Id-mutant mice. However, the CCR2−/− mice had significantly increased numbers of myogenic progenitor cells in the injured skeletal muscle at days 3 and 7 post-cardiotoxin injury, while we have seen significantly fewer proliferating myogenic progenitors at day 3 in the Id-mutant muscle (Fig. 5) (54). This difference suggests that we are not observing the same phenomenon as in the CCR2−/− mouse model of muscle regeneration.
Another possibility is that the Id1 and/or Id3 genes may be involved in the growth/proliferation of tissue resident endothelial and smooth muscle cells (10, 11, 38, 59). This could also contribute to muscle regeneration defects in the Id-mutant mice. In fact, we have also observed that Id1 is upregulated in endothelial cells of arteries, veins, and capillaries in the FAE model by IF microscopy, while Id3 staining is very apparent in vascular smooth muscle cells of remodeling small arteries (J. L. Clever, unpublished observation). We believe that, after cardiotoxin injection, the majority of the Id3 signal we are seeing by Western blotting is coming from the expanding population of Pax7+ myoblasts, since cardiotoxin injection does not cause extensive large-vessel vascular injuries. Some of the Id1 protein signal in the Western blotting of cardiotoxin-injected muscle may originate from proliferating endothelial cells of the regenerating capillary beds. However, by IF microscopy, we see about one-half (47%) of Pax7+ cells expressing detectable Id1, as well. Whether Id1 protein expression in capillary endothelial cells results from BMP signaling remains to be determined. The cross talk between proliferating endothelial cells and satellite cells may be extensive, based on their proximity in regenerating muscle, but little is known about these interactions (7). We cannot rule out the possibility that resident endothelial cell dysfunction might contribute, in part, to the muscle regeneration defect in the Id-mutants.
Based on our observations that Id1 and Id3 expression can be reduced in C2C12 myoblasts with the BMP antagonist Noggin, and that these cells secrete mature BMP-4, it is tempting to speculate that BMP-4 may be responsible for Id1 and Id3 expression in the mouse muscle as well. However, this remains to be determined, because it has proven difficult to detect BMP-4 in mouse muscle lysates. However, we have presented evidence that BMP signaling is responsible for the upregulation of Id1 and Id3 in the mouse hindlimb by Noggin injection at the time of cardiotoxin injury (Fig. 2, E and F). The determination of the exact BMPs responsible for Id1 and Id3 upregulation in skeletal muscle awaits further studies. Interestingly, the group that recently reported that Id3 was expressed in flow cytometry purified mouse satellite cells also found that BMP-4 mRNA was expressed in these cells (27).
The exact mode of action of Id1 and Id3 in proliferating myoblasts is not clear. The Id proteins interact with the E class of bHLH transcription factors and prevent them from binding with the tissue-specific bHLH MRFs MyoD, Myf5, Mrf4, and myogenin (47). Although the Id proteins have a high affinity for the E-class transcription factors, it has also been reported that Id1 has a relatively high affinity for MyoD and Myf5, while Id3 has a weak affinity for all MRF proteins in a yeast two-hybrid system (28). Therefore, the Id proteins probably exert their effects through a complex interaction/interchanging of transcription factor binding during the course of muscle regeneration in vivo. However, there is little data concerning these interactions in animal tissues. Interestingly, adult Myf5-null mice have been reported to display a muscle regeneration defect that is quite similar to what we are describing here in the Id-mutant mice (16). The Myf5-null mice were reported to have a reduced number of myoblasts at days 3 and 4 after muscle injury, similar to what we are seeing at day 3 post-cardiotoxin injury. Another group also recently reported that Myf5 is involved in the transient amplification of myoblasts (58).
It may seem somewhat surprising that BMP signaling is involved in skeletal muscle regeneration. However, it is notable that BMP signaling is known to be involved in embryonic skeletal muscle formation. Noggin has been reported to act as an inducer of MRF expression, while BMP-4 inhibits their expression in the dermomyotome of the developing embryo (6, 43, 50). Interestingly, another recent report has provided evidence that BMP-4 regulates the proliferation of fetal myogenic progenitors during human development (14). In addition, BMP signaling has been shown to be an important regulator of proliferation in both the adult intestinal- and hair follicle stem cell systems (reviewed in Ref. 36). However, ectopic expression of BMP-4 in skeletal muscle is known to cause heterotopic ossification, and it is also well established that exogenous BMP-2 and BMP-4 cause C2C12 and primary satellite cells to transdifferentiate into an osteoblastic lineage (1, 17, 22, 23, 41, 60). We have shown here that the concentration of BMP-4 secreted by C2C12 myoblasts is quite low compared with the amounts that are routinely used in transdifferentiation studies that are thousands of times higher. In addition, when we added high concentrations of BMP-2 or BMP-4 (50 ng/ml) to C2C12 cells for 6 days in low serum, we found that the BMPR-II still became undetectable by immunoblotting, suggesting that the signaling may be occurring through an alternate receptor. This may lead the cells to acquire an osteoblastic cell fate. Resolution of these possibilities awaits further studies. Therefore, we do not believe that our results are at odds with these previous observations. Our data suggest that the BMP-Id pathway is important for postnatal muscle regeneration and indicate that BMP signaling after muscle injury is tightly, temporally regulated and coincides with the peak proliferative phase of muscle regeneration, which is known to be maximal between days 2 and 4 after cardiotoxin injury (6, 50).
The results presented here may have relevance to the rare, disabling genetic disease fibrodysplasia ossificans progressive, which is characterized by progressive heterotopic bone formation in muscle tissue. A single amino acid change in ALK2, a type I BMP receptor, is responsible for this condition, along with an inflammatory milieu, and appears to produce a constitutively active receptor (15, 52). The cell types that express this mutant receptor, which lead to development of fibrodysplasia ossificans progressive, are under intense investigation and have recently been reported to include vascular progenitors identified by Tie2-Cre lineage tracing methods, but not skeletal muscle stem cells identified by MyoD-Cre expression (31). Another group has recently reported that total Smad1 and Smad5 are both markedly upregulated at day 3, and to a lesser extent day 7, after habu venom-induced mouse muscle injury, and can act together with the mutant ALK2 to induce osteoblastic differentiation in C2C12 cells (15). Although we found marked upregulation of pSmad1/5/8 at day 1 and day 3 post-cardiotoxin injection, we found that total Smad1 and Smad5 were both reduced at 6 h, day 1, and day 3 and were already robustly expressed in undamaged hindlimb skeletal muscle of WT mice (Fig. 2, A and B). The reasons for these conflicting results on total Smad1 and Smad5 in undamaged vs. damaged skeletal muscle are unclear, although we used different antibodies and a different toxin than Fukuda et al. (15).
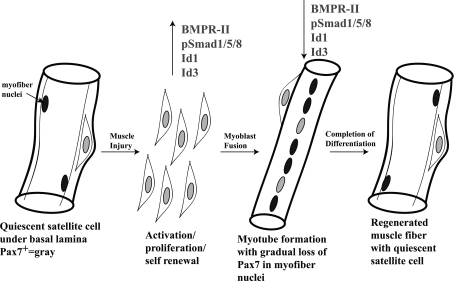
In conclusion, our results suggest a model in which BMP signaling is responsible for Id1 and Id3 expression in damaged mouse skeletal muscle, particularly in proliferating myoblasts (Fig. 6). We believe that Id1 and Id3 expression allows for the proper proliferation and expansion of the activated satellite cell pool before their fusion into myotubes and terminal differentiation into skeletal muscle (Fig. 6). We believe that the lack of proper Pax7+ cell proliferation in the Id-mutant mice led, at least in part, to the reduced or delayed muscle regeneration we observed in vivo and suggests a hitherto unappreciated role of BMP signaling during adult muscle regeneration.
Fig. 6.
Model of skeletal muscle regeneration showing when BMP-Id pathway-associated protein expression peaks during the satellite cell activation/proliferation phase. p-S1/5/8, Id1, and Id3 were observed in activated Pax7+ cell nuclei by immunofluorescence microscopy. BMPR-II, p-S1/5/8, Id1, and Id3 expression were subsequently reduced during terminal skeletal muscle differentiation in immunoblots. Id-mutants (Id1+/−Id3−/−) had less proliferating Pax7+ cells than WT mice at D3 post-cardiotoxin injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant K08 HL076390, a grant from the Lifeline foundation, and the Foundation for Accelerated Vascular Research (Formerly the Pacific Vascular Research Foundation) (to D. B. Schneider).
DISCLOSURES
The authors declare no competing financial interests.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. John Chen of Biocheck (Foster City, CA) for supplying us with the rabbit monoclonal anti-Id1 and -Id3 antibodies and for helpful discussions about their use. We also thank Korey Griffin of Santa Cruz Biotechnology for supplying us with many sample antibodies for testing.
REFERENCES
- 1.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Atherton GT, Travers H, Deed R, Norton JD. Regulation of cell differentiation in C2C12 myoblasts by the Id3 helix-loop-helix protein. Cell Growth Differ 7: 1059–1066, 1996 [PubMed] [Google Scholar]
- 3.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science 230: 758–766, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Buckingham M, Montarras D. Skeletal muscle stem cells. Curr Opin Genet Dev 18: 330–336, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Carlson BM, Faulkner JA. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc 15: 187–198, 1983 [PubMed] [Google Scholar]
- 6.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol 292: C953–C967, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development 130: 6089–6099, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Forrest S, McNamara C. Id family of transcription factors and vascular lesion formation. Arterioscler Thromb Vasc Biol 24: 2014–2020, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Forrest ST, Taylor AM, Sarembock IJ, Perlegas D, McNamara CA. Phosphorylation regulates Id3 function in vascular smooth muscle cells. Circ Res 95: 557–559, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science 306: 247–252, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank DB, Abtahi A, Yamaguchi DJ, Manning S, Shyr Y, Pozzi A, Baldwin HS, Johnson JE, de Caestecker MP. Bone morphogenetic protein 4 promotes pulmonary vascular remodeling in hypoxic pulmonary hypertension. Circ Res 97: 496–504, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, Ramoni MF, Frank MH, Kohane IS, Gussoni E. Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin. J Cell Biol 175: 99–110, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284: 7149–7156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 312: 13–28, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gonda K, Nakaoka T, Yoshimura K, Otawara-Hamamoto Y, Harrii K. Heterotopic ossification of degenerating rat skeletal muscle induced by adenovirus-mediated transfer of bone morphogenetic protein-2 gene. J Bone Miner Res 15: 1056–1065, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren's syndrome in Id3 knockout mice. Immunology 122: 73–79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274: 19838–19845, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Jen Y, Manova K, Benezra R. Each member of the Id gene family exhibits a unique expression pattern in mouse gastrulation and neurogenesis. Dev Dyn 208: 92–106, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev 6: 1466–1479, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Kaplan FS, Fiori JLSDLP, Ahn J, Billings PC, Shore EM. Dysregulation of the BMP-4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci 1068: 54–65, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127: 1755–1766, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172: 103–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2: 22–31, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129: 999–1010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar D, Shadrach JL, Wagers AJ, Lassar AB. Id3 is a direct transcriptional target of Pax7 in quiescent satellite cells. Mol Biol Cell 20: 3170–3177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langlands K, Yin X, Anand G, Prochownik EV. Differential interactions of Id proteins with basic-helix-loop-helix transcription factors. J Biol Chem 272: 19785–19793, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460: 627–631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren's syndrome. Immunity 21: 551–560, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am 91: 652–663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7: 1194–1201, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401: 670–677, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 1: 169–178, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE 2002: PE40, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Moore KA, Lemischka IR. Stem cells and their niches. Science 311: 1880–1885, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol 26: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama K, Takaji K, Kataoka K, Kurihara Y, Yoshimura M, Kato A, Ogawa H, Kurihara H. Id1 gene transfer confers angiogenic property on fully differentiated endothelial cells and contributes to therapeutic angiogenesis. Circulation 112: 2840–2850, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, Shireman PK. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–R661, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23: 3430–3439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. Alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res 312: 4162–4180, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan L, Sato S, Frederick JP, Sun XH, Zhuang Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol 19: 5969–5980, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reshef R, Maroto M, Lassar AB. Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev 12: 290–303, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera RR, Johns CP, Quan J, Johnson RS, Murre C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity 12: 17–26, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, Nerlov C. A CREB-C/EBP beta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A 106: 17475–17480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 13: 410–418, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol 292: H1–H18, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene 414: 95–105, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev 20: 1692–1708, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Sugai M, Gonda H, Nambu Y, Yokota Y, Shimizu A. Role of Id proteins in B lymphocyte activation: new insights from knockout mouse studies. J Mol Med 82: 592–599, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takakura N. Role of hematopoietic lineage cells as accessory components in blood vessel formation. Cancer Sci 97: 568–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takayama K, Suzuki A, Manaka T, Taguchi S, Hashimoto Y, Imai Y, Wakitani S, Takaoka K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab 27: 402–411, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Ustanina S, Carvajal J, Rigby P, Braun T. The myogenic factor Myf5 supports efficient skeletal muscle regeneration by enabling transient myoblast amplification. Stem Cells 25: 2006–2016, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 106: 2263–2270, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development 129: 2987–2995, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.